Dasatinib-d8
Overview
Description
Dasatinib-d8 is a deuterium-labeled isotopologue of the tyrosine kinase inhibitor (TKI) dasatinib, where eight hydrogen atoms are replaced with deuterium. This modification retains nearly identical chemical and physical properties to the parent compound while providing a distinct mass signature for analytical differentiation. This compound is primarily employed as an internal standard (IS) in liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify dasatinib and structurally similar small-molecule kinase inhibitors (SMKIs) in biological matrices such as plasma and cerebrospinal fluid (CSF) . Its use ensures precision by compensating for matrix effects, extraction variability, and instrument fluctuations during pharmacokinetic (PK) or pharmacodynamic (PD) studies .
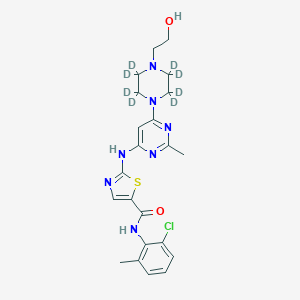
The molecular formula of this compound is C₂₂H₁₈D₈ClN₇O₂S, with a molecular weight of 496.06 g/mol . It is validated under U.S. Food and Drug Administration (FDA) guidelines for bioanalytical methods, demonstrating linearity, accuracy (95.3–113.2%), and precision (0.72–11.7%) across concentrations from 2.50 to 500 ng/mL .
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of Dasatinib-d8 involves the incorporation of deuterium atoms into the dasatinib molecule. This is typically achieved through a series of chemical reactions that replace hydrogen atoms with deuterium. One common method involves the use of deuterated reagents in the synthesis process . For example, the use of deuterated solvents and deuterated reducing agents can facilitate the incorporation of deuterium into the final product.
Industrial Production Methods
Industrial production of this compound follows similar principles but on a larger scale. The process involves the use of high-purity deuterated reagents and solvents to ensure the consistent incorporation of deuterium atoms. The final product is then purified using techniques such as liquid chromatography to achieve the desired purity and isotopic enrichment .
Chemical Reactions Analysis
Types of Reactions
Dasatinib-d8, like its non-deuterated counterpart, undergoes various chemical reactions, including oxidation, reduction, and substitution reactions . These reactions are essential for its metabolism and function within biological systems.
Common Reagents and Conditions
Common reagents used in the reactions involving this compound include oxidizing agents like hydrogen peroxide, reducing agents such as sodium borohydride, and various nucleophiles for substitution reactions . The conditions for these reactions typically involve controlled temperatures and pH levels to ensure the desired reaction pathways.
Major Products Formed
The major products formed from the reactions of this compound depend on the specific reaction conditions. For example, oxidation reactions may produce hydroxylated metabolites, while reduction reactions can lead to the formation of deuterated analogs of dasatinib .
Scientific Research Applications
Pharmacokinetics and Drug Metabolism
Dasatinib-d8 serves as an important tool in pharmacokinetic studies due to its stable isotope labeling. The deuteration allows for differentiation between the parent compound and its metabolites during analysis, facilitating a more accurate understanding of drug absorption, distribution, metabolism, and excretion (ADME).
- Enhanced Stability : The presence of deuterium increases the metabolic stability of dasatinib, potentially leading to prolonged action and reduced dosing frequency.
- Analytical Techniques : this compound is often used in liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods to quantify dasatinib levels in biological samples. This is critical for therapeutic drug monitoring (TDM) to optimize dosing regimens based on individual patient responses .
Therapeutic Drug Monitoring (TDM)
TDM is increasingly recognized as a valuable approach for personalizing dasatinib therapy. This compound plays a crucial role in this context by providing a reliable reference standard for measuring plasma concentrations.
- Exposure-Response Relationships : Studies have demonstrated that plasma levels of dasatinib correlate with clinical outcomes. For instance, achieving specific pharmacokinetic parameters such as Area Under the Curve (AUC) can predict major molecular responses (MMR) in patients with CML .
- Case Studies : In one study involving patients treated with dasatinib, monitoring AUC and peak plasma concentrations (Cmax) helped identify optimal dosing strategies that minimized toxicity while maximizing therapeutic efficacy .
Preclinical Studies
This compound has been utilized in various preclinical models to investigate its efficacy and mechanism of action.
