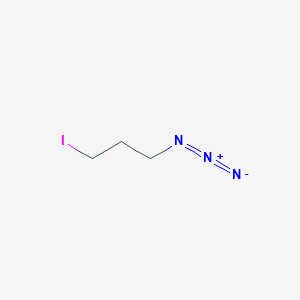
1-Azido-3-iodopropane
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Overview
Description
1-Azido-3-iodopropane (C₃H₆IN₃) is a bifunctional compound containing an azide group (-N₃) and an iodine atom at terminal positions. It is synthesized via a two-step halogen exchange reaction starting from 1-bromo-3-chloropropane. Sodium azide (NaN₃) replaces the bromine atom to form 1-azido-3-chloropropane, which undergoes subsequent substitution with sodium iodide (NaI) to yield the iodo derivative . The compound is a pale-yellow oil and is widely utilized in click chemistry, particularly in copper-catalyzed azide-alkyne cycloaddition (CuAAC) reactions to synthesize 1,2,3-triazoles . Its applications extend to protein labeling , fluorescent probe synthesis , and alkylation reactions in natural product synthesis .
Scientific Research Applications
Applications in Click Chemistry
Click chemistry refers to a set of powerful reactions that allow for the rapid and efficient formation of covalent bonds. 1-Azido-3-iodopropane is particularly useful in:
- Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) : This reaction involves the reaction of azides with terminal alkynes to form 1,2,3-triazoles. The use of this compound facilitates the synthesis of various triazole derivatives, which have applications ranging from drug development to materials science .
| Reaction Type | Description | Applications |
|---|---|---|
| CuAAC | Reaction between azides and alkynes | Drug synthesis, materials science |
| Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) | Copper-free alternative for biological applications | Bioconjugation in living systems |
Bioconjugation and Protein Labeling
This compound is employed for bioconjugation processes, particularly in labeling proteins with fluorescent tags. For instance, bovine serum albumin (BSA) can be modified with azide groups using this compound, allowing for subsequent labeling with fluorophores through click chemistry . This method is advantageous due to its specificity and efficiency.
Case Study: Fluorescent Labeling of Proteins
A study demonstrated the use of this compound to label BSA with fluorescent dyes. The process involved:
- Treating BSA with this compound in a TRIS buffer.
- Irradiating the mixture to facilitate the reaction.
- Analyzing the resulting fluorescently labeled protein using spectroscopic methods.
This technique highlights the potential for real-time imaging and tracking of proteins in biological systems .
Pharmaceutical Applications
The compound has been explored for its potential in developing new pharmaceuticals. Its ability to form stable triazole linkages makes it a candidate for synthesizing bioactive compounds.
Case Study: Antifungal Activity
Research has indicated that derivatives synthesized from this compound exhibit antifungal properties. A series of compounds were prepared through click chemistry involving this azide, leading to promising results against various fungal strains .
| Compound | Activity | Target Organisms |
|---|---|---|
| Triazole Derivative A | Moderate | Candida albicans |
| Triazole Derivative B | High | Aspergillus niger |
Material Science Applications
In material science, this compound is utilized for modifying surfaces and creating functional materials. Its reactivity allows for the incorporation of azide functionalities into polymers, which can then undergo click reactions to create crosslinked networks or functionalized surfaces.
Case Study: Surface Functionalization
A study demonstrated the functionalization of polymer surfaces with azide groups using this compound, followed by subsequent reactions with alkyne-functionalized compounds. This approach enabled the creation of bioactive surfaces suitable for tissue engineering applications .
Chemical Reactions Analysis
Cu(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC)
The azido group undergoes regioselective 1,3-dipolar cycloaddition with terminal alkynes under Cu(I) catalysis, forming stable 1,4-disubstituted 1,2,3-triazoles. This reaction is central to click chemistry applications .
Example Reaction:
Substrates : 1-Azido-3-iodopropane + Terminal alkyne (e.g., phenylacetylene)
Conditions :
-
Catalyst: CuI (0.1–1.0 equiv)
-
Base: Triethylamine (TEA) or DIPEA
-
Solvent: THF, room temperature
Product : 1,4-Disubstituted triazole (e.g., 1-(3-iodopropyl)-4-phenyl-1,2,3-triazole)
Yield : 74–82%
Key Observations:
Formation of 5-Iodo-1,2,3-Triazoles
Under oxidative conditions, electrophilic iodine species participate in cycloadditions, yielding 5-iodo-1,4,5-trisubstituted triazoles .
