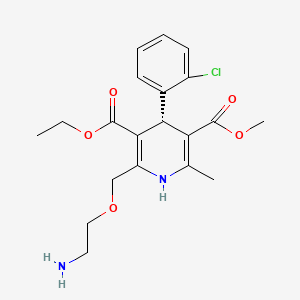
(R)-Amlodipine
Overview
Description
(R)-Amlodipine is the less pharmacologically active enantiomer of the racemic calcium channel blocker amlodipine, a dihydropyridine derivative widely used to treat hypertension and angina. Amlodipine exists as a 1:1 racemic mixture of (R)- and (S)-enantiomers. The (S)-enantiomer (levamlodipine) is responsible for 90–95% of the therapeutic activity, acting via selective inhibition of L-type calcium channels in vascular smooth muscle cells . This compound is primarily regarded as an impurity formed during degradation or synthesis, with minimal intrinsic efficacy.
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of ®-Amlodipine typically involves the resolution of racemic amlodipine or asymmetric synthesis. One common method is the chiral resolution of racemic amlodipine using chiral acids or bases to separate the enantiomers. Another approach is the asymmetric synthesis, which involves the use of chiral catalysts or chiral auxiliaries to produce the desired enantiomer directly.
Industrial Production Methods
In industrial settings, the production of ®-Amlodipine often involves large-scale chiral resolution techniques. These methods are optimized for high yield and purity, ensuring that the final product meets pharmaceutical standards. The use of advanced chromatographic techniques and crystallization methods is common in the industrial production of ®-Amlodipine.
Chemical Reactions Analysis
Derivatization Reactions
(R)-Amlodipine undergoes functional group transformations to form derivatives with enhanced stability or bioactivity:
Salt Formation
Reaction with benzene sulfonic acid under solid-phase conditions produces amlodipine besylate :
Urea/Thiourea Derivatives
The amine group reacts with isocyanates/isothiocyanates to form urea/thiourea analogs:
textThis compound + R-NCO/R-NCS → Urea/Thiourea derivatives (e.g., 4a–4k)
-
Conditions : Et₃N in THF at 50°C.
-
Applications : Improved antibacterial activity against Gram-positive and Gram-negative strains .
Imine Conjugates
Schiff base formation with aldehydes/ketones yields 15 novel conjugates (6a–6o):
textThis compound + R-CHO → Imine derivatives
Metabolic and Enzymatic Reactions
This compound exhibits unique interactions with cytochrome P450 (CYP) enzymes:
| Enzyme | Inhibition Type | Kᵢ (µM) | K<sub>inact</sub> (min⁻¹) | Source |
|---|---|---|---|---|
| CYP3A4 | Time-dependent inhibition | 8.22 | 0.065 | |
| CYP2C9 | Competitive inhibition | 12.11 | N/A |
-
Mechanism : Time-dependent inhibition involves reactive intermediate formation or quasi-irreversible enzyme binding .
-
Stereoselectivity : this compound shows stronger CYP2C9/CYP2C19 inhibition than the S-enantiomer .
Stability and Degradation
-
Hydrolysis : The ester groups in this compound undergo slow hydrolysis in aqueous solutions, forming inactive metabolites (e.g., pyridine derivatives) .
-
Oxidation : Hepatic CYP3A4 mediates oxidation of the amine group, contributing to its long half-life (30–50 hours) .
Comparative Reactivity with S-Amlodipine
| Property | This compound | (S)-Amlodipine |
|---|---|---|
| CYP3A4 inhibition | Kᵢ = 8.22 µM | Kᵢ = 14.06 µM |
| Nitric oxide release | 45 ± 5 pmol/mg/20 min | No activity |
| Calcium channel blockade | Minimal | Potent (L-type) |
-
Key Finding : this compound’s nitric oxide release is kinin-dependent and blocked by HOE-140 (B2 receptor antagonist) .
Industrial-Scale Production
A modified Hantzsch process achieves high-purity this compound for pharmaceutical use:
Scientific Research Applications
Pharmacological Properties
(R)-Amlodipine is a dihydropyridine calcium channel blocker that selectively inhibits voltage-dependent L-type calcium channels. This inhibition leads to relaxation of vascular smooth muscle, resulting in decreased peripheral vascular resistance and lowered blood pressure. Its prolonged half-life allows for once-daily dosing, enhancing patient compliance .