- Animal Models : Research involving mice has shown that this compound can effectively inhibit tumor growth in xenograft models. The incorporation of deuterium allows researchers to trace the compound's bioavailability and pharmacodynamics more accurately .
- Cell Viability Assays : In vitro studies have assessed the cytotoxic effects of this compound on leukemia cell lines. Results indicate that this compound maintains similar potency to its non-deuterated counterpart while providing insights into metabolic pathways involved in resistance mechanisms .
Clinical Applications
The clinical implications of using this compound extend beyond pharmacokinetics to encompass treatment optimization.
- Individualized Treatment Plans : By utilizing this compound in TDM, clinicians can tailor treatment plans based on real-time pharmacokinetic data, improving patient outcomes through personalized medicine approaches .
- Research on Resistance Mechanisms : this compound is also instrumental in studying resistance mechanisms in CML patients. Understanding how metabolic pathways differ between responders and non-responders can inform future therapeutic strategies .
Summary Table of Key Findings
| Application Area | Key Findings |
|---|---|
| Pharmacokinetics | Enhanced stability and differentiation in metabolic studies using LC-MS/MS |
| Therapeutic Monitoring | Correlation between plasma levels and clinical outcomes; optimization of dosing regimens |
| Preclinical Studies | Efficacy demonstrated in animal models; insights into resistance mechanisms |
| Clinical Applications | Tailored treatment plans based on TDM; improved understanding of drug metabolism |
Mechanism of Action
Dasatinib-d8 exerts its effects by inhibiting multiple tyrosine kinases, including BCR-ABL, SRC family kinases, c-KIT, EPHA2, and PDGFRβ . These kinases play crucial roles in cell signaling pathways that regulate cell growth, proliferation, and survival. By inhibiting these kinases, this compound disrupts these signaling pathways, leading to the inhibition of cancer cell growth and proliferation . The molecular targets and pathways involved in the mechanism of action of this compound are similar to those of dasatinib, making it a valuable tool in studying the pharmacodynamics of dasatinib .
Comparison with Similar Compounds
Dasatinib-d8 is compared to other isotope-labeled internal standards used in SMKI quantification. Key criteria include molecular properties, target analytes, and methodological advantages.
Table 1: Comparison of Isotope-Labeled Internal Standards for SMKIs
*Estimated based on parent compound alectinib (C₃₀H₃₄N₄O₄).
Key Research Findings:
Universal Internal Standard : this compound’s structural similarity to multiple SMKIs (e.g., ceritinib, osimertinib) allows it to serve as a single IS for multi-analyte panels, reducing costs and preparation time compared to analyte-specific IS .
CSF Quantification : In CSF studies, this compound enabled simultaneous quantification of lorlatinib and osimertinib with minimal matrix interference, achieving a lower limit of quantification (LLOQ) of 2.50 ng/mL .
Stability and Precision: this compound demonstrated superior stability in pre-treated BSA-coated vials, mitigating non-specific binding issues common in CSF collection .
Limitations of Alternatives:
- Alectinib-d8: Limited to alectinib analysis, requiring additional IS for multi-drug panels .
- Imatinib-d8 : Specific to imatinib metabolism studies, lacking cross-reactivity with newer SMKIs .
Methodological Advantages of this compound
- Cost Efficiency : Replaces the need for multiple isotope-labeled IS, saving ~30–50% in reagent costs .
- Streamlined Workflow : Simplified sample preparation and reduced run time (10 minutes per sample) in UPLC-MS/MS methods .
- Clinical Validation : Used in prospective trials (e.g., START-TKI study, NCT05221372) for NSCLC patients, confirming its reliability in real-world PK analyses .
Biological Activity
Dasatinib-d8 is a deuterated analog of dasatinib, a potent tyrosine kinase inhibitor (TKI) primarily used in the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). The incorporation of deuterium in this compound alters its pharmacokinetic properties, potentially enhancing its therapeutic efficacy and safety profile. This article explores the biological activity of this compound, including its mechanism of action, pharmacokinetics, clinical implications, and case studies.
