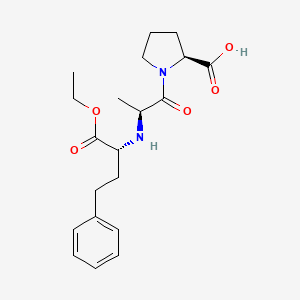
enalapril
Overview
Description
Enalapril is an angiotensin-converting enzyme (ACE) inhibitor widely used for hypertension, heart failure, and diabetic nephropathy. It acts as a prodrug, metabolized in the liver to its active form, enalaprilat, which inhibits ACE, reducing angiotensin II production and aldosterone secretion. This mechanism lowers blood pressure (BP) and decreases cardiac afterload, making it effective in managing hypertension and heart failure with reduced ejection fraction (HFrEF) .
This compound’s pharmacokinetics include a half-life of 11 hours (prolonged in renal impairment) and bioavailability of ~60%. It is often administered once daily (5–40 mg), with dose adjustments based on clinical response and tolerability .
Preparation Methods
Synthetic Routes and Reaction Conditions
enalapril is synthesized through a multi-step process involving the reaction of L-proline with ethyl 4-phenylbutanoate to form an intermediate, which is then coupled with L-alanine . The reaction conditions typically involve the use of organic solvents and catalysts to facilitate the formation of the desired product.
Industrial Production Methods
In industrial settings, the production of this compound involves large-scale synthesis using optimized reaction conditions to ensure high yield and purity. The process includes rigorous quality control measures to meet pharmaceutical standards .
Chemical Reactions Analysis
Metabolic Activation to Enalaprilat
Enalapril is a prodrug requiring enzymatic hydrolysis to release its active metabolite, enalaprilat . This transformation occurs via cleavage of the ethyl ester group (-OCH2CH3) (Fig. 1), facilitated by hepatic and intestinal esterases .
Key Reaction Steps:
Structural Determinants:
-
Enalaprilat’s carboxylate group enables potent ACE inhibition, contrasting with Captopril’s thiol-based mechanism .
Solid-State Degradation via Intramolecular Cyclization
Under thermal stress (>120°C), this compound maleate undergoes intramolecular cyclization to form This compound diketopiperazine (DKP) , a major degradation product .
Kinetic Parameters (Isothermal Study):
| Temperature (°C) | Induction Period (min) | Rate Constant (k) | Activation Energy (Eₐ) |
|---|---|---|---|
| 120 | 25.2 | 0.024 h⁻¹ | 195 ± 12 kJ/mol |
| 130 | 10.5 | 0.042 h⁻¹ |
Mechanism:
-
Autocatalytic Reaction: Sigmoidal decomposition curves indicate self-accelerating kinetics .
-
FT-IR Evidence: Peaks at 1649 cm⁻¹ (this compound) diminish, while 1672 cm⁻¹ (DKP) and 3250 cm⁻¹ (water) emerge .
Reactivity with Oxidizing Agents
This compound formulations may react with strong oxidizing agents, though specific products are not well-characterized. Hazardous conditions include:
pH-Dependent Stability
This compound’s ester group renders it susceptible to hydrolysis in aqueous environments:
Comparative Stability with Other ACE Inhibitors
Analytical Detection of Degradation
Scientific Research Applications
FDA-Approved Indications
Enalapril is approved by the U.S. Food and Drug Administration (FDA) for several key indications:
- Hypertension : this compound effectively lowers blood pressure in patients with essential and renovascular hypertension.
- Heart Failure : It is utilized in managing chronic heart failure, particularly in patients with reduced ejection fraction (EF).
- Asymptomatic Left Ventricular Dysfunction : this compound helps prevent the progression to symptomatic heart failure in patients with asymptomatic left ventricular dysfunction .
Hypertension Management
This compound is effective in managing both essential and secondary hypertension. It has demonstrated significant reductions in systolic and diastolic blood pressure across various patient populations, including those with low-renin hypertension .
Table 1: Efficacy of this compound in Hypertension
| Study | Population | Dose | Outcome |
|---|---|---|---|
| Study A | Newly diagnosed hypertensives | 10 mg/day | Significant BP reduction |
| Study B | Patients with chronic kidney disease | 5-20 mg/day | Improved renal function |
Heart Failure Treatment
In heart failure management, this compound has been shown to improve symptoms and reduce hospitalizations. A landmark study demonstrated that this compound reduces mortality in patients with heart failure with reduced EF .
Table 2: Effects of this compound on Heart Failure
| Study | Population | Outcome Measure | Result |
|---|---|---|---|
| Study C | Heart failure patients | Mortality rate | Reduced by 20% |
| Study D | Congestive heart failure | Quality of life score | Improved significantly |
Cardiovascular Protection
This compound has been associated with improved endothelial function compared to other ACE inhibitors like lisinopril. A recent study highlighted its superior effects on flow-mediated dilation (FMD), which is crucial for cardiovascular health .