Hypertension Management
This compound is widely recognized as a first-line treatment for hypertension. It has demonstrated significant efficacy in lowering blood pressure across various demographics, including patients with comorbid conditions such as diabetes and chronic kidney disease. Clinical trials have shown that it effectively reduces cardiovascular events compared to other antihypertensive agents .
Angina Pectoris
In patients with chronic stable angina, this compound improves exercise tolerance and reduces the frequency of anginal attacks. Its mechanism of action not only alleviates symptoms but also contributes to long-term cardiovascular health by preventing atherosclerosis progression .
Renal Protection
Studies indicate that this compound may offer protective benefits for renal function in hypertensive patients. In the ACCOMPLISH trial, it was associated with a reduced risk of progression to end-stage renal disease compared to hydrochlorothiazide .
Impact on Heart Failure
While this compound is not typically used as a primary treatment for heart failure, it has shown potential benefits in patients with preserved ejection fraction and can be safely used alongside other heart failure therapies .
Case Study 1: Amlodipine Overdose
A case study documented an adolescent girl who ingested 150 mg of amlodipine with suicidal intent. The management involved decontamination and the administration of calcium and glucagon, highlighting the challenges associated with calcium channel blocker overdoses. This case emphasizes the importance of safe storage practices for medications .
Case Study 2: COVID-19 Outcomes
A retrospective clinical investigation suggested that patients treated with amlodipine besylate had a significantly lower case fatality rate from COVID-19 compared to those not receiving the medication. This finding prompts further research into the potential protective effects of amlodipine against severe viral infections .
Comparative Efficacy
The following table summarizes key findings from various studies comparing this compound with other antihypertensive agents:
Mechanism of Action
®-Amlodipine exerts its effects by inhibiting the influx of calcium ions through L-type calcium channels in vascular smooth muscle and cardiac muscle. This inhibition leads to vasodilation, reduced peripheral resistance, and decreased blood pressure. The molecular targets of ®-Amlodipine include the alpha-1 subunit of the L-type calcium channel. By binding to this subunit, ®-Amlodipine stabilizes the channel in its inactive state, preventing calcium entry and subsequent muscle contraction.
Comparison with Similar Compounds
Enantiomeric Comparison: (R)-Amlodipine vs. (S)-Amlodipine
Key Findings :
- Enantioselective binding to human serum albumin (HSA) influences pharmacokinetics: (S)-amlodipine exhibits stronger binding, prolonging its half-life compared to the (R)-form .
- Capillary electrophoresis (CE) methods reliably resolve (R)- and (S)-amlodipine during stability testing, critical for detecting enantiomeric impurities in degraded formulations .
Comparison with Other Calcium Channel Blockers
Mibefradil vs. Amlodipine
Key Findings :
- Both drugs show comparable antihypertensive efficacy, but mibefradil’s T-type channel action and heart rate reduction limit its use due to adverse interactions .
Bioisosteres of Amlodipine
Novel 1,4-dihydropyridine derivatives designed as bioisosteres aim to optimize amlodipine’s pharmacokinetic and safety profiles. For example:
- Amlodipine Maleate : Similar bioavailability but improved stability in formulation .
- FVA03 (Slow-Release Formulation) : Demonstrates distinct dissolution profiles (f2 = 32 vs. reference), affecting in vivo absorption .
Pharmacokinetic and Bioequivalence Comparisons
Key Findings :
- Generic amlodipine formulations may fail to meet dissolution efficiency criteria, risking subtherapeutic plasma levels despite chemical equivalence .
- Single-pill combinations (SPCs) like ramipril/amlodipine improve patient adherence by 20–30% compared to free-dose combinations, enhancing therapeutic outcomes .
Degradation and Stability Profiles
This compound is a major degradation product under stress conditions:
Biological Activity
(R)-Amlodipine, a calcium channel blocker (CCB), is primarily used in the treatment of hypertension and angina. This article explores its biological activity, focusing on its pharmacological properties, mechanisms of action, clinical implications, and case studies.
Overview of Amlodipine
Amlodipine exists as two enantiomers: this compound and (S)-amlodipine. The (R)-enantiomer is generally considered more pharmacologically active. Amlodipine operates by inhibiting calcium ion influx through L-type calcium channels, leading to vasodilation and reduced vascular resistance.
