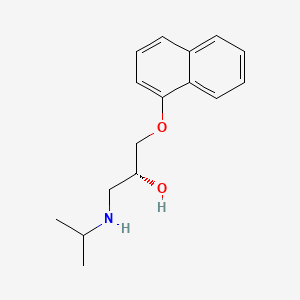
Dexpropranolol
Overview
Description
Dexpropranolol, chemically known as (R)-propranolol hydrochloride, is the dextrorotatory enantiomer of the non-selective β-adrenergic receptor antagonist propranolol . While propranolol exists as a racemic mixture of (S)-(-)-propranolol (active form) and (R)-(+)-propranolol (this compound), the latter exhibits significantly lower β-blocking potency—less than 1% of the (S)-enantiomer’s activity at β1 and β2 receptors . Despite this, this compound retains high affinity for β-adrenergic receptors, with Ki values of 1.8 nM for β1AR and 0.8 nM for β2AR . It has been studied in experimental models for its effects on noradrenaline uptake inhibition and arrhythmia prevention, though it lacks the therapeutic β-blocking efficacy of its racemic counterpart in clinical settings .
Preparation Methods
Dexpropranolol can be synthesized through several synthetic routes. One common method involves the resolution of racemic propranolol using chiral agents to separate the dextrorotatory enantiomer. The reaction conditions typically involve the use of solvents like dimethyl sulfoxide (DMSO) and specific chiral catalysts . Industrial production methods may involve large-scale resolution processes and purification techniques to ensure the high enantiomeric purity of this compound.
Chemical Reactions Analysis
Dexpropranolol undergoes various chemical reactions, including:
Oxidation: It can be oxidized using reagents like potassium permanganate or chromium trioxide to form corresponding naphthoquinones.
Reduction: Reduction reactions can be carried out using hydrogen gas in the presence of palladium on carbon to yield reduced derivatives.
Substitution: Nucleophilic substitution reactions can occur at the naphthalene ring, often using reagents like sodium hydride and alkyl halides.
The major products formed from these reactions depend on the specific reagents and conditions used.
Scientific Research Applications
Pharmacological Profile
Dexpropranolol exhibits local anesthetic properties similar to those of propranolol but with negligible beta-adrenergic receptor blocking activity. This distinct profile allows for potential applications in areas where traditional beta-blockers may not be suitable.
Key Characteristics of this compound
| Property | Description |
|---|---|
| Type | Beta-adrenergic antagonist |
| Isomer | Dextro isomer of propranolol |
| Local Anesthetic Action | Present, similar to propranolol |
| Beta-Blockade | Negligible |
Clinical Applications
-
Cardiovascular Conditions
- This compound has been studied in the context of angina pectoris and exercise tolerance. A comparative study indicated that while this compound did not significantly affect exercise time, propranolol and practolol improved exercise tolerance in patients with angina . This suggests that this compound may not be as effective as its racemic counterpart in managing certain cardiovascular symptoms.
- Migraine Prophylaxis
-
Psychological Effects
- Emerging studies suggest that propranolol can influence emotional responses and implicit biases, raising questions about whether this compound could have similar effects without the associated beta-blocking properties. Research indicates that propranolol reduces implicit racial bias, potentially due to its effects on the autonomic nervous system . Further exploration into this compound's impact on psychological conditions could yield valuable insights.
- Post-Traumatic Stress Disorder (PTSD)
Case Study 1: Exercise Tolerance in Angina Patients
A study comparing this compound with racemic propranolol and practolol revealed that while both propranolol and practolol improved exercise tolerance, this compound did not show significant effects. This highlights the importance of understanding the specific actions of each isomer in clinical settings .
Case Study 2: Psychological Impact
In a study examining the effects of propranolol on implicit racial bias, participants receiving propranolol scored lower on measures of subconscious bias compared to those receiving a placebo. This raises questions about whether this compound could modulate such biases without the cardiovascular implications of traditional beta-blockers .
Mechanism of Action
Dexpropranolol exerts its effects by blocking beta-adrenergic receptors, which are involved in the response to catecholamines like adrenaline and noradrenaline. This blockade leads to a decrease in heart rate, blood pressure, and myocardial oxygen demand. The molecular targets include beta-1 and beta-2 adrenergic receptors, and the pathways involved are related to the inhibition of cyclic AMP production and subsequent downstream signaling .
Comparison with Similar Compounds
Propranolol (Racemic Mixture)
- Structural Similarity: Dexpropranolol shares identical molecular structure (C16H21NO2) with propranolol but differs in stereochemistry at the chiral center .
- Pharmacological Activity: β-Blocking Potency: Propranolol’s (S)-enantiomer is responsible for >99% of β-blockade, while this compound contributes minimally . Noradrenaline Uptake Inhibition: Both enantiomers inhibit noradrenaline uptake equally, but only propranolol potentiates nerve-evoked vasoconstriction due to β-blockade . Clinical Effects: Propranolol reduces blood pressure and heart rate, whereas this compound lacks antihypertensive effects despite similar noradrenaline-blocking activity .
