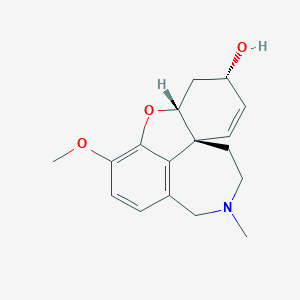
(+)-Galanthamine
Overview
Description
(+)-Galanthamine is a naturally occurring tertiary alkaloid belonging to the Amaryllidaceae family, first isolated from Galanthus (snowdrop) species . It is a reversible acetylcholinesterase (AChE) inhibitor and allosteric nicotinic acetylcholine receptor modulator, making it a cornerstone in the treatment of mild-to-moderate Alzheimer’s disease (AD) . Clinically, it improves cognitive function by enhancing cholinergic neurotransmission, with additional neuroprotective and neurogenic properties observed in preclinical studies .
Despite its efficacy, commercial production relies heavily on plant extraction from Leucojum aestivum (summer snowflake), Narcissus spp., and Lycoris radiata . However, wild populations of these plants are increasingly endangered, driving efforts to optimize in vitro cultivation and explore synthetic routes . Chemical synthesis remains economically unviable due to low yields and structural complexity, though biotechnological advances in nutrient medium optimization have significantly boosted galanthamine yields in shoot cultures (e.g., modified MS medium increased relative galanthamine content to 88.5% of the alkaloid mixture) .
Preparation Methods
Classical Total Synthesis Approaches
Magnus Lactol Route
The Magnus synthesis, a benchmark in galanthamine preparation, achieves racemic narwedine in seven steps, which is subsequently resolved into enantiopure (+)-galanthamine. The key step involves an intramolecular alkylation of a phenol derivative to construct the ABC-ring system . A modified approach by Nugent and Banwell introduces an iodinated isovanillin derivative subjected to Mitsunobu coupling with 2-cyclohexen-1-ol, followed by an intramolecular Heck cyclization (85% yield) to form the tetracyclic lactol intermediate . Hydrolysis and reductive amination with methylamine cyanoborohydride afford narwedine, which undergoes L-Selectride®-mediated reduction to yield (±)-galanthamine .
Trost Intramolecular Heck Reaction
Trost’s methodology employs a palladium-catalyzed intramolecular Heck reaction to assemble the quaternary carbon center and ABC rings in a single step . This strategy, utilizing a brominated precursor, achieves a 78% yield of the tetracyclic framework, which is then functionalized via reductive amination and demethylation . While efficient, the route requires chiral resolution to isolate this compound, limiting overall enantiomeric excess (ee) to 92–95% .
Enantioselective Synthesis via Dynamic Kinetic Resolution
Fröhlich-Jordis Dynamic Resolution
Fröhlich and Jordis developed a pilot-scale process leveraging dynamic kinetic resolution of racemic narwedine. Exposing (±)-narwedine to catalytic this compound induces enantioselective crystallization, achieving >99% ee for the (+)-enantiomer . This method, integrated into industrial workflows, reduces reliance on chromatographic purification, enhancing throughput to multi-kilogram scales .
Chiral Acid-Mediated Resolution
Patent WO2006072818A2 discloses resolving racemic galanthamine using di-p-toluoyl-D-tartaric acid, forming diastereomeric salts separable by fractional crystallization . This approach elevates ee from 85% to >99.5% and is adaptable to galanthamine hydrobromide or tartrate salts . Reprocessing protocols recover excess enantiomers, ensuring <1% epi-galanthamine contamination .
Catalytic Asymmetric Reductive Amination
Borohydride-Mediated Amination
A scalable route from norgalanthamine employs sodium cyanoborohydride and formaldehyde in acetic acid, achieving 92% yield of (±)-galanthamine . Enantiopure (+)-norgalanthamine, obtained via chiral HPLC, undergoes methylation with methyl triflate (33% yield) to furnish this compound .
L-Selectride®-Controlled Ketone Reduction
Critical to stereochemical fidelity, L-Selectride® selectively reduces the C4 ketone of narwedine derivatives to the cis-diol, avoiding epimerization . For example, reducing dienone XXVIII with L-Selectride® (THF, –78°C) provides the enol intermediate in 84% yield, which is further reduced with LiAlH4 to this compound .
Industrial-Scale Manufacturing Processes
Narwedine-Free Synthesis
To circumvent narwedine’s allergenic risks, industrial methods (e.g., Sanochemia Pharmazeutika AG) synthesize this compound via 3,4-dihydro-6,7-dimethoxy-4'-oxo-spiro[5H]-2-benzazepine intermediates . Bromination with benzyl trimethyl ammonium tribromide (75% yield) and subsequent AlCl3-mediated O-demethylation form the apogalanthamine core, which undergoes L-Selectride® reduction and methylation .