This compound functions by inhibiting multiple tyrosine kinases involved in the proliferation of cancer cells. Its primary target is the BCR-ABL fusion protein, which is responsible for the uncontrolled growth of CML cells. Dasatinib is approximately 325 times more potent than imatinib in inhibiting unmutated BCR-ABL kinase in vitro and exhibits activity against most imatinib-resistant BCR-ABL mutants . Additionally, this compound also inhibits other kinases such as c-KIT, PDGFRβ, and ephrin receptor kinases, contributing to its broad-spectrum anti-cancer activity .
Pharmacokinetics
This compound's pharmacokinetic profile is characterized by high variability among patients. It is extensively bound to plasma proteins (approximately 96% ) and has a large apparent volume of distribution (2505 L with 93% CV) . The metabolism of this compound primarily occurs via cytochrome P450 3A4 (CYP3A4), leading to several active and inactive metabolites, including M4, M5, M6, M20, and M24 . Notably, the active metabolite M4 has been shown to possess antiproliferative activity similar to that of dasatinib itself .
Table 1: Pharmacokinetic Parameters of this compound
| Parameter | Value |
|---|---|
| Protein Binding | 96% |
| Volume of Distribution | 2505 L (93% CV) |
| Main Metabolic Pathway | CYP3A4 |
| Active Metabolites | M4, M5, M6, M20, M24 |
Clinical Implications
This compound's clinical application has been explored in various studies. A randomized trial comparing dasatinib with imatinib in newly diagnosed CML patients demonstrated superior molecular response rates for dasatinib at early time points . Specifically, at six months, the major molecular response (MR 3.0) rate was 68% for dasatinib versus 17% for imatinib . This efficacy highlights the potential for this compound to achieve faster and deeper responses in CML treatment.
Case Studies
- Nephrotoxicity Observations : A study reported that patients treated with dasatinib showed a higher incidence of proteinuria compared to those on other TKIs. In one case involving a 40-year-old male patient who developed heavy proteinuria after three months on dasatinib therapy, kidney function improved significantly after switching to nilotinib . This suggests that while this compound may offer enhanced efficacy, careful monitoring for renal side effects is necessary.
- Population Studies : In a cohort study involving 101 patients treated with various TKIs, those on dasatinib exhibited a greater likelihood of developing albuminuria compared to those on imatinib or nilotinib. Out of the dasatinib cohort, approximately 50% experienced increased albuminuria levels . This reinforces the need for individualized therapeutic monitoring when using dasatinib or its derivatives.
Q & A
Basic Research Questions
Q. What is the role of Dasatinib-d8 in LC-MS/MS bioanalysis, and how does it improve data accuracy?
this compound is a deuterated internal standard used to correct for matrix effects, ion suppression, and variability during sample preparation and instrument analysis. By spiking a known concentration of this compound into plasma samples, researchers can normalize analyte recovery and instrument response, ensuring precise quantification of Dasatinib . For example, in validated LC-MS/MS methods, this compound compensates for losses during protein precipitation, enabling a linear calibration range of 1–400 ng/mL with a lower limit of quantification (LLOQ) of 1 ng/mL .
Q. How should researchers validate an LC-MS/MS method incorporating this compound?
Key validation parameters include:
- Linearity : Assessed via calibration curves (e.g., 1–400 ng/mL for Dasatinib) .
- Precision and Accuracy : Intra- and inter-day variability should meet FDA/EMA criteria (e.g., ≤15% CV for precision, 85–115% accuracy) .
- Recovery and Matrix Effects : Compare analyte response in pre-extraction vs. post-extraction spiked samples to quantify matrix interference .
- Stability : Evaluate freeze-thaw cycles, short-term storage (ice bath), and long-term storage (-80°C) .
Q. What are the best practices for preparing and storing plasma samples when using this compound?
- Immediately centrifuge blood samples and store plasma in an ice bath to prevent degradation .
- Ship samples on dry ice to maintain stability and avoid thawing during transit .
- Minimize freeze-thaw cycles to preserve analyte integrity; aliquot samples before storage .
Q. How does protein precipitation compare to solid-phase extraction (SPE) for this compound-based assays?
Protein precipitation (e.g., using acetonitrile) is cost-effective and rapid but may introduce more matrix interference. SPE offers cleaner extracts but requires optimization of sorbent chemistry and elution solvents. For high-throughput studies, precipitation is preferred due to shorter run times (e.g., 5-minute chromatography vs. 15-minute SPE methods) .
