Clopidogrel Camphorsulfonate
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Overview
Description
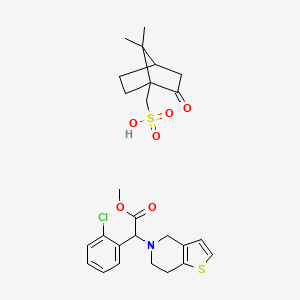
Clopidogrel camphorsulfonate (CSA) is a salt form of clopidogrel, a thienopyridine-class antiplatelet agent used to prevent thrombotic events in cardiovascular diseases. The camphorsulfonate counterion improves the physicochemical properties of clopidogrel, such as stability and crystallinity, which are critical for pharmaceutical formulation and manufacturing. CSA is chemically defined as (S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid methyl ester (1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]heptane-1-methanesulfonate, with the molecular formula C₁₆H₁₆ClNO₂S·C₁₀H₁₆O₄S and CAS number 120202-68-8 . Its synthesis often involves resolving clopidogrel enantiomers using L-camphorsulfonic acid, as described in patents and preformulation studies .
Scientific Research Applications
Pharmacological Properties
Clopidogrel is an inhibitor of platelet aggregation, primarily acting by blocking the binding of adenosine diphosphate (ADP) to its receptor on platelets. This mechanism reduces the likelihood of thrombus formation, which is crucial in preventing cardiovascular events such as myocardial infarction and stroke . The camphorsulfonate salt form enhances the stability and solubility of clopidogrel, making it suitable for pharmaceutical formulations .
Key Characteristics
- Stability : Clopidogrel camphorsulfonate exhibits improved stability against moisture and heat compared to other salt forms .
- Solubility : It has satisfactory water solubility, approximately 4.0 mg/ml, which facilitates its dissolution in pharmaceutical compositions .
- Optical Purity : The salt can achieve high optical purity (≥98.5% enantiomeric excess), crucial for ensuring therapeutic efficacy .
Clinical Applications
This compound is primarily indicated for the prevention of atherothrombotic events in various clinical scenarios:
- Acute Coronary Syndrome : It is used in combination with acetylsalicylic acid (ASA) for patients experiencing unstable angina or non-ST-segment elevation myocardial infarction .
- Post-Myocardial Infarction : Administered to prevent further thrombotic events within 35 days following an acute myocardial infarction .
- Ischemic Stroke : Effective in preventing recurrent strokes within six months after an initial event .
- Peripheral Arterial Disease : Used for patients with established peripheral arterial disease to reduce cardiovascular complications .
Efficacy in Post-PCI Patients
A study highlighted that patients undergoing percutaneous coronary intervention (PCI) benefit significantly from clopidogrel therapy, showing a relative risk reduction of 30-85% in major adverse cardiovascular events compared to placebo . This underscores the importance of this compound in high-risk populations.
Pharmacogenetic Considerations
Research indicates that genetic variations affecting the CYP2C19 enzyme can influence the efficacy of clopidogrel. Patients with loss-of-function alleles may experience reduced therapeutic benefits, emphasizing the need for genotype-guided therapy in specific populations .
Comparison with Other Antiplatelet Agents
Clopidogrel has been compared with other antiplatelet medications like ticagrelor and aspirin. Evidence suggests that clopidogrel remains a critical option for patients who may not tolerate newer agents due to side effects or contraindications .
Comparison with Similar Compounds
Clopidogrel is commonly administered as acid addition salts to enhance bioavailability and processability. Below is a detailed comparison of CSA with other clopidogrel salts:
Physicochemical Properties
Table 1: Comparative Physicochemical Properties of Clopidogrel Salts
Key Findings:
- Solubility: CSA and besylate exhibit lower aqueous solubility compared to hydrogen sulfate, hydrochloride, and hydrobromide salts . This may influence dissolution rates and bioavailability in formulation design.
- Thermal Stability: Hydrogen sulfate salts have higher melting points due to hydrogen bonding between bisulfate anions, forming stable crystalline chains .
- Hygroscopicity: Hydrogen sulfate polymorphs (Forms 1 and 2) are highly hygroscopic, necessitating controlled packaging to prevent moisture uptake. CSA and besylate are less hygroscopic, simplifying storage .
- Crystalline Forms: CSA is anhydrous, whereas hydrobromide exists as a monohydrate, affecting stability under humid conditions .
Clinical and Formulation Considerations
Its lower solubility may necessitate formulation adjustments, such as using surfactants or co-solvents, to match the dissolution profile of bisulfate salts .
Properties
Molecular Formula |
C26H32ClNO6S2 |
|---|---|
Molecular Weight |
554.1 g/mol |
IUPAC Name |
(7,7-dimethyl-2-oxo-1-bicyclo[2.2.1]heptanyl)methanesulfonic acid;methyl 2-(2-chlorophenyl)-2-(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate |
InChI |
InChI=1S/C16H16ClNO2S.C10H16O4S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-5,7,9,15H,6,8,10H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14) |
InChI Key |
XEENARPWPCQXST-UHFFFAOYSA-N |
Canonical SMILES |
CC1(C2CCC1(C(=O)C2)CS(=O)(=O)O)C.COC(=O)C(C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 |
Origin of Product |
United States |
Synthesis routes and methods
Procedure details





Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.














