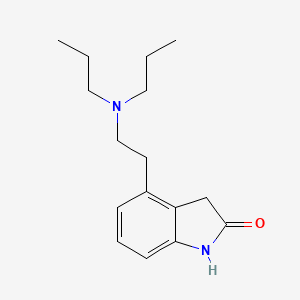
Ropinirole
Overview
Description
Ropinirole is a non-ergoline dopamine agonist approved for the treatment of Parkinson’s disease (PD) and moderate-to-severe restless legs syndrome (RLS) . It selectively activates D2 and D3 dopamine receptors with minimal affinity for D1, adrenergic, or serotonergic receptors . Clinically, it is used as monotherapy in early PD or as an adjunct to levodopa in advanced PD to manage motor fluctuations and reduce OFF-time . Sustained-release (prolonged-release, PR) and immediate-release (IR) formulations are available, with PR offering once-daily dosing and comparable efficacy to IR while delaying dyskinesia onset .
Preparation Methods
Synthetic Routes and Reaction Conditions
Ropinirole can be synthesized through several methods. One common method involves the reduction of a 2-nitrophenyl acetic acid precursor followed by spontaneous cyclization. Another method involves the reductive cyclization of 2-(2’-bromoethyl) β-nitrostyrene using palladium on carbon (Pd/C) as a catalyst .
Industrial Production Methods
In industrial settings, this compound hydrochloride is often prepared by dissolving the compound in solvents such as ethanol, methanol, or ethyl acetate, followed by the addition of Pd/C. The mixture is then reacted to obtain the desired product .
Chemical Reactions Analysis
Types of Reactions
Ropinirole undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized to form different metabolites.
Reduction: The reduction of nitro groups to amines is a key step in its synthesis.
Substitution: Halogenation and sulfonation reactions are also involved in its synthesis.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Catalysts like palladium on carbon (Pd/C) are used for reductive cyclization.
Substitution: Reagents such as p-methylbenzenesulfonyl chloride and pyridine are used in substitution reactions.
Major Products Formed
The major products formed from these reactions include various intermediates that eventually lead to the formation of this compound hydrochloride .
Scientific Research Applications
Treatment of Parkinson's Disease
Ropinirole has been extensively studied for its effectiveness in managing Parkinson's disease, particularly in early stages. A significant clinical trial demonstrated that patients treated with this compound showed substantial improvements in motor function as measured by the Unified Parkinson's Disease Rating Scale (UPDRS). In this study, this compound-treated patients had a 24% improvement in UPDRS motor scores compared to a -3% change in the placebo group (p < 0.001) .
Efficacy and Safety Data
- Study Design : A double-blind, placebo-controlled trial involving 241 patients.
- Duration : 12 months.
- Results :
Management of Restless Legs Syndrome
This compound is also approved for treating restless legs syndrome. Its mechanism as a dopamine agonist helps alleviate symptoms by enhancing dopaminergic transmission in the central nervous system. Clinical studies have shown that this compound significantly reduces the severity of symptoms and improves sleep quality in affected individuals .
Psychotic Symptoms Induced by this compound
While this compound is effective for various conditions, it has been associated with inducing or exacerbating psychotic symptoms in some patients. For instance, a case study reported a patient who developed acute psychosis after starting this compound for restless legs syndrome. Symptoms resolved rapidly upon discontinuation of the drug, highlighting the need for careful monitoring of psychiatric symptoms during treatment .
Potential Applications Beyond Parkinson's Disease
Recent research has explored additional applications of this compound:
- Emotional Regulation : Studies indicate that this compound may influence emotionality and exhibit anxiolytic and antidepressant-like effects without affecting motor functions or cognition .
- Urease Inhibition : Investigations into the compound's pharmacological properties revealed potential urease inhibitory activity, suggesting its utility in treating conditions related to urease activity .
