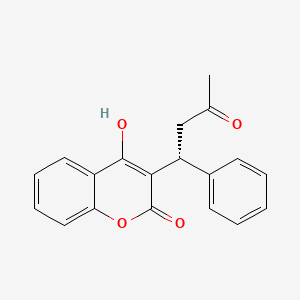
(R)-Warfarin
Overview
Description
(R)-Warfarin is the dextrorotatory enantiomer of the racemic anticoagulant warfarin, a vitamin K antagonist (VKA) widely used for thromboembolic prophylaxis. Unlike its more potent enantiomer, (S)-warfarin, this compound exhibits significantly lower anticoagulant activity, contributing only ~30–50% of the total pharmacological effect of racemic warfarin .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of ®-Warfarin typically involves the condensation of 4-hydroxycoumarin with benzylideneacetone under basic conditions. The reaction is carried out in the presence of a base such as sodium hydroxide or potassium hydroxide, and the product is isolated through crystallization.
Industrial Production Methods: In industrial settings, the production of ®-Warfarin involves similar synthetic routes but on a larger scale. The process is optimized for yield and purity, often involving additional purification steps such as recrystallization and chromatography to ensure the desired enantiomeric purity.
Chemical Reactions Analysis
Types of Reactions: ®-Warfarin undergoes various chemical reactions, including:
Oxidation: ®-Warfarin can be oxidized to form hydroxy derivatives.
Reduction: The compound can be reduced to form dihydro derivatives.
Substitution: ®-Warfarin can undergo nucleophilic substitution reactions, particularly at the benzylidene moiety.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and chromium trioxide.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Nucleophiles such as amines and thiols can be used under basic conditions.
Major Products:
Oxidation: Hydroxywarfarin derivatives.
Reduction: Dihydrowarfarin derivatives.
Substitution: Various substituted warfarin derivatives depending on the nucleophile used.
Scientific Research Applications
Clinical Applications
Anticoagulation Therapy
(R)-Warfarin is primarily utilized as an anticoagulant to prevent and treat conditions such as venous thrombosis, pulmonary embolism, and thromboembolic events associated with atrial fibrillation. Its efficacy in reducing the risk of stroke and systemic embolism has made it a staple in anticoagulation therapy. Studies have shown that this compound can be administered alone or in combination with (S)-warfarin to enhance therapeutic outcomes .
Pharmacokinetics and Pharmacodynamics
Research indicates that this compound has a longer half-life compared to (S)-warfarin and exhibits different metabolic pathways. It is less potent than (S)-warfarin but plays a crucial role in achieving the desired International Normalized Ratio (INR) levels when used in conjunction with its enantiomer. A study demonstrated that the pharmacokinetic parameters for both enantiomers were similar, but the therapeutic effects varied based on genetic factors such as VKORC1 genotype .
Pharmacogenomic Considerations
The metabolism of this compound is significantly influenced by genetic polymorphisms in cytochrome P450 enzymes, particularly CYP1A2 and CYP3A4. Variations in these genes can affect drug response and necessitate personalized dosing strategies. A comprehensive understanding of these genetic factors allows for tailored anticoagulation therapy, minimizing adverse effects while maximizing therapeutic efficacy .
Herb-Drug Interactions
This compound's interaction with herbal supplements is an area of concern due to potential alterations in pharmacokinetics. For instance, compounds from Salvia miltiorrhiza have been shown to affect the binding of this compound to human serum albumin, potentially increasing its free concentration and enhancing anticoagulant effects. This interaction underscores the importance of monitoring patients who use herbal products concurrently with warfarin .
Case Study: Warfarin and Herbal Interactions
A study investigated the interaction between this compound and Salvia miltiorrhiza, revealing that certain compounds significantly decreased the binding constant of warfarin to human serum albumin, leading to increased free drug concentrations in vivo. This finding emphasizes the need for careful consideration of herbal therapies in patients undergoing treatment with warfarin .
Clinical Trials on Efficacy
Clinical trials have demonstrated that administering this compound alongside (S)-warfarin can yield improved INR control compared to using either enantiomer alone. One trial highlighted that patients with specific VKORC1 genotypes responded more favorably to combined therapy, suggesting a personalized approach based on genetic testing could optimize treatment outcomes .
