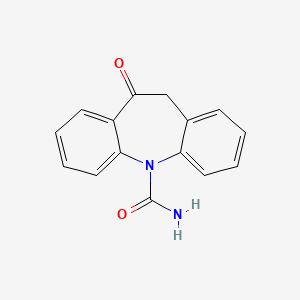
Oxcarbazepine
Vue d'ensemble
Description
Oxcarbazepine (OXC) is a second-generation antiepileptic drug (AED) approved for partial seizures and generalized tonic-clonic seizures. Structurally, it is the 10-keto analog of carbamazepine (CBZ), sharing a dibenzazepine backbone . OXC primarily blocks voltage-gated sodium channels, stabilizing hyperexcited neuronal membranes . Unlike CBZ, OXC is metabolized via cytosolic enzymes to its active metabolite, 10-hydroxycarbazepine (MHD), bypassing cytochrome P450-mediated oxidation and reducing drug interactions . This pharmacokinetic advantage contributes to its improved tolerability and safety profile, making it a preferred alternative to CBZ in patients with hypersensitivity or intolerance .
Méthodes De Préparation
Structural and Pharmacological Context of Oxcarbazepine
This compound (C₁₅H₁₂N₂O₂; IUPAC name: H-benzo[b]benzazepine-11-carboxamide) features a dibenzazepine core with a carboxamide substituent at position 11. Unlike carbamazepine, its ketone group minimizes hepatic autoinduction, reducing drug-drug interactions. The metabolite 10,11-dihydro-10-hydroxycarbamazepine (MHD) mediates primary anticonvulsant effects through voltage-gated sodium channel modulation.
Industrial Synthesis Methodologies
Hydrolysis of 10-Methoxycarbamazepine
This two-step approach dominates large-scale production due to operational simplicity and high yields.
Carbamoylation of 10-Methoxyiminostilbene
10-Methoxyiminostilbene reacts with sodium cyanate (NaOCN) in dichloromethane/toluene at reflux (80–110°C) using mandelic acid (C₆H₅CH(OH)COOH) as catalyst. The α-hydroxy acid facilitates imine activation, achieving 88–91% conversion to 10-methoxycarbamazepine.
Reaction conditions :
- Molar ratio: 1:1.75 (substrate:NaOCN)
- Catalyst: 2.4 eq mandelic acid
- Solvent: Dichloromethane (250 mL/g substrate)
- Time: 6–8 hours
Acidic Hydrolysis to this compound
10-Methoxycarbamazepine undergoes hydrolysis in aqueous oxalic acid (C₂H₂O₄·2H₂O) at 90°C for 17 hours, achieving 90% yield. Oxalic acid’s dual role as proton donor and chelating agent prevents diketone formation.
Optimization parameters :
- Acid concentration: 20–36.5% w/w
- Temperature: 85–95°C
- Workup: Isopropanol/water (1:1) recrystallization
Hydrogenation-Hydrolysis of 5-Cyano-10-Nitro Intermediate
Patent EP2311812A1 details a nitro-to-amine reduction pathway using Raney nickel under hydrogen pressure (2–20 atm).
Catalytic Hydrogenation
5-Cyano-10-nitro-5H-dibenz[b,f]azepine undergoes exothermic hydrogenation (40–120°C) in tetrahydrofuran. Raney nickel (5–15% w/w) selectively reduces nitro groups while preserving the cyano function.
Critical parameters :
- H₂ pressure: 10–15 atm
- Reaction monitoring: Disappearance of nitro IR stretch (1520 cm⁻¹)
- Yield: 86–89%
Hydrochloric Acid-Mediated Hydrolysis
The resulting amine intermediate reacts with concentrated HCl (20–36.5%) at reflux, with subsequent cooling crystallization yielding this compound (mp 215–217°C). This method minimizes cyanide byproducts versus conventional nitrile hydrolysis.
Carbamoylation of Iminostilbene Derivatives
A one-pot synthesis converts iminostilbene to carbamazepine analogs using alkali cyanates. Mandelic acid (2.7 eq) in toluene enables direct carbamoylation at 110°C for 10 hours, bypassing methoxy intermediates.