Green Chemistry Innovations
Recent protocols replace toxic reagents like LiAlH4 with Vitride® (sodium bis(2-methoxyethoxy)aluminum hydride), improving safety profiles without compromising yield (82% vs. 78%) . Solvent systems transition from chlorinated solvents to ethanol/water mixtures, aligning with EPA guidelines .
Comparative Analysis of Synthetic Methodologies
Table 1: Key Metrics for this compound Synthesis
Chemical Reactions Analysis
(+)-Galanthamine undergoes various chemical reactions, including:
Oxidation: It can be oxidized to form galanthamine N-oxide.
Reduction: Reduction of this compound can yield dihydrogalanthamine.
Substitution: It can undergo substitution reactions, particularly at the nitrogen atom, to form various derivatives.
Common reagents and conditions used in these reactions include oxidizing agents like hydrogen peroxide for oxidation, reducing agents like sodium borohydride for reduction, and alkylating agents for substitution reactions. The major products formed from these reactions include galanthamine N-oxide, dihydrogalanthamine, and various substituted derivatives.
Scientific Research Applications
Alzheimer's Disease Treatment
The most prominent application of (+)-galanthamine is in the treatment of Alzheimer's disease. It was approved by the FDA in 2001 for managing mild to moderate Alzheimer's disease. Clinical studies have demonstrated that galantamine can significantly improve cognitive function and daily living activities in patients with Alzheimer's.
- Efficacy : A meta-analysis revealed that galantamine treatment resulted in a significant reduction in cognitive decline as measured by the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) and the Mini-Mental State Examination (MMSE) scores compared to placebo groups .
- Long-term Benefits : Research indicates that continuous treatment with galantamine for up to 36 months can sustain cognitive benefits, reducing the expected decline in cognitive function by approximately 50% compared to untreated patients .
Other Neurological and Psychiatric Conditions
Beyond Alzheimer's disease, this compound has been investigated for various other conditions:
- Cognitive Impairment : It has shown promise in treating cognitive impairment associated with Lewy body dementia, frontotemporal dementia, and multiple sclerosis .
- Schizophrenia : Galantamine may alleviate both positive and negative symptoms of schizophrenia when used alongside antipsychotics .
- Bipolar Disorder : Studies suggest that galantamine can improve cognitive dysfunction linked to bipolar disorder .
- Post-Traumatic Nerve Palsy : It has been effective in treating nerve palsies related to trauma .
Innovative Uses
Recent research has explored novel applications for this compound:
- Lucid Dreaming : Galantamine is being studied for its potential to induce lucid dreaming and enhance dream recall by increasing REM sleep duration .
- Antioxidant Properties : Its neuroprotective effects are attributed to its ability to scavenge reactive oxygen species, which may help mitigate oxidative stress-related neuronal damage .
Clinical Trials
Numerous clinical trials have assessed the efficacy and safety of this compound:
- A randomized controlled trial involving 2,045 patients found that long-term treatment with galantamine significantly reduced mortality rates and cognitive decline compared to placebo .
- Another study confirmed that doses of 24 mg daily resulted in significant improvements in global assessments of cognitive function over six months .
Case Studies
Several case studies highlight the therapeutic potential of this compound:
- A study involving patients with chronic post-stroke aphasia demonstrated improved language skills following treatment with galantamine .
- Research on adult autism indicated that galantamine could enhance expressive language and communication abilities .
Production Techniques
The traditional extraction of this compound from plants is labor-intensive and subject to environmental variability. Recent advancements aim to develop more efficient production methods:
Microbial Fermentation
Mechanism of Action
The primary mechanism of action of (+)-Galanthamine is the inhibition of acetylcholinesterase, an enzyme responsible for breaking down acetylcholine in the synaptic cleft. By inhibiting this enzyme, this compound increases the concentration of acetylcholine, enhancing cholinergic neurotransmission. This is particularly beneficial in conditions like Alzheimer’s disease, where acetylcholine levels are reduced. Additionally, this compound has been found to interact with nicotinic acetylcholine receptors, further contributing to its therapeutic effects.