Comparative Data Table
| Application Area | Description | Key Findings |
|---|---|---|
| Anticoagulation Therapy | Used for preventing thromboembolic events | Effective alone or with (S)-warfarin |
| Pharmacogenomics | Influenced by CYP1A2 and CYP3A4 genetic polymorphisms | Personalized dosing improves outcomes |
| Herb-Drug Interaction | Interacts with herbal supplements like Salvia miltiorrhiza | Alters binding to serum albumin, increasing free drug levels |
| Clinical Trials | Combined use with (S)-warfarin enhances INR control | Genotype-specific responses noted |
Mechanism of Action
®-Warfarin exerts its anticoagulant effects by inhibiting the enzyme vitamin K epoxide reductase (VKOR). This inhibition prevents the conversion of vitamin K epoxide to its active form, thereby reducing the synthesis of vitamin K-dependent clotting factors (II, VII, IX, and X). The reduction in these clotting factors leads to a decrease in blood coagulation.
Comparison with Similar Compounds
Key Characteristics:
- Metabolism : (R)-Warfarin is primarily metabolized via cytochrome P450 (CYP) enzymes, including CYP3A4 and CYP1A2, forming 6- and 8-hydroxy metabolites. This contrasts with (S)-warfarin, which is predominantly metabolized by CYP2C9 to 7-hydroxywarfarin .
- Pharmacokinetics : this compound has a longer half-life (~45 hours) compared to (S)-warfarin (~30 hours) due to slower clearance rates .
- Glucuronidation : this compound undergoes extensive glucuronidation, which introduces a bulky sugar moiety that reduces its affinity for vitamin K epoxide reductase (VKOR), further diminishing its anticoagulant activity .
Comparison with Enantiomer (S)-Warfarin
| Parameter | This compound | (S)-Warfarin |
|---|---|---|
| Potency | 1× (Baseline) | 3–5× more potent |
| Primary Metabolic Enzymes | CYP3A4, CYP1A2 | CYP2C9 |
| Major Metabolites | 6-hydroxywarfarin, 8-hydroxywarfarin | 7-hydroxywarfarin |
| Clearance Rate | Slower (1.5–3.0% per hour) | Faster (2.3–3.1% per hour) |
| Drug Interaction Profile | Affected by CYP3A4/1A2 inhibitors | Highly sensitive to CYP2C9 inhibitors |
Key Findings :
- (S)-Warfarin is responsible for ~85% of warfarin’s anticoagulant effect due to its superior binding to VKOR .
- Drug interactions with warfarin are often enantiomer-specific. For example, phenylbutazone inhibits (S)-warfarin metabolism (increasing bleeding risk) while accelerating this compound clearance, masking net changes in racemic warfarin .
Comparison with Direct Oral Anticoagulants (DOACs)
| Parameter | This compound | Edoxaban | Rivaroxaban |
|---|---|---|---|
| Target | VKOR | Factor Xa | Factor Xa |
| Half-Life | ~45 hours | 10–14 hours | 5–13 hours |
| Dosing Frequency | Once daily | Once daily | Once daily |
| Monitoring | INR required | None | None |
| Major Bleeding Risk | 3.43% annual risk | 2.75% (high-dose), 1.61% (low-dose) | 14.9% annual risk |
| Stroke Prevention | 1.50% annual risk (warfarin) | 1.18% (high-dose) | 1.7% annual risk |
Key Findings :
- Efficacy: Edoxaban and rivaroxaban demonstrated non-inferiority to warfarin in stroke prevention, with annualized stroke rates of 1.18% (high-dose edoxaban) and 1.7% (rivaroxaban) versus 1.50% for warfarin .
- Safety : DOACs significantly reduce intracranial hemorrhage risk (0.5% for rivaroxaban vs. 0.7% for warfarin) and eliminate the need for routine INR monitoring .
- Drug Interactions : Warfarin interacts with >120 drugs (e.g., antibiotics, antifungals), whereas DOACs have fewer pharmacokinetic interactions .
Comparison with Novel Vitamin K Antagonists
Novel chroman-2,4-dione derivatives (e.g., compounds 2a, 2f) were developed to mimic warfarin’s mechanism while improving safety:
| Parameter | This compound | Compound 2a | Compound 2f |
|---|---|---|---|
| VKORC1 Inhibition | Moderate | Comparable to warfarin | Comparable to warfarin |
| Serum Albumin Binding | High (similar to warfarin) | High | High |
| Prothrombin Time | 56–60 s (warfarin) | 56.63 s | 60.08 s |
| Metabolic Stability | Low (extensive glucuronidation) | Higher (resistant to CYP-mediated metabolism) | Higher |
Key Findings :
- Derivatives like 2a and 2f retain warfarin’s binding to serum albumin and VKORC1 but show comparable or improved anticoagulant activity (prothrombin times >56 s) .