Table 1: Key Pharmacological Differences
4-Hydroxypropranolol (Metabolite)
- Metabolic Relationship: 4-Hydroxypropranolol is an active metabolite of propranolol with intrinsic β-blocking activity .
- Comparison: Potency: 4-Hydroxypropranolol has ~40% of propranolol’s β-blocking potency but longer half-life due to hepatic recirculation . Hemodynamic Effects: In dogs, 4-hydroxypropranolol reduces cardiac output less markedly than propranolol, making it a weaker negative inotrope .
Practolol (Cardioselective β1 Blocker)
- Selectivity: Practolol is β1-selective, unlike this compound’s non-selectivity .
- Clinical Utility: Practolol was historically used for angina but withdrawn due to side effects (e.g., oculomucocutaneous syndrome). This compound, while safer, lacks therapeutic β-blocking utility .
Atenolol (β1-Selective Blocker)
- Mechanism: Atenolol selectively blocks β1 receptors, reducing sympathetic nerve activity without affecting β2-mediated bronchodilation .
Research Findings and Clinical Implications
- Arrhythmia Models: Both this compound and propranolol prevent adrenaline-induced arrhythmias in cats, but the (S)-enantiomer requires 7.5-fold lower doses .
- Vascular Responses: this compound (50 mg/kg) fails to potentiate vascular responses to sympathetic nerve stimulation, unlike racemic propranolol, confirming its minimal β-blocking role .
Biological Activity
Dexpropranolol is a selective beta-adrenergic antagonist derived from propranolol, primarily used in the management of various cardiovascular conditions. This article explores the biological activity of this compound, including its pharmacokinetics, mechanisms of action, therapeutic applications, and safety profile, supported by data tables and relevant case studies.
Pharmacokinetics
This compound exhibits distinct pharmacokinetic properties compared to its racemic counterpart, propranolol. Key pharmacokinetic parameters include:
This compound is cleared more rapidly than propranolol, primarily because it does not significantly affect hepatic blood flow, leading to a more predictable pharmacokinetic profile .
This compound functions by selectively blocking beta-adrenergic receptors, particularly β1 receptors in the heart. This blockade results in:
- Reduced heart rate : By inhibiting the effects of catecholamines on the heart.
- Decreased myocardial contractility : Leading to lower oxygen demand during stress.
- Vasodilation : Although less pronounced than with other beta-blockers.
These actions contribute to its therapeutic efficacy in managing hypertension and preventing angina pectoris .
Therapeutic Applications
This compound has been studied for various clinical applications:
- Hypertension : Clinical trials have demonstrated its effectiveness in lowering blood pressure compared to placebo and other antihypertensives.
- Anxiety Disorders : It is used off-label for performance anxiety due to its ability to mitigate physical symptoms like tachycardia and tremors.
- Migraine Prophylaxis : Some studies suggest it may help reduce the frequency of migraine attacks.
A meta-analysis indicated that this compound significantly reduces the risk of disease progression in infants with retinopathy of prematurity (ROP), showing a relative risk (RR) of 0.65 for stage progression compared to controls .
Safety Profile
While generally well-tolerated, this compound can cause adverse effects similar to other beta-blockers:
| Adverse Effect | Incidence Rate |
|---|---|
| Bradycardia | 11.42 RR [95% CI, 0.66–196.40] |
| Hypotension | 7.27 RR [95% CI, 0.39–133.95] |
| Hypoglycemia | 3.10 RR [95% CI, 0.33–29.27] |
The increased risk of adverse events was noted in a meta-analysis involving infants treated with propranolol, indicating a need for careful monitoring during treatment .
Case Studies
Several case studies highlight the clinical efficacy of this compound:
- Case Study 1 : A 30-year-old male with generalized anxiety disorder reported significant improvement in symptoms after initiating treatment with this compound, particularly during public speaking engagements.
- Case Study 2 : An infant diagnosed with ROP showed marked improvement in disease stage after receiving this compound, reducing the need for laser therapy.
Q & A
Basic Research Questions
Q. What are the key pharmacological properties of Dexpropranolol that distinguish it from its enantiomer, (S)-propranolol, in β-adrenergic receptor antagonism?
- Methodological Answer : Comparative studies should assess binding affinities (Ki values) for β1AR and β2AR using radioligand displacement assays. For example, this compound exhibits Ki values of 1.8 nM (β1AR) and 0.8 nM (β2AR), whereas (S)-propranolol shows higher potency . Dose-response curves in isolated tissue models (e.g., guinea pig atria for β1AR, trachea for β2AR) can further validate enantiomer-specific efficacy. Data should be analyzed using nonlinear regression to calculate EC50 values and statistical significance (e.g., t-tests for inter-group differences) .
Q. How should researchers design in vivo experiments to evaluate this compound’s cardiovascular effects while minimizing confounding variables?