Data Summary
| Application | Efficacy Evidence | Safety Profile |
|---|---|---|
| Parkinson's Disease | Significant UPDRS improvement (+24%) | Low incidence of adverse effects |
| Restless Legs Syndrome | Reduces symptom severity | Generally well tolerated |
| Psychosis | Induction of acute psychotic symptoms | Requires monitoring |
| Urease Inhibition | In vitro studies show significant activity | Not fully established |
Mechanism of Action
Ropinirole exerts its effects by selectively stimulating dopamine D2 receptors within the caudate-putamen system in the brain. This stimulation helps to compensate for the dopamine deficiency observed in conditions like Parkinson’s disease. This compound has a high affinity for D2 and D3 receptors, with a higher affinity for D3 receptors .
Comparison with Similar Compounds
Structural and Pharmacological Differences
Ropinirole vs. Pramipexole
- Chemical Structure : this compound is a substituted oxindole, whereas pramipexole is a cyclohexyl-thiazoline with a chiral center. Both share an alkylamine group critical for receptor binding .
- Receptor Selectivity : Both target D2/D3 receptors, but structural differences influence pharmacokinetics and side-effect profiles.
| Compound | Core Structure | Receptor Affinity | Key Structural Feature |
|---|---|---|---|
| This compound | Substituted oxindole | D2/D3 selective | Alkylamine side chain |
| Pramipexole | Cyclohexyl-thiazoline | D2/D3 selective | Chiral center, thiazoline moiety |
This compound vs. Piribedil and Rotigotine
- Piribedil (a non-ergoline agonist with D2/D3 and α2-adrenergic antagonism) demonstrates superior efficacy in early PD compared to this compound IR in network meta-analyses .
- Rotigotine (transdermal patch) offers continuous dopaminergic stimulation but lacks head-to-head comparisons with this compound PR .
Efficacy in Parkinson’s Disease
Early PD
- This compound vs. Levodopa (LD) : A 5-year study (N=268) showed LD provided greater UPDRS motor score improvement (+4 points vs. This compound), but this compound delayed dyskinesia. LD supplementation in this compound groups reduced efficacy gaps .
- This compound IR vs. PR : Both formulations show similar UPDRS motor score improvements (mean reduction: ~4–6 points) .
Advanced PD
- Adjunct Therapy : this compound PR reduced OFF-time by 2.1 hours vs. placebo, outperforming IR in maintaining ON-period stability .
- Comparison with Sumanirole: Sumanirole (D2/D3 agonist) demonstrated non-inferiority to this compound in UPDRS II + III scores (difference: 1.17 points, 90% CI: -0.56–2.89) with comparable tolerability .
Pharmacokinetics and Drug Interactions
- Ciprofloxacin Interaction : Ciprofloxacin increases this compound AUC (1.84x) and Cmax (1.6x) via CYP1A2 inhibition .
- Dose Optimization : Population PK models suggest 8–12 mg/day achieves clinical benefit in early PD, while higher doses (>12 mg) improve OFF-time in advanced PD .
- Renal Impairment: No dose adjustment required for mild-to-moderate renal impairment .
Key Clinical Findings and Controversies
- Early LD Initiation : Studies debate whether delaying LD in favor of this compound sacrifices long-term motor benefits .
- Generic vs. Branded PR : Generic this compound PR showed comparable efficacy but increased gastrointestinal AEs (e.g., 3-hour shorter ON-time without dyskinesia) .
- Antioxidant Activity : this compound exhibits poor antioxidant efficacy compared to ascorbic acid and thiazolidine derivatives .
Biological Activity
Ropinirole is a non-ergoline dopamine agonist primarily used in the treatment of Parkinson's disease and Restless Legs Syndrome (RLS). Its biological activity is characterized by its interaction with dopamine receptors, pharmacokinetics, and its effects on various neurological and psychological parameters. This article delves into the detailed biological activity of this compound, supported by data tables and relevant case studies.
