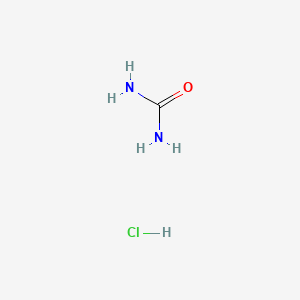
Urea hydrochloride
Overview
Description
Urea hydrochloride (CH₄N₂O·HCl) is a salt formed by the reaction of urea with hydrochloric acid. It is widely used in industrial processes, including petroleum well acidization, metal cleaning, and pH adjustment, due to its reduced corrosivity compared to pure hydrochloric acid . Its efficacy in dissolving water-insoluble metal salts (e.g., CaCO₃, FeS) and stabilizing acidic solutions makes it a safer alternative to traditional acids .
Preparation Methods
Synthetic Routes and Reaction Conditions: Urea hydrochloride can be synthesized by reacting urea with hydrochloric acid. The reaction is typically carried out in an aqueous solution, where urea is dissolved in water and hydrochloric acid is added gradually. The reaction proceeds as follows:
CO(NH2)2+HCl→CO(NH2)2HCl
Industrial Production Methods: In industrial settings, this compound is produced by mixing urea and hydrochloric acid in large reactors. The reaction is controlled to ensure complete conversion of urea to this compound. The product is then crystallized, filtered, and dried to obtain the final product.
Types of Reactions:
Hydrolysis: this compound undergoes hydrolysis in the presence of water, leading to the formation of ammonia and carbon dioxide.
Decomposition: Upon heating, this compound decomposes to release ammonia and hydrochloric acid.
Substitution: this compound can participate in substitution reactions with various organic compounds, forming substituted urea derivatives.
Common Reagents and Conditions:
Hydrolysis: Water is the primary reagent, and the reaction is typically carried out at room temperature.
Decomposition: Heating is required, usually at temperatures above 100°C.
Substitution: Organic reagents such as amines or alcohols are used, often in the presence of a catalyst.
Major Products Formed:
Hydrolysis: Ammonia and carbon dioxide.
Decomposition: Ammonia and hydrochloric acid.
Substitution: Substituted urea derivatives.
Scientific Research Applications
Isolation of Helicobacter pylori
Urea hydrochloride is used effectively in the isolation of Helicobacter pylori, a bacterium linked to gastric ulcers. A study demonstrated that pretreatment with a mixture of 0.06 N hydrochloric acid and 0.08 M urea significantly enhanced the recovery rates of H. pylori from saliva samples. The optimal conditions allowed for reproducible isolation even at low inocula levels (≤10² CFU/ml) .
Table 1: Survival Rates of H. pylori under Various Conditions
| HCl Concentration (N) | Urea Concentration (mM) | pH | Survival Rate (%) |
|---|---|---|---|
| 0.24 | 1000 | 1.2 | 4.75 |
| 0.12 | 500 | 1.2 | 5.5 |
| 0.06 | 200 | 1.2 | 3.45 |
| 0.03 | 100 | 1.2 | 1 |
This method proved more effective than traditional culture methods, highlighting the utility of this compound in microbiological research.
Moisturizing and Keratolytic Effects
In dermatology, this compound is recognized for its emollient properties and effectiveness as a keratolytic agent. It enhances skin barrier function by increasing water retention in the stratum corneum, making it beneficial for conditions like ichthyosis and psoriasis . Clinical trials have shown that formulations containing urea at concentrations ranging from 10% to 50% significantly improve skin hydration and reduce symptoms of dryness and scaling.
Table 2: Clinical Trials on Urea-Based Formulations
| Condition | Urea Concentration (%) | Outcome |
|---|---|---|
| Ichthyosis vulgaris | 10 | Reduced flaking and roughness |
| Nail disorders | 40-50 | Improved nail permeability and reduced discomfort |
| Tinea pedis | 10 | Enhanced response to antifungal treatments |
Cleaning Agent for Membranes
This compound has been explored as a cleaning agent for biofouling control in reverse osmosis membrane systems. A pilot-scale study indicated that using urea combined with hydrochloric acid effectively increased the normalized permeate flux compared to conventional cleaning agents .
Table 3: Performance Comparison of Cleaning Agents
| Cleaning Agent | Normalized Permeate Flux (Lm²/h) | Reduction in Active Biomass (pg ATP/cm²) |
|---|---|---|
| NaOH + HCl | X | Y |
| Urea + HCl | X + Δ | Y - Δ |
This application underscores the potential of this compound in enhancing operational efficiency in water treatment facilities.