- Methodological Answer : Use controlled animal models (e.g., rodents or cats) with standardized anesthesia protocols to isolate adrenergic responses. For instance, measure heart rate variability and arrhythmia incidence post-ouabain or cocaine administration, as this compound reverses ventricular tachycardia in these models . Randomize treatment groups, include vehicle controls, and blind outcome assessments to reduce bias. Data should be normalized to baseline measurements and analyzed via ANOVA with post-hoc corrections .
Q. What are the best practices for validating this compound’s enantiomeric purity in experimental samples?
- Methodological Answer : Employ chiral chromatography (e.g., HPLC with a chiral stationary phase) coupled with mass spectrometry to quantify enantiomeric excess. Calibrate methods using reference standards and report resolution factors (Rs > 1.5). Cross-validate with circular dichroism spectroscopy to confirm optical activity .
Advanced Research Questions
Q. How can contradictory findings regarding this compound’s enantiomer-specific activity in different species be systematically analyzed?
- Methodological Answer : Conduct a meta-analysis of existing data (e.g., cat vs. dog studies ) to identify species-dependent receptor expression or metabolic differences. Use sensitivity analysis to weigh confounding factors like dosage (e.g., (−)-propranolol requires lower doses for efficacy). Apply mixed-effects models to account for interspecies variability and publish negative results to mitigate publication bias .
Q. What experimental frameworks are optimal for investigating this compound’s off-target effects on non-adrenergic pathways?
- Methodological Answer : Utilize high-throughput screening (e.g., kinase panels or GPCR profiling) to identify off-target interactions. Combine with transcriptomic analysis (RNA-seq) of treated cell lines to detect downstream signaling perturbations. Validate hits using CRISPR knockouts or pharmacological inhibitors and report effect sizes with 95% confidence intervals .
Q. How should researchers address discrepancies between in vitro binding data and in vivo functional outcomes for this compound?
- Methodological Answer : Reconcile discrepancies by evaluating tissue-specific receptor density (e.g., β2AR dominance in bronchial tissue) and pharmacokinetic factors (e.g., plasma protein binding). Use physiologically based pharmacokinetic (PBPK) modeling to simulate tissue exposure levels and correlate with functional assays. Report limitations in translational relevance explicitly in discussions .
Q. Data Analysis & Interpretation
Q. What statistical approaches are recommended for analyzing dose-dependent responses in this compound studies with small sample sizes?
- Methodological Answer : Use non-parametric tests (e.g., Mann-Whitney U) for non-normal distributions and apply bootstrapping to estimate confidence intervals. For dose-response curves, Bayesian hierarchical models can improve precision in small datasets. Report effect sizes (e.g., Cohen’s d) and power calculations to contextualize findings .
Q. How can researchers ensure reproducibility when comparing this compound’s effects across heterogeneous experimental systems?
- Methodological Answer : Adopt FAIR data principles (Findable, Accessible, Interoperable, Reusable). Standardize protocols using guidelines like ARRIVE 2.0 for animal studies. Share raw data (e.g., electrophysiology traces, chromatograms) in public repositories and document metadata (e.g., batch numbers, instrument calibration) .
Q. Contradiction & Limitations
Q. What strategies are effective for resolving contradictions between historical and contemporary findings on this compound’s clinical potential?
- Methodological Answer : Perform systematic reviews to identify temporal trends (e.g., evolving diagnostic criteria or improved analytical techniques). Use GRADE criteria to assess evidence quality and highlight studies with robust blinding or randomization. Discuss how historical limitations (e.g., lack of enantiomer-specific assays) may skew interpretations .
Q. How should researchers frame the limitations of this compound studies in grant proposals or publications?
- Methodological Answer : Explicitly address limitations (e.g., translational gaps between animal models and humans) in the discussion section. Propose follow-up experiments (e.g., human induced pluripotent stem cell-derived cardiomyocytes) to mitigate these gaps. Use structured frameworks like PICOS (Population, Intervention, Comparison, Outcomes, Study Design) to align limitations with research objectives .
Q. Tables of Key Data
| Parameter | This compound | (S)-Propranolol | Source |
|---|---|---|---|
| β1AR Ki (nM) | 1.8 | 0.5 | |
| β2AR Ki (nM) | 0.8 | 0.3 | |
| Effective Dose in Arrhythmia | 0.5–1.0 mg/kg (cats) | 0.1–0.3 mg/kg (cats) |
Properties
IUPAC Name |
(2R)-1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3/t14-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AQHHHDLHHXJYJD-CQSZACIVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC(COC1=CC=CC2=CC=CC=C21)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)NC[C@H](COC1=CC=CC2=CC=CC=C21)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H21NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3045304 | |
| Record name | Dexpropranolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3045304 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
259.34 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
5051-22-9, 13071-11-9 | |
| Record name | (+)-Propranolol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=5051-22-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexpropranolol [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0005051229 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dexpropranolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB03322 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dexpropranolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3045304 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Dexpropranolol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.023.409 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | (R)-[2-hydroxy-3-(naphthyloxy)propyl]isopropylammonium chloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.032.677 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DEXPROPRANOLOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/PG6KY07UD7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details







Synthesis routes and methods IV
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













