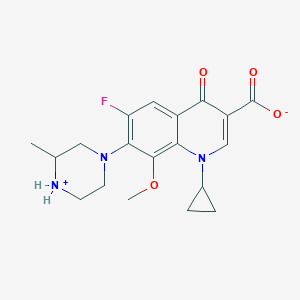
Gatifloxacin
Description
Evolution of Fluoroquinolone Antibiotics
The development of fluoroquinolone antibiotics has progressed through several generations, marked by structural modifications aimed at improving antibacterial spectrum, potency, and pharmacokinetic properties. The earliest quinolone, nalidixic acid, discovered in the 1960s, exhibited narrow-spectrum activity primarily against Gram-negative bacteria. bocsci.comnih.gov Subsequent generations, starting with the introduction of the fluorine atom, led to compounds like ciprofloxacin and norfloxacin (second generation), which showed broader activity against both Gram-negative and some Gram-positive bacteria. bocsci.comnih.govnih.gov Third-generation fluoroquinolones, such as levofloxacin, were developed with enhanced activity against Gram-positive organisms, including Streptococcus pneumoniae. nih.govnih.gov
Gatifloxacin within Fourth-Generation Fluoroquinolone Research
This compound is classified as a fourth-generation fluoroquinolone. asianjpr.comdrugbank.com This generation, which also includes moxifloxacin and trovafloxacin, was developed with a focus on further enhancing activity against Gram-positive bacteria, including those resistant to earlier fluoroquinolones, while retaining broad-spectrum activity against Gram-negative pathogens. nih.govresearchgate.netcrstoday.com A key structural feature contributing to the enhanced activity of some fourth-generation fluoroquinolones, including this compound and moxifloxacin, is the presence of a C-8 methoxy group. crstoday.comreviewofophthalmology.comnih.gov This group has been shown to contribute to enhanced activity against resistant gyrase and wild-type topoisomerase IV. nih.gov
This compound exerts its bactericidal action by inhibiting two critical bacterial enzymes: DNA gyrase (also known as topoisomerase II) and topoisomerase IV. asianjpr.comdrugbank.compatsnap.com These enzymes are essential for bacterial DNA replication, transcription, repair, and recombination. asianjpr.compatsnap.com DNA gyrase is primarily involved in introducing negative supercoils into bacterial DNA, a process necessary for replication and transcription, while topoisomerase IV is crucial for separating interlinked daughter DNA molecules after replication. patsnap.comoup.com By inhibiting both enzymes, this compound disrupts these vital DNA processes, leading to bacterial cell death. patsnap.com While older quinolones often had a primary target preference (gyrase for Gram-negative, topoisomerase IV for Gram-positive), research on newer agents like this compound indicates significant activity against both targets, contributing to their broader spectrum. oup.com Studies have shown that this compound has a high affinity for bacterial DNA gyrase. drugbank.comasianjpr.com
Current Academic Research Landscape of this compound
Current academic research on this compound continues to explore its efficacy against various bacterial pathogens and investigate mechanisms related to its activity and potential resistance. Research highlights its broad spectrum of activity against both Gram-positive and Gram-negative bacteria. drugbank.comasianjpr.com Studies have compared the in vitro activity of this compound with other fluoroquinolones against a range of bacterial isolates, including those from ocular infections. nih.govnih.gov
Data from in vitro studies provide insights into the minimum inhibitory concentrations (MICs) of this compound against specific pathogens. For instance, a study evaluating ocular pathogens found that this compound exhibited activity against Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Bacillus cereus, and Enterococcus faecalis, with MIC90 values comparable to previously published values for systemic infections. nih.gov The same study reported MIC90 values for this compound against Streptococcus viridans and Pseudomonas aeruginosa. nih.gov
| Bacterial Species | This compound MIC90 (mg/mL) | Source of Isolates |
|---|---|---|
| Staphylococcus epidermidis | 0.08 - 0.57 | Ocular nih.gov |
| Staphylococcus aureus | 0.08 - 0.57 | Ocular nih.gov |
| Streptococcus pneumoniae | 0.08 - 0.57 | Ocular nih.gov |
| Streptococcus pyogenes | 0.08 - 0.57 | Ocular nih.gov |
| Bacillus cereus | 0.08 - 0.57 | Ocular nih.gov |