Comparison with Similar Compounds
Acetylcholinesterase Inhibitors: Galanthamine vs. Physostigmine and Tacrine
Galanthamine is often compared to other AChE inhibitors like physostigmine (a carbamate alkaloid) and tacrine (a synthetic acridine). Key differences include:
Table 1: AChE Inhibitory Activity in Human Tissues
| Compound | IC50 (Postmortem Brain Frontal Cortex) | IC50 (Erythrocytes) | Selectivity for Brain vs. Peripheral Tissues |
|---|---|---|---|
| Physostigmine | 14 nM | 15 nM | Non-selective |
| Tacrine | 1.0 µM | 1.1 µM | Non-selective |
| Galanthamine | 3.2 µM | 0.28 µM | 10-fold less potent in brain |
- Mechanistic Differences : Unlike physostigmine and tacrine, galanthamine exhibits dual action—AChE inhibition and nicotinic receptor modulation—which may enhance its clinical efficacy in AD .
- Clinical Preference : Despite lower potency, galanthamine is favored over tacrine due to fewer hepatotoxic side effects and over physostigmine due to longer half-life and better tolerability .
NMDA Receptor Antagonists: Galanthamine vs. Memantine
Memantine, an NMDA receptor antagonist, is another AD therapeutic but operates via a distinct mechanism:
Table 2: Mechanism and Pharmacological Profiles
Plant-Derived Alkaloids: Haemanthamine, Lycorine, and Narciclasine
Co-occurring Amaryllidaceae alkaloids complicate galanthamine isolation but have distinct pharmacological profiles:
Table 3: Co-Occurring Alkaloids in Galanthamine Sources
- Biosynthetic Optimization : Modified nutrient media suppress haemanthamine and lycorine synthesis, simplifying galanthamine purification .
Key Findings from Arylaminopropanone Derivatives :
- Potency : Piperidine-substituted compounds showed AChE IC50 values of ~2.5 µM (vs. galanthamine’s 3.2 µM in brain tissue).
- Binding Mode : Molecular docking revealed similar active-site interactions as galanthamine but weaker affinity due to altered spatial orientation of nitrogen groups.
Biological Activity
(+)-Galanthamine, a natural alkaloid derived from the snowdrop plant (Galanthus spp.), is widely recognized for its significant biological activity, particularly in the treatment of Alzheimer's disease (AD). This article explores its mechanisms of action, efficacy in clinical studies, and recent advancements in derivative research.
This compound functions primarily as an acetylcholinesterase (AChE) inhibitor , which increases acetylcholine levels in the brain, thereby enhancing cholinergic neurotransmission. Additionally, it acts as an allosteric modulator of nicotinic acetylcholine receptors (nAChRs), promoting their activation and providing neuroprotective effects against β-amyloid toxicity, a hallmark of AD pathology .
Efficacy in Clinical Studies
Clinical trials have demonstrated this compound's effectiveness in improving cognitive function and daily living activities among patients with mild to moderate Alzheimer's disease. A notable study involving 75 Thai patients reported significant improvements in cognitive scores (ADAS-cog) and overall functioning (CIBIC-plus) after 24 weeks of treatment. The results indicated a well-tolerated profile with mild adverse effects such as nausea and dizziness .
Summary of Clinical Findings
Recent Advances in Derivatives Research
Recent studies have focused on synthesizing new derivatives of this compound to enhance its therapeutic potential while reducing toxicity. For instance, new galanthamine-peptide hybrids exhibited up to 100 times lower toxicity compared to the parent compound while maintaining or improving AChE inhibitory activity . These derivatives are being explored for their antioxidant properties and potential applications in treating neurodegenerative diseases.
Case Studies
- Efficacy in Alzheimer's Disease : A multicenter trial assessed the impact of this compound on cognitive function and quality of life in AD patients. Results indicated a statistically significant enhancement in cognitive performance and daily living activities over six months .
- Toxicity Reduction : Research on newly synthesized galanthamine derivatives highlighted their potential to inhibit AChE with significantly lower toxicity profiles. These findings suggest promising avenues for developing safer therapeutic options for AD .
Q & A
Basic Research Questions
Q. What is the primary mechanism of action of (+)-Galanthamine in Alzheimer’s disease (AD) research?
this compound acts as a reversible acetylcholinesterase (AChE) inhibitor and an allosteric potentiator of nicotinic acetylcholine receptors (nAChRs). Its dual mechanism enhances cholinergic neurotransmission by slowing acetylcholine degradation and amplifying receptor responsiveness. X-ray crystallography of the this compound-AChE complex reveals binding at the base of the enzyme’s active site gorge, interacting with Trp84 and Phe330 residues . Clinical trials prioritize dose-response studies to balance efficacy (cognitive improvement) and tolerability (nausea, gastrointestinal effects) .
Q. Which plant species are validated sources of this compound, and how is its yield quantified?
this compound is biosynthesized in Galanthus (snowdrop), Leucojum aestivum (summer snowflake), and Narcissus species. Quantification employs high-performance liquid chromatography (HPLC) coupled with hydrophilic interaction liquid chromatography (HILIC) columns and electrospray ionization time-of-flight mass spectrometry (ESI-oaTOF-MS), achieving detection limits of ~43 fg . For field studies, ¹H NMR metabolite profiling distinguishes galanthamine content from co-occurring alkaloids (e.g., lycorine) .