- Calcium Channel Blockade : Amlodipine selectively inhibits L-type calcium channels in vascular smooth muscle and cardiac muscle.
- Vasodilation : This inhibition causes relaxation of vascular smooth muscle, leading to decreased peripheral vascular resistance and lower blood pressure.
- Cardiac Effects : While primarily a vasodilator, amlodipine also reduces myocardial oxygen demand by decreasing afterload.
Enantiomeric Differences
Research indicates that this compound exhibits different inhibitory effects on cytochrome P450 enzymes compared to its counterpart:
| Enzyme | (µM) for this compound | (µM) for (S)-Amlodipine |
|---|---|---|
| CYP3A4 | 8.22 | 14.06 |
| CYP2C9 | 12.11 | 21.45 |
| CYP2C19 | 7.12 | 10.30 |
This stereoselectivity highlights the importance of considering enantiomers in drug interactions and efficacy .
Efficacy in Hypertension
This compound has been shown to effectively lower blood pressure in hypertensive patients. In clinical trials, it demonstrated significant reductions in cardiovascular events compared to other antihypertensive therapies .
- Long-term Outcomes : Studies such as the ACCOMPLISH trial found that amlodipine significantly reduced the risk of chronic kidney disease progression and cardiovascular events compared to hydrochlorothiazide .
Case Studies
- Amlodipine Overdose : A notable case involved an 18-year-old girl who ingested 150 mg of amlodipine with suicidal intent. She exhibited severe hypotension and required multiple interventions including hyperinsulinemic-euglycemic therapy for recovery . This case emphasizes the need for careful monitoring and management of overdose situations involving CCBs.
- Vascular Health : Another study indicated that amlodipine not only lowers blood pressure but also inhibits vascular cell senescence, contributing to atherosclerosis prevention . This suggests potential benefits beyond mere blood pressure control.
In Vitro Studies
In vitro studies have demonstrated that this compound can inhibit oxidative stress-induced damage to cellular membranes, which may contribute to its protective cardiovascular effects . Additionally, it has been shown to exert anti-inflammatory effects by modulating cytokine production in various cell types.
Comparative Effectiveness
Comparative studies have shown that while both amlodipine and candesartan are effective in managing hypertension, amlodipine has a higher incidence of new-onset diabetes compared to candesartan . This highlights the importance of considering metabolic side effects when prescribing antihypertensive medications.
Q & A
Basic Research Questions
Q. Q1.1: How do the pharmacological properties of (R)-Amlodipine differ from its (S)-enantiomer, and what experimental methods are used to validate these differences?
Methodological Answer: Comparative studies require enantiomer separation via chiral chromatography (e.g., HPLC with chiral stationary phases) followed by pharmacodynamic assays (e.g., calcium channel binding affinity tests). Dose-response curves and molecular docking simulations can further elucidate stereospecific interactions . Ensure purity validation using FTIR and NMR to confirm enantiomeric integrity .
Q. Q1.2: What are the standard protocols for synthesizing this compound with high enantiomeric excess?
Methodological Answer: Asymmetric synthesis using chiral catalysts (e.g., Sharpless epoxidation) or enzymatic resolution methods are common. Monitor reaction progress via chiral HPLC and optimize reaction conditions (temperature, solvent polarity) to maximize yield. Report enantiomeric excess (ee) using polarimetry or circular dichroism (CD) spectroscopy, adhering to IUPAC guidelines for stereochemical reporting .
Q. Q1.3: How can researchers ensure reproducibility in pharmacokinetic studies of this compound?
Methodological Answer: Adopt standardized protocols for animal models (e.g., Sprague-Dawley rats) and human trials (dose normalization by body weight). Use validated LC-MS/MS methods for plasma concentration analysis. Predefine inclusion/exclusion criteria and document deviations meticulously. Share raw data and analysis scripts (e.g., R/Python) in supplementary materials to enable replication .
Advanced Research Questions
Q. Q2.1: How can contradictory data on the cardiovascular efficacy of this compound versus racemic mixtures be systematically analyzed?
Methodological Answer: Conduct a scoping review using PRISMA guidelines to aggregate preclinical/clinical studies. Apply meta-regression to assess heterogeneity sources (e.g., dosage, patient demographics). Use the FINER framework (Feasible, Interesting, Novel, Ethical, Relevant) to design follow-up experiments addressing gaps, such as head-to-head trials with blinded endpoint adjudication .
