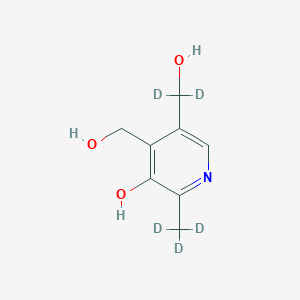
Pyridoxine-d5
Overview
Description
Pyridoxine-d5 (Pyridoxol-d5) is a deuterium-labeled isotopologue of pyridoxine (vitamin B6), a water-soluble vitamin essential for amino acid metabolism, neurotransmitter synthesis, and antioxidant pathways. Its chemical formula is C8H6D5NO3 (molecular weight: 174.21 g/mol), where five hydrogen atoms are replaced with deuterium at specific positions . This isotopic modification enhances its utility in pharmacokinetic and metabolic studies, particularly for tracing vitamin B6 pathways via mass spectrometry or NMR. This compound is used exclusively in research, such as investigating its antioxidant effects in Alzheimer’s disease models via the Nrf-2/HO-1 pathway .
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of Pyridoxine-d5 involves the incorporation of deuterium into the Pyridoxine molecule. One common method is the catalytic hydrogenation of Pyridoxine in the presence of deuterium gas. This process replaces the hydrogen atoms in the molecule with deuterium atoms. The reaction typically occurs under mild conditions, using a palladium or platinum catalyst.
Industrial Production Methods
Industrial production of this compound follows similar synthetic routes but on a larger scale. The process involves the use of high-pressure reactors and specialized equipment to handle deuterium gas. The final product is purified through crystallization or chromatography to achieve the desired purity and isotopic labeling.
Chemical Reactions Analysis
Types of Reactions
Pyridoxine-d5 undergoes various chemical reactions similar to those of natural Pyridoxine. These include:
Oxidation: this compound can be oxidized to Pyridoxal-d5, a form of Vitamin B6.
Reduction: Pyridoxal-d5 can be reduced back to this compound.
Substitution: The hydroxyl group in this compound can be substituted with other functional groups.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Reduction: Reducing agents such as sodium borohydride or lithium aluminum hydride are used.
Substitution: Various reagents like alkyl halides or acyl chlorides can be used for substitution reactions.
Major Products Formed
Oxidation: Pyridoxal-d5
Reduction: this compound
Substitution: Various substituted this compound derivatives
Scientific Research Applications
Pharmacological Applications
Pyridoxine-d5 is primarily utilized in pharmacological studies to investigate the metabolism and bioavailability of vitamin B6 compounds. Its deuterated nature allows researchers to trace metabolic pathways and study the pharmacokinetics of pyridoxine in human subjects.
Metabolic Studies
Research indicates that deuterated compounds like this compound can be used to assess the bioavailability and metabolic pathways of vitamin B6. A study comparing the bioavailability of pyridoxine-5'-beta-D-glucoside with pyridoxine revealed that the latter had a significantly higher bioavailability, which is crucial for understanding how different forms of vitamin B6 are absorbed and utilized in the body .
Clinical Applications
This compound is also instrumental in clinical settings, particularly in understanding conditions related to vitamin B6 deficiency. It is used in studies examining the effectiveness of pyridoxine supplementation in treating conditions such as peripheral neuropathy induced by isoniazid, a common tuberculosis medication .
Biochemical Research
The biochemical role of this compound extends to its function as a coenzyme in various enzymatic reactions.
Enzyme Co-factor
Pyridoxine is converted into pyridoxal 5'-phosphate (PLP), an active coenzyme involved in over 100 enzymatic reactions, including amino acid metabolism and neurotransmitter synthesis . The use of this compound allows for the tracking of these reactions through mass spectrometry, providing insights into metabolic disorders and enzyme kinetics.
Neurotransmitter Synthesis
Studies have shown that vitamin B6 plays a crucial role in synthesizing neurotransmitters such as serotonin and dopamine. Research utilizing this compound has helped elucidate the pathways involved in neurotransmitter production, which is vital for understanding mood disorders and neurodegenerative diseases .
