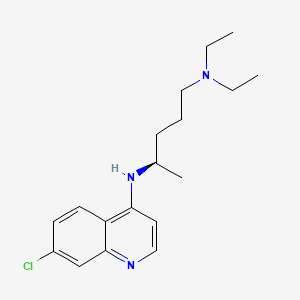
(R)-chloroquine
Overview
Description
(R)-Chloroquine is the enantiomer of chloroquine, a 4-aminoquinoline compound historically used as an antimalarial agent. Chloroquine and its derivatives, including hydroxychloroquine, function by inhibiting hemozoin formation in Plasmodium parasites, leading to toxic heme accumulation .
Biological Activity
(R)-Chloroquine, a stereoisomer of chloroquine, is an aminoquinoline compound originally developed for the treatment of malaria. Its biological activity extends beyond antimalarial effects, demonstrating antiviral properties and potential applications in various diseases. This article explores the biological activity of this compound, focusing on its mechanisms of action, efficacy in clinical studies, and potential therapeutic applications.
This compound exerts its biological effects through several mechanisms:
- Antimalarial Activity : It inhibits heme polymerase in Plasmodium species, preventing the conversion of toxic heme into hemazoin. This leads to the accumulation of toxic heme within the parasite, ultimately resulting in its death .
- Antiviral Effects : Recent studies have highlighted this compound's ability to inhibit viral replication by raising endosomal pH, which is crucial for viral entry into host cells. It interferes with the glycosylation of ACE2, a receptor utilized by coronaviruses for cell entry . In vitro studies have shown effective concentrations (EC50 and EC90) against SARS-CoV-2 at 1.13 μM and 6.90 μM, respectively .
- Immunomodulatory Effects : The compound has been noted to modulate immune responses by inhibiting pro-inflammatory cytokines and reducing T cell activation, which may be beneficial in autoimmune diseases .
Antimalarial Studies
This compound remains effective against malaria, particularly strains resistant to other treatments. In a study evaluating new chloroquine analogs against P. falciparum, several compounds demonstrated improved efficacy with IC50 values significantly lower than that of chloroquine itself (e.g., 0.21 µM for compound 7h) .
COVID-19 Research
The use of this compound in COVID-19 treatment has been controversial but noteworthy:
- In Vitro Studies : Research indicated that this compound could reduce viral loads in infected cells and improve outcomes when administered early in the infection process .
- Clinical Trials : A small randomized controlled trial reported that patients treated with this compound showed a faster reduction in viral carriage compared to controls. Specifically, all patients in the chloroquine group tested negative for the virus by day 13, while a significant proportion in the control group remained positive . However, concerns regarding methodology and bias were raised about these findings.
Case Studies
Several case studies have illustrated the potential benefits and risks associated with this compound:
- Malaria Treatment : A cohort study involving patients with malaria demonstrated a high cure rate with this compound treatment, reinforcing its role as a first-line therapy despite emerging resistance .
- COVID-19 Management : In a clinical setting, a group of 120 patients treated with this compound showed an average time to negative nucleic acid test results of 4.4 days post-treatment. Notably, no cases progressed to severe disease during this period .
Comparative Efficacy Table
Scientific Research Applications
Antimalarial Properties
(R)-Chloroquine was originally developed for the treatment of malaria caused by Plasmodium species. It functions by increasing the levels of heme in the blood, which is toxic to the malaria parasite. Its efficacy against malaria remains a significant aspect of its application, particularly in regions where malaria is endemic.
Rheumatological Applications
This compound has been widely utilized in treating autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis. The drug exhibits immunomodulatory effects, inhibiting antigen presentation and cytokine production in immune cells. Its role in managing these conditions is well-documented:
- Systemic Lupus Erythematosus : Chloroquine is effective in reducing flares and managing symptoms.
- Rheumatoid Arthritis : It is often part of combination therapies to enhance treatment efficacy and reduce cardiovascular risks associated with the disease .
Oncology
Recent studies have highlighted the potential of this compound as an adjunct therapy in cancer treatment. Its ability to induce autophagy and apoptosis makes it a candidate for enhancing the effectiveness of certain chemotherapeutic agents. Applications include:
- Breast Cancer : Research indicates that chloroquine can sensitize cancer cells to chemotherapy by inhibiting autophagy.
- Lymphomas : It has shown promise in treating various forms of lymphoma through similar mechanisms .
Viral Infections
The emergence of viral diseases has led to renewed interest in this compound for its antiviral properties. Notably, it has been studied for its effects against coronaviruses, including SARS-CoV and SARS-CoV-2:
- SARS-CoV-2 : In vitro studies revealed that chloroquine inhibits viral replication by altering endosomal pH and interfering with glycosylation of cellular receptors . Clinical trials during the COVID-19 pandemic suggested that chloroquine could reduce hospitalization duration and improve clinical outcomes, although results were mixed and further research is needed .
- Other Viral Infections : Chloroquine has also been investigated for its potential against other viral pathogens, including Zika virus and HIV .
Other Medical Applications
Beyond its primary uses, this compound has been explored for various other medical applications:
- Chronic Kidney Disease : Evidence suggests that chloroquine may provide benefits in managing chronic kidney disease through its anti-inflammatory properties .
