Isoniazid-d4
Overview
Description
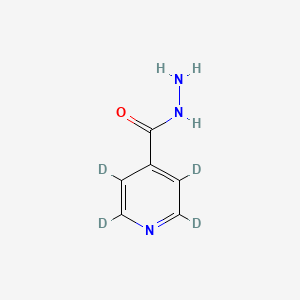
Isoniazid-d4 is the deuterium-labeled isotopologue of Isoniazid (INH), a first-line antitubercular agent. Its molecular formula is C₅H₄D₄N₂O, with deuterium atoms replacing four hydrogens in the parent compound . As a stable isotope-labeled internal standard, this compound is critical in bioanalytical methods, such as liquid chromatography-mass spectrometry (LC-MS), for quantifying Isoniazid and its metabolites in biological matrices . Structurally identical to Isoniazid except for isotopic substitution, it retains the parent drug's bactericidal activity against Mycobacterium tuberculosis by inhibiting mycolic acid synthesis after activation by bacterial catalase-peroxidase KatG .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of isoniazid-d4 typically involves the introduction of deuterium atoms into the isoniazid molecule. One common method is the catalytic exchange of hydrogen with deuterium in the presence of a deuterium source such as deuterium oxide (D2O). The reaction is usually carried out under controlled conditions to ensure the selective incorporation of deuterium atoms.
Industrial Production Methods: Industrial production of this compound follows similar principles but on a larger scale. The process involves the use of specialized reactors and catalysts to achieve high yields and purity. The reaction conditions, such as temperature, pressure, and the concentration of deuterium source, are optimized to maximize the efficiency of the deuteration process.
Chemical Reactions Analysis
Types of Reactions: Isoniazid-d4 undergoes various chemical reactions similar to its non-deuterated counterpart. These include:
Oxidation: this compound can be oxidized to form isonicotinic acid.
Reduction: It can be reduced to form hydrazine derivatives.
Substitution: The compound can undergo nucleophilic substitution reactions, particularly at the hydrazide group.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Reduction: Reducing agents such as sodium borohydride or lithium aluminum hydride are used.
Substitution: Nucleophiles like amines or thiols can be used under basic conditions.
Major Products Formed:
Oxidation: Isonicotinic acid.
Reduction: Hydrazine derivatives.
Substitution: Various substituted hydrazides.
Scientific Research Applications
Tuberculosis Treatment
Isoniazid-d4 retains the pharmacological properties of isoniazid, making it relevant in the treatment of tuberculosis (TB). The compound's mechanism involves inhibiting the synthesis of mycolic acids in the bacterial cell wall, which is critical for the survival of Mycobacterium tuberculosis.
Pharmacokinetic Studies
This compound is employed as an internal standard in pharmacokinetic studies to quantify the concentration of isoniazid and its metabolites in biological samples. A study utilized liquid chromatography-tandem mass spectrometry (LC-MS/MS) to analyze serum levels of isoniazid using this compound as a stable isotope-labeled internal standard, enhancing the accuracy of quantification .
Translational Pharmacology
Recent research has demonstrated the potential of zebrafish larvae as a model for studying TB pharmacodynamics using this compound. By quantifying internal exposure and correlating it with bacterial burden, researchers established a translational model that links findings from zebrafish to human responses . This innovative approach aids in understanding drug efficacy and optimizing dosing regimens.
Bioanalytical Methods
This compound has been integral in developing bioanalytical methods for measuring drug concentrations in various biological matrices. For instance, a validated assay was developed to quantify isoniazid and acetyl-isoniazid in human urine, utilizing this compound to improve measurement reliability . This method underscores the importance of stable isotopes in enhancing analytical precision.
Dried Blood Spot Analysis
The application of this compound in dried blood spot (DBS) analysis has been explored to assess drug stability and concentration. A study developed a UPLC-MS/MS method for measuring isoniazid concentrations from DBS samples, demonstrating the feasibility of using this compound for accurate drug monitoring .
Case Studies
| Study | Objective | Methodology | Findings |
|---|---|---|---|
| Study 1 | Evaluate pharmacokinetics of isoniazid | LC-MS/MS with this compound | Accurate quantification in serum samples |
| Study 2 | Assess TB treatment efficacy using zebrafish | Pharmacokinetic-pharmacodynamic modeling | Correlation between zebrafish and human data |
| Study 3 | Analyze drug concentrations from DBS | UPLC-MS/MS method development | Reliable measurement of isoniazid levels |
Mechanism of Action
Isoniazid-d4, like isoniazid, is a prodrug that requires activation by bacterial catalase-peroxidase enzyme. Once activated, it inhibits the synthesis of mycolic acids, essential components of the mycobacterial cell wall. This inhibition leads to the bactericidal effect against Mycobacterium tuberculosis. The primary molecular target is the enzyme InhA, which is involved in the fatty acid synthesis pathway.