Q. What analytical methods are recommended for purity assessment of this compound in synthetic or extracted samples?
Purity validation requires a multi-technique approach:
- HPLC-UV/ESI-MS : Baseline separation on Atlantis HILIC silica columns (3 µm) with mobile phases optimized for alkaloids.
- TLC-bioautography : Detects AChE inhibitors at picogram levels, distinguishing this compound from derivatives like 1,2-dihydrogalanthamine .
- X-ray crystallography : Resolves stereochemical integrity, critical for activity .
Advanced Research Questions
Q. How can artificial neural networks (ANNs) optimize enzymatic synthesis of this compound derivatives?
ANNs predict optimal reaction parameters for lipase-catalyzed esterification (e.g., Candida antarctica lipase B). Key variables include temperature (50–90°C), enzyme load (2–5 wt%), and molar ratio of this compound to acyl donor (2:1–5:1). The batch back propagation (BBP) algorithm with a 4-7-1 node configuration achieved 60.36% experimental yield, validating ANN utility in process scaling .
Q. What in vitro cultivation strategies maximize this compound production in Leucojum aestivum shoot cultures?
A 2⁴ full factorial design modifies Murashige and Skoog (MS) basal medium components:
- Ammonium (X₁) : 10–40 mM NH₄⁺.
- Nitrate (X₂) : 20–80 mM NO₃⁻.
- Phosphate (X₃) : 0.5–2.0 mM KH₂PO₄.
- Sucrose (X₄) : 1–4% (w/v). Regression models (R² > 0.88) indicate NH₄⁺ and sucrose as critical for intracellular galanthamine (EndoGal) accumulation. Optimized medium yields 1.2 mg/g dry weight .
Q. How do molecular interactions between this compound and Aβ peptides influence its anti-fibrillogenic activity?
Circular dichroism (CD) and fluorescence assays demonstrate that this compound stabilizes Aβ₁–42 in unfolded conformations, preventing β-sheet aggregation. Computational docking identifies electrostatic interactions between its protonated tertiary amine and Aβ’s Asp23/Lys28 residues. Synergy with Cu²⁺ enhances inhibitory effects by chelating metal ions involved in oxidative stress .
Q. What metabolic shifts occur in Narcissus bulbs under nitrogen fertilization, and how do they affect this compound biosynthesis?
¹H NMR-based profiling reveals that doubling standard nitrogen (N) fertilizer increases amino acids (e.g., asparagine) and citric acid cycle intermediates but does not elevate this compound. Standard N/K levels (50 kg N/ha, 100 kg K₂O/ha) maximize alkaloid yield (0.05% dry weight), suggesting biosynthetic pathways are nitrate-sufficient but sensitive to excess N .
Q. How do hydrogen-bonding properties of this compound inform its pharmacokinetic optimization?
Crystallographic and ab initio studies identify NH⁺ and OH groups as primary hydrogen-bond donors (HBDs), while methoxy and ether oxygens act as acceptors (HBAs). Molecular electrostatic potential (MEP) analysis shows protonation at physiological pH enhances HBD capacity, guiding derivatization for blood-brain barrier penetration .
Q. Data Contradiction Analysis
Q. Why do some studies report conflicting results on fertilizer effects for this compound accumulation?
Discrepancies arise from species-specific nutrient thresholds (e.g., Narcissus vs. Leucojum) and methodological variability in alkaloid extraction (e.g., solvent polarity, hydrolysis protocols). Multivariate analysis of ¹H NMR data is essential to disentangle primary metabolism (e.g., amino acids) from secondary alkaloid pathways .
Q. How can researchers reconcile variability in enzymatic synthesis yields of this compound derivatives?
Yield inconsistencies stem from lipase source (e.g., Thermomyces lanuginosus vs. C. antarctica), solvent polarity (e.g., hexane vs. tert-butanol), and water activity control. ANN-driven parameter optimization and real-time reaction monitoring (e.g., FTIR) improve reproducibility .
Properties
IUPAC Name |
(1R,12R,14S)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ASUTZQLVASHGKV-SUYBPPKGSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCC23C=CC(CC2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CC[C@]23C=C[C@H](C[C@H]2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H21NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
287.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
60384-53-4 | |
| Record name | Galanthamine, (+)- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0060384534 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | GALANTAMINE, (+)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/8L3T05AQ82 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details












Synthesis routes and methods II
Procedure details











Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.