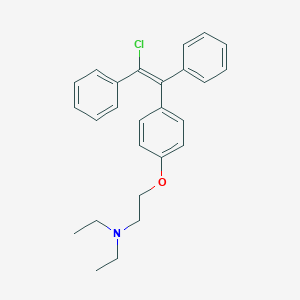
Enclomiphene
Overview
Description
Enclomiphene is the trans-isomer of clomiphene citrate, a selective estrogen receptor modulator (SERM). It acts primarily as an estrogen receptor antagonist in the hypothalamus, stimulating the release of gonadotropin-releasing hormone (GnRH), which increases luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This cascade enhances endogenous testosterone production in men, making it a therapeutic option for secondary hypogonadism . Unlike testosterone replacement therapy (TRT), this compound preserves or improves sperm counts, addressing both hypogonadism and fertility concerns .
Clinical trials demonstrate its efficacy in raising testosterone levels (median increase: 166 ng/dL) with fewer estrogenic side effects compared to clomiphene citrate . This compound is under development as a targeted treatment for hormonal imbalances while avoiding the suppression of spermatogenesis associated with TRT .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of enclomiphene involves the preparation of clomiphene, which is a mixture of cis-clomiphene (zuclomiphene) and trans-clomiphene (this compound). One method for synthesizing clomiphene uses a single solvent, such as dichloromethane, in a one-pot reaction . The process involves the reaction of 2-chloro-1,2-diphenylethene with 4-(2-chloro-1,2-diphenylethenyl)phenol in the presence of a base, followed by purification to isolate the trans isomer, this compound .
Industrial Production Methods: Industrial production of this compound typically follows similar synthetic routes but on a larger scale. The process involves optimizing reaction conditions to maximize yield and purity, followed by large-scale purification techniques to isolate this compound from the mixture of isomers .
Chemical Reactions Analysis
Primary Reaction Types
Enclomiphene undergoes three principal reaction types under controlled laboratory conditions :
| Reaction Type | Reagents/Conditions | Major Products |
|---|---|---|
| Oxidation | KMnO₄, CrO₃ | Quinones (via aromatic oxidation) |
| Reduction | LiAlH₄, NaBH₄ | Alcohol derivatives |
| Nucleophilic Substitution | NaOCH₃, KOtBu | Chlorine replaced by nucleophiles (e.g., methoxy groups) |
-
Oxidation : The compound’s phenolic rings are susceptible to oxidation, forming quinones under strong oxidizing agents like potassium permanganate.
-
Reduction : The double bond in the chlorinated diphenylethylene moiety is reduced to an alcohol using hydride donors .
-
Substitution : The chlorine atom undergoes nucleophilic displacement, particularly in polar aprotic solvents, yielding derivatives like methoxy-enclomiphene .
Synthetic Routes
This compound citrate is synthesized via a multi-step process :
-
Horner-Wadsworth-Emmons Reaction :
-
Citrate Salt Formation :
Key Industrial Conditions :
-
Chlorinating Agent: Dichlorodimethylhydantoin (0.51 mol equivalents) under anhydrous conditions to minimize impurities .
Metabolic Pathways
In vivo, this compound is metabolized primarily by hepatic enzymes :
-
Phase I Metabolism : CYP2D6 and CYP3A4 mediate oxidative demethylation and hydroxylation.
-
Phase II Metabolism : Glucuronidation enhances water solubility for renal excretion.
Metabolite Stability :
Stability and Decomposition
Thermal Stability :
-
Melting point: 147°C (onset) with decomposition at >200°C, releasing toxic fumes .
-
Critical Storage Conditions :
Photodegradation :
Comparative Reaction Kinetics
Data from enantiomerically pure synthesis :
| Parameter | This compound Citrate | Clomiphene Citrate |
|---|---|---|
| Trans:cis isomer ratio | 98:2 | 60:40 |
| Chlorination yield | 85% | 72% |
Functional Group Reactivity
Scientific Research Applications
Enclomiphene has a wide range of scientific research applications:
Chemistry: Used as a model compound for studying selective estrogen receptor modulators.
Biology: Investigated for its effects on the hypothalamic-pituitary-gonadal axis.
Medicine: Primarily used in the treatment of male hypogonadism to increase testosterone levels.
Industry: Utilized in the development of new therapeutic agents targeting estrogen receptors.
Mechanism of Action
Enclomiphene acts by antagonizing estrogen receptors in the pituitary gland. This disruption of the negative feedback loop by estrogen leads to an increase in gonadotropin secretion, which in turn stimulates the gonads to produce more testosterone . The molecular targets include estrogen receptors in the hypothalamus and pituitary gland, and the pathways involved are the hypothalamic-pituitary-gonadal axis .
Comparison with Similar Compounds
Enclomiphene vs. Zuclomiphene
Zuclomiphene, the cis-isomer of clomiphene, constitutes ~40% of clomiphene citrate formulations. Key differences include:
- Antiviral Efficacy : Both isomers inhibit Ebola virus (EBOV) entry with similar potency (IC50: ~1.0–1.4 µM) .
