Loperamide hydrochloride
Overview
Description
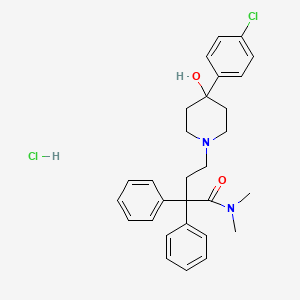
Loperamide hydrochloride, chemically designated as 4-(p-chlorophenyl)-4-hydroxy-N,N-dimethyl-diphenyl-1-piperidine butyramide hydrochloride, is a synthetic opioid agonist that selectively targets μ-opioid receptors in the gastrointestinal tract (Ki = 0.16 nM) . Its primary mechanism of action involves slowing intestinal motility by inhibiting peristalsis and enhancing fluid and electrolyte reabsorption, making it a cornerstone in managing acute and chronic diarrhea . It is available in tablets, capsules, and oral solutions, with molecular formula C₂₉H₃₄Cl₂N₂O₂ and molecular weight 513.5 g/mol .
Preparation Methods
Synthetic Routes and Reaction Conditions: Loperamide hydrochloride is synthesized through a multi-step process involving the reaction of 4-chlorobenzhydryl chloride with 4-hydroxypiperidine to form 4-(4-chlorophenyl)-4-hydroxypiperidine. This intermediate is then reacted with N,N-dimethyl-2,2-diphenylbutanamide to yield loperamide .
Industrial Production Methods: In industrial settings, this compound is produced using high-pressure liquid chromatography (HPLC) for purification. The process involves potentiometric titration and the use of mobile phases containing potassium phosphate monobasic and acetonitrile .
Chemical Reactions Analysis
Types of Reactions: Loperamide hydrochloride undergoes various chemical reactions, including:
Oxidation: Loperamide can be oxidized to form its N-oxide derivative.
Reduction: Reduction reactions can convert loperamide to its corresponding amine.
Substitution: Substitution reactions can occur at the piperidine ring or the phenyl groups.
Common Reagents and Conditions:
Oxidation: Hydrogen peroxide or other oxidizing agents.
Reduction: Sodium borohydride or lithium aluminum hydride.
Substitution: Halogenating agents or nucleophiles.
Major Products Formed:
Oxidation: Loperamide N-oxide.
Reduction: Loperamide amine.
Substitution: Various substituted derivatives depending on the reagents used.
Scientific Research Applications
Approved Medical Uses
Loperamide hydrochloride is FDA-approved for treating several forms of diarrhea, including:
- Acute nonspecific diarrhea
- Traveler's diarrhea
- Chronic diarrhea associated with irritable bowel syndrome
- Reduction of ileostomy output
Recent studies have also highlighted its effectiveness in managing chemotherapy-related diarrhea, particularly in patients undergoing immune checkpoint inhibitor therapy, where it is recommended to rule out infections before administration .
Off-Label Uses and Misuse
Increasingly, loperamide has been used off-label for purposes that raise concerns about safety:
- Opioid Withdrawal Management : Some individuals with opioid dependence use loperamide to alleviate withdrawal symptoms due to its action on mu-opioid receptors in the gastrointestinal tract. This has led to reports of misuse and dependence on loperamide itself .
- Euphoria Induction : There has been a rise in cases where loperamide is used recreationally to achieve euphoric effects, leading to high doses that pose significant health risks .
Health Risks and Cardiotoxicity
The misuse of loperamide can lead to severe cardiac events. The FDA has documented numerous cases of serious heart problems associated with high doses of loperamide, including:
- QT interval prolongation
- Torsades de Pointes
- Cardiac arrest
Between 1976 and 2015, 48 cases of serious cardiac events were reported, with some resulting in death. These events often occurred in the context of misuse or concurrent use with drugs that inhibit loperamide metabolism .
