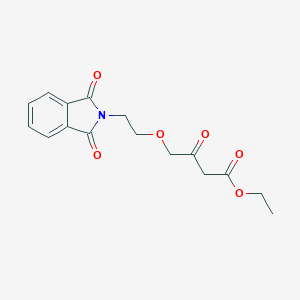
Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate
Overview
Description
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate typically involves the condensation of an aromatic primary amine with a maleic anhydride derivative, leading to the formation of the isoindoline-1,3-dione scaffold . Another approach involves the reaction of phthalic anhydride with glycine and acetic acid in the presence of triethylamine in toluene as a solvent .
Industrial Production Methods
Industrial production methods for this compound are not extensively documented, but they likely involve similar synthetic routes with optimization for large-scale production. This includes the use of efficient catalysts and solvent systems to maximize yield and purity.
Chemical Reactions Analysis
Types of Reactions
Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate undergoes various types of chemical reactions, including:
Oxidation: This compound can be oxidized to introduce additional functional groups.
Reduction: Reduction reactions can modify the isoindoline-1,3-dione moiety.
Substitution: Electrophilic and nucleophilic substitution reactions can occur at various positions on the molecule.
Common Reagents and Conditions
Common reagents used in these reactions include oxidizing agents like potassium permanganate, reducing agents such as lithium aluminum hydride, and various nucleophiles and electrophiles for substitution reactions .
Major Products
The major products formed from these reactions depend on the specific conditions and reagents used. For example, oxidation can lead to the formation of carboxylic acids, while reduction can yield amines or alcohols.
Scientific Research Applications
Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate has a wide range of scientific research applications:
Mechanism of Action
The mechanism of action of Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate involves its interaction with specific molecular targets and pathways. The isoindoline-1,3-dione moiety is known to interact with enzymes and receptors, modulating their activity and leading to various biological effects . The exact pathways and targets depend on the specific application and context of use.
Comparison with Similar Compounds
Chemical Identity :
- IUPAC Name: Ethyl 4-[2-(1,3-dioxoisoindol-2-yl)ethoxy]-3-oxobutanoate
- CAS Number : 88150-75-8
- Molecular Formula: C₁₆H₁₇NO₆
- Molecular Weight : 319.31 g/mol .
- Synonyms: Ethyl-4(2-Phthalimido Ethoxy)Acetoacetate, 4-(2-Phthalimidoethoxy)acetoacetic Acid Ethyl Ester .
Structural Features: The compound consists of a phthalimide (1,3-dioxoisoindoline) group linked via an ethoxy chain to a 3-oxobutanoate ethyl ester. This structure confers both lipophilic (phthalimide) and reactive (β-keto ester) properties, making it a versatile intermediate in organic synthesis .
Applications :
Primarily used as a synthetic intermediate in pharmaceuticals (e.g., related to Amlodipine impurities) and isotopomer synthesis .
Physical Properties :
Structural Analogues
(a) Ethyl 3-Oxobutanoate
- Formula : C₆H₁₀O₃
- Molecular Weight : 130.14 g/mol.
- Key Differences : Lacks the phthalimidoethoxy group. Simpler structure with a single β-keto ester functionality.
- Applications: Precursor for synthesizing chlorinated derivatives (e.g., ethyl 2-chloro-3-oxobutanoate) used in isotopomer production .
(b) Ethyl 4-[2-(Dimethylamino)ethoxy]-3-oxobutanoate
- CAS : 84157-65-3
- Formula: C₁₀H₁₉NO₄
- Molecular Weight : 217.26 g/mol.
- Key Differences: Substitutes phthalimide with a dimethylamino group, introducing basicity. This enhances solubility in acidic media but reduces thermal stability compared to the phthalimide analogue .
(c) Ethyl 4-(2,4-Difluorophenyl)-4-oxobutyrate
- CAS : 1041553-00-7
- Formula : C₁₂H₁₂F₂O₃
- Molecular Weight : 242.22 g/mol.
- Key Differences : Contains a fluorinated aromatic ring instead of phthalimide. Fluorine atoms increase electronegativity, improving metabolic stability in pharmaceutical contexts .
Functional Analogues
(a) Ethyl 2-Chloro-3-oxobutanoate
- Formula : C₆H₉ClO₃
- Molecular Weight : 164.59 g/mol.
- Key Differences : Chlorine substituent at the α-position enhances electrophilicity, facilitating nucleophilic substitutions. Used in synthesizing isotopically labeled 1-chloroacetone .
