Pyridinium bromide
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Description
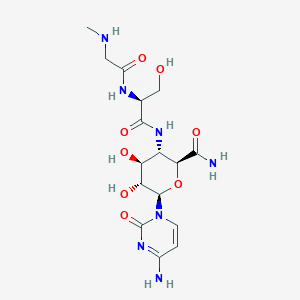
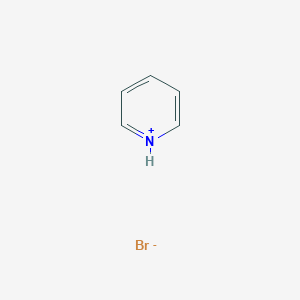
Pyridinium bromide (C₅H₅NH⁺Br⁻) is a quaternary ammonium salt consisting of a pyridinium cation paired with a bromide anion. It is a strong electrolyte that fully dissociates in aqueous solutions, yielding a pH of 2.95 due to the acidic nature of the pyridinium ion . This compound is hygroscopic and requires careful handling to avoid moisture absorption . This compound serves as a precursor in synthesizing ionic liquid crystals (ILCs) and biologically active derivatives, with applications in catalysis, corrosion inhibition, and cancer therapy .
Q & A
Q. Basic: What are the recommended methods for synthesizing alkyl-substituted pyridinium bromide ionic liquids, and how do alkyl chain lengths influence their physicochemical properties?
Answer:
Alkyl-substituted pyridinium bromides (e.g., 1-dodecylthis compound, C₁₂PyBr) are synthesized by quaternizing pyridine with alkyl bromides. For example, 1-hexadecylthis compound (C₁₆PyBr) is prepared by reacting pyridine with 1-bromohexadecane under reflux conditions in anhydrous solvents . The alkyl chain length significantly impacts properties such as melting point, solubility, and surface activity. Longer chains (e.g., C₁₈) enhance hydrophobicity and reduce critical micelle concentration (CMC), making them suitable for applications like surfactants or electrolytes . Structural confirmation requires ¹H NMR (e.g., using deuterated DMSO for resolving alkyl and pyridinium proton signals) and elemental analysis .
Q. Advanced: How can researchers design experiments to evaluate the cytotoxic efficacy of this compound derivatives against cancer cell lines, and what factors contribute to variability in IC₅₀ values?
Answer:
Cytotoxicity assays involve treating cancer cells (e.g., A-549 lung carcinoma) with this compound derivatives at varying concentrations (10–140 mg/mL) and using the MTT assay to measure cell viability after 24 hours . Advanced protocols include:
- Morphological analysis : Phase-contrast microscopy to observe apoptosis-related changes (e.g., cell shrinkage, detachment) .
- DAPI staining : Fluorescence microscopy to detect chromatin condensation and nuclear fragmentation .
Variability in IC₅₀ values arises from structural factors: - Monomeric vs. dimeric structures : Dimeric pyridinium bromides (e.g., m-xylene-linked derivatives) often show lower IC₅₀ (20 mg/mL) compared to flexible dimers (100 mg/mL) due to enhanced membrane interaction .
- Substituent effects : Electron-withdrawing groups (e.g., nitro) increase electrophilicity, enhancing cytotoxicity .
Q. Basic: What spectroscopic and thermal analysis techniques are critical for characterizing the structural and phase behavior of this compound compounds?
Answer:
- ¹H NMR : Resolves pyridinium ring protons (δ 8.5–9.5 ppm) and alkyl chain protons (δ 0.5–2.5 ppm) using deuterated DMSO .
- DSC (Differential Scanning Calorimetry) : Identifies phase transitions, such as solid-solid transitions in (bis)thiourea this compound at 150 K (first-order) and 180 K (second-order) .
- Dielectric spectroscopy : Measures ion dynamics and dielectric relaxation to study order-disorder transitions in crystalline phases .
Q. Advanced: In studying phase transitions in this compound complexes, how should dielectric spectroscopy data be interpreted alongside differential scanning calorimetry results?
Answer:
Dielectric spectroscopy reveals ion mobility changes during phase transitions. For example, in (bis)thiourea this compound:
- T₁ = 180 K (second-order transition) : Dielectric permittivity shows a gradual increase, correlating with DSC-detected latent heat absence .
- T₂ = 150 K (first-order transition) : A sharp permittivity drop indicates restricted ion motion due to structural ordering, confirmed by DSC enthalpy changes . Cross-referencing dielectric anomalies with DSC thermograms ensures accurate assignment of transition mechanisms.
Q. Basic: What are the best practices for ensuring reproducibility in the synthesis of this compound derivatives, particularly regarding purity and structural confirmation?
Answer:
- Purification : Recrystallization from ethanol/acetone mixtures removes unreacted alkyl bromides .
- Purity assessment : Melting point analysis (e.g., this compound perbromide melts at 188–190°C) and HPLC .
- Structural validation : ¹H/¹³C NMR, FT-IR (C-Br stretch at ~600 cm⁻¹), and comparison with literature data .
- Documentation : Detailed experimental protocols (solvent ratios, reaction times) as per Beilstein Journal of Organic Chemistry guidelines to enable replication .
Q. Advanced: What molecular docking approaches are employed to investigate the interaction mechanisms between this compound derivatives and biological targets?
