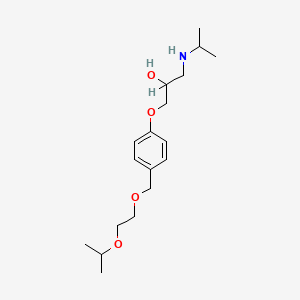
Bisoprolol
Übersicht
Beschreibung
Bisoprolol is a highly selective β₁-adrenoceptor antagonist widely used in managing hypertension, chronic heart failure (HF), and angina pectoris. Its β₁-selectivity, pharmacokinetic stability, and favorable safety profile distinguish it from other beta-blockers. This article provides a detailed comparison of this compound with similar compounds, focusing on pharmacological properties, clinical efficacy, pharmacokinetics, and safety.
Vorbereitungsmethoden
Conventional Synthesis Framework
Historical Context and Initial Methodologies
The foundational synthesis of bisoprolol, as described in BE859425 and US4258062, involves three sequential stages:
-
Etherification of 4-hydroxybenzyl alcohol with 2-isopropoxyethanol using protonic acids or cationic resins
-
Epoxidation with epichlorohydrin to form 2-[[4-(2-isopropoxyethoxy)methyl]phenoxymethyl]oxirane
-
Amination with isopropylamine to yield this compound base, followed by fumarate salt formation
This route suffered from low overall yields (15–27%) due to multiple high-vacuum distillations required to purify liquid intermediates . For instance, the epoxidation step generated 10–15% residual 1-[p-(2-isopropoxyethoxy)methyl]phenoxy]-3-chloro-propan-2-ol, necessitating energy-intensive purification .
Novel Industrial Synthesis Routes
Amberlyst-15 Catalyzed Process (WO2007069266A2)
This patent describes a streamlined method emphasizing impurity control at each stage:
Intermediate I: 4-[(2-Isopropoxyethoxy)methyl]phenol Synthesis
-
Reagents : 4-Hydroxybenzyl alcohol, 2-isopropoxyethanol, Amberlyst-15 resin
-
Conditions : 0–5°C for 2 h → 15–20°C for 10 h
-
Purification : Toluene/water partitioning removes dimer impurities (2–3% → <0.5%)
Intermediate II: 2-[[4-(2-Isopropoxyethoxy)methyl]phenoxymethyl]oxirane
-
Epoxidation : Sodium hydroxide-mediated reaction with epichlorohydrin
-
Key Improvement : In-situ monitoring reduces chloro-propanol residuals from 15% to <1%
-
Distillation : Eliminated via aqueous extraction (CHCl₃/water)
Final Amination and Salt Formation
-
Amination : Reflux with isopropylamine in methanol (3 h)
-
Chromatography : Neutral alumina filtration traps 4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]benzenemethanol
Oxazolidinone-Based Route (CN103664657A)
Chinese Patent CN103664657A circumvents traditional epoxidation through a novel heterocyclic intermediate:
Intermediate III: 5-[4-(2-Isopropoxyethoxymethyl)-phenoxy]-3-isopropyloxazolidin-2-one
-
Coupling Reaction : Toluene-4-sulfonic acid ester + 4-(2-isopropoxyethoxymethyl)phenol
-
Catalyst : K₂CO₃ in DMF at 80°C (12 h)
-
Yield : 89% after extraction (vs. 72% in conventional method)
Ring-Opening and Salt Formation
-
Hydrolysis : HCl/ethanol at 60°C (4 h) releases this compound free base
-
Fumarate Crystallization : Ethanol/acetone system achieves 93% yield (Example 8)
Comparative Analysis of Methodologies
Critical Process Optimization Strategies
Catalytic System Design
-
Amberlyst-15 vs. Homogeneous Acids : The macroreticular resin enables 98% conversion at 20°C versus 85% with H₂SO₄ at 50°C, minimizing thermal degradation
-
Alkali Metal Carbonates : K₂CO₃ in DMF increases coupling reaction rates by 3× compared to NaHCO₃ (TOF 0.42 vs. 0.15 min⁻¹)
Impurity Control Mechanisms
-
Dimer Suppression : Toluene/water partitioning reduces 4-hydroxybenzyl alcohol dimers from 2.3% to 0.4%
-
Chromatographic Trapping : Neutral alumina beds adsorb 92% of benzenemethanol impurities during amination
-
Crystallization Optimization : Acetone-mediated fumarate recrystallization lowers aldehyde content from 0.15% to 0.06%
Analyse Chemischer Reaktionen
Bisoprolol unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann zu verschiedenen Metaboliten oxidiert werden.
Reduktion: Reduktionsreaktionen sind weniger häufig, können aber unter bestimmten Bedingungen auftreten.
Substitution: this compound kann Substitutionsreaktionen eingehen, insbesondere unter Beteiligung seiner phenolischen und Aminogruppen.