(b) Cetirizine Ethyl Ester
- Structure : Contains a piperazine-ethoxy-acetic acid ethyl ester backbone.
- Key Differences : Piperazine group introduces basic nitrogen atoms, altering pharmacokinetics (e.g., antihistamine activity). The absence of a phthalimide reduces lipophilicity .
Comparative Data Table
| Compound | Molecular Formula | Molecular Weight (g/mol) | Key Functional Groups | Applications |
|---|---|---|---|---|
| Ethyl 4-(2-(1,3-Dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate | C₁₆H₁₇NO₆ | 319.31 | Phthalimide, β-keto ester | Pharmaceutical intermediates, isotopomers |
| Ethyl 3-Oxobutanoate | C₆H₁₀O₃ | 130.14 | β-keto ester | Precursor for chlorinated derivatives |
| Ethyl 4-[2-(Dimethylamino)ethoxy]-3-oxobutanoate | C₁₀H₁₉NO₄ | 217.26 | Dimethylamino, β-keto ester | Basic intermediates, solubility studies |
| Ethyl 4-(2,4-Difluorophenyl)-4-oxobutyrate | C₁₂H₁₂F₂O₃ | 242.22 | Fluorophenyl, β-keto ester | Agrochemicals, bioactive molecules |
Biological Activity
Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate is a compound with significant potential in medicinal chemistry, particularly in the context of anticancer research. This article explores its biological activity, focusing on its synthesis, cytotoxic effects, and structure-activity relationships.
- Chemical Formula : CHNO
- Molecular Weight : 319.31 g/mol
- CAS Number : 88150-75-8
- IUPAC Name : Ethyl 4-[2-(1,3-dioxoisoindolin-2-yl)ethoxy]-3-oxobutanoate
- PubChem CID : 9840027
The synthesis of this compound typically involves the reaction of ethyl chloroacetate with various heterocycles. The resulting derivatives are evaluated for their cytotoxic properties against different cancer cell lines, including MCF-7 (breast cancer) and HeLa (cervical cancer) cells. The mechanism of action is believed to involve the inhibition of key growth factors and enzymes that are crucial for cancer cell proliferation.
Cytotoxic Activity
Recent studies have highlighted the cytotoxic activity of this compound, demonstrating its effectiveness against various cancer cell lines. For instance, a study evaluated several derivatives containing phthalimide moieties and reported significant cytotoxic effects:
Table 1: Cytotoxic Activity of Related Compounds
| Compound | IC (μM) MCF-7 | IC (μM) HeLa |
|---|---|---|
| 3a | 87 ± 3.5 | 77 ± 3.3 |
| 3b | 86 ± 3.9 | 71 ± 3.6 |
| 3c | 92 ± 3.3 | 67 ± 4.1 |
| 3d | 73 ± 3 | 29 ± 2.9 |
| Paclitaxel | 5.25 - 11.03 (μg/mL) | 7.76 (μg/mL) |
Compound 3d , which contains two phthalimide structures, exhibited the highest cytotoxicity with an IC value of 29 μM against the HeLa cell line, indicating a strong potential for further development as an anticancer agent .
Structure-Activity Relationship (SAR)
The structure-activity relationship studies indicate that the presence of specific functional groups significantly enhances the biological activity of these compounds. The incorporation of phthalimide moieties appears to increase lipophilicity, which may facilitate better interaction with biological targets .
Key Findings:
- Lipophilicity : Increased lipophilic character correlates with enhanced cytotoxicity.
- Functional Groups : The presence of electron-withdrawing groups often improves potency by stabilizing reactive intermediates.
- Heterocyclic Scaffolds : Five-membered heterocycles are crucial for maintaining biological activity due to their ability to mimic natural substrates.
Case Studies
Research has demonstrated that derivatives of this compound can induce apoptosis in cancer cells through various pathways:
- Apoptosis Induction : Compounds were shown to activate caspase pathways leading to programmed cell death.
- Inhibition of Growth Factors : Studies indicate that these compounds can inhibit key signaling pathways involved in tumor growth and metastasis.
Q & A
Q. Basic: What are the recommended synthetic routes for Ethyl 4-(2-(1,3-dioxoisoindolin-2-yl)ethoxy)-3-oxobutanoate, and how can purity be optimized?