Mechanistic insight :
- Iminostilbene protonation at the imine nitrogen
- Cyanate (OCN⁻) nucleophilic attack
- Tautomerization stabilized by α-hydroxy acid hydrogen bonding
Yield comparison :
| Substrate | Product | Yield (%) |
|---|---|---|
| Iminostilbene | Carbamazepine | 78 |
| 10-Methoxyiminostilbene | 10-Methoxycarbamazepine | 91 |
Purification and Polymorph Control
Pharmaceutical-grade this compound (≥99.5% purity) requires solvent-mediated recrystallization.
Methanol/Methylene Dichloride Recrystallization
Patent WO2009139001A2 optimizes crystal habit using a 1:1 methanol/methylene dichloride mixture:
- Dissolution at reflux (64°C)
- Slow cooling (0.5°C/min) to 15–20°C
- Anti-solvent addition (hexane) for crystal seeding
- Vacuum drying at 50°C (residual solvents <0.1%)
Purity enhancement :
Aqueous Isopropanol Slurry
Alternate protocol for thermolabile batches:
- Slurry in 70% isopropanol/water
- High-shear mixing (500 rpm) for 2 hours
- Filtration through 0.45 μm PTFE membranes
Comparative Analysis of Synthetic Routes
Table 1. Methodological Comparison
The hydrolysis route provides optimal balance between yield and operational safety, though methylene dichloride usage necessitates solvent recovery systems. Emerging methods explore enzymatic cyanate transferases to replace harsh acid conditions, though industrial viability remains unproven.
Analyse Des Réactions Chimiques
Types de réactions : L’oxcarbazépine subit diverses réactions chimiques, notamment :
Oxydation : Conversion de l’oxcarbazépine en son métabolite actif, la 10,11-dihydro-10-hydroxycarbamazépine (MHD).
Réduction : Les réactions de réduction peuvent ramener l’oxcarbazépine à ses formes précurseurs.
Substitution : Les réactions de substitution peuvent se produire aux positions amide ou aromatique
Réactifs et conditions courants :
Agents oxydants : Peroxyde d’hydrogène, peracides.
Agents réducteurs : Borohydrure de sodium, hydrure de lithium et d’aluminium.
Réactifs de substitution : Halogènes, agents alkylants
Principaux produits :
10,11-Dihydro-10-hydroxycarbamazépine (MHD) : Le principal métabolite actif.
Divers dérivés substitués : Selon les réactifs utilisés dans les réactions de substitution
4. Applications de la recherche scientifique
L’oxcarbazépine a un large éventail d’applications dans la recherche scientifique :
Chimie : Utilisée comme composé modèle pour étudier les réactions d’oxydation et de réduction.
Biologie : Étudiée pour ses effets sur l’activité neuronale et la libération de neurotransmetteurs.
Médecine : Étudiée de manière approfondie pour ses propriétés anticonvulsivantes et son utilisation potentielle dans le traitement du trouble bipolaire.
Industrie : Utilisée dans le développement de nouvelles formulations pharmaceutiques et de systèmes d’administration de médicaments .
Applications De Recherche Scientifique
Antiepileptic Use
Oxcarbazepine is effective in treating partial-onset seizures and primary generalized tonic-clonic seizures . It functions by blocking voltage-dependent sodium channels, which reduces abnormal electrical activity in the brain. The drug is indicated for use as both monotherapy and adjunctive therapy in adults and children aged four years and older .
Efficacy Studies
- Clinical Trials : Numerous studies have demonstrated that this compound has comparable efficacy to other antiepileptic drugs such as carbamazepine, valproate, and phenytoin, with advantages in terms of side effects and pharmacokinetics .
- Meta-Analysis : A meta-analysis indicated that this compound could effectively decrease seizure frequency in patients with drug-resistant epilepsy when used as an add-on therapy .