Häufig verwendete Reagenzien in diesen Reaktionen sind Oxidationsmittel wie Wasserstoffperoxid und Reduktionsmittel wie Natriumborhydrid. Die Hauptprodukte, die aus diesen Reaktionen entstehen, sind typischerweise Metaboliten, die entweder ausgeschieden oder im Körper weiter verstoffwechselt werden .
Wissenschaftliche Forschungsanwendungen
Pharmaceutical Applications
1.1 Drug Delivery Systems
The amphiphilic nature of 2-Methylene-beta-alanine allows it to form micelles and liposomes, which are effective in encapsulating hydrophobic drugs. This enhances the solubility and bioavailability of these compounds, making them more effective in therapeutic applications. The ability to modify drug release profiles through the use of this compound can lead to improved patient outcomes in various treatments.
1.2 Antioxidant Properties
While 2-Methylene-beta-alanine itself does not exhibit antioxidant properties, it contributes to increased levels of carnosine in tissues. Carnosine is known for its ability to scavenge reactive oxygen species (ROS), thereby potentially mitigating oxidative stress and enhancing cellular protection against damage caused by free radicals.
Sports Nutrition
2.1 Performance Enhancement
Research indicates that supplementation with beta-alanine can enhance athletic performance by increasing muscle carnosine content. This results in improved buffering capacity during high-intensity exercise, which can delay fatigue and enhance endurance. A study on recreationally trained men demonstrated that a sustained-release formulation of beta-alanine improved performance metrics and reduced symptoms of paraesthesia commonly associated with beta-alanine supplementation .
2.2 Case Study: Equestrian Performance
In a study involving Yili horses participating in speed racing, beta-alanine supplementation resulted in a significant improvement in performance, with test group horses completing races faster than control group horses. The supplementation also led to increased levels of antioxidants in the blood, suggesting a dual benefit of enhanced performance and reduced oxidative stress during exercise .
Biochemical Research
3.1 Modulation of Enzymatic Activity
2-Methylene-beta-alanine has been studied for its potential to modulate the activity of various enzymes, including cytochrome c oxidase. Research indicates that increasing concentrations of this compound can significantly decrease enzyme function, suggesting applications in metabolic disorders where modulation of mitochondrial respiration is desired.
3.2 Biosynthesis of Natural Products
The incorporation of beta-amino acids like 2-Methylene-beta-alanine into natural products is an area of active research. The ability to swap beta-amino acid moieties with different side chains could lead to the development of novel bioactive compounds with therapeutic potential .
Summary Table of Applications
Wirkmechanismus
Bisoprolol exerts its effects by selectively blocking beta-1 adrenergic receptors in the heart. This action reduces the heart rate and the force of contraction, leading to a decrease in cardiac output and blood pressure. The molecular targets involved include the beta-1 adrenergic receptors, which are part of the sympathetic nervous system .
Vergleich Mit ähnlichen Verbindungen
Pharmacological Properties
Receptor Selectivity and Molecular Mechanisms
Bisoprolol exhibits >100-fold higher affinity for β₁-adrenoceptors compared to β₂-receptors, minimizing bronchoconstrictive and metabolic side effects . In contrast:
- Atenolol: At 50 mg, shows similar β₁-selectivity to this compound 10 mg but requires higher doses for equivalent β₁-blockade .
- Carvedilol: Non-selective β-blocker with additional α₁-blocking activity, increasing vasodilation but raising hypotension risk .
- Nebivolol : β₁-selective with nitric oxide-mediated vasodilation, offering unique hemodynamic benefits .
Table 1: Receptor Selectivity and Key Mechanisms
| Compound | β₁-Selectivity | Additional Mechanisms |
|---|---|---|
| This compound | >100:1 | None |
| Metoprolol | 20:1 | None |
| Carvedilol | Non-selective | α₁-blockade, antioxidant effects |
| Nebivolol | 300:1 | Nitric oxide vasodilation |
| Propranolol | Non-selective | Membrane-stabilizing activity |
Ion Channel Effects
This contrasts with propranolol, which primarily blocks Na⁺ channels .
Pharmacokinetic Profile
Metabolism and Elimination
- This compound : Hepatic metabolism (CYP3A4) and renal excretion (50% unchanged). Half-life: 10–12 hours , enabling once-daily dosing .
- Metoprolol : Metabolized by CYP2D6, leading to variability in poor metabolizers .
- Carvedilol : CYP2D6/2C9-dependent metabolism; shorter half-life (6–8 hours) necessitates twice-daily dosing .
Table 2: Pharmacokinetic Comparison
| Compound | Half-Life (h) | Metabolism Enzymes | Renal Excretion (%) |
|---|---|---|---|
| This compound | 10–12 | CYP3A4 | 50 |
| Metoprolol CR | 7–9 | CYP2D6 | <10 |
| Carvedilol | 6–8 | CYP2D6/2C9 | <2 |
| Nebivolol | 11–30 | CYP2D6 | 38 |
Genetic Polymorphisms
In contrast, metoprolol’s efficacy is highly dependent on CYP2D6 status .
