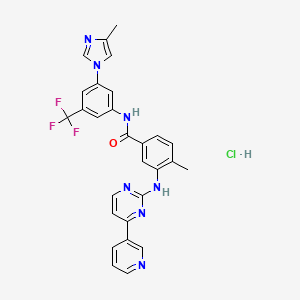
Nilotinib hydrochloride
Overview
Description
Nilotinib hydrochloride is a medication primarily used to treat chronic myelogenous leukemia (CML) that is positive for the Philadelphia chromosome. It is a second-generation tyrosine kinase inhibitor developed to overcome resistance to imatinib, another tyrosine kinase inhibitor. This compound is marketed under the brand name Tasigna and is known for its efficacy in treating both newly diagnosed and imatinib-resistant CML .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of nilotinib hydrochloride involves multiple steps, starting from the preparation of the core structure, which includes a benzamide derivative. The process typically involves the following steps:
Formation of the Core Structure: The core structure is synthesized by reacting 4-methyl-3-nitrobenzoic acid with 4-methyl-1H-imidazole in the presence of a coupling agent.
Formation of the Hydrochloride Salt: The final step involves converting the intermediate compound into this compound by reacting it with hydrochloric acid.
Industrial Production Methods: Industrial production of this compound follows a similar synthetic route but is optimized for large-scale production. The process ensures the formation of a stable crystalline form of this compound, which is essential for its pharmaceutical formulation .
Chemical Reactions Analysis
Types of Reactions: Nilotinib hydrochloride undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions, leading to the formation of degradation products.
Reduction: The nitro group in the intermediate compound is reduced to an amine during synthesis.
Substitution: The synthesis involves substitution reactions to introduce functional groups into the core structure.
Common Reagents and Conditions:
Catalytic Hydrogenation: Raney nickel in methanol is used for the reduction step.
Hydrochloric Acid: Used to form the hydrochloride salt.
Major Products Formed:
This compound: The primary product formed from the synthesis.
Degradation Products: Formed during oxidation and other stress conditions
Scientific Research Applications
Clinical Applications
1.1 Treatment of Chronic Myelogenous Leukemia
Nilotinib is primarily indicated for:
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase : It has shown superior efficacy compared to imatinib in achieving cytogenetic and molecular remission in newly diagnosed patients .
- Chronic phase and accelerated phase CML resistant or intolerant to prior therapy : This includes patients who have not responded to imatinib or other tyrosine kinase inhibitors .
Pharmacological Mechanism
Nilotinib functions by inhibiting the Bcr-Abl tyrosine kinase, which is responsible for the proliferation of CML cells. Its structural modifications enhance its binding affinity to the Bcr-Abl fusion protein, making it more potent than imatinib against both wild-type and many imatinib-resistant Bcr-Abl mutations .
Side Effects and Safety Profile
Common side effects include:
- Hematological effects: Thrombocytopenia, neutropenia, and anemia.
- Non-hematological effects: Rash, nausea, diarrhea, and fatigue.
Serious adverse effects can include QT prolongation and liver enzyme abnormalities . Regular monitoring is essential to manage these risks.
Case Studies
Case Study 1: Pediatric Patient with CML
A 16-year-old female patient with chronic myeloid leukemia was treated with nilotinib at a dose of 150 mg twice daily. After one month, due to liver enzyme derangements, her dose was adjusted. The patient demonstrated significant clinical improvement, highlighting nilotinib's effectiveness even in pediatric populations .
Case Study 2: Nilotinib in Parkinson's Disease
Recent studies have explored nilotinib's potential beyond oncology, particularly in neurodegenerative diseases like Parkinson's disease. A randomized trial evaluated nilotinib's safety and tolerability in Parkinson's patients, noting some adverse events but no significant differences compared to placebo groups . This suggests a potential role for nilotinib in modifying disease progression.
Emerging Research Areas
5.1 Combination Therapies
Research is ongoing into combining nilotinib with other agents to enhance therapeutic efficacy while minimizing side effects. For instance, studies are assessing its use alongside immunotherapies and other targeted therapies in various malignancies .
5.2 Other Cancer Types
While primarily used for CML, nilotinib is being investigated for its efficacy against other cancers such as acute lymphoblastic leukemia (ALL) and gastrointestinal stromal tumors (GISTs), particularly those harboring specific mutations that confer resistance to other therapies .