Comparison with Similar Compounds
Structural and Functional Analogues
Sulfameter-d4
- Structure : Deuterated derivative of Sulfameter, a sulfonamide antibiotic.
- Function : Used for urinary tract infections and leprosy, unlike Isoniazid-d4's antitubercular focus.
- Mechanism : Inhibits dihydropteroate synthase (DHPS), contrasting with this compound's mycolic acid inhibition .
Acetylthis compound
- Structure : Deuterated metabolite of Isoniazid.
- Function : Serves as an internal standard for acetylated Isoniazid in pharmacokinetic studies.
- Comparison : Both this compound and Acetylthis compound are used in tandem for quantifying parent drug and metabolite levels, with identical lower quantification limits (0.05 µg/mL) .
Hydrazide Derivatives (Drugs A–D)
- Structure : Hydrazides with aromatic or aliphatic chains (e.g., Drug C and D share this compound's single aromatic ring; Drugs A and B have aliphatic chains) .
- Function : Varied targets, including antimicrobial and anticancer activities.
- Key Difference : this compound's specificity for mycobacterial KatG activation distinguishes it from broader-spectrum hydrazides .
Clinical and Research Utility
- This compound : Validated in bioanalytical assays per European Medicines Agency guidelines, enabling precise therapeutic drug monitoring in tuberculosis patients .
- Non-Deuterated Comparators: Isoniazid: Requires activation by KatG, limiting utility in KatG-deficient resistant strains. Kaempferol (): A flavonoid with anticancer activity, structurally unrelated but highlights the diversity of hydrazide/non-hydrazide pharmacophores.
Challenges and Considerations
- Isotopic Interference: While deuterated standards like this compound improve assay accuracy, cross-talk with non-deuterated analogs must be mitigated via chromatographic separation .
- Synthetic Complexity : Deuterium incorporation at specific sites (e.g., aromatic vs. aliphatic) affects synthesis cost and isotopic purity .
Biological Activity
Isoniazid-d4 (INH-d4) is a deuterated analog of isoniazid, a first-line drug used in the treatment of tuberculosis (TB). The introduction of deuterium in the molecular structure alters its pharmacokinetic and metabolic properties, potentially enhancing its therapeutic efficacy and reducing toxicity. This article explores the biological activity of INH-d4, focusing on its pharmacodynamics, pharmacokinetics, and potential mechanisms of action.
Chemical Structure and Properties
This compound has the chemical formula and is characterized by the substitution of four hydrogen atoms with deuterium. This modification can influence the drug's metabolism and interaction with biological systems.
Pharmacodynamics
-
Mechanism of Action :
- Isoniazid functions as a prodrug that requires activation by the KatG enzyme in Mycobacterium tuberculosis. The activated form inhibits the synthesis of mycolic acids, essential components of the bacterial cell wall, leading to cell death .
- INH-d4's deuteration may enhance its stability against metabolic degradation, thereby prolonging its action against TB bacteria .
- Histone Modification :
Pharmacokinetics
Pharmacokinetic parameters are crucial for understanding the efficacy and safety profile of INH-d4. The following table summarizes key pharmacokinetic data for isoniazid and its deuterated form:
| Parameter | Isoniazid | This compound |
|---|---|---|
| Dose (mg) | 300 | 300 |
| C_max (mg/L) | 4.8 | TBD |
| T_max (h) | 1 | TBD |
| AUC_0–24 (mg·hour/L) | 16.4 | TBD |
| Half-life (h) | 2.2 | TBD |
| Volume of distribution (L) | 57.6 | TBD |
| Clearance (L/h) | 20.8 | TBD |
Note : TBD indicates that specific data for INH-d4 is still under investigation or not available in current literature.
Case Studies and Research Findings
- Metabolic Stability :
- Toxicity Profile :
- In Vivo Efficacy :
Q & A
Basic Research Questions
Q. How is Isoniazid-d4 synthesized and characterized in experimental settings?
- Methodological Answer : this compound is synthesized via deuterium exchange or isotopic labeling using deuterated precursors (e.g., D₂O or deuterated hydrazine). Characterization involves nuclear magnetic resonance (¹H/²H NMR) to confirm deuterium incorporation and liquid chromatography–mass spectrometry (LC-MS) to assess isotopic purity (>98%). Purity is validated via high-performance liquid chromatography (HPLC), and stability under storage conditions (e.g., -20°C in inert atmospheres) is monitored over time .
Q. What analytical techniques are critical for distinguishing this compound from its non-deuterated counterpart in pharmacokinetic studies?
- Methodological Answer : Mass spectrometry (MS) with selected reaction monitoring (SRM) is essential for differentiating this compound from Isoniazid based on mass shifts caused by deuterium substitution. Isotopic peak ratios and retention time consistency in ultra-high-performance liquid chromatography (UHPLC) further validate specificity. Calibration curves using deuterated vs. non-deuterated standards ensure quantitation accuracy in biological matrices .