| Enterococcus faecalis | 0.08 - 0.57 | Ocular nih.gov |
| Streptococcus viridans | 0.22 | Ocular nih.gov |
| Pseudomonas aeruginosa | 1.28 | Ocular nih.gov |
Research also examines the activity of this compound against bacteria resistant to earlier-generation fluoroquinolones. In vitro studies have indicated that fourth-generation fluoroquinolones, including this compound, appear to cover bacterial resistance seen with second and third-generation agents and demonstrate greater potency against Gram-positive bacteria compared to these older classes. nih.gov
However, the emergence of bacterial resistance to fluoroquinolones, including this compound, remains a significant area of research. Resistance can develop through mechanisms such as mutations in the genes encoding DNA gyrase (gyrA, gyrB) and topoisomerase IV (parC, parE), particularly in the quinolone resistance-determining regions (QRDR), or through increased efflux of the antibiotic from the bacterial cell. bocsci.comoup.comnih.gov Recent studies highlight increasing resistance to this compound in certain bacterial groups, such as Staphylococcus, Streptococcus, and Corynebacterium, particularly in the context of ophthalmic infections. nih.gov This underscores the ongoing need for surveillance and research into resistance mechanisms and strategies to mitigate their impact. nih.gov
Academic research also explores novel formulations and delivery methods for this compound, particularly for ophthalmic use, to enhance local concentration and minimize systemic exposure. researchgate.netresearchandmarkets.com Studies have investigated the potential for this compound, in combination with excipients like benzalkonium chloride (BAK), to demonstrate enhanced efficacy against certain resistant strains in vitro. reviewofophthalmology.com
Furthermore, this compound has been investigated in clinical trials for various indications, including tuberculosis, to explore potential treatment regimen shortenings, although demonstrating noninferiority to standard regimens has presented challenges. researchgate.net The academic landscape continues to investigate the therapeutic potential of this compound in light of evolving resistance patterns and the need for effective antimicrobial agents. researchandmarkets.comgiiresearch.com
Properties
IUPAC Name |
1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-4-ium-1-yl)-4-oxoquinoline-3-carboxylate | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H22FN3O4/c1-10-8-22(6-5-21-10)16-14(20)7-12-15(18(16)27-2)23(11-3-4-11)9-13(17(12)24)19(25)26/h7,9-11,21H,3-6,8H2,1-2H3,(H,25,26) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XUBOMFCQGDBHNK-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CN(CC[NH2+]1)C2=C(C=C3C(=C2OC)N(C=C(C3=O)C(=O)[O-])C4CC4)F | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H22FN3O4 | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
375.4 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
6.31e-01 g/L | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
112811-59-3 | |
| Record name | Gatifloxacin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=112811-59-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
182 - 185 °C | |
| Record name | Gatifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015178 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Gatifloxacin's Molecular Mechanism of Antimicrobial Action
Inhibition of Bacterial DNA Gyrase (Topoisomerase II)
Bacterial DNA gyrase, a type II topoisomerase, plays a vital role in controlling DNA topology by introducing negative supercoils into bacterial DNA. patsnap.commdpi.com This process is essential for DNA replication and transcription, as it enables the unwinding of the double helix and facilitates the progression of the replication fork. patsnap.com Gatifloxacin targets DNA gyrase, inhibiting its function. drugbank.comasianjpr.compatsnap.com
Elucidation of Inhibitory Activities Against Bacterial DNA Gyrase
Studies have determined the inhibitory activities of this compound against bacterial DNA gyrase. For instance, this compound demonstrated potent inhibitory activity against Escherichia coli DNA gyrase with a 50% inhibitory concentration (IC50) of 0.109 μg/ml. nih.govasm.orgnih.govmedchemexpress.com This highlights its effectiveness in inhibiting this essential bacterial enzyme.