Answer:
- Software : AutoDock Tools (ADT) and AutoDock Vina for docking studies .
- Protocol :
- Optimize ligand geometry (e.g., this compound derivatives) using ChemDraw and Gaussian.
- Prepare protein targets (e.g., EGFR kinase) by removing water molecules and adding polar hydrogens.
- Use genetic algorithms to explore binding poses, with binding affinity calculated via the Lamarckian model .
- Validation : Compare docking scores (e.g., binding energy < −6 kcal/mol) with experimental IC₅₀ values to identify structure-activity relationships .
Q. Advanced: How are this compound derivatives utilized as additives in bromine-generating electrochemical cells, and what synthesis optimizations enhance their performance?
Answer:
Alkyl 3-methyl pyridinium bromides (e.g., 3-methyl-1-octylthis compound) act as bromine-complexing agents in Zn/Br₂ batteries, preventing bromine diffusion . Synthesis optimizations include:
- Alkyl chain tuning : Longer chains (C₁₀–C₁₈) improve bromine sequestration but may reduce ionic conductivity .
- Concentration : 0.5–1.0 M aqueous solutions achieve optimal electrolyte stability .
Performance is evaluated via cyclic voltammetry and charge-discharge cycling .
Q. Basic: What safety protocols should be followed when handling this compound derivatives in laboratory settings?
Answer:
- Hazard mitigation : Use PPE (gloves, goggles) due to skin/eye irritation risks (R36/37/38) .
- Ventilation : Handle in fume hoods to avoid inhalation of fine powders .
- Storage : Keep in airtight containers away from oxidizers (e.g., dichromates) to prevent combustion .
- Waste disposal : Neutralize bromide residues with NaHCO₃ before aqueous disposal .
Comparison with Similar Compounds
Comparison with Structurally Similar Compounds
Monomeric vs. Dimeric Pyridinium Bromides
Dimeric pyridinium bromides, such as m-xylene-linked or flexible aliphatic chain-linked derivatives, exhibit enhanced anticancer activity compared to monomeric forms. For example:
- Monomeric pyridinium bromide 3 (IC₅₀: 20 µg/mL) showed superior cytotoxicity against lung cancer cells (A-549) compared to dimeric analogs 1 and 2 (IC₅₀: 100 µg/mL) .
- Dimeric derivatives, however, demonstrate higher thermal stability and unique columnar phase behavior in ILCs due to increased molecular rigidity .
Table 1: Anticancer Activity of Mono- and Dimeric Pyridinium Bromides
Counterion Effects: Br⁻ vs. PF₆⁻
Replacing bromide with hexafluorophosphate (PF₆⁻) alters physicochemical properties:
- NMR Shifts : The protons near the pyridinium nitrogen in Br⁻ salts resonate at 9.17–9.24 ppm, while PF₆⁻ analogs show upfield shifts (8.47–8.62 ppm) due to weaker hydrogen bonding .
- Phase Transitions : PF₆⁻ salts exhibit lower transition temperatures to isotropic phases compared to Br⁻ derivatives, enhancing their utility in low-temperature ILC applications .
Table 2: Counterion Impact on Pyridinium Salts
| Property | Br⁻ Salts | PF₆⁻ Salts |
|---|---|---|
| ¹H-NMR Shift (ppm) | 9.17–9.24 | 8.47–8.62 |
| Phase Transition Temp | Higher (~180 K) | Lower (~150 K) |
| Hygroscopicity | High | Low |
Alkyl Chain-Length Variants
Alkyl chain length modulates micellar and biological properties:
- 1-Butylthis compound (C₄ chain): Used in micellization studies, with a critical micelle concentration (CMC) influenced by temperature and hydrophilic groups .
- Dodecylthis compound (C₁₂ chain): Demonstrates surfactant properties with a CMC of 0.8 mM, effective in corrosion inhibition for mild steel .
- Hexadecylthis compound (C₁₆ chain): Exhibits higher surface excess (Γ_max) due to stronger hydrophobic interactions .
Specialized Derivatives
- 1-(1-Adamantyl)this compound : A bulky adamantane-substituted derivative with a melting point of 245°C, used in organic synthesis and drug design .
- Pyridinium tribromide: A brominating agent for ketones and phenols, distinct from this compound due to its tribromide counterion (Br₃⁻) .
Key Research Findings
- Anticancer Mechanisms: Dimeric pyridinium bromides activate Ca²⁺ signaling pathways, while monomeric forms induce apoptosis via chromatin condensation .
- Corrosion Inhibition : this compound derivatives (C1–C3) achieve >90% inhibition efficiency for mild steel in acidic environments through adsorption .
- Synthetic Flexibility : Microwave-assisted synthesis enables solvent-free preparation of nitrobenzyl-substituted derivatives with high yields (~95%) .
Properties
Molecular Formula |
C5H6BrN |
|---|---|
Molecular Weight |
160.01 g/mol |
IUPAC Name |
pyridin-1-ium;bromide |
InChI |
InChI=1S/C5H5N.BrH/c1-2-4-6-5-3-1;/h1-5H;1H |
InChI Key |
BBFCIBZLAVOLCF-UHFFFAOYSA-N |
Canonical SMILES |
C1=CC=[NH+]C=C1.[Br-] |
Origin of Product |
United States |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.