The compound is synthesized via multicomponent reactions , such as coupling phthalimide derivatives with ethyl acetoacetate intermediates. A key step involves introducing the 1,3-dioxoisoindolin-2-yl ethoxy group through nucleophilic substitution or esterification under anhydrous conditions . To optimize purity:
- Use column chromatography (silica gel, ethyl acetate/hexane eluent) for separation.
- Monitor reaction progress via thin-layer chromatography (TLC) .
- Recrystallize from ethanol or methanol to achieve >95% purity, as reported by suppliers .
Q. Basic: How should researchers handle and store this compound to ensure stability?
Conflicting storage recommendations exist:
- Refrigeration (2–8°C) is advised by Fujifilm Wako to prevent decomposition of the β-ketoester moiety .
- Room temperature (dry conditions) is suggested by other suppliers, assuming short-term stability .
Methodological guidance : - Conduct accelerated stability studies (e.g., 40°C/75% RH for 1 week) to determine degradation kinetics.
- Store under inert gas (N₂/Ar) to minimize hydrolysis of the ester group .
Q. Intermediate: What spectroscopic techniques are critical for characterizing this compound?
Essential methods :
- ¹H/¹³C NMR : Confirm the presence of the phthalimide ring (δ 7.8–8.0 ppm for aromatic protons) and β-ketoester (δ 3.5–4.5 ppm for ethoxy groups) .
- FT-IR : Verify ester C=O stretches (~1730 cm⁻¹) and phthalimide C=O (~1770 cm⁻¹) .
- HRMS : Validate molecular weight (C₁₆H₁₇NO₆, [M+H]⁺ = 320.1128) .
Q. Advanced: How can researchers resolve contradictions in reported melting points and boiling points?
reports a boiling point of 479.7±30.0°C but no melting point , while other sources omit thermal data.
Approach :
- Perform differential scanning calorimetry (DSC) to determine decomposition temperature.
- Use thermogravimetric analysis (TGA) to assess volatility under reduced pressure.
- Compare results with computational predictions (e.g., COSMO-RS for boiling point estimation).
Q. Advanced: What mechanistic insights exist for the reactivity of the β-ketoester group in this compound?
The β-ketoester moiety undergoes:
- Knoevenagel condensation with aldehydes, forming α,β-unsaturated esters.
- Michael addition with nucleophiles (e.g., amines), useful for constructing heterocycles .
Key considerations : - Stabilize enolate intermediates using Lewis acids (e.g., MgCl₂).
- Monitor pH to avoid hydrolysis of the ester group during reactions .
Q. Advanced: How can computational modeling guide the design of derivatives for biological applications?
Strategies :
- Perform docking studies to evaluate interactions with targets like tyrosyl-DNA phosphodiesterase 1 (TDP1), leveraging its phthalimide scaffold’s DNA-binding affinity .
- Use QSAR models to predict solubility (LogP = 1.2) and bioavailability.
- Simulate metabolic pathways (e.g., esterase-mediated hydrolysis) using ADMET predictors .
Q. Advanced: What are the implications of conflicting purity grades (85% vs. 97%) across suppliers?
Impact : Lower purity (85%) may introduce side products (e.g., hydrolyzed β-ketoester) affecting reaction yields.
Mitigation :
- HPLC-PDA analysis to quantify impurities.
- Pre-purify commercial batches via flash chromatography if purity <95% .
Q. Cross-Disciplinary: How is this compound applied in materials science or catalysis?
- Coordination chemistry : The phthalimide group chelates metals (e.g., Cu²⁺), enabling use in catalytic systems .
- Polymer synthesis : Acts as a monomer in step-growth polymerization for functional polyesters.
Properties
IUPAC Name |
ethyl 4-[2-(1,3-dioxoisoindol-2-yl)ethoxy]-3-oxobutanoate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H17NO6/c1-2-23-14(19)9-11(18)10-22-8-7-17-15(20)12-5-3-4-6-13(12)16(17)21/h3-6H,2,7-10H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RIGKLAOKQFKWNN-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)CC(=O)COCCN1C(=O)C2=CC=CC=C2C1=O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H17NO6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90431687 | |
| Record name | Ethyl 4-[2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethoxy]-3-oxobutanoate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90431687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
319.31 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
88150-75-8 | |
| Record name | Ethyl 4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxobutanoate | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=88150-75-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ethyl 4-[2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethoxy]-3-oxobutanoate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90431687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Butanoic acid, 4-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethoxy]-3-oxo-, ethyl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details










Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