Case Studies and Clinical Insights
Several case studies illustrate the risks associated with loperamide misuse:
- A 28-year-old male with a history of heroin abuse took over 100 capsules daily for six months to manage withdrawal symptoms. He experienced severe cardiac arrhythmias as a result .
- Another study discussed a patient requiring methadone management due to protracted withdrawal symptoms from excessive loperamide use .
These cases underscore the importance of monitoring and educating patients about the potential dangers of self-medication with loperamide.
Research Findings on Efficacy and Safety
Recent research has focused on the pharmacokinetics and safety profile of loperamide:
Mechanism of Action
Loperamide hydrochloride exerts its effects by binding to the mu-opioid receptors in the gut wall. This binding leads to the recruitment of G-protein receptor kinases and the activation of downstream molecular cascades that inhibit enteric nerve activity. By suppressing the excitability of enteric neurons, loperamide reduces intestinal motility and increases the absorption of fluids and electrolytes, thereby decreasing the frequency of diarrhea .
Comparison with Similar Compounds
Comparative Analysis with Similar Compounds
Loperamide Hydrochloride vs. Lidamidine Hydrochloride
- Pharmacological Profile :
- Clinical Efficacy: A double-blind study comparing loperamide (8 mg/day) and lidamidine (16 mg/day) in acute diarrhea found loperamide superior in reducing stool frequency and achieving faster symptom resolution (p < 0.05) . Both drugs were well-tolerated, but lidamidine showed a marginally better safety profile in patients with hypertension due to its non-opioid mechanism .
This compound vs. Bismuth Subsalicylate
- Mechanism of Action :
- Efficacy in Acute Diarrhea :
This compound vs. Diphenoxylate
- Pharmacokinetics: Loperamide: Minimal systemic absorption; peak plasma concentration at 4–6 hours .
- Clinical Outcomes: A systematic review (n = 1,400) found loperamide reduced diarrhea duration by 55% vs. placebo, while diphenoxylate showed inconsistent efficacy and higher rates of drowsiness .
This compound vs. Loperamide Oxide
- Formulation Differences :
- Efficacy :
Research Advancements and Formulation Innovations
- Solubility Enhancement : Cocrystallization with glutaric acid improved loperamide’s solubility at intestinal pH (7.4), enhancing dissolution rates by 40% compared to commercial formulations .
- Nanoformulations: Casein-based nanoparticles increased loperamide’s oral bioavailability by 2.5-fold while masking its bitter taste .
Biological Activity
Loperamide hydrochloride, commonly known by its brand name Imodium, is an opioid receptor agonist primarily used to manage diarrhea. Its biological activity is multifaceted, involving interactions with various receptors and physiological pathways. This article explores the biological mechanisms, pharmacokinetics, therapeutic effects, and case studies related to this compound.
Loperamide acts primarily as a μ-opioid receptor agonist in the myenteric plexus of the large intestine. This action leads to:
- Decreased Intestinal Motility : By reducing the activity of the myenteric plexus, loperamide decreases the tone of both longitudinal and circular smooth muscles in the intestinal wall. This prolongs the time fecal material remains in the intestine, allowing for increased water absorption from stool .
- Inhibition of Gastrocolic Reflex : Loperamide suppresses colonic mass movements and the gastrocolic reflex, further contributing to its antidiarrheal effects .
Pharmacokinetics
Loperamide is characterized by low systemic bioavailability due to extensive first-pass metabolism in the liver. Key pharmacokinetic parameters include:
- Bioavailability : Approximately 0.3% due to hepatic metabolism .
- Half-Life : The mean biological half-life is about 10.8 hours .
- Peak Plasma Concentration : Achieved within 2.5 hours for syrup formulations and 5 hours for capsule forms .
Biological Activities
In addition to its primary use as an antidiarrheal agent, loperamide exhibits several other biological activities:
- Calcium Channel Blocking : At low micromolar concentrations, loperamide blocks high-voltage activated (HVA) calcium channels, while at higher concentrations, it reduces calcium flux through NMDA receptor-operated channels .