Nutritional Studies
This compound is utilized in nutritional studies to evaluate the effectiveness of dietary interventions aimed at improving vitamin B6 status.
Dietary Bioavailability
Research on the bioavailability of different forms of vitamin B6 often employs deuterated compounds to accurately measure absorption rates and metabolic conversion . This is particularly important for developing dietary guidelines and supplements aimed at preventing deficiencies.
Clinical Trials
Clinical trials assessing the impact of pyridoxine supplementation on various health outcomes frequently use this compound as a tracer to monitor absorption and utilization in participants . For instance, its role in alleviating symptoms of premenstrual syndrome has been investigated using this compound.
Case Studies
Several notable case studies highlight the applications of this compound:
Mechanism of Action
Pyridoxine-d5 exerts its effects through its conversion to Pyridoxal 5’-phosphate, the active coenzyme form of Vitamin B6. This coenzyme is involved in various biochemical reactions, including amino acid metabolism, neurotransmitter synthesis, and hemoglobin production. The molecular targets include enzymes such as aminotransferases, decarboxylases, and racemases, which require Pyridoxal 5’-phosphate as a cofactor.
Comparison with Similar Compounds
Comparison with Structurally Similar Compounds
Pyridoxine (Non-Deuterated)
- Chemical Formula: C8H11NO3 (MW: 169.18 g/mol)
- Key Differences :
- Pyridoxine-d5 has five deuterium substitutions, increasing molecular weight by ~5 atomic mass units.
- Solubility: Pyridoxine dissolves readily in water (50 mg/mL), while this compound requires specific solvents (e.g., DMSO) and controlled storage (-80°C for stability) .
- Applications : Pyridoxine is used clinically for deficiency treatment, whereas this compound serves as a tracer in metabolic studies .
4-Deoxy Pyridoxine Hydrochloride
- Chemical Formula: C8H12ClNO2 (MW: 189.64 g/mol)
- Key Differences :
Other B6 Vitamers: PL, PLP, PM, PMP
Functional Contrast : this compound is a tracer, while PLP and PMP are coenzymes. Deuterated forms (e.g., PLP-d5) are used to study enzyme mechanisms, but this compound is preferred for whole-organism metabolic tracing .
Comparison with Isotopically Labeled Analogs
Riboflavin-d3 (Vitamin B2-d3)
- Formula : C17H17D3N4O6 (MW: 379.36 g/mol)
- Contrast: Riboflavin-d3 has three deuteriums, while this compound has five. Both are used as internal standards in LC-MS, but Riboflavin-d3 targets flavoprotein metabolism, unlike this compound’s focus on amino acid pathways .
Vitamin B12-13C7
- Formula : C63H88CoN14O14P (with seven 13C substitutions)
- Contrast :
Pharmacokinetic Studies
- This compound’s deuterium substitution reduces metabolic degradation rates compared to non-deuterated pyridoxine, enhancing its utility in long-term tracer studies .
- In Alzheimer’s disease models, this compound demonstrated enhanced antioxidant activity via Nrf-2/HO-1 upregulation, a property shared with non-deuterated pyridoxine but quantifiable more precisely due to isotopic labeling .
Analytical Chemistry
- This compound is critical in distinguishing endogenous vs. exogenous vitamin B6 in mass spectrometry, avoiding matrix interference common with non-deuterated analogs .
Data Tables
Table 1: Structural and Isotopic Comparison
| Compound | Formula | MW (g/mol) | Deuterium/Isotope Substitutions | Key Application |
|---|---|---|---|---|
| This compound | C8H6D5NO3 | 174.21 | 5 H → D | Metabolic tracing |
| Pyridoxine | C8H11NO3 | 169.18 | None | Clinical supplementation |
| PLP-d5 | C8H5D5NO6P | 252.18 | 5 H → D | Enzyme mechanism studies |
| Riboflavin-d3 | C17H17D3N4O6 | 379.36 | 3 H → D | Flavoprotein quantification |
Table 2: Solubility and Stability
| Compound | Solubility (mg/mL) | Storage Conditions | Stability Duration |
|---|---|---|---|
| This compound | 10 (DMSO) | -80°C (6 months) | 1 month at -20°C |
| Pyridoxine | 50 (water) | Room temperature (dry) | Indefinite |
| 4-Deoxy Pyridoxine | Limited data | -20°C (recommended) | Unstable in solution |
Biological Activity
Pyridoxine-d5, a deuterated form of pyridoxine (vitamin B6), has garnered attention in research due to its potential therapeutic applications and biological activities. This article explores the biological activity of this compound, highlighting its mechanisms, effects on metabolic pathways, and implications for clinical use.