- Dermatological Conditions : It is effective in treating skin disorders like discoid lupus erythematosus and psoriasis due to its immunomodulatory effects .
Data Table: Summary of Applications
| Application Area | Specific Uses | Mechanism of Action |
|---|---|---|
| Antimalarial | Treatment of malaria | Increases heme levels; toxic to Plasmodium species |
| Rheumatology | Systemic lupus erythematosus, rheumatoid arthritis | Immunomodulation; inhibits cytokine production |
| Oncology | Adjunct therapy for breast cancer, lymphomas | Induces autophagy; sensitizes cells to chemotherapy |
| Viral Infections | SARS-CoV-2, Zika virus | Alters endosomal pH; interferes with receptor binding |
| Chronic Kidney Disease | Management of CKD | Anti-inflammatory properties |
| Dermatological Conditions | Discoid lupus erythematosus, psoriasis | Immunomodulation |
Case Study 1: Chloroquine in COVID-19 Treatment
A clinical trial involving 120 patients treated with this compound showed promising results where patients had negative nucleic acid tests after treatment. However, methodological concerns were raised regarding the study's design and sample size .
Case Study 2: Chloroquine for Rheumatoid Arthritis
In a cohort study involving rheumatoid arthritis patients, those treated with this compound exhibited significant improvements in joint symptoms compared to those receiving standard care alone. The study highlighted the drug's role as a safe alternative to more expensive biologics .
Q & A
Basic Research Questions
Q. How do stereoselective differences between (R)- and (S)-chloroquine enantiomers influence pharmacokinetic and pharmacodynamic outcomes in preclinical models?
Methodological guidance: To assess stereoselectivity, use chiral chromatography (e.g., HPLC with chiral stationary phases) to separate enantiomers in plasma and tissue samples. Compare pharmacokinetic parameters (e.g., volume of distribution, half-life) and dose-response curves in rodent models. Evidence from rat studies shows (S)-chloroquine exhibits lower ED₅₀ values than (R)-enantiomers, suggesting metabolic or receptor-binding disparities .
Q. What experimental designs are recommended for characterizing chloroquine's tissue distribution and elimination kinetics in vivo?
Methodological guidance: Conduct single-dose pharmacokinetic studies with serial sampling of plasma, erythrocytes, and urine over extended periods (≥30 days) due to chloroquine’s prolonged half-life (146–333 hours). Use LC-MS/MS for quantification, noting erythrocyte-to-plasma concentration ratios (2–5:1) to evaluate compartmental accumulation . Incorporate radiolabeled chloroquine in autoradiography to map tissue-specific binding .
Q. How can researchers mitigate confounding factors when evaluating chloroquine’s antiviral efficacy in cell culture models?
Methodological guidance: Standardize viral load quantification (e.g., plaque assays, RT-qPCR) across multiple cell lines (e.g., Vero E6, Calu-3) and control for pH variations, as chloroquine’s lysosomotropic effects are pH-dependent. Include cytotoxicity assays (e.g., MTT) to differentiate antiviral activity from cell death. EC₅₀ values should be validated against positive controls (e.g., remdesivir) .
Advanced Research Questions
Q. What mechanisms underlie the discordance between in vitro antiviral activity and clinical inefficacy of chloroquine in COVID-19?
Methodological guidance: Analyze pharmacokinetic-pharmacodynamic (PK/PD) mismatches using physiologically based modeling. For example, lung tissue concentrations in humans may not reach inhibitory levels despite in vitro efficacy (EC₅₀ = 1–5 µM). Incorporate human bronchial epithelial cell models and compare free drug concentrations in alveolar lining fluid . Retrospective analysis of clinical trial data (e.g., dose-dependent QT prolongation) should inform safety ceilings .
Q. How can researchers address chloroquine resistance in Plasmodium falciparum while preserving (R)-chloroquine’s therapeutic potential?
Methodological guidance: Investigate resistance-reversal agents (e.g., verapamil) that inhibit chloroquine efflux via PfCRT mutations. Use isogenic parasite lines to compare chloroquine accumulation rates in resistant vs. susceptible strains. Evaluate this compound’s binding affinity to heme detoxification pathways using surface plasmon resonance (SPR) .
Q. What strategies optimize the design of randomized controlled trials (RCTs) for repurposing chloroquine in autoimmune or inflammatory diseases?
Methodological guidance: Implement adaptive trial designs with pre-specified interim analyses for efficacy/safety. Use biomarkers (e.g., angiotensin-converting enzyme levels in sarcoidosis) to stratify patients. For chronic use, monitor retinal toxicity via optical coherence tomography (OCT) and adjust dosing based on CYP2D6/CYP3A4 genotyping to minimize metabolic variability .
Q. How does chloroquine interfere with transfection efficiency in molecular biology experiments, and how can this be controlled?
Methodological guidance: Chloroquine inhibits lysosomal degradation of nucleic acids, enhancing transfection in short-term assays (e.g., 4–6 hours post-transfection). For gene editing (CRISPR) or viral vector studies, titrate chloroquine concentrations (5–25 µM) and include no-treatment controls. Note that prolonged exposure (>24 hours) may induce autophagy, confounding results .