- Immunomodulatory Effects : Loperamide has been shown to enhance antibacterial responses in macrophages against Mycobacterium tuberculosis, increasing the production of antimicrobial peptides and cytokines such as IL1β and IL10 .
- Antiviral Activity : In vitro studies indicate that loperamide inhibits replication of MERS-CoV and SARS-CoV .
Cardiac Events Associated with Loperamide Use
Recent literature has documented cases of serious cardiac events linked to excessive loperamide use. For instance:
- A 28-year-old male with a history of heroin abuse took over 100 capsules daily for withdrawal symptoms, resulting in severe cardiac arrhythmias and cardiogenic shock. The patient required intensive treatment including intralipid emulsion therapy and showed significant recovery after management .
Formulation Studies
Research has focused on improving the bioavailability of loperamide through novel formulations:
- Orally Disintegrating Tablets (ODTs) : A study developed ODTs using superdisintegrants to enhance dissolution rates. The best formulation released 95% of the drug within five minutes, significantly improving patient compliance and therapeutic outcomes .
Summary of Research Findings
| Study Focus | Key Findings |
|---|---|
| Mechanism of Action | μ-opioid receptor agonist; decreases intestinal motility; calcium channel blocker |
| Pharmacokinetics | Bioavailability ~0.3%; half-life ~10.8 hours; peak plasma concentration at 2.5 hours (syrup) and 5 hours (capsule) |
| Immunomodulatory Effects | Enhances macrophage response against M. tuberculosis; increases cytokine production |
| Cardiac Events | Documented cases of arrhythmias linked to high doses; recovery observed with appropriate medical intervention |
| Novel Formulations | ODTs show rapid dissolution and improved bioavailability |
Q & A
Basic Research Questions
Q. What experimental models are suitable for studying loperamide hydrochloride’s antidiarrheal mechanism?
Loperamide’s μ-opioid receptor agonism (Ki = 0.16 nM) and δ-opioid receptor activity (Ki = 50 nM) can be studied using in vitro receptor binding assays with isolated intestinal tissues or transfected cell lines expressing opioid receptors . For in vivo models, rodent diarrhea induction (e.g., castor oil-induced diarrhea) is common. Measure intestinal transit time via charcoal meal tests or assess water/electrolyte transport in intestinal segments using Ussing chambers .
Q. How can solubility limitations of this compound in aqueous media be addressed in preclinical studies?
Loperamide HCl has low water solubility (1 mg/mL or 1.95 mM) but dissolves in DMSO (50 mg/mL). For in vivo dosing, prepare suspensions using 0.5% carboxymethylcellulose or administer via oral gavage after sonication at 37°C to enhance dispersion . Validate solubility using HPLC or spectrophotometry (λ = 220–230 nm) .
Advanced Research Questions
Q. What methodological considerations are critical for validating analytical assays (e.g., HPLC, UV-spectrophotometry) for loperamide quantification?
- Linearity : Use concentrations spanning 50–150% of expected levels (e.g., 2–10 µg/mL for UV).
- Specificity : Confirm no interference from excipients or degradation products via peak purity analysis .
- Accuracy/Precision : Spike recovery tests (e.g., 98–102% recovery) and inter-day variability <2% RSD .
- Degradation Studies : Acid/alkali hydrolysis (e.g., 0.1 M HCl/NaOH at 80°C) to identify degradation pathways .
Q. How can cocrystallization strategies improve loperamide’s dissolution rate for enhanced intestinal efficacy?
Cocrystallization with glutaric acid increases solubility at neutral pH (intestinal conditions). Synthesize cocrystals via solvent evaporation and validate using DSC (endotherm shifts), PXRD (new diffraction peaks), and FTIR (hydrogen-bond formation). In vitro dissolution tests in pH 6.8 buffer show 2–3x faster release compared to pure loperamide HCl .