Psychiatric Applications
This compound has been explored as a mood stabilizer for conditions such as bipolar disorder . Although not FDA-approved for this indication, it is used off-label with some success.
Case Studies
- A case report documented a 53-year-old male with schizoaffective disorder who developed hyponatremia during treatment with this compound, highlighting both its psychiatric application and potential side effects .
- Another study observed significant improvements in symptoms of bipolar disorder when this compound was administered, suggesting its utility in managing mood disorders .
Neuropathic Pain Management
While evidence supporting this compound's effectiveness in treating neuropathic pain is limited, some studies have suggested it may provide relief for conditions such as trigeminal neuralgia.
Research Findings
- A review indicated that this compound could be beneficial for neuropathic pain management; however, the overall evidence remains inconclusive due to low patient numbers and event rates in studies .
Oncological Applications
Recent research has identified this compound as a potential pro-apoptotic agent in certain cancer cell lines, particularly those with IDH mutations.
Experimental Findings
- In vitro studies showed that this compound could inhibit the growth of glioma stem cells, suggesting a dual role as an antiepileptic and an antineoplastic agent . The treated cells exhibited significant reductions in size and increased apoptosis rates.
Adverse Effects and Considerations
Despite its therapeutic benefits, this compound is associated with several adverse effects:
Mécanisme D'action
L’oxcarbazépine et son métabolite actif, la MHD, exercent leurs effets en bloquant les canaux sodiques sensibles au voltage. Cette action stabilise les membranes neuronales hyperexcitables, inhibe le tir neuronal répétitif et réduit la propagation des impulsions synaptiques. Ces mécanismes sont essentiels pour empêcher la propagation des crises .
Composés similaires :
Carbamazépine : Le composé parent dont l’oxcarbazépine est dérivée.
Eslicarbazepine : Un autre dérivé avec des propriétés anticonvulsivantes similaires.
Lamotrigine : Un anticonvulsivant avec une structure chimique différente mais des utilisations thérapeutiques similaires .
Comparaison :
Oxcarbazépine contre carbamazépine : L’oxcarbazépine a un meilleur profil d’effets secondaires et moins d’interactions médicamenteuses que la carbamazépine.
Oxcarbazépine contre eslicarbazepine : Les deux ont des mécanismes d’action similaires, mais l’eslicarbazepine est commercialisée comme un promédicament avec une pharmacocinétique potentiellement améliorée.
Oxcarbazépine contre lamotrigine : Bien que tous deux soient utilisés pour traiter l’épilepsie, la lamotrigine est également utilisée pour le trouble bipolaire et a un mécanisme d’action différent impliquant l’inhibition de la libération de glutamate.
L’oxcarbazépine se distingue par sa faible propension aux interactions médicamenteuses et son efficacité dans la gestion des crises partielles avec un profil d’effets secondaires relativement favorable .
Comparaison Avec Des Composés Similaires
Comparison with Carbamazepine
Mechanism of Action
- Similarities : Both OXC and CBZ inhibit voltage-gated sodium channels, preventing seizure propagation .
- Differences :
Pharmacokinetics
| Parameter | Oxcarbazepine (OXC) | Carbamazepine (CBZ) |
|---|---|---|
| Metabolism | Cytosolic reduction to MHD (non-CYP) | CYP3A4 oxidation to epoxide metabolite |
| Half-Life | MHD: 8–14 hours | CBZ: 25–65 hours; auto-induces metabolism |
| Drug Interactions | Minimal | High (CYP3A4 induction) |
| Bioavailability | >95% | 75–85% |
| Protein Binding | MHD: 40% | CBZ: 70–80% |
Efficacy
- Epilepsy: No significant difference in seizure control between OXC and CBZ in monotherapy .
- Trigeminal Neuralgia (TN) : Comparable efficacy, but OXC is better tolerated .