Mechanism of Action
Urea hydrochloride exerts its effects through various mechanisms depending on its application. In biological systems, it acts as a denaturant by disrupting hydrogen bonds in proteins and nucleic acids. This leads to the unfolding of these macromolecules, making them more accessible for study or modification. In industrial applications, this compound acts as a catalyst or reactant, facilitating chemical reactions and improving product yields.
Comparison with Similar Compounds
Guanidine Hydrochloride (GdnHCl)
GdnHCl (CH₆N₃·HCl) is another widely used denaturant and chaotropic agent. Key differences include:
Example : For β-lactoglobulin, ΔGapp (free energy of unfolding) in water is 11.7 ± 0.8 kcal/mol with urea vs. 3.5 M GdnHCl required for similar destabilization .
Urea
While urea (CH₄N₂O) shares structural similarities with this compound, key differences arise from the absence of HCl:
Example : this compound reduces the hydrophobicity of aliphatic side chains (e.g., Val, Leu) more effectively than urea alone .
Other Hydrochloride Salts
Compounds like Amiloride Hydrochloride (C₆H₈ClN₇O) and Eucatropine Hydrochloride (C₁₇H₂₆ClNO₃) differ in application but share structural features:
Protein Denaturation
Biological Activity
Urea hydrochloride is a compound that has garnered attention in various fields, particularly in medicinal chemistry and biochemistry. This article delves into the biological activities associated with this compound, highlighting its applications, mechanisms of action, and relevant case studies.
Overview of this compound
This compound is formed by the reaction of urea with hydrochloric acid, resulting in a white crystalline substance that is soluble in water. It has been used in various medical and biochemical applications due to its ability to interact with proteins and other biological molecules.
This compound exhibits its biological activity primarily through the following mechanisms:
- Hydrogen Bonding : The urea moiety can form multiple stable hydrogen bonds with protein targets, which is crucial for drug-target interactions. This property enhances the bioactivity of compounds containing urea derivatives, making them effective in medicinal applications .
- Protein Stabilization : As a chaotropic agent, urea can disrupt hydrophobic interactions within proteins, leading to denaturation or stabilization depending on the context. This property is leveraged in various biochemical assays and therapeutic applications .
- Antiproliferative Activity : Recent studies have shown that certain urea derivatives possess significant antiproliferative effects against cancer cell lines. For instance, a study identified a novel urea derivative that induced cell cycle arrest at the G0/G1 phase in colorectal cancer cells .
1. Antiproliferative Effects
A study evaluated several urea derivatives for their antiproliferative activity against various cancer cell lines, including HCT116 (colorectal), A375 (melanoma), and MiaPaca-2 (pancreatic). The findings indicated that:
- Compound 14 demonstrated the highest activity across all tested cancer cell lines.
-
The IC50 values for selected compounds were as follows:
Compound IC50 (µM) Cell Line 13 14 HCT116 14 9.8 HCT116 16 12.0 HCT116
These compounds exhibited better antiproliferative activity than cisplatin, indicating their potential as therapeutic agents .
2. Urea Hydrolysis Studies
Research on urea hydrolysis in human urine has shown that urea can be converted into ammonia and bicarbonate through enzymatic reactions. This process is significant in understanding how urea interacts with biological systems:
- In experiments simulating urea hydrolysis, it was observed that approximately 25% of urea was transformed into ammonia over a period of 240 minutes. This transformation is critical for various physiological processes and waste management .
3. Clinical Applications
This compound has been explored for its role in enhancing the isolation of Helicobacter pylori, a bacterium linked to gastric ulcers. A study found that pretreatment with a solution containing this compound significantly improved recovery rates of H. pylori compared to controls:
| HCl Concentration (N) | Survival Rate (%) at pH |
|---|---|
| 0.06 | 15.7 |
| 0.12 | 5.5 |
| 0.24 | 3.2 |
This suggests that this compound can be effectively utilized in microbiological techniques to isolate specific pathogens .
Q & A
Basic Research Questions
Q. What are the standard experimental protocols for synthesizing urea hydrochloride with high purity, and how can researchers validate the synthesis process?
- Methodological Answer : this compound synthesis typically involves the reaction of urea with hydrochloric acid under controlled stoichiometric conditions. Researchers should monitor pH and temperature during crystallization to ensure purity . Post-synthesis validation requires techniques like nuclear magnetic resonance (NMR) for structural confirmation and high-performance liquid chromatography (HPLC) to assess purity (>99%) . Titration against standardized base solutions can quantify residual acid impurities .