Overview of this compound
Pyridoxine, or vitamin B6, exists in several forms, including pyridoxal and pyridoxamine, which are crucial for various biochemical processes in the body. The active form, pyridoxal 5-phosphate (PLP), functions as a coenzyme in over 100 enzymatic reactions involved in amino acid metabolism, neurotransmitter synthesis, and lipid metabolism . The introduction of deuterium (d5) into the molecular structure alters its pharmacokinetics and potentially enhances its metabolic stability.
1. Enzymatic Co-factor Role:
- This compound is converted into PLP, which acts as a coenzyme for numerous enzymes. These include:
2. Modulation of Metabolic Pathways:
- Research indicates that this compound may influence metabolic pathways related to lysine catabolism and neurotransmitter regulation. In particular, it has been studied in the context of pyridoxine-dependent epilepsy (PDE), where deficiencies lead to severe neurological symptoms .
Biological Activity and Clinical Implications
1. Case Studies:
- In patients with PDE caused by ALDH7A1 mutations, administration of pyridoxine has shown variable efficacy. While it can control seizures in many cases, some patients continue to experience breakthrough seizures despite treatment . This highlights the need for further research into deuterated forms like this compound that may offer improved outcomes.
2. Metabolomic Insights:
- Recent studies utilizing untargeted metabolomics have identified novel biomarkers associated with PDE, such as 2-OPP and 6-oxoPIP, which are significantly elevated in affected patients compared to controls . The relationship between these biomarkers and the biological activity of this compound remains an area for future exploration.
Pharmacokinetics
The pharmacokinetic profile of this compound differs from that of non-deuterated pyridoxine due to the presence of deuterium:
- Absorption: Similar to other forms of vitamin B6, absorbed primarily in the small intestine.
- Distribution: Deuterated compounds may exhibit altered distribution patterns due to changes in lipophilicity.
- Metabolism: The metabolic pathways remain largely unchanged; however, the incorporation of deuterium may slow down metabolic degradation.
- Excretion: Primarily excreted through urine as metabolites and unchanged forms .
Data Table: Comparison of Biological Effects
| Parameter | Pyridoxine | This compound |
|---|---|---|
| Active Form | PLP | PLP (deuterated) |
| Enzymatic Reactions | >100 | Similar |
| Seizure Control Efficacy | Variable | Potentially improved |
| Biomarker Association | Standard markers | Novel markers (e.g., 2-OPP) |
| Metabolic Stability | Standard | Enhanced |
Q & A
Q. What are the key considerations when synthesizing Pyridoxine-d5 for metabolic research?
Basic
Synthesis involves deuteration of Pyridoxine via catalytic exchange or chemical synthesis. Critical steps include selecting deuterium sources (e.g., D₂O) and optimizing reaction conditions to maximize isotopic incorporation. Post-synthesis, characterization via ¹H NMR (to identify non-deuterated protons) and high-resolution mass spectrometry (HRMS) is essential to confirm deuteration sites and isotopic purity (>98%). Document synthetic protocols rigorously to ensure reproducibility, adhering to standards for experimental transparency .
Q. How can researchers validate the isotopic purity of this compound in experimental settings?
Basic
Isotopic purity is validated using:
- HRMS : Measures exact mass-to-charge ratios to quantify deuterium enrichment.
- LC-MS/MS with MRM : Monitors specific transitions (e.g., m/z 170 → 152 for this compound) and compares peak ratios to non-deuterated controls.
- Isotopic dilution assays : Spiking known quantities into biological matrices to assess recovery rates. Regular batch testing ensures consistency, with deviations >2% warranting re-purification .