Summary Table of Nilotinib Applications
| Application Area | Description |
|---|---|
| Primary Use | Treatment of Philadelphia chromosome-positive chronic myelogenous leukemia (CML) |
| Indications | Newly diagnosed CML; CML resistant or intolerant to imatinib |
| Mechanism of Action | Inhibition of Bcr-Abl tyrosine kinase |
| Common Side Effects | Thrombocytopenia, neutropenia, rash, nausea |
| Emerging Uses | Investigated for use in Parkinson's disease and potential application in other cancers |
Mechanism of Action
Nilotinib hydrochloride exerts its effects by inhibiting the tyrosine kinase activity of the BCR-ABL protein, which is produced by the Philadelphia chromosome. It binds to the ATP-binding site of the BCR-ABL protein, preventing its phosphorylation activity. This inhibition blocks the downstream signaling pathways that are crucial for the survival and proliferation of leukemia cells .
Comparison with Similar Compounds
Imatinib: The first-generation tyrosine kinase inhibitor used to treat CML. Nilotinib hydrochloride was developed to overcome resistance to imatinib.
Dasatinib: Another second-generation tyrosine kinase inhibitor used to treat CML.
Bosutinib: A third-generation tyrosine kinase inhibitor used for CML treatment.
Uniqueness of this compound: this compound is unique due to its higher potency and specificity for the BCR-ABL protein compared to imatinib. It is also effective against several imatinib-resistant BCR-ABL mutations, making it a valuable option for patients who do not respond to imatinib .
Biological Activity
Nilotinib hydrochloride is a potent second-generation tyrosine kinase inhibitor (TKI) primarily used in the treatment of chronic myelogenous leukemia (CML). It specifically targets the BCR-ABL fusion protein, which is responsible for the pathogenesis of CML. This article explores its biological activity, mechanisms of action, clinical implications, and relevant case studies.
Nilotinib exerts its therapeutic effects by selectively inhibiting the tyrosine kinase activity of the BCR-ABL oncoprotein. This inhibition prevents downstream signaling pathways that promote cell proliferation and survival, leading to apoptosis in malignant cells. Nilotinib binds to the ATP-binding site of BCR-ABL with higher affinity than imatinib, making it effective against many imatinib-resistant mutations .
In Vitro Studies
Recent studies have demonstrated that nilotinib not only inhibits BCR-ABL but also affects other kinases such as c-KIT and PDGF, suggesting broader therapeutic potential beyond CML. For instance, a study evaluated novel nilotinib analogues and found that they exhibited significant antiplatelet activity and inhibited cancer cell proliferation in vitro. The analogues were shown to induce apoptosis in cancer cells more effectively than nilotinib itself .
Clinical Trials
A randomized clinical trial investigated nilotinib's safety and effects on biomarkers in patients with Parkinson's disease, revealing that it altered cerebrospinal fluid biomarkers related to dopamine metabolism and showed potential as a disease-modifying therapy . The trial indicated that doses of 150 mg and 300 mg were reasonably safe and effective in modifying disease markers without significant adverse effects.
Case Studies
- Child with Chronic Myeloid Leukemia : A notable case involved a 16-year-old female diagnosed with CML who achieved complete remission after three months of nilotinib treatment. This case highlights nilotinib's efficacy even in pediatric patients, although it was used off-label due to lack of specific pediatric guidelines .
- Safety Profile : Another study assessed the safety profile of nilotinib in various patient populations, noting common side effects such as thrombocytopenia, neutropenia, and hepatotoxicity. Despite these side effects, nilotinib's benefits in achieving remission rates superior to imatinib were emphasized .
Summary of Findings
The following table summarizes key findings from various studies on nilotinib's biological activity:
Q & A
Basic Research Questions
Q. What is the molecular mechanism of nilotinib hydrochloride in inhibiting Bcr-Abl tyrosine kinase, and how does this inform experimental design for in vitro studies?
this compound selectively binds to the ATP-binding site of Bcr-Abl, stabilizing the inactive conformation of the kinase domain. This inhibition blocks downstream signaling pathways critical for leukemic cell proliferation. For in vitro studies, researchers should use dose-response assays (e.g., IC50 determination in murine bone marrow progenitor cells, where IC50 <30 nM) and validate target engagement via phospho-Bcr-Abl Western blotting . Include controls with imatinib-resistant cell lines (e.g., T315I mutants) to confirm specificity .
Q. What are the standard protocols for synthesizing this compound in a laboratory setting, and how can purity be ensured?
Laboratory-scale synthesis typically involves coupling intermediates (e.g., Intermediate-I and Intermediate-II) in polar aprotic solvents like N-methyl pyrrolidone, followed by HCl salt formation. Key steps include refluxing with thionyl chloride and recrystallization in toluene. Purity (>99%) is verified via HPLC with UV detection at 254 nm, referencing pharmacopeial standards (e.g., USP monographs). Ensure residual solvents comply with ICH Q3C guidelines by gas chromatography .