Pharmacokinetic and Pharmacodynamic Considerations
- Genetic Influences : CYP2C9 and VKORC1 polymorphisms account for ~40% of warfarin dose variability. However, this compound’s metabolism is less affected by these variants compared to (S)-warfarin .
- Drug Interactions : this compound’s clearance is accelerated by CYP3A4 inducers (e.g., rifampin), whereas (S)-warfarin is highly sensitive to CYP2C9 inhibitors (e.g., fluconazole) .
- Monitoring : Thromboelastography (TEG) studies show poor correlation between warfarin’s INR and TEG parameters (e.g., R-value), limiting utility in periprocedural bleeding risk assessment .
Clinical Implications and Guidelines
- Dosing Algorithms: Pharmacogenetic-guided dosing (incorporating CYP2C9/VKORC1 genotypes) improves warfarin dose accuracy, particularly for patients requiring ≤21 mg/week or ≥49 mg/week .
- Therapeutic Switching : DOACs are preferred over warfarin in atrial fibrillation due to predictable pharmacokinetics and reduced bleeding risk. However, warfarin remains first-line for mechanical heart valves and antiphospholipid syndrome .
Biological Activity
(R)-Warfarin, a well-known anticoagulant, is one of the two enantiomers of warfarin, the other being (S)-warfarin. This compound is primarily used for the prevention and treatment of thromboembolic disorders. Understanding its biological activity is crucial for optimizing therapeutic outcomes and minimizing adverse effects.
Pharmacokinetics and Pharmacodynamics
Pharmacokinetics refers to how the body absorbs, distributes, metabolizes, and excretes a drug. In contrast, pharmacodynamics involves the effects of the drug on the body, including the mechanism of action.
- Absorption and Distribution : this compound is rapidly absorbed from the gastrointestinal tract, with peak plasma concentrations occurring within 1 to 4 hours post-administration. It is highly protein-bound (approximately 99%) to albumin and alpha-1 acid glycoprotein, which influences its distribution and bioavailability.
-
Metabolism : The metabolism of this compound is complex and involves several cytochrome P450 enzymes:
- CYP1A2 : Primarily responsible for 6-hydroxylation.
- CYP2C19 : Minor role in 8-hydroxylation.
- CYP3A4 : Exclusively responsible for 10-hydroxylation.
- Unlike (S)-warfarin, which is predominantly metabolized by CYP2C9, this compound undergoes multiple metabolic pathways leading to various hydroxylated metabolites .
- Elimination : The elimination half-life of this compound ranges from 20 to 60 hours, depending on individual patient factors such as age, liver function, and genetic polymorphisms affecting metabolism.
This compound exerts its anticoagulant effect by inhibiting vitamin K epoxide reductase complex 1 (VKORC1), an enzyme critical for the regeneration of reduced vitamin K. This inhibition leads to a decrease in the synthesis of vitamin K-dependent clotting factors II, VII, IX, and X in the liver . The potency of this compound is approximately 2-5 times greater than that of (S)-warfarin due to its higher affinity for VKORC1 .
Dosing Variability
The dosing of this compound can vary significantly among individuals due to several factors:
- Genetic Variability : Genetic polymorphisms in CYP2C9 and VKORC1 significantly affect warfarin metabolism and response. For example, patients with certain VKORC1 genotypes may require lower doses due to increased sensitivity to warfarin .
- Drug Interactions : Various medications and dietary factors can influence warfarin activity. For instance, drugs that inhibit CYP enzymes can increase warfarin levels and enhance its anticoagulant effect .
Case Studies
Several studies have highlighted the clinical implications of this compound's biological activity:
- In a cohort study involving patients with atrial fibrillation treated with warfarin, it was found that those with genetic variants requiring lower doses had a significantly reduced risk of bleeding complications compared to those on standard dosing regimens .
- A case report documented a patient who developed warfarin resistance due to non-compliance and high vitamin K intake, emphasizing the importance of monitoring dietary habits alongside pharmacotherapy .
Efficacy Compared to Other Anticoagulants
Recent studies have compared this compound's efficacy with newer oral anticoagulants like rivaroxaban. In a clinical trial involving patients at high risk for stroke, rivaroxaban demonstrated non-inferiority to warfarin while exhibiting a lower incidence of major bleeding events . This raises questions about the long-term use of warfarin given its complexity in management.