Comparative Selectivity for Bacterial DNA Gyrase versus Mammalian Topoisomerase II
This compound exhibits a high selectivity for bacterial type II topoisomerases compared to mammalian topoisomerase II. nih.govasm.orgnih.gov The IC50 of this compound for HeLa cell topoisomerase II was significantly higher (265 μg/ml) than its IC50 values for bacterial enzymes like S. aureus topoisomerase IV (13.8 μg/ml) and E. coli DNA gyrase (0.109 μg/ml). nih.govasm.orgnih.govmedchemexpress.com The ratio of the IC50 for HeLa cell topoisomerase II to that for bacterial enzymes was more than 2,400 times higher, indicating a substantially greater selectivity for bacterial targets. nih.govasm.orgnih.gov This differential activity is a key factor in the drug's ability to target bacterial cells with less impact on host cells.
Here is a table summarizing the inhibitory activities:
| Enzyme | Species | IC50 (μg/ml) | Source |
| Topoisomerase IV | S. aureus | 13.8 | nih.govasm.orgnih.govmedchemexpress.com |
| DNA Gyrase | E. coli | 0.109 | nih.govasm.orgnih.govmedchemexpress.com |
| Topoisomerase II | HeLa cells | 265 | nih.govasm.orgnih.govmedchemexpress.com |
Molecular Interaction with GyrA Subunit and Bacterial DNA
This compound targets the A subunit of DNA gyrase. patsnap.compatsnap.com By binding to the GyrA subunit, this compound inhibits the enzyme's ability to re-ligate cleaved DNA strands. patsnap.compatsnap.com This interaction stabilizes the transient double-strand breaks introduced by DNA gyrase, leading to the accumulation of double-stranded breaks in the bacterial chromosome. patsnap.com Fluoroquinolones, including this compound, poison cells by trapping DNA gyrase and topoisomerase IV on chromosomes as quinolone-enzyme-DNA complexes. nih.gov These complexes block the movement of replication forks and transcription complexes. researchgate.net Fluoroquinolones bind to a region near tyrosine-122 in the N-terminal domain of the GyrA subunits, forming a ternary complex that blocks movement over the replication fork. mdpi.com
Allele-Specific Enhancement of Inhibition Against Resistant Gyrase Mutants
The C-8-methoxy group of this compound contributes to enhanced activity against resistant gyrase mutants. nih.govasm.org Studies using isogenic sets of quinolone-resistant gyrA mutants of Escherichia coli have shown that the C-8-methoxy group lowered the resistance due to mutations, particularly those mapping in α-helix 4 of the GyrA subunit, which is thought to interact with DNA. nih.govasm.org This suggests that the C-8-methoxy group facilitates the attack of this compound on these resistant gyrase variants. nih.gov
Influence of the C-8 Methoxy Group on DNA Gyrase Inhibition
The presence of a methoxy group at the C-8 position of the quinolone ring in this compound is associated with enhanced inhibitory activity against bacterial DNA gyrase. researchgate.netoup.comnih.govnih.gov This structural feature appears to contribute to increased potency against Gram-positive bacteria and improved activity against DNA gyrase mutants. oup.comresearchgate.net Studies comparing this compound (C-8-methoxy) with compounds lacking this group (C-8-H), such as ciprofloxacin and AM1121, have shown that the C-8-methoxy compounds exhibit higher inhibitory activity against DNA gyrase. nih.govnih.gov Specifically, the inhibitory activities of C-8-methoxy compounds against DNA gyrase were approximately six times higher than those of their C-8-H counterparts in Staphylococcus aureus. nih.gov This enhancement of DNA gyrase inhibition is considered a possible mechanism underlying the potent antibacterial activity of this compound. nih.gov
In Silico Modeling and Docking Studies of this compound-DNA Gyrase Complexes
In silico studies, including molecular modeling and docking, have been conducted to investigate the interaction between this compound and bacterial DNA gyrase. These studies aim to understand the binding modes and affinities of this compound with the enzyme. academicjournals.orgacademicjournals.orgresearchgate.nettandfonline.com Docking calculations have been used to predict the interaction between this compound and the "Quinolone Resistance Determining Region" (QRDR) of E. coli DNA Gyrase-A. researchgate.netitmedicalteam.pl These studies can help identify critical amino acid residues involved in the binding of quinolones to DNA gyrase, such as Asp87, Thr88, Arg91, and Met92 in E. coli. jppres.com Molecular modeling has also been used to elucidate the hypothetical structures of enzymes like Mycobacterium tuberculosis DNA gyrase and investigate the binding affinities of this compound analogs. tandfonline.com These computational approaches provide valuable insights into the molecular basis of this compound's interaction with its target enzyme.