Q. Q2.2: What experimental designs are optimal for studying the tissue-specific distribution of this compound in hypertensive models?
Methodological Answer: Use radiolabeled this compound (e.g., ^14C labeling) combined with autoradiography or PET imaging. Pair with mass spectrometry imaging (MSI) for spatial resolution. Control for vascular permeability differences using knockout models (e.g., eNOS-deficient mice). Include sham-operated controls and blinded histopathological analysis to reduce bias .
Q. Q2.3: How can computational modeling improve the prediction of this compound’s off-target effects?
Methodological Answer: Develop QSAR (Quantitative Structure-Activity Relationship) models using datasets from PubChem or ChEMBL. Train machine learning algorithms (e.g., random forests) on binding affinity data for non-target receptors (e.g., potassium channels). Validate predictions via high-throughput screening (HTS) and patch-clamp electrophysiology. Address overfitting by cross-validation and external test sets .
Q. Data Analysis and Reporting
Q. Q3.1: What statistical approaches resolve variability in enantiomer stability studies under different pH conditions?
Methodological Answer: Apply multivariate ANOVA to assess pH, temperature, and buffer composition effects. Use principal component analysis (PCA) to visualize degradation pathways. Report confidence intervals and effect sizes (e.g., Cohen’s d) for stability comparisons. Follow STROBE guidelines for transparent reporting of experimental conditions .
Q. Q3.2: How should researchers handle missing data in longitudinal studies of this compound’s renal effects?
Methodological Answer: Implement multiple imputation (MI) techniques with sensitivity analyses to test assumptions about missingness (e.g., MAR vs. MNAR). Use mixed-effects models to account for individual variability. Pre-register analysis plans to mitigate post hoc bias. Document imputation methods in supplementary materials .
Q. Ethical and Methodological Pitfalls
Q. Q4.1: What ethical considerations arise when designing clinical trials for this compound in pediatric populations?
Methodological Answer: Obtain informed assent/consent with age-appropriate documentation. Use adaptive trial designs (e.g., Bayesian methods) to minimize patient exposure to ineffective doses. Partner with ethics committees to address vulnerability concerns. Include data safety monitoring boards (DSMBs) for interim analyses .
Q. Q4.2: How can researchers avoid confirmation bias when interpreting this compound’s neuroprotective effects?
Methodological Answer: Predefine primary/secondary endpoints in protocols registered on platforms like ClinicalTrials.gov . Use blinded outcome assessors and automated data pipelines to reduce human intervention. Perform negative control experiments (e.g., sham-treated cohorts) and report all results, including null findings, to uphold transparency .
Q. Literature and Collaboration
Q. Q5.1: What strategies ensure comprehensive literature reviews on this compound’s metabolic interactions?
Methodological Answer: Combine systematic searches (PubMed, Scopus) with citation chaining using tools like Connected Papers. Annotate findings in reference managers (e.g., Zotero) with tags for CYP450 enzymes and drug-drug interactions. Engage in preprint platforms (e.g., bioRxiv) to capture emerging studies not yet indexed .
Q. Q5.2: How can interdisciplinary teams optimize this compound formulation studies?
Methodological Answer: Establish clear roles (e.g., medicinal chemists for synthesis, pharmacologists for in vivo testing). Use collaborative tools (e.g., GitHub for code sharing, LabArchives for electronic lab notebooks). Schedule cross-disciplinary workshops to align on objectives and troubleshoot methodological conflicts (e.g., nanoparticle stability vs. bioavailability) .
Properties
IUPAC Name |
3-O-ethyl 5-O-methyl (4R)-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H25ClN2O5/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h5-8,17,23H,4,9-11,22H2,1-3H3/t17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HTIQEAQVCYTUBX-QGZVFWFLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC(=O)C1=C(NC(=C([C@H]1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H25ClN2O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90430938 | |
| Record name | (R)-Amlodipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90430938 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
408.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
103129-81-3 | |
| Record name | (+)-Amlodipine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=103129-81-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amlodipine, (+)- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0103129813 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (R)-Amlodipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90430938 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | AMLODIPINE, (R)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/YUH55G7ZTY | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details






Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details




Synthesis routes and methods IV
Procedure details





Synthesis routes and methods V
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