Q. What experimental designs mitigate confounding factors in assessing loperamide’s cardiac toxicity in animal models?
Use telemetry-implanted rodents to monitor ECG (e.g., QTc prolongation) under controlled dosing (0.3–10 mg/kg). Include positive controls (e.g., cisapride) and negative controls (vehicle-only). Measure plasma levels via LC-MS to correlate toxicity with pharmacokinetics. Histopathological analysis of myocardial tissue post-mortem is critical .
Q. How do pharmacological interactions between loperamide and gut microbiota influence experimental outcomes?
Co-administer broad-spectrum antibiotics (e.g., vancomycin) in rodent models to assess microbiome-dependent effects. Measure fecal bile acid composition (LC-MS) and serotonin levels (ELISA), as loperamide alters microbial bile acid metabolism and enteroendocrine signaling .
Q. What methodologies optimize bioavailability studies of this compound in preclinical models?
Use crossover designs with radiolabeled loperamide (³H or ¹⁴C) to track absorption. Collect serial blood samples for LC-MS/MS analysis. Calculate bioavailability (F) using AUC₀–t ratios (oral vs. IV). Account for first-pass metabolism by sampling portal venous blood .
Q. How should researchers resolve contradictions in reported data on loperamide’s µ-opioid receptor binding affinity?
Replicate assays under standardized conditions (e.g., 25°C, pH 7.4, 1 nM radioligand). Use homologous competition binding with [³H]DAMGO. Validate cell membrane preparation protocols (e.g., HEK293 vs. CHO cells) and correct for non-specific binding with 10 µM naloxone .
Q. What quality control standards are essential for synthesizing this compound reference materials?
Follow USP guidelines: purity ≥98% (HPLC), residual solvents <ICH limits (e.g., toluene <890 ppm), and chloride content 6.8–7.2% (argentometric titration). Characterize impurities (e.g., 4-(4-chlorophenyl)piperidine) via LC-MS and quantify ≤0.15% .
Q. Which advanced techniques are used for impurity profiling in this compound formulations?
- Forced Degradation : Expose to UV light (ICH Q1B) and acidic/oxidative conditions to generate degradants.
- LC-QTOF-MS : Identify unknown impurities using high-resolution mass spectra and molecular formula prediction.
- NMR : Confirm structural changes in degradation products (e.g., N-oxide formation) .
Properties
IUPAC Name |
4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-N,N-dimethyl-2,2-diphenylbutanamide;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H33ClN2O2.ClH/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23;/h3-16,34H,17-22H2,1-2H3;1H | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PGYPOBZJRVSMDS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)C(=O)C(CCN1CCC(CC1)(C2=CC=C(C=C2)Cl)O)(C3=CC=CC=C3)C4=CC=CC=C4.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H34Cl2N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
53179-11-6 (Parent) | |
| Record name | Loperamide hydrochloride [USAN:USP:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034552835 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID00880006 | |
| Record name | 4-(4-Chlorophenyl)-4-hydroxy-N,N-dimethyl-alpha,alpha-diphenylpiperidine-1-butyramide monohydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID00880006 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
513.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
>77 [ug/mL] (The mean of the results at pH 7.4) | |
| Record name | SID11533030 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
CAS No. |
34552-83-5 | |
| Record name | Loperamide hydrochloride | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=34552-83-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Loperamide hydrochloride [USAN:USP:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034552835 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Loperamide hydrochloride | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759568 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 4-(4-Chlorophenyl)-4-hydroxy-N,N-dimethyl-alpha,alpha-diphenylpiperidine-1-butyramide monohydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID00880006 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 4-(4-chlorophenyl)-4-hydroxy-N,N-dimethyl-α,α-diphenylpiperidine-1-butyramide monohydrochloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.047.333 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LOPERAMIDE HYDROCHLORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/77TI35393C | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.
