- Metabolism : this compound is metabolized to 4-hydroxy-enclomiphene, while zuclomiphene forms 4-hydroxy-zuclomiphene, both retaining antiviral activity .
This compound vs. Clomiphene Citrate
Clomiphene citrate is a racemic mixture (~60:40 this compound:zuclomiphene) used off-label for male hypogonadism. Critical distinctions include:
- Mechanistic Overlap : Both inhibit EBOV entry via cholesterol trafficking disruption, with clomiphene’s IC50 (~1.2 µM) nearly matching this compound’s (~1.0 µM) .
- Clinical Preference : this compound is favored for long-term male therapy due to its safety profile, whereas clomiphene’s zuclomiphene component raises estrogenic risks .
This compound vs. Tamoxifen
Tamoxifen, another SERM, is used in breast cancer and infertility.
Research Findings and Data Tables
Table 1: Hormonal Changes in Hypogonadal Men (Clomiphene vs. This compound)
| Parameter | This compound | Clomiphene | P-value |
|---|---|---|---|
| Testosterone (Δ ng/dL) | +166 | +98 | 0.20 |
| Estradiol (Δ pg/mL) | -5.92 | +17.50 | 0.001 |
| Adverse Event Rate | 12% | 35% | 0.02 |
Table 2: Antiviral Potency Against EBOV Entry
| Compound | IC50 (µM) | Cell Type |
|---|---|---|
| This compound | 1.0–1.2 | HEK293T/17, Vero E6 |
| Clomiphene | 1.2–1.4 | HEK293T/17, Vero E6 |
| Zuclomiphene | 1.1–1.3 | HEK293T/17 |
Source:
Biological Activity
Enclomiphene, a selective estrogen receptor modulator (SERM), is primarily recognized for its role in treating male hypogonadism and infertility. Its biological activity is characterized by both estrogenic and anti-estrogenic properties, influencing various hormonal pathways and physiological responses.
This compound acts on estrogen receptors in a manner that can be both agonistic and antagonistic, depending on the tissue context. This duality allows it to stimulate the hypothalamic-pituitary-gonadal (HPG) axis, leading to increased production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn promotes testosterone synthesis in the testes.
- Agonist Activity : this compound exhibits agonistic effects on certain tissues, enhancing estrogen receptor activity, which can lead to increased body weight and food intake in animal models .
- Antagonist Activity : Conversely, at higher doses, it can block estrogen's effects, particularly in reproductive tissues, thus inhibiting processes like sexual receptivity and prolactin secretion .
Testosterone Restoration
Recent studies have highlighted this compound's effectiveness in restoring testosterone levels in men with secondary hypogonadism. A notable clinical trial involved administering varying doses of this compound citrate over a period of six weeks:
| Treatment Group | Dose (mg/day) | Total Testosterone (ng/dL) Day 42 | LH Levels (mIU/mL) |
|---|---|---|---|
| This compound Citrate | 6.25 | 604 ± 160 | Increased |
| This compound Citrate | 25 | 604 ± 160 | Increased |
| Transdermal Testosterone | 5 g | 500 ± 278 | Decreased |
Results showed that this compound significantly increased serum testosterone levels without disproportionately raising dihydrotestosterone (DHT), contrasting with transdermal testosterone treatment which led to higher DHT levels .
Hormonal Effects
The hormonal profile following this compound treatment demonstrated significant increases in both LH and FSH levels, confirming its role in stimulating endogenous testosterone production:
- Total Testosterone : Increased from baseline to therapeutic range.
- LH/FSH Ratio : Elevated LH levels were observed post-treatment, indicating effective stimulation of the testes .
Pharmacokinetics
This compound is rapidly absorbed with an elimination half-life of approximately 10 hours. Steady-state concentrations are achieved at doses around 25 mg, reflecting its pharmacodynamic stability and efficacy .
Case Studies
- Study on Older Men : A study involving older men with low testosterone levels demonstrated that this compound citrate effectively normalized testosterone levels within two weeks without significant side effects or changes in DHT levels .
- Animal Models : Research on ovariectomized rats indicated that this compound could inhibit estrogen-induced changes in reproductive tissues while promoting weight gain through its agonistic effects on specific tissues .
Q & A
Basic Research Questions
Q. What are the key considerations for designing a controlled study to evaluate enclomiphene’s efficacy in restoring testosterone levels in hypogonadal males?
- Methodological Guidance : Use a randomized, single-blind design with dose-ranging arms (e.g., 6.25–25 mg/day) and an active comparator (e.g., transdermal testosterone). Include baseline hormonal profiling (total testosterone, LH, FSH) and standardized assays (e.g., immunoassays for hormone quantification). Monitor covariates like BMI and age, as these may influence metabolic responses . Ensure exclusion criteria address confounding factors (e.g., diabetes, CYP2D6 polymorphisms) .