Table 3: Endothelial Function Improvement
| Comparison | This compound FMD Improvement (%) | Lisinopril FMD Improvement (%) |
|---|---|---|
| Baseline vs Post-Treatment | 15% | 10% |
Case Study 1: Hypertension Management
A 55-year-old male with stage 2 hypertension was treated with this compound (10 mg daily). After six weeks, his blood pressure decreased from 160/100 mmHg to 130/85 mmHg without significant side effects.
Case Study 2: Heart Failure
A 70-year-old female with heart failure and an EF of 30% was initiated on this compound (5 mg daily). Over six months, her symptoms improved significantly, evidenced by a reduction in NYHA class from III to II.
Mechanism of Action
enalapril exerts its effects by inhibiting the angiotensin-converting enzyme, which is responsible for converting angiotensin I to angiotensin II, a potent vasoconstrictor . By inhibiting this enzyme, this compound reduces the levels of angiotensin II, leading to vasodilation and decreased blood pressure. The molecular targets include the angiotensin-converting enzyme and the renin-angiotensin-aldosterone system .
Comparison with Similar Compounds
Comparison with Other ACE Inhibitors
Spirapril
A 1997 double-blind study compared spirapril (6 mg/day) with enalapril (5–20 mg/day) in mild-to-moderate hypertension. Both drugs significantly reduced diastolic BP (DBP) and systolic BP (SBP) versus placebo. However, spirapril showed superior DBP reduction at peak (-17.4 mmHg vs. This compound’s -14.8 mmHg) and trough (-14.7 mmHg vs. -12.4 mmHg). Trough/peak ratios were similar (84% vs. 82%), but spirapril’s greater DBP reduction suggests enhanced efficacy at equivalent doses .
Imidapril
A 2006 study compared imidapril with this compound in essential hypertension. While both achieved comparable BP control, imidapril exhibited a significantly lower incidence of cough (0.9% vs. 7.0% with this compound), a common ACE inhibitor side effect linked to bradykinin accumulation. This suggests imidapril may offer better tolerability in cough-sensitive patients .
Structural Homologues: Lisinopril, Ramipril, and Trandolapril
- Both drugs share similar clinical applications but differ in dosing (lisinopril: 10–40 mg/day) .
- Ramipril: Structurally analogous to this compound, ramipril is also a prodrug.
- Trandolapril : Metabolized to trandolaprilat, it has a longer half-life (~24 hours), permitting once-daily dosing. Studies suggest comparable efficacy to this compound in hypertension .
Comparison with Angiotensin Receptor Blockers (ARBs)
Losartan
In a 1998 renal ablation model, losartan (ARB) and this compound (ACE inhibitor) showed equivalent renoprotection, reducing glomerulosclerosis and interstitial fibrosis.
Olmesartan
Olmesartan (ARB) has a half-life (12 hours) and antihypertensive potency comparable to this compound.
Comparison with ARNI (Sacubitril/Valsartan)
Sacubitril/valsartan (ARNI) outperformed this compound in the PARADIGM-HF and PIONEER-HF trials. In HFrEF patients, ARNI reduced cardiovascular mortality by 20% and HF hospitalizations by 21% versus this compound. It also lowered NT-proBNP levels faster post-acute HF, supporting its superiority in HF management .
Combination Therapies
This compound + Hydrochlorothiazide (HCTZ)
A 1998 study demonstrated that this compound/HCTZ (20/6 mg) reduced albuminuria more effectively than atenolol (50 mg) in hypertensive patients, despite similar BP control. This highlights synergistic renal benefits with low-dose thiazide combinations .
This compound + Losartan
Structural and Pharmacological Similarities
SuperPred identified six compounds structurally similar to this compound (Tanimoto coefficient >0.8), including cilazapril and ramipril, all sharing ACE-inhibiting effects . QueryChem highlighted homologues like lisinopril and ramipril, which share clinical applications but differ in pharmacokinetics (e.g., prodrug status) .
Biological Activity
Enalapril is a widely used angiotensin-converting enzyme (ACE) inhibitor known for its antihypertensive properties and role in treating heart failure. This article explores the biological activity of this compound, focusing on its mechanisms of action, therapeutic effects, and recent research findings.
This compound functions primarily by inhibiting the ACE, an enzyme responsible for converting angiotensin I to angiotensin II, a potent vasoconstrictor. This inhibition leads to several physiological effects:
- Decreased Blood Pressure : By reducing angiotensin II levels, this compound promotes vasodilation and decreases vascular resistance, resulting in lower blood pressure .