Summary Table: Key Characteristics of this compound
| Characteristic | Details |
|---|---|
| Molecular Formula | C19H16O4 |
| Mechanism of Action | VKORC1 inhibition |
| Half-Life | 20-60 hours |
| Bioavailability | High (>90%) |
| Protein Binding | ~99% |
| Primary Metabolizing Enzymes | CYP1A2, CYP2C19, CYP3A4 |
Q & A
Basic Research Questions
Q. How do CYP2C9 and VKORC1 genotypes influence (R)-warfarin pharmacokinetics and dosing requirements?
Genetic polymorphisms in CYP2C9 (e.g., CYP2C92, 3) and VKORC1 significantly alter this compound metabolism and dosing. CYP2C9 variants reduce enzymatic activity, leading to slower clearance of this compound metabolites (e.g., 4'-, 6-, 8-OH-R-warfarin) and lower maintenance doses. VKORC1 polymorphisms (e.g., 1639G>A) reduce vitamin K epoxide reductase activity, increasing sensitivity to warfarin. Population pharmacokinetic models integrating these genotypes improve dose prediction accuracy by up to 30% compared to clinical algorithms alone .
Q. What clinical and genetic variables are prioritized in pharmacogenetic algorithms for this compound dosing?
Key variables include age, weight, CYP2C9/VKORC1 genotypes, and concomitant medications. For example, the International Warfarin Pharmacogenetics Consortium algorithm incorporates these factors using multivariate regression to estimate therapeutic doses. Validation studies show genetic data improve dose prediction for extreme dose requirements (e.g., ≤21 mg/week or ≥49 mg/week) by 16–17% .
Q. What analytical methods are used to separate and quantify this compound enantiomers in pharmacokinetic studies?
Chiral HPLC using glycopeptide-based columns (e.g., CHIROBIOTIC® V2) enables high-resolution separation. Mobile phases with polar organic modifiers (e.g., methanol/acetonitrile/glacial acetic acid) optimize retention and sensitivity. LC-MS/MS further enhances specificity for low-concentration metabolites in plasma, with validation parameters meeting FDA bioanalytical guidelines .
Advanced Research Questions
Q. How do population pharmacokinetic models resolve CYP2C9 genotype-dependent drug interactions with this compound?
Nonlinear mixed-effects modeling (NONMEM) quantifies metabolic pathway alterations. For example, fluconazole inhibits CYP2C9-mediated 6-/7-OH-R-warfarin formation (73–75% CL reduction), while rifampin induces CYP3A4-mediated 10-OH-R-warfarin clearance (355% increase). Covariate analysis identifies genotype-specific interaction magnitudes (e.g., CYP2C91B/*1B enhances rifampin induction by 15–20%) .
Q. What structural mechanisms underlie this compound's binding to human serum albumin (HSA), and how does this affect distribution?
Crystal structures (2.5 Å resolution) reveal this compound binds HSA's Site I (subdomain IIA) via hydrophobic interactions with Trp214 and hydrogen bonds with Lys199/Lys195. Fatty acids (e.g., myristate) induce conformational changes in HSA, increasing warfarin binding affinity by 40–60%. This interaction prolongs half-life (25–45 hours) and reduces free drug availability .
Q. How do metabolic pathway discrepancies between (R)- and (S)-warfarin inform genotype-phenotype association studies?
this compound is primarily metabolized by CYP1A2/3A4 (vs. CYP2C9 for S-warfarin), leading to distinct metabolite profiles. Model-based analysis shows CYP2C9 variants minimally affect this compound clearance (IIV <20%), whereas (S)-warfarin clearance decreases by 72–85% in CYP2C92/*3 carriers. This divergence necessitates enantiomer-specific modeling to avoid confounding in DDI and pharmacogenetic studies .
Properties
IUPAC Name |
4-hydroxy-3-[(1R)-3-oxo-1-phenylbutyl]chromen-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H16O4/c1-12(20)11-15(13-7-3-2-4-8-13)17-18(21)14-9-5-6-10-16(14)23-19(17)22/h2-10,15,21H,11H2,1H3/t15-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PJVWKTKQMONHTI-OAHLLOKOSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)CC(C1=CC=CC=C1)C2=C(C3=CC=CC=C3OC2=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(=O)C[C@H](C1=CC=CC=C1)C2=C(C3=CC=CC=C3OC2=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H16O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
308.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
5543-58-8, 40281-89-8 | |
| Record name | (R)-Warfarin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=5543-58-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | (R)-Warfarin | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0005543588 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (R)-warfarin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08496 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | (R)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2-benzopyrone | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.024.463 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | WARFARIN, (R)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/09CC5J5C8A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details




















Synthesis routes and methods II
Procedure details





Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