Q. How should researchers standardize hormone measurement protocols to ensure reproducibility across this compound studies?
- Methodological Guidance : Employ centralized laboratories for consistency in assays (e.g., ADVIA® systems for lipid panels, ELISA for osteocalcin). Report detailed methodologies for blood collection timing (e.g., morning sampling to account for diurnal testosterone variations) and validation parameters (e.g., intra-assay coefficients of variation) . Cross-reference with ICH guidelines for bioanalytical method validation.
Q. What are the recommended dosages for this compound in preclinical vs. clinical research, and how are these determined?
- Methodological Guidance : Preclinical studies often use rodent models with doses adjusted for metabolic scaling (e.g., 1–5 mg/kg). For clinical trials, start with 12.5–25 mg/day based on phase II data showing dose-dependent increases in testosterone without severe adverse effects. Justify deviations using pharmacokinetic modeling (e.g., AUC comparisons between isomers) .
Advanced Research Questions
Q. How can researchers resolve contradictions in this compound’s metabolic clearance rates observed in outlier subjects (e.g., prolonged serum detection)?
- Methodological Guidance : Conduct pharmacogenetic analyses (e.g., CYP2D6 isoform profiling) to identify metabolic outliers. Use longitudinal sampling to track this compound and zuclomiphene levels over time. Apply mixed-effects models to account for interindividual variability in pharmacokinetic parameters . Include raw data in supplementary materials for transparency .
Q. What experimental strategies differentiate this compound’s anti-estrogenic effects from zuclomiphene’s estrogenic activity in dual-isomer formulations?
- Methodological Guidance : Use chiral chromatography to isolate isomers and perform receptor-binding assays (e.g., competitive ERα/β binding). Compare transcriptional activity in estrogen-responsive cell lines (e.g., MCF-7) using luciferase reporters. Reference isomer-specific pharmacokinetic data to contextualize in vivo effects .
Q. How should long-term studies address this compound’s potential estrogen rebound effects after discontinuation?
- Methodological Guidance : Design washout phases with frequent hormonal monitoring (e.g., weekly estradiol/total testosterone measurements). Use crossover designs to compare rebound kinetics against baseline. Incorporate patient-reported outcomes (e.g., mood scales) to assess clinical relevance of biochemical fluctuations .
Q. What statistical approaches are optimal for analyzing this compound’s dose-response relationships in heterogeneous populations?
- Methodological Guidance : Apply dose-response meta-analysis to pooled data from multiple trials. Use Bayesian hierarchical models to adjust for covariates (e.g., BMI, age). Report effect sizes with 95% credible intervals and sensitivity analyses for outlier exclusion .
Q. Data Management & Reporting
Q. How should this compound researchers structure supplemental data to enhance reproducibility?
- Methodological Guidance : Follow FAIR principles:
- Raw Data : Include hormone assay raw outputs (e.g., plate reader values) and pharmacokinetic time-series.
- Metadata : Document assay conditions (e.g., lot numbers, calibration curves).
- Code : Share scripts for statistical analysis (e.g., R/Python) .
Q. What ethical considerations are critical when designing this compound trials involving human subjects?
- Methodological Guidance : Address informed consent for genetic testing (e.g., CYP2D6). Implement DSMB oversight for adverse events (e.g., vision disturbances). Adhere to Declaration of Helsinki principles for vulnerable populations (e.g., fertility patients) .
Q. Contradictions & Gaps
Q. How do conflicting findings on this compound’s impact on gonadotropins (LH/FSH) inform future research directions?
- Methodological Guidance : Reconcile discrepancies by comparing study designs (e.g., dosing duration, population BMI). Conduct mechanistic studies using hypothalamic-pituitary explants to isolate this compound’s central vs. peripheral effects .
Properties
IUPAC Name |
2-[4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GKIRPKYJQBWNGO-OCEACIFDSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)CCOC1=CC=C(C=C1)C(=C(C2=CC=CC=C2)Cl)C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCN(CC)CCOC1=CC=C(C=C1)/C(=C(\C2=CC=CC=C2)/Cl)/C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C26H28ClNO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID201318048 | |
| Record name | Enclomiphene | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201318048 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
406.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
1.5 [ug/mL] (The mean of the results at pH 7.4), SLIGHTLY SOL IN WATER (1 IN 900), ETHANOL (1 IN 40) AND CHLOROFORM (1 IN 800); FREELY SOL IN METHANOL; PRACTICALLY INSOL IN DIETHYL ETHER /CITRATE/ | |
| Record name | SID50085975 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
15690-57-0, 911-45-5 | |
| Record name | Enclomiphene | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=15690-57-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Enclomiphene [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015690570 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Enclomiphene | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06735 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Enclomiphene | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201318048 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clomifene | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.011.826 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ENCLOMIPHENE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/R6D2UI4FLS | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
MP: 116.5-118 °C /CITRATE/ | |
| Record name | CLOMIPHENE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3039 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Synthesis routes and methods
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