Q. How should this compound be stored to maintain stability in experimental conditions?
Crystalline this compound is stable at 2–8°C in desiccated, light-protected containers. For short-term use in cell culture, prepare stock solutions in DMSO (10 mM), aliquot to avoid freeze-thaw cycles, and store at -80°C. Thermal stability studies indicate degradation above 150°C, necessitating avoidance of high-temperature processing .
Advanced Research Questions
Q. How can researchers investigate and overcome nilotinib resistance in CML cell lines, particularly regarding kinase domain mutations?
Resistance mechanisms (e.g., T315I, Y253H mutations) are studied using mutagenesis screens and structural modeling of Bcr-Abl-nilotinib interactions. To overcome resistance, combine nilotinib with allosteric inhibitors (e.g., asciminib) or autophagy inducers (e.g., AMPK activators). Use isogenic cell lines expressing mutant Bcr-Abl and assess viability via MTT assays. Cross-reference clinical trial data on resistance rates (e.g., 7% progression to blast crisis over 5 years in imatinib studies) .
Q. What methodological approaches are used to assess nilotinib's off-target effects in kinase profiling studies?
Employ kinome-wide selectivity screens using recombinant kinase panels (e.g., Eurofins KinaseProfiler). Prioritize kinases with >50% inhibition at 1 µM nilotinib. Validate off-target hits in primary cells (e.g., cardiac fibroblasts for PDGFR inhibition) using functional assays (e.g., collagen contraction assays). Correlate findings with clinical adverse event profiles (e.g., QTc prolongation) .
Q. How should researchers design experiments to evaluate nilotinib in combination with other TKIs for synergistic effects?
Use a factorial design with nilotinib and partner drugs (e.g., dasatinib) at multiple concentrations. Calculate synergy via the Chou-Talalay combination index (CI <1 indicates synergy). Include single-agent controls and measure apoptosis (Annexin V/PI flow cytometry) and clonogenic survival. Preclinical in vivo models should use NSG mice engrafted with patient-derived CML cells, monitoring BCR-ABL transcript levels via qRT-PCR .
Q. What advanced analytical techniques are recommended for quantifying nilotinib and its degradation products in pharmacokinetic studies?
Develop a stability-indicating UPLC method with a C18 column (2.1 × 50 mm, 1.7 µm), mobile phase of 0.1% formic acid/acetonitrile, and UV detection at 280 nm. Validate per ICH Q2(R1) guidelines for linearity (1–100 µg/mL), precision (RSD <2%), and LOQ (0.1 µg/mL). For degradation studies, stress samples under acidic/alkaline hydrolysis, oxidation (H2O2), and photolysis, identifying impurities via LC-MS/MS .
Q. How should contradictory data on nilotinib-induced autophagy be reconciled in preclinical studies?
Discrepancies may arise from cell type-specific AMPK activation thresholds. Replicate experiments in multiple models (e.g., K562 vs. primary CD34+ cells) using autophagy flux assays (LC3-II turnover via Western blot with bafilomycin A1). Control for off-target effects via CRISPR knockout of AMPK subunits. Cross-validate findings with lysosomal inhibitors (e.g., chloroquine) .
Q. Methodological Best Practices
- Data Reproducibility : Follow NIH preclinical guidelines for reporting experimental conditions (e.g., cell line authentication, passage numbers, statistical methods) .
- Statistical Analysis : Use two-tailed t-tests for pairwise comparisons and ANOVA with Tukey post-hoc correction for multi-group studies. Report exact p-values and effect sizes .
- Ethical Compliance : For in vivo work, adhere to ARRIVE 2.0 guidelines, including randomization, blinding, and power calculations .
Properties
IUPAC Name |
4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C28H22F3N7O.ClH/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38;/h3-16H,1-2H3,(H,35,39)(H,33,36,37);1H | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VTGGYCCJUPYZSX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C=C(C=C1)C(=O)NC2=CC(=CC(=C2)C(F)(F)F)N3C=C(N=C3)C)NC4=NC=CC(=N4)C5=CN=CC=C5.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C28H23ClF3N7O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60238968 | |
| Record name | Nilotinib hydrochloride anhydrous | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60238968 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
566.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
923288-95-3 | |
| Record name | Nilotinib hydrochloride anhydrous | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0923288953 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nilotinib hydrochloride anhydrous | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60238968 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 4-Methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide hydrochloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NILOTINIB HYDROCHLORIDE ANHYDROUS | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/K37N7BYX3X | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.