Q. Data Contradiction and Validation
Q. How should researchers reconcile conflicting reports on chloroquine’s immunomodulatory effects in autoimmune encephalomyelitis models?
Methodological guidance: Replicate studies using standardized dosing regimens (e.g., 50 mg/kg intraperitoneal in mice) and validate outcomes with flow cytometry (Treg quantification) and histopathology. Discrepancies may arise from differences in disease induction protocols or chloroquine’s dual pro-/anti-inflammatory roles depending on exposure timing .
Q. What statistical approaches are robust for analyzing censored pharmacokinetic data from chloroquine studies with long-term follow-up?
Methodological guidance: Use non-compartmental analysis (NCA) with WinNonlin or Phoenix for terminal half-life estimation. For multi-exponential decay, apply nonlinear mixed-effects modeling (NONMEM) to account for inter-individual variability. Censor urinary recovery data beyond 119 days due to assay sensitivity limits .
Q. How can meta-analyses address heterogeneity in chloroquine’s clinical trial outcomes for COVID-19?
Methodological guidance: Perform living systematic reviews with frequent updates (e.g., Cochrane’s L·OVE platform) and subgroup analyses by disease severity, dosing, and comorbidities. Use GRADE criteria to assess evidence quality, noting retracted studies (e.g., Lancet 2020) and publication bias .
Comparison with Similar Compounds
Comparison with Structurally Similar Compounds
Chloroquine vs. Hydroxychloroquine
- Structural Differences : Hydroxychloroquine differs from chloroquine by a hydroxyl group substitution, altering binding interactions. Molecular docking reveals distinct binding residues for chloroquine (MET 165, GLN 189) versus hydroxychloroquine (CYS 145, MET 165) .
- Efficacy : Both compounds showed similar ototoxicity in zebrafish and mouse cochlear models, but hydroxychloroquine demonstrated marginally lower cytotoxicity in vitro .
Reversed Chloroquine Analogs (e.g., DM1157)
- Design : DM1157 incorporates a "reversal agent" to counteract chloroquine resistance mechanisms.
- Binding Properties : Despite sharing similar thermodynamic profiles with chloroquine (ΔHB ≈ 60 kcal/mol, ΔTm ≈ 1°C), DM1157 exhibits distinct binding constants to human serum albumin, suggesting stereochemical impacts on target affinity .
Chalcone Derivatives
- Activity on Resistant Strains: Chalcone derivatives (e.g., B14, B17) showed superior efficacy against chloroquine-resistant P. falciparum isolates compared to chloroquine, likely due to divergent mechanisms (e.g., non-hemozoin pathways) .
- Structural Advantage: The absence of a quinoline backbone in chalcones avoids resistance mediated by PfCRT mutations, a common chloroquine resistance mechanism .
Pharmacodynamic and Resistance Profiles
Antimalarial Activity
†CQ-S: Chloroquine-sensitive strains; ‡CQ-R: Chloroquine-resistant strains .
Resistance Mechanisms
- Efflux Pumps : Chloroquine-resistant P. falciparum expel the drug 40–50× faster via ATP-dependent transporters (e.g., PfCRT). Resistance is reversed by calcium channel blockers (verapamil) or tricyclic compounds (desipramine) .
- Cross-Resistance: 4-Aminoquinoline analogs (e.g., amodiaquine) face cross-resistance due to shared PfCRT-mediated efflux, whereas chalcones and reversed chloroquine derivatives remain effective .
Toxicity and Pharmacokinetics
Ototoxicity
Both chloroquine and hydroxychloroquine caused dose-dependent hair cell damage in zebrafish and mice, with outer cochlear cells being particularly vulnerable .
Cardiac Risks
Chloroquine derivatives prolong QT intervals, but enantiomer-specific cardiac toxicity data are lacking. Hydroxychloroquine’s hydroxyl group may reduce cardiotoxicity compared to chloroquine .
Pharmacokinetic Behavior
- Albumin Binding : Chloroquine and DM1157 bind human serum albumin with similar ΔHB (~60 kcal/mol) but differ in binding constants, implying stereochemistry-driven disparities in distribution .
- Metabolites : Bisdesethylchloroquine (a chloroquine metabolite) shows comparable cheminformatics profiles to the parent drug, suggesting retained bioavailability .
Properties
IUPAC Name |
(4R)-4-N-(7-chloroquinolin-4-yl)-1-N,1-N-diethylpentane-1,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21)/t14-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WHTVZRBIWZFKQO-CQSZACIVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCN(CC)CCC[C@@H](C)NC1=C2C=CC(=CC2=NC=C1)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H26ClN3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID401316905 | |
| Record name | (-)-Chloroquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID401316905 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
319.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
58175-87-4 | |
| Record name | (-)-Chloroquine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=58175-87-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | (-)-Chloroquine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0058175874 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (-)-Chloroquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID401316905 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | CHLOROQUINE, (R)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/WE58C2WV1T | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details




Synthesis routes and methods II
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