Pharmacogenomic Considerations
| HLA Allele | This compound (OXC) | Carbamazepine (CBZ) |
|---|---|---|
| HLA-B*15:02 | Avoid in carriers (SJS/TEN risk) | Contraindicated in carriers |
| HLA-A*31:01 | Potential risk (limited evidence) | Strong association with SCARs |
CPIC guidelines recommend HLA-B15:02 testing for both drugs; HLA-A31:01 testing is optional for OXC
Comparison with Other Antiepileptic Drugs
Levetiracetam (LEV)
- Efficacy : Similar seizure-free rates in focal epilepsy (48-week study: 72% for OXC vs. 70% for LEV) .
- Mechanism : LEV modulates synaptic vesicle protein SV2A, distinct from OXC’s sodium channel blockade .
- Tolerability : LEV has fewer metabolic interactions, but higher rates of behavioral side effects (e.g., irritability) .
Lamotrigine (LTG)
- Combination Therapy : OXC + LTG is used for refractory epilepsy or bipolar disorder, leveraging LTG’s mood-stabilizing properties .
- Safety : LTG carries a higher risk of rash (10% vs. 5% for OXC) .
Clinical Implications and Special Populations
Hormonal and Bone Effects
- Androgens : OXC increases androgen levels and polycystic ovary risk, similar to CBZ .
- Bone Health : Both drugs reduce 25-hydroxyvitamin D and bone density, necessitating supplementation .
Pediatric Use
- OXC is effective in children with intellectual disability and epilepsy, showing 50% seizure reduction in localization-related cases .
Activité Biologique
Oxcarbazepine (OXC) is an anticonvulsant medication primarily used for the treatment of epilepsy. It is a derivative of carbamazepine and functions by stabilizing neuronal membranes and inhibiting repetitive neuronal firing. This article explores the biological activity of this compound, focusing on its mechanisms of action, therapeutic efficacy, and emerging research findings.
This compound acts through several mechanisms:
- Sodium Channel Blockade : OXC inhibits voltage-gated sodium channels, which reduces the excitability of neurons. This action is crucial in preventing seizures by decreasing abnormal electrical activity in the brain .
- Potassium Conductance : The drug enhances potassium conductance, contributing to its anticonvulsant properties .
- Calcium Channel Modulation : OXC modulates voltage-activated calcium channels, which may play a secondary role in its efficacy against seizures .
- Neurotransmitter Effects : Although initially thought to inhibit glutamatergic activity, this effect has not been consistently replicated in vivo .
Efficacy in Epilepsy Treatment
Numerous studies have evaluated the effectiveness of this compound in treating various seizure types:
- Clinical Trials : A double-blind trial comparing this compound with phenytoin (PHT) found no significant differences in seizure frequency between the two drugs. However, OXC demonstrated better tolerability with fewer severe side effects .
- Monotherapy vs. Add-on Therapy : In a study involving children and adolescents, OXC was shown to be effective as both a first-line monotherapy and an add-on therapy for partial seizures (PS) and generalized tonic-clonic seizures (GTCS) with comparable efficacy to carbamazepine .
- Long-Term Outcomes : A six-month follow-up study indicated that patients on OXC had significant improvements in mood and anxiety scales (SAS and SDS), suggesting additional psychological benefits beyond seizure control .
Proapoptotic Effects in Cancer Research
Recent research has identified this compound's potential role beyond epilepsy treatment. A study published in 2023 highlighted its proapoptotic effects on IDH-mutant glioma cells, showing that OXC significantly reduced cell viability and induced apoptosis in tumor spheroids. The treated spheroids were found to be 82% smaller than controls after 72 hours, indicating substantial growth inhibition .
Table 1: Summary of Biological Activities of this compound
Case Studies and Emerging Research
- Efficacy in Bipolar Disorder : A pilot study suggested that this compound may help prevent impulsivity and depressive episodes in bipolar patients when used as an adjunctive therapy to lithium. The results indicated a lower relapse rate among those treated with OXC compared to placebo .
- Retinoprotective Properties : Preliminary findings suggest that this compound may have retinoprotective effects, showing no cytotoxicity in retinal cells while promoting cell proliferation under certain conditions .