- Increased Bradykinin Levels : ACE also degrades bradykinin, a peptide that promotes vasodilation. Inhibition of ACE leads to increased bradykinin levels, which may contribute to some of the drug's beneficial effects but also to side effects such as cough .
- Renal Effects : this compound has natriuretic and uricosuric properties, promoting sodium excretion and potentially benefiting patients with fluid retention .
Pharmacokinetics
This compound is a prodrug that is converted in the liver to its active form, enalaprilat. Key pharmacokinetic characteristics include:
- Absorption : this compound is well absorbed orally, with peak plasma concentrations occurring within one hour.
- Distribution : The active metabolite enalaprilat penetrates various tissues, particularly the kidneys and vascular tissues, but shows limited ability to cross the blood-brain barrier .
- Metabolism : Approximately 60% of the absorbed dose is converted to enalaprilat. No significant metabolism occurs beyond this conversion .
- Elimination : The drug is primarily eliminated through renal pathways, necessitating dosage adjustments in patients with renal impairment.
Therapeutic Applications
This compound is indicated for various cardiovascular conditions:
- Hypertension : It effectively lowers blood pressure across all grades of essential hypertension.
- Heart Failure : this compound improves symptoms and outcomes in patients with heart failure by reducing preload and afterload .
- Diabetic Nephropathy : It offers renal protective effects in diabetic patients by reducing glomerular pressure .
Recent Research Findings
Recent studies have explored additional biological activities and potential therapeutic applications of this compound beyond traditional uses:
- Anti-Cancer Properties : A study indicated that this compound may enhance the anti-tumor effects of 5-fluorouracil (5-FU) in colorectal cancer cells by increasing apoptosis and modulating oxidative stress markers. This compound inhibited cell proliferation in a dose-dependent manner and affected migration through modulation of matrix metalloproteinases (MMPs) .
- Cardiovascular Outcomes : The PARADIGM-HF trial highlighted this compound's efficacy compared to newer agents like LCZ696 (sacubitril/valsartan) in managing heart failure with reduced ejection fraction (HFrEF), underscoring its importance in contemporary heart failure management protocols .
- Respiratory Effects : Research has shown that this compound inhibits aminopeptidase P activity in the mouse trachea, which may relate to its side effects such as cough. This differential effect compared to other ACE inhibitors suggests unique interactions within respiratory pathways .
Table 1: Pharmacological Properties of this compound
| Property | Description |
|---|---|
| Drug Class | ACE Inhibitor |
| Prodrug | Yes (converted to enalaprilat) |
| Primary Action | Inhibition of angiotensin II production |
| Secondary Effects | Increased bradykinin levels |
| Bioavailability | ~60% |
| Elimination Half-Life | 11 hours (enalaprilat) |
Table 2: Clinical Applications of this compound
| Condition | Evidence Level | Notes |
|---|---|---|
| Hypertension | Strong | First-line treatment for hypertension |
| Heart Failure | Strong | Improves morbidity and mortality |
| Diabetic Nephropathy | Moderate | Renoprotective effects observed |
| Colorectal Cancer | Emerging | Potential adjunct therapy with chemotherapy agents |
Q & A
Q. Basic: What are the primary mechanisms of action of enalapril in managing heart failure, and how are these effects quantified in clinical trials?
This compound, an angiotensin-converting enzyme (ACE) inhibitor, reduces angiotensin II production and aldosterone secretion, mitigating vasoconstriction and sodium retention. In heart failure trials (e.g., SOLVD and CONSENSUS), its efficacy is quantified using endpoints like cardiovascular mortality, hospitalization rates, and changes in ejection fraction. For example, the SOLVD trial demonstrated a 16% risk reduction in all-cause mortality with this compound compared to placebo, analyzed via Cox proportional hazards models .
Q. Advanced: How does this compound compare to angiotensin receptor-neprilysin inhibitors (ARNIs) like sacubitril/valsartan (LCZ696) in reducing cardiovascular mortality?
The PARADIGM-HF trial (double-blind, randomized, 8442 patients) compared LCZ696 (200 mg twice daily) to this compound (10 mg twice daily). LCZ696 reduced the composite risk of cardiovascular death or heart failure hospitalization by 20% (HR 0.80, 95% CI 0.73–0.87; P<0.001). Methodologically, the trial used prespecified stopping rules for overwhelming efficacy and adjusted for covariates like renal function and baseline NYHA class. Subgroup analyses confirmed consistency across demographics .
Q. Advanced: What methodological approaches are used to assess this compound’s renoprotective effects in diabetic patients?