This compound exhibits a high affinity for post-synaptic dopamine D2 receptors, which are G-protein-coupled receptors located in the central nervous system. These receptors play a crucial role in modulating neurotransmission and motor control. The activation of D2 receptors by this compound inhibits adenylyl cyclase activity, which decreases cyclic AMP levels, leading to reduced neuronal excitability and modulation of various physiological processes .
Pharmacokinetics
Absorption and Bioavailability:
- This compound is rapidly absorbed after oral administration, reaching peak plasma concentrations within 1 to 2 hours.
- The absolute bioavailability ranges from 45% to 55%, indicating significant first-pass metabolism .
Distribution:
- The volume of distribution is approximately 7.5 L/kg, with about 40% of the drug bound to plasma proteins .
Metabolism:
- This compound is extensively metabolized in the liver, primarily through N-despropylation and hydroxylation. The major metabolic enzyme involved is CYP1A2 .
Elimination:
- Approximately 88% of a radiolabeled dose is recovered in urine, with less than 10% excreted as unchanged drug .
Neurological Impact
This compound has been shown to improve motor function in various models of Parkinson's disease. Clinical studies indicate that it effectively alleviates symptoms associated with both Parkinson's disease and RLS .
Table 1 summarizes key findings from clinical trials regarding the efficacy of this compound:
| Study Type | Population | Treatment Duration | Key Findings |
|---|---|---|---|
| Randomized Controlled | Early-stage PD patients | 12 weeks | Significant improvement in motor function scores |
| Open-label Study | RLS patients | 6 months | Reduced frequency and severity of leg restlessness |
| Case Study | PD patient with nocturnal symptoms | 3 months | Successful management of nocturnal tremors |
Psychological Effects
Recent studies have highlighted this compound's influence on emotional regulation. In animal models, doses ranging from 1 to 10 mg/kg have demonstrated anxiolytic and antidepressant-like effects without significantly impacting locomotion or cognition. These effects are associated with alterations in neurotransmitter receptor phosphorylation patterns in the brain .
Case Studies
-
Case Study on Nocturnal Tremors:
A patient with Parkinson's disease exhibited violent nocturnal tremors unresponsive to standard treatment. The introduction of a this compound patch resulted in significant symptom relief, underscoring its utility beyond daytime symptom management . -
Long-term Efficacy:
A longitudinal study followed Parkinson's disease patients over five years while on this compound therapy. Results indicated sustained improvement in quality of life metrics and motor function, with manageable side effects .
Q & A
Basic Research Questions
Q. What experimental design considerations are critical for evaluating the dissolution profile of ropinirole extended-release tablets?
- Methodological Answer : Follow FDA dissolution protocols (e.g., USP Apparatus 2 with sinkers) to ensure tablets remain submerged, reducing variability in release rates . Include sinkers to stabilize tablet positioning, improving RSD values (e.g., RSD <10%) while minimally affecting overall dissolution kinetics. Use HPLC with UV detection (250 nm, L7 column) for quantifying this compound and related impurities .
- Data Reference : Studies show sinkers reduce RSD from 15% to 5% in early dissolution phases without altering cumulative release .
Q. How do researchers validate the purity of this compound hydrochloride in preclinical formulations?
- Methodological Answer : Utilize USP reference standards (e.g., USP this compound Hydrochloride RS) for chromatographic calibration. Employ LC-UV systems (e.g., 4.6-mm × 25-cm column, 1 mL/min flow rate) with system suitability criteria: resolution ≥2.0 between this compound and related compounds (e.g., 3-oxo this compound), and S/N ratio ≥10 . Preclinical studies must adhere to NIH guidelines for reproducibility, including detailed reporting of animal models and statistical methods .
Q. What are the standard pharmacokinetic parameters assessed in early-phase this compound trials?
- Methodological Answer : Focus on bioavailability (AUC, Cmax), elimination half-life (t½), and metabolite profiling. Use validated HPLC-MS methods with SPE extraction (C18 columns, methanol-water eluents) to achieve detection limits of 5 ng/mL in plasma . Compare results against healthy baselines to identify deviations in patients with Parkinson’s disease .