Q. Which spectroscopic and chromatographic techniques are optimal for characterizing this compound in complex mixtures?
- Methodological Answer : Fourier-transform infrared spectroscopy (FTIR) identifies functional groups (e.g., NH and Cl bonds), while X-ray diffraction (XRD) confirms crystalline structure . For mixtures, gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS) can distinguish this compound from co-solvents or degradation products . Differential scanning calorimetry (DSC) quantifies thermal stability and phase transitions .
Q. How can researchers assess this compound’s stability under varying pH and temperature conditions?
- Methodological Answer : Accelerated stability studies should be conducted at elevated temperatures (e.g., 40°C, 60°C) and pH ranges (2–12) to simulate degradation pathways. Kinetic modeling (e.g., Arrhenius plots) predicts shelf life, while UV-Vis spectroscopy tracks absorbance changes indicative of decomposition . For hydrolytic stability, monitor chloride ion release via ion-selective electrodes .
Advanced Research Questions
Q. How do researchers resolve contradictions in this compound’s thermodynamic data (e.g., solubility, enthalpy) across studies?
- Methodological Answer : Discrepancies often arise from solvent polarity, ionic strength, or impurities. Systematic replication under controlled conditions (e.g., standardized buffer systems) is critical . Advanced computational models, such as molecular dynamics simulations, can predict solubility parameters and validate experimental data . Meta-analyses of existing literature should account for methodological differences (e.g., calorimetry vs. gravimetric methods) .
Q. What experimental designs minimize interference from this compound’s denaturation effects in protein studies?
- Methodological Answer : this compound’s chaotropic effects can be mitigated by using lower concentrations (<4 M) or combining it with stabilizing agents (e.g., glycerol). Analytical ultracentrifugation (AUC) or circular dichroism (CD) spectroscopy can differentiate between reversible and irreversible protein denaturation . Control experiments with guanidine hydrochloride or thiourea help isolate urea-specific effects .
Q. How can researchers optimize this compound’s role in nucleic acid precipitation without compromising yield or purity?
- Methodological Answer : Ethanol or isopropanol co-precipitation protocols require pH adjustment (e.g., sodium acetate buffer at pH 5.2) to enhance nucleic acid-urea interactions. Quantify RNA/DNA recovery using spectrophotometric A260/A280 ratios and gel electrophoresis. Compare efficiency against traditional chaotropic salts (e.g., guanidinium thiocyanate) to identify trade-offs .
Q. What strategies address conflicting results in this compound’s catalytic efficiency in organic synthesis?
- Methodological Answer : Contradictions may stem from solvent polarity or substrate specificity. Design a factorial experiment varying this compound concentration (0.1–2 M), solvent (aqueous vs. polar aprotic), and temperature. Reaction progress can be tracked via thin-layer chromatography (TLC) or in-situ FTIR. Compare kinetic data (e.g., turnover frequency) with computational density functional theory (DFT) models to identify mechanistic outliers .
Q. Data Analysis and Validation
Q. How should researchers validate this compound’s interaction with metal ions in coordination chemistry studies?
- Methodological Answer : Use isothermal titration calorimetry (ITC) to quantify binding constants (Kd) and stoichiometry. X-ray absorption spectroscopy (XAS) or inductively coupled plasma mass spectrometry (ICP-MS) confirms metal-urea complex formation. Control experiments with EDTA or competing ligands (e.g., chloride ions) isolate specific interactions .
Q. What statistical approaches are recommended for analyzing this compound’s dose-response effects in cell culture studies?
- Methodological Answer : Dose-response curves should use nonlinear regression models (e.g., sigmoidal fits) to calculate EC50/IC50 values. Account for cytotoxicity via MTT assays and normalize data to untreated controls. Principal component analysis (PCA) identifies confounding variables (e.g., pH shifts from HCl release) .
Properties
IUPAC Name |
urea;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/CH4N2O.ClH/c2-1(3)4;/h(H4,2,3,4);1H | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VYWQTJWGWLKBQA-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C(=O)(N)N.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
CH5ClN2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6060139 | |
| Record name | Urea hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6060139 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
96.52 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Liquid; Water or Solvent Wet Solid, White to slightly yellow deliquescent solid; [Merck Index] Yellowish-white odorless powder; [Redox Pty MSDS] | |
| Record name | Urea, hydrochloride (1:1) | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Urea hydrochloride | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/18581 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
CAS No. |
506-89-8 | |
| Record name | Urea hydrochloride | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=506-89-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Urea hydrochloride | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000506898 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Urea, hydrochloride (1:1) | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Urea hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6060139 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Urea hydrochloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.007.327 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | UREA HYDROCHLORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/RCE1061F6A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.
