Q & A
Basic Research Questions
Q. How should researchers design pharmacokinetic studies to compare immediate-release (OXC-IR) and extended-release (OXC-XR) formulations of oxcarbazepine?
- Methodological Answer : Utilize crossover study designs to minimize inter-individual variability, and employ population pharmacokinetic models to account for covariates like age, weight, and renal/hepatic function. Parameters such as AUC, Cmax, and t1/2 should be analyzed under both fed and fasted conditions. Reference adult and pediatric population data from clinical trials (e.g., studies 804P103, 804P301, and 804P107) to validate dose-response relationships .
Q. What are the key considerations for validating analytical methods to quantify this compound and its metabolites in plasma?
- Methodological Answer : Ensure validation parameters include specificity, linearity (e.g., 0.1–20 µg/mL), accuracy (recovery ≥95%), precision (CV <15%), and stability under long-term storage. Cross-validate assays using techniques like HPLC or LC-MS/MS, and reference established protocols for metabolite quantification (e.g., MDH) .
Q. How can researchers systematically review literature on this compound’s efficacy in refractory epilepsy?
- Methodological Answer : Follow PRISMA guidelines, search databases (MEDLINE, Cochrane Library, EMBASE) using Boolean operators (e.g., "this compound AND (epilepsy OR seizure)"), and include both published/unpublished trials. Assess heterogeneity using chi-squared tests and I² statistics, and prioritize studies with intention-to-treat (ITT) analysis to minimize attrition bias .
Q. What frameworks are recommended for formulating research questions on this compound’s mechanisms in bipolar disorder?
- Methodological Answer : Apply PICO (Population: bipolar patients; Intervention: OXC; Comparison: placebo/standard therapy; Outcome: manic/depressive episodes) and FINER criteria (Feasible, Interesting, Novel, Ethical, Relevant). Prioritize hypotheses that address gaps in double-blind RCTs versus case reports .
Advanced Research Questions
Q. How should conflicting efficacy data between this compound and carbamazepine in partial seizures be analyzed?
- Methodological Answer : Conduct meta-analyses using hazard ratios (HRs) for time-to-treatment withdrawal and odds ratios (ORs) for adverse events. Address heterogeneity via subgroup analysis (e.g., dosing regimens, study blinding) and sensitivity analysis to exclude high-risk-of-bias trials. Note that wide confidence intervals (e.g., HR 0.78–1.39 for withdrawal) may indicate underpowered studies .
Q. What methodologies resolve contradictions in this compound’s therapeutic drug monitoring (TDM) for pediatric populations?
- Methodological Answer : Develop population pharmacokinetic models incorporating covariates (e.g., age, CYP3A4 activity) and validate using bootstrap resampling. Compare TDM outcomes (efficacy/toxicity) against historical controls, and adjust for protein binding variations in free drug assays .
Q. Data Presentation and Reporting Guidelines
Propriétés
IUPAC Name |
5-oxo-6H-benzo[b][1]benzazepine-11-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C15H12N2O2/c16-15(19)17-12-7-3-1-5-10(12)9-14(18)11-6-2-4-8-13(11)17/h1-8H,9H2,(H2,16,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CTRLABGOLIVAIY-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C2=CC=CC=C2N(C3=CC=CC=C3C1=O)C(=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C15H12N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0045703 | |
| Record name | Oxcarbazepine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0045703 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
252.27 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble in chloroform, dichloromethane, acetone, and methanol and practically insoluble in ethanol, ether, and water., 1.60e-01 g/L | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Crystals from ethanol, White to faintly orange crystalline powder | |
CAS No. |
28721-07-5 | |
| Record name | Oxcarbazepine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=28721-07-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Oxcarbazepine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0028721075 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Oxcarbazepine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00776 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | OXCARBAZEPINE | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758693 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Oxcarbazepine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0045703 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Oxcarbazepine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.044.702 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | OXCARBAZEPINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/VZI5B1W380 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
215-216 °C, 215.5 °C | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details







Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details





Synthesis routes and methods V
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.