Example Reaction:
Substrates : this compound + Ethynyl-BODIPY
Conditions :
-
Catalyst: CuI (1.0 equiv)
-
Oxidant: N-Bromosuccinimide (NBS, 1.2 equiv)
-
Base: DIPEA (1.0 equiv)
-
Solvent: THF, inert atmosphere
Product : 5-Iodo-1,4,5-trisubstituted triazole
Yield : 69%
Mechanistic Insight:
Oxidation of iodide (I⁻) to electrophilic iodine (I⁺) enables iodination at the triazole’s 5-position .
One-Pot Cycloaddition-Sonogashira Coupling
Integrated CuAAC and Pd-catalyzed Sonogashira coupling enables synthesis of 1,4,5-trisubstituted triazoles with alkynyl substituents .
Example Reaction:
Substrates : this compound + 1-Ethynylpyrene
Conditions :
-
Catalysts: CuI (0.2 equiv) + Pd(PPh₃)₂Cl₂ (0.1 equiv)
-
Solvent: THF, 16 h
Product : 1,4,5-Trisubstituted triazole (e.g., 1-(3-iodopropyl)-4-(pyren-1-yl)-5-ethynyl-1,2,3-triazole)
Yield : 7–27%
Comparative Reaction Pathways
| Reaction Type | Conditions | Product | Yield | Key Feature |
|---|---|---|---|---|
| CuAAC | CuI, TEA, THF | 1,4-Disubstituted triazole | 74–82% | Regioselective, mild conditions |
| Oxidative iodination | CuI, NBS, DIPEA | 5-Iodo-triazole | 33–69% | Requires electrophilic iodine |
| Sonogashira coupling | CuI, Pd(PPh₃)₂Cl₂ | 1,4,5-Trisubstituted triazole | 7–27% | Modular chromophore integration |
Substitution Reactions
The iodo group participates in nucleophilic substitution (SN2), enabling derivatization .
Example Reaction:
Substrate : this compound
Reagent : Sodium azide (NaN₃)
Conditions : DMF, 60°C
Product : 1,5-Diazidopentane (via intermediate displacement)
Yield : 87%
Q & A
Basic Research Questions
Q. What are the established protocols for synthesizing 1-Azido-3-iodopropane, and how can reproducibility be ensured across laboratories?
- Methodology : Follow factorial design principles to optimize reaction parameters (e.g., temperature, solvent ratio, catalyst loading). Document purification steps (e.g., column chromatography, distillation) and characterize intermediates using NMR or IR spectroscopy. Reproducibility requires explicit reporting of solvent purity, reaction time, and quenching methods, as per standardized experimental sections in peer-reviewed journals .
- Example Table :
| Variable | Optimal Range | Impact on Yield |
|---|---|---|
| Temperature | 0–5°C | Prevents azide decomposition |
| Solvent | THF/Water (3:1) | Maximizes solubility |
| Reaction Time | 2–4 hours | Balances conversion vs. side reactions |
Q. Which spectroscopic/chromatographic methods are most effective for characterizing this compound, and how should conflicting spectral data be resolved?
- Methodology : Use 1H/13C NMR to confirm molecular structure, IR for azide (2100 cm−1) and iodide (500 cm−1) groups. For purity, employ GC-MS or HPLC with a polar stationary phase. Conflicting data (e.g., unexpected peaks) should be addressed via spiking experiments, deuterated solvent controls, or repeating analyses under inert atmospheres to rule out degradation .
Q. What safety protocols are critical when handling this compound due to its reactive functional groups?
- Methodology : Conduct small-scale reactions in fume hoods with blast shields. Use secondary containment for azide-containing waste. Monitor thermal stability via DSC (Differential Scanning Calorimetry) to identify decomposition thresholds. Emergency protocols should include dilute sodium thiosulfate solutions for iodide neutralization .
Advanced Research Questions
Q. How can computational methods like DFT elucidate reaction mechanisms involving this compound in click chemistry?
- Methodology : Perform density functional theory (DFT) calculations using software like Gaussian or ORCA. Optimize geometries at the B3LYP/6-31G* level, and calculate transition states with intrinsic reaction coordinate (IRC) analysis. Validate results against experimental kinetics (e.g., Arrhenius plots) to confirm mechanistic pathways .
Q. What experimental designs are optimal for assessing the thermal and photolytic stability of this compound under varying storage conditions?
- Methodology : Use accelerated stability studies with controlled variables (temperature, UV exposure). Apply Arrhenius modeling to predict shelf life. For photolysis, employ quartz reactors with monochromatic light sources (e.g., 254 nm). Monitor degradation via in-situ FTIR or Raman spectroscopy .
Q. What statistical approaches resolve contradictions in yield or selectivity data for reactions using this compound?
- Methodology : Conduct root-cause analysis using Fishbone diagrams to identify variables (e.g., impurity profiles, moisture levels). Apply ANOVA to compare datasets across labs. Use Bayesian inference to assess the likelihood of outlier results being systematic vs. random .