Q. What experimental design frameworks are optimal for tracer studies using this compound?
Advanced
Apply the PICO framework :
- Population : Cell lines or animal models with defined vitamin B6 status.
- Intervention : Administer this compound at physiologically relevant doses.
- Comparison : Use non-deuterated Pyridoxine controls to isolate isotope effects.
- Outcome : Quantify metabolic flux via kinetic modeling (e.g., compartmental analysis of LC-MS data).
Ensure alignment with FINER criteria (Feasible, Novel, Ethical, Relevant), particularly addressing detection limits of analytical methods and relevance to gaps in vitamin B6 metabolism .
Q. How should discrepancies in deuterium enrichment data be addressed?
Advanced
Systematically analyze potential sources of error:
- Technical variability : Recalibrate instruments and standardize sample preparation.
- Biological variability : Use longitudinal sampling to account for metabolic turnover rates.
- Cross-validation : Employ orthogonal techniques (e.g., NMR for positional deuteration vs. LC-MS for bulk enrichment).
Apply mixed-effects models to distinguish between inter-individual variability and experimental noise, referencing prior kinetic parameters from similar studies .
Q. What analytical techniques are most effective for quantifying this compound in biological matrices?
Basic
- LC-MS/MS with deuterated internal standards : Achieves sensitivity (LOQ <1 ng/mL) and corrects for matrix effects.
- Stable isotope dilution assays : Use this compound as an internal standard to normalize recovery rates.
- HPLC-UV with isotopic ratio monitoring : Less sensitive but cost-effective for high-concentration samples. Validate methods per FDA bioanalytical guidelines , including precision (CV <15%) and accuracy (80–120%) .
Q. When should this compound be preferred over ¹³C- or ¹⁵N-labeled analogs?
Advanced
- Deuterium : Ideal for studying hydrogen exchange-stable pathways (e.g., pyridoxal phosphate (PLP) cofactor recycling in aqueous environments).
- ¹³C : Better for tracking carbon skeleton rearrangements (e.g., transamination reactions).
Selection depends on the detection method (e.g., NMR for ¹³C vs. MS for deuterium) and the metabolic pathway’s susceptibility to isotopic effects .
Q. What ethical considerations apply to this compound studies in humans or animals?
Basic
- Safety thresholds : Ensure deuterium exposure remains below 0.5% body water enrichment to avoid toxicity.
- Ethics approval : Justify tracer use in protocols, emphasizing minimal risk and informed consent.
- Control groups : Include non-deuterated cohorts to control for isotope-specific effects on enzyme kinetics .
Q. How can this compound elucidate vitamin B6’s role in neurological disorders?
Advanced
- Kinetic modeling : Correlate deuterium enrichment in PLP-dependent enzymes (e.g., glutamic acid decarboxylase) with activity changes in disease models.
- Stratified analysis : Control for confounders (e.g., dietary B6 intake) using regression models.
- Longitudinal studies : Track enrichment decay rates to assess turnover abnormalities in disorders like epilepsy or autism, referencing clinical findings on pyridoxine-dependent seizures .
Properties
IUPAC Name |
5-[dideuterio(hydroxy)methyl]-4-(hydroxymethyl)-2-(trideuteriomethyl)pyridin-3-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H11NO3/c1-5-8(12)7(4-11)6(3-10)2-9-5/h2,10-12H,3-4H2,1H3/i1D3,3D2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LXNHXLLTXMVWPM-WNWXXORZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NC=C(C(=C1O)CO)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])C1=NC=C(C(=C1O)CO)C([2H])([2H])O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H11NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID10574085 | |
| Record name | 4-(Hydroxymethyl)-5-[hydroxy(~2~H_2_)methyl]-2-(~2~H_3_)methylpyridin-3-ol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10574085 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
174.21 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
688302-31-0 | |
| Record name | 4-(Hydroxymethyl)-5-[hydroxy(~2~H_2_)methyl]-2-(~2~H_3_)methylpyridin-3-ol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10574085 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details











Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.