Advanced Research Questions
Q. How can researchers troubleshoot discrepancies in Dasatinib quantification when using this compound?
- Isotopic Interference : Verify the mass transitions (e.g., m/z 488.1 → 401.1 for Dasatinib vs. 496.15 → 406.1 for this compound) to avoid cross-talk .
- Internal Standard Degradation : Monitor this compound stability under storage conditions; re-validate if deviations exceed 20% .
- Sample Hemolysis : Hemolyzed plasma may alter ionization efficiency; include hemolysis tests during method validation .
Q. How can chromatographic conditions be optimized to separate Dasatinib from its deuterated analog?
- Use a reversed-phase column (e.g., Waters Atlantis dC18, 75 × 4.6 mm, 3.5 µm) with a mobile phase of 0.1% formic acid in water and acetonitrile .
- Adjust gradient elution (e.g., 40% to 95% organic phase over 5 minutes) to resolve Dasatinib and this compound while maintaining baseline separation .
Q. What strategies enable multiplexed quantification of kinase inhibitors using this compound?
Al Shirity et al. developed a method to simultaneously quantify 21 kinase inhibitors (including Dasatinib) using deuterated analogs. Key steps include:
- MRM Optimization : Assign unique transitions for each analyte/internal standard pair .
- Cross-Validation : Ensure no interference between co-eluting analytes (e.g., Gefitinib-d8 and Ceritinib-d7) .
- Batch Analysis : Validate precision and accuracy for all analytes in a single run to reduce variability .
Q. How can matrix effects be mitigated when analyzing diverse biological matrices (e.g., serum vs. plasma) with this compound?
- Perform post-column infusion studies to identify ion suppression/enhancement zones .
- Use matrix-matched calibration standards and quality controls (QCs) for each biological matrix .
- Validate method robustness across matrices (e.g., heparin vs. EDTA plasma) .
Q. What statistical approaches are critical for interpreting data from this compound-based pharmacokinetic studies?
- Non-compartmental Analysis (NCA) : Calculate AUC, Cmax, and t1/2 using validated software (e.g., Phoenix WinNonlin) .
- Population Pharmacokinetics : Use nonlinear mixed-effects modeling (NONMEM) to account for inter-individual variability .
- Report confidence intervals and p-values for key parameters (e.g., bioequivalence studies require 90% CI within 80–125%) .
Q. How should researchers document methods using this compound to ensure regulatory compliance and reproducibility?
- Follow EMA guidelines for bioanalytical method validation, including detailed descriptions of sample preparation, instrumentation, and validation results .
- Adhere to journal-specific formatting (e.g., Beilstein Journal of Organic Chemistry requires experimental details in the main text or supplementary materials) .
- Provide raw data and processing scripts in repositories like Zenodo to enhance transparency .
Properties
IUPAC Name |
N-(2-chloro-6-methylphenyl)-2-[[2-methyl-6-[2,2,3,3,5,5,6,6-octadeuterio-4-(2-hydroxyethyl)piperazin-1-yl]pyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27)/i6D2,7D2,8D2,9D2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZBNZXTGUTAYRHI-COMRDEPKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C(=CC=C1)Cl)NC(=O)C2=CN=C(S2)NC3=CC(=NC(=N3)C)N4CCN(CC4)CCO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C1(C(N(C(C(N1CCO)([2H])[2H])([2H])[2H])C2=NC(=NC(=C2)NC3=NC=C(S3)C(=O)NC4=C(C=CC=C4Cl)C)C)([2H])[2H])[2H] | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H26ClN7O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID30649385 | |
| Record name | N-(2-Chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)(~2~H_8_)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30649385 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
496.1 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1132093-70-9 | |
| Record name | N-(2-Chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)(~2~H_8_)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30649385 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-(2-chloro-6-methyl-phenyl)-2-[[2-methyl-6-[2,2,3,3,5,5,6,6-octadeuterio-4-(2-hydroxyethyl)piperazin-1-yl]pyrimidin-4-yl]methyl]thiazole-5-carboxamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details


Synthesis routes and methods IV
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.