Q. How can this compound be strategically utilized in multi-step syntheses to construct complex heterocyclic or polymeric architectures?
- Methodology : Employ azide-alkyne cycloaddition (CuAAC) for regioselective triazole formation. Optimize stoichiometry via Design of Experiments (DoE) to balance reactivity and steric effects. For polymers, use RAFT polymerization with iodopropyl initiators, characterized by GPC and MALDI-TOF .
Q. What validation strategies are recommended when using molecular dynamics (MD) simulations to predict solvent interactions of this compound?
- Methodology : Simulate solvation in explicit solvents (e.g., water, DMSO) using GROMACS or AMBER. Validate force fields by comparing simulated radial distribution functions with experimental X-ray scattering data. Calibrate dielectric constants against empirical measurements .
Q. Methodological Notes
- Data Contradictions : Cross-validate findings using orthogonal techniques (e.g., XRD for crystallinity vs. DSC for thermal behavior) .
- Experimental Design : Prioritize fractional factorial designs to reduce variable combinations while maintaining statistical power .
- Computational Validation : Always benchmark computational results against experimental controls to ensure predictive accuracy .
Comparison with Similar Compounds
Comparison with Structurally Similar Compounds
1-Azido-3-chloropropane (C₃H₆ClN₃)
- Synthesis : Prepared directly from 1-bromo-3-chloropropane and NaN₃ via nucleophilic substitution .
- Reactivity : The chlorine atom exhibits lower leaving-group ability compared to iodine, making it less reactive in SN₂ substitutions. However, it serves as an intermediate for synthesizing 1-azido-3-iodopropane via halogen exchange .
- Applications: Primarily used as a precursor for the iodo analog. Limited direct applications in click chemistry due to slower reaction kinetics.
Key Differences :
| Property | This compound | 1-Azido-3-chloropropane |
|---|---|---|
| Molecular Weight (g/mol) | 226.99 | 119.55 |
| Halogen Reactivity | High (I⁻ is a better leaving group) | Moderate (Cl⁻) |
| Typical Use | Direct applications in synthesis | Precursor for iodo derivative |
3-Azidopropylamine (C₃H₈N₄)
- Structure : Contains an azide and a primary amine (-NH₂) group.
- Reactivity : The amine enables conjugation with carboxylic acids or carbonyl groups, expanding utility in bioconjugation.
- Applications : Used in click chemistry for synthesizing peptide-fluorescent dye conjugates .
Key Differences :
| Property | This compound | 3-Azidopropylamine |
|---|---|---|
| Functional Groups | Azide, iodide | Azide, amine |
| Key Reactivity | Halogen substitution | Amide bond formation |
| Applications | Triazole synthesis, alkylation | Bioconjugation, probes |
1-Azidopropane (C₃H₇N₃)
- Structure : A simpler analog lacking the iodine atom.
- Reactivity: Limited utility in substitution reactions due to the absence of a leaving group.
- Applications : Used in strain-promoted azide-alkyne cycloaddition (SPAAC) where steric bulk is minimized .
Key Differences :
| Property | This compound | 1-Azidopropane |
|---|---|---|
| Molecular Complexity | Bifunctional | Monofunctional |
| Reactivity | Versatile in SN₂ reactions | Restricted to azide-specific reactions |
1-Iodopropyne (C₃H₃I)
- Structure : An alkyne with a terminal iodine atom.
- Reactivity : Participates in Cadiot-Chodkiewicz coupling for carbon-carbon bond formation .
- Applications : Key intermediate in synthesizing acetylenic natural products.
Key Differences :
| Property | This compound | 1-Iodopropyne |
|---|---|---|
| Functional Groups | Azide, iodide | Alkyne, iodide |
| Primary Use | Triazole formation | Alkyne coupling reactions |
1-Azido-2,3-epoxypropane (C₃H₅N₃O)
- Structure : Combines an azide with an epoxide ring.
- Reactivity : The epoxide enables ring-opening reactions with nucleophiles (e.g., amines, thiols).
- Applications : Used in polymer chemistry and crosslinking agents .
Key Differences :
| Property | This compound | 1-Azido-2,3-epoxypropane |
|---|---|---|
| Reactivity | SN₂ substitutions | Epoxide ring-opening |
| Applications | Small-molecule synthesis | Polymer networks |
Properties
Molecular Formula |
C3H6IN3 |
|---|---|
Molecular Weight |
211.00 g/mol |
IUPAC Name |
1-azido-3-iodopropane |
InChI |
InChI=1S/C3H6IN3/c4-2-1-3-6-7-5/h1-3H2 |
InChI Key |
GTBCENNLSAZFSM-UHFFFAOYSA-N |
Canonical SMILES |
C(CN=[N+]=[N-])CI |
Origin of Product |
United States |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.