The RASS trial (n=285, normoalbuminuric type 1 diabetes) employed renal biopsy to measure mesangial fractional volume and glomerular filtration rate (GFR) via iohexol clearance. Despite no significant nephropathy progression reduction, retinopathy progression was reduced by 65% with this compound (OR 0.35, 95% CI 0.14–0.85), analyzed using logistic regression adjusted for blood pressure changes. These findings highlight the need for multimodal endpoints in diabetic trials .
Q. Advanced: How do racial differences influence this compound’s efficacy in heart failure patients?
A matched-cohort analysis of SOLVD data revealed this compound reduced heart failure hospitalization risk by 44% in white patients (HR 0.56, 95% CI 0.43–0.73) but showed no benefit in Black patients (HR 0.95, 95% CI 0.72–1.26). The study matched cohorts by ejection fraction, age, and sex, with sensitivity analyses confirming robustness. This underscores the need for pharmacogenomic studies to address racial disparities .
Q. Basic: What statistical methods are appropriate for analyzing this compound’s effect on composite endpoints like mortality and hospitalization?
Large-scale trials (e.g., PARADIGM-HF, CSPPT) use Cox proportional hazards models to analyze time-to-event outcomes, adjusting for covariates like baseline risk factors. For continuous outcomes (e.g., blood pressure), linear mixed-effects models or ANOVA with post-hoc tests (e.g., Dunnett’s) are applied. The CSPPT trial also utilized intention-to-treat analysis with stratification by MTHFR genotype .
Q. Advanced: What molecular interactions underlie this compound’s potential anti-arthritis effects?
Network pharmacology and molecular docking studies identified this compound’s binding to TNF-α (ΔG = -5.749 kcal/mol), CASP3 (ΔG = -5.85 kcal/mol), and MMP9 (ΔG = -8.18 kcal/mol). Hydrogen bonding with Lys89 (TNF-α) and His401 (MMP9) suggests inhibition of pro-inflammatory pathways. These computational findings were validated using in vitro assays and gene enrichment analysis of overlapping RA-related targets .
Q. Advanced: How does this compound modulate the humoral immune response in preclinical models?
In C57Bl/6 mice immunized with OVA, this compound increased IgG2c titers (Th1-associated) without altering IgG1 (Th2-associated). Flow cytometry and ELISA revealed no changes in systemic IFN-γ, suggesting localized Th2 inhibition. This aligns with this compound’s role in macrophage polarization (M1/M2 balance), assessed via renal tissue analysis in diabetic models .
Q. Advanced: Why does this compound reduce retinopathy but not nephropathy progression in type 1 diabetes?
The RASS trial attributed this divergence to retinopathy’s sensitivity to ACE-independent pathways (e.g., oxidative stress). While mesangial volume (kidney) showed no improvement (mean difference vs placebo: -0.011, P=0.38), retinopathy progression was reduced via blood pressure-independent mechanisms, possibly involving angiotensin-(1-7) modulation .
Q. Basic: What key biomarkers are monitored in this compound trials to assess safety and efficacy?
Common biomarkers include:
- Serum potassium (hyperkalemia risk, ~19.8% with this compound vs 21% with LCZ696) .
- Urinary albumin-to-creatinine ratio (e.g., in diabetic trials) .
- Plasma renin activity and aldosterone (CONSENSUS trial linked aldosterone reduction to mortality benefits) .
Q. Advanced: How does combining this compound with folic acid impact stroke risk in hypertensive patients?
The CSPPT trial (n=20,702) used a double-blind design to compare this compound + folic acid vs this compound alone. The combination reduced first stroke risk by 21% (HR 0.79, 95% CI 0.68–0.93), with effect modification by baseline folate levels. Interaction tests confirmed homogeneity across subgroups, analyzed via stratified Cox models .
Properties
IUPAC Name |
(2S)-1-[(2S)-2-[[(2R)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16+,17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GBXSMTUPTTWBMN-UAGQMJEPSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2CCCC2C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC(=O)[C@@H](CCC1=CC=CC=C1)N[C@@H](C)C(=O)N2CCC[C@H]2C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H28N2O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID601181936 | |
| Record name | N-[(1R)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-L-proline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601181936 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
376.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
76420-74-1 | |
| Record name | N-[(1R)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-L-proline | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=76420-74-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Enalapril maleate impurity A | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0076420741 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | N-[(1R)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-L-proline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601181936 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (2S)-1-((2S)-2-(((1R)-1-(ETHOXYCARBONYL)-3-PHENYLPROPYL)AMINO)PROPANOYL)PYRROLIDINE-2-CARBOXYLIC ACID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/S7IJ7OBG9V | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods
Procedure details













Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