Clinical Efficacy Comparison
Hypertension
- This compound vs. Metoprolol CR/ZOK: In the CREATIVE study, this compound provided superior HR reduction (ΔHR: −11.2 vs. −8.5 bpm) and non-inferior BP control .
- This compound vs. Nifedipine : Comparable BP reduction, but this compound showed more consistent 24-hour control .
Heart Failure (HF)
- CIBIS-II Trial : this compound reduced all-cause mortality by 34% in HF patients vs. placebo .
- CARNEBI Trial : this compound, carvedilol, and nebivolol showed similar improvements in ejection fraction, but carvedilol had higher hypotension rates .
Table 3: Clinical Outcomes in Key Trials
| Trial | Compound | Mortality Reduction | HR Reduction (Δbpm) | Notable Side Effects |
|---|---|---|---|---|
| CIBIS-II | This compound | 34% | −12.1 | Fatigue (6%) |
| MERIT-HF | Metoprolol CR | 34% | −11.3 | Bradycardia (5%) |
| CARNEBI | Carvedilol | 35% | −10.8 | Hypotension (12%) |
| SENIORS | Nebivolol | 14% | −9.5 | Headache (4%) |
Sources:
Biologische Aktivität
Bisoprolol is a selective beta-1 adrenergic antagonist primarily used in the management of hypertension and heart failure. Its pharmacological properties, mechanisms of action, and clinical efficacy have been extensively studied, revealing significant insights into its biological activity. This article delves into the biological activity of this compound, supported by data tables, case studies, and detailed research findings.
This compound exhibits its therapeutic effects through competitive inhibition of beta-1 adrenergic receptors located predominantly in the heart. By blocking these receptors, this compound reduces cardiac output and lowers heart rate, which decreases myocardial oxygen demand. Additionally, it is believed to lower renin secretion from the kidneys, further contributing to its antihypertensive effects .
Pharmacokinetics
The pharmacokinetics of this compound are characterized by moderate lipophilicity and high bioavailability. It is primarily metabolized in the liver via cytochrome P450 enzymes, particularly CYP2D6 and CYP3A4. Variations in these enzymes due to genetic polymorphisms can influence this compound's plasma concentrations and therapeutic outcomes .
Heart Failure
The Cardiac Insufficiency this compound Study II (CIBIS-II) demonstrated that this compound significantly reduces mortality in patients with chronic heart failure. Key findings include:
- All-Cause Mortality : A relative reduction of 29.3% compared to placebo (hazard ratio 0.66; 95% CI 0.54–0.81) was observed .
- Sudden Death : Fewer sudden deaths occurred in the this compound group (3.6% vs. 6.3% in placebo) with a hazard ratio of 0.56 .
Table 1: Summary of CIBIS-II Findings
| Outcome | This compound Group | Placebo Group | Hazard Ratio (95% CI) |
|---|---|---|---|
| All-Cause Mortality | 156 (11.8%) | 228 (17.3%) | 0.66 (0.54–0.81) |
| Sudden Death | 48 (3.6%) | 83 (6.3%) | 0.56 (0.39–0.80) |
| Hospitalization for HF | Reduced | Increased | - |
Hypertension
In patients with mild to moderate hypertension, this compound has shown significant reductions in blood pressure and heart rate:
- Blood Pressure Reduction : After six weeks of treatment, systolic blood pressure decreased by an average of 14.3 mmHg and diastolic by 8.4 mmHg .
- Heart Rate Reduction : The average reduction in heart rate was noted to be approximately 6 BPM .
BISOCOR Observational Study
This study evaluated the long-term effects of this compound on patients with heart failure over nine months:
- Ejection Fraction Improvement : An increase of 0.06 in ejection fraction was recorded.
- Adverse Effects : Approximately 10% of patients discontinued due to adverse effects, indicating a need for careful monitoring during treatment .
This compound in COPD Study (BICS)
A recent randomized clinical trial assessed this compound's efficacy in patients with chronic obstructive pulmonary disease (COPD):
Eigenschaften
IUPAC Name |
1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy]propan-2-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H31NO4/c1-14(2)19-11-17(20)13-23-18-7-5-16(6-8-18)12-21-9-10-22-15(3)4/h5-8,14-15,17,19-20H,9-13H2,1-4H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VHYCDWMUTMEGQY-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC(COC1=CC=C(C=C1)COCCOC(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H31NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6022682 | |
| Record name | Bisoprolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
325.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Bisoprolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014750 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
445.0±45.0 | |
| Record name | Bisoprolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00612 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
7.07e-02 g/L | |
| Record name | Bisoprolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014750 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
66722-44-9 | |
| Record name | Bisoprolol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=66722-44-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bisoprolol [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0066722449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bisoprolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00612 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bisoprolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2-Propanol, 1-[4-[[2-(1-methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino] | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.108.941 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BISOPROLOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/Y41JS2NL6U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Bisoprolol | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8316 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Bisoprolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014750 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
100-103, 100 °C | |
| Record name | Bisoprolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00612 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bisoprolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014750 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.