Advanced Research Questions
Q. How can contradictory efficacy data between this compound and newer dopamine agonists (e.g., D-512) be reconciled in preclinical models?
- Methodological Answer : Apply comparative dose-response studies in animal models (e.g., MPTP-induced Parkinsonism) to assess symptom relief duration and neuroprotective effects. Use ANOVA and post-hoc tests to analyze differences in motor function scores and neuronal survival rates . Address variability by standardizing disease progression metrics (e.g., Unified Parkinson’s Disease Rating Scale) and controlling for pharmacokinetic confounders (e.g., blood-brain barrier penetration) .
- Data Reference : D-512 showed 30% greater efficacy in symptom relief and 50% lower dyskinesia incidence compared to this compound in rodent models .
Q. What mechanisms underlie this compound’s potential neuroprotective effects in motor neuron disease (MND)?
- Methodological Answer : Use patient-derived iPSCs differentiated into motor neurons to study axonal integrity and cholesterol synthesis pathways. Perform RNA-seq to identify suppressed genes (e.g., 29 genes linked to cholesterol metabolism) and validate via qPCR . Compare dendritic length and synaptic connectivity in this compound-treated vs. control cells using immunofluorescence and morphometric analysis .
Q. How should researchers design studies to resolve discrepancies in this compound’s long-term tolerability across populations?
- Methodological Answer : Implement longitudinal, double-blind RCTs with stratified randomization by age, disease stage, and genetic biomarkers (e.g., DRD2 polymorphisms). Use mixed-effects models to analyze adverse event rates (e.g., nausea, hypotension) and adjust for covariates like CYP1A2 metabolic activity . Include pre/post-test assessments of non-motor symptoms (e.g., sleep disturbances) to capture holistic tolerability .
Q. What analytical strategies optimize detection of this compound-related impurities in stability studies?
- Methodological Answer : Apply forced degradation (e.g., heat, light, pH extremes) followed by LC-MS/MS to identify degradation products (e.g., 4-[2-(dipropylamino)ethyl]indoline-2,3-dione). Use orthogonal methods (e.g., NMR for structural confirmation) and adhere to ICH Q3B guidelines for reporting thresholds .
Q. Methodological Frameworks for this compound Research
- PICO Framework : For clinical trials, structure questions around Population (e.g., advanced Parkinson’s patients), Intervention (this compound XR), Comparison (placebo/alternative agonists), and Outcome (e.g., UPDRS score change) .
- FINER Criteria : Ensure questions are Feasible (e.g., accessible patient cohorts), Interesting (addressing gaps in neuroprotection mechanisms), Novel (exploring MND applications), Ethical (IRB-approved protocols), and Relevant (aligning with NIH priorities) .
Properties
IUPAC Name |
4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H24N2O/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15/h5-7H,3-4,8-12H2,1-2H3,(H,17,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UHSKFQJFRQCDBE-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCN(CCC)CCC1=C2CC(=O)NC2=CC=C1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H24N2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
91374-20-8 (hydrochloride) | |
| Record name | Ropinirole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0091374219 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8045195 | |
| Record name | Ropinirole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8045195 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
260.37 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
410.5±45.0 °C at 760 mmHg | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
3.53e-01 g/L | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
91374-21-9 | |
| Record name | Ropinirole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=91374-21-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ropinirole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0091374219 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ropinirole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758917 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Ropinirole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8045195 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2H-Indol-2-one, 4-[2-(dipropylamino)ethyl]-1,3-dihydro | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.110.353 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ROPINIROLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/030PYR8953 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ROPINIROLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
243-250 °C, 243 - 250 °C | |
| Record name | Ropinirole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00268 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ropinirole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014413 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