Q. How should researchers design experiments to evaluate this compound’s stability under varying physiological conditions?
- Methodological Answer : Stability studies should simulate physiological pH (1.2–7.4), temperature (37°C), and enzymatic environments (e.g., liver microsomes). Degradation kinetics are analyzed via time-course sampling, with LC-MS quantifying intact this compound. Parallel experiments with non-deuterated Isoniazid control for deuterium-specific effects. Statistical tools like ANOVA assess significance of degradation differences .
Advanced Research Questions
Q. What mechanistic insights can be gained by studying deuterium isotope effects in this compound’s activation by KatG?
- Methodological Answer : Kinetic isotope effect (KIE) studies compare the reaction rates of this compound and Isoniazid during KatG-mediated activation. A primary KIE (kH/kD > 1) suggests hydrogen/deuterium transfer is rate-limiting. Stopped-flow spectroscopy monitors intermediate formation (e.g., isonicotinoyl radical), while electron paramagnetic resonance (EPR) detects radical species. Computational modeling (DFT) further elucidates bond cleavage energetics .
Q. How can researchers resolve contradictions in reported bactericidal activity data for this compound across mycobacterial strains?
- Methodological Answer : Discrepancies may arise from strain-specific KatG expression levels or metabolic variability. Standardized protocols for minimum inhibitory concentration (MIC) assays (e.g., broth microdilution under controlled O₂ levels) are critical. Genomic sequencing of katG in tested strains identifies mutations affecting activation. Meta-analysis of published MIC data with standardized normalization (e.g., log₂ fold-change) clarifies strain-dependent trends .
Q. What strategies optimize the use of this compound as an internal standard in quantitative proteomic studies of tuberculosis drug resistance?
- Methodological Answer : this compound is spiked into mycobacterial lysates at fixed concentrations to normalize LC-MS/MS data. Parallel reaction monitoring (PRM) targets proteotypic peptides of resistance markers (e.g., inhA, katG). Signal drift is corrected using this compound’s stable isotopic signature. Multivariate analysis (e.g., PCA) identifies co-variates affecting quantification, such as ion suppression .
Q. How do deuterium substitution patterns in this compound influence its metabolic fate in cytochrome P450 (CYP) enzyme systems?
- Methodological Answer : Comparative metabolism studies using human liver microsomes and recombinant CYP isoforms (e.g., CYP2E1) identify deuteration-sensitive pathways. Metabolites are profiled via high-resolution MS (HRMS), with deuterium retention tracked using mass defect filtering. Kinetic parameters (Km, Vmax) for deuterated vs. non-deuterated substrates reveal isotopic effects on enzyme binding and turnover .
Q. Data Analysis and Reproducibility
Q. What statistical frameworks are recommended for analyzing dose-response relationships in this compound efficacy studies?
- Methodological Answer : Non-linear regression models (e.g., log-logistic curves) fit dose-response data, with IC₅₀ values calculated using software like GraphPad Prism. Bootstrap resampling estimates confidence intervals. Replicability is ensured by reporting raw data, normalization methods, and outlier exclusion criteria in supplementary materials .
Q. How should researchers document experimental protocols for this compound studies to ensure reproducibility?
- Methodological Answer : Protocols must detail deuterium source, synthetic routes, and characterization data (NMR/LC-MS spectra). For biological assays, document strain identifiers, growth conditions, and instrument calibration parameters. Follow FAIR data principles: store datasets in repositories (e.g., Zenodo) with unique DOIs and machine-readable metadata .
Q. Tables for Key Experimental Parameters
(Note: Described here as per guidelines; actual tables would be included in supplementary materials.)
- Table 1 : Comparison of this compound and Isoniazid in MIC assays against M. tuberculosis H37Rv and clinical isolates. Includes katG mutation status, MIC values, and KIE calculations .
- Table 2 : Stability parameters of this compound under physiological conditions (pH, temperature), with degradation half-lives and statistical significance (p-values) .
Properties
IUPAC Name |
2,3,5,6-tetradeuteriopyridine-4-carbohydrazide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H7N3O/c7-9-6(10)5-1-3-8-4-2-5/h1-4H,7H2,(H,9,10)/i1D,2D,3D,4D | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QRXWMOHMRWLFEY-RHQRLBAQSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CN=CC=C1C(=O)NN | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C1=C(N=C(C(=C1C(=O)NN)[2H])[2H])[2H] | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H7N3O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60662042 | |
| Record name | (~2~H_4_)Pyridine-4-carbohydrazide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60662042 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
141.16 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
774596-24-6 | |
| Record name | (~2~H_4_)Pyridine-4-carbohydrazide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60662042 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details









Synthesis routes and methods II
Procedure details








Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