Inhibition of Bacterial Topoisomerase IV
Topoisomerase IV, a type II topoisomerase, plays a crucial role in bacterial cell division, primarily by decatenating (separating) the interlinked daughter chromosomes after DNA replication. patsnap.compatsnap.com Inhibition of this enzyme by this compound disrupts this essential segregation process, contributing significantly to the drug's bactericidal activity. patsnap.compatsnap.com
Elucidation of Inhibitory Activities Against Bacterial Topoisomerase IV
Research has elucidated the inhibitory activities of this compound against bacterial type II topoisomerases, including topoisomerase IV. Studies comparing this compound with other quinolones have shown its potent inhibitory effects on bacterial topoisomerase IV. nih.gov this compound binds to the B subunit of topoisomerase IV, preventing its ability to relax supercoiled DNA and decatenate chromosomes. patsnap.com The inhibitory activities of quinolones against bacterial type II topoisomerases, such as Staphylococcus aureus topoisomerase IV and Escherichia coli DNA gyrase, have been shown to correlate significantly with their antibacterial activities. nih.gov
Comparative Inhibition of Topoisomerase IV Across Diverse Bacterial Species
This compound demonstrates inhibitory activity against topoisomerase IV in a range of bacterial species. While fluoroquinolones generally target DNA gyrase as the primary target in Gram-negative bacteria and topoisomerase IV in Gram-positive bacteria, the specific preference can vary depending on the quinolone and bacterial species. asm.orgoup.com For instance, while some compounds like this compound target gyrase in Streptococcus pneumoniae, they appear to target topoisomerase IV in Staphylococcus aureus. asm.org Studies have shown this compound to be highly active against various bacterial species, including Staphylococcus aureus and Escherichia coli, with demonstrated minimum inhibitory concentrations (MICs). ontosight.aipsu.edu
Data illustrating the comparative inhibitory activities of this compound against topoisomerase IV in different bacterial species, often measured by IC50 (50% inhibitory concentration) values, highlights its potency. For example, this compound has shown potent inhibitory activity against S. aureus topoisomerase IV. nih.gov
| Bacterial Species | Target Enzyme | IC50 (μg/ml) | Source |
| Staphylococcus aureus | Topoisomerase IV | 13.8 | nih.gov |
| Escherichia coli | DNA Gyrase | 0.109 | nih.gov |
Note: While the table includes DNA Gyrase data for comparison as per the source, the focus remains on Topoisomerase IV inhibition.
The Dual-Targeting Strategy in Fluoroquinolones and this compound's Role
Fluoroquinolones, including this compound, employ a dual-targeting strategy, inhibiting both bacterial DNA gyrase and topoisomerase IV. patsnap.comnih.govwikipedia.orgwikidata.orgontosight.ai This dual action is crucial for their broad-spectrum activity and helps mitigate the development of bacterial resistance. patsnap.comdovepress.com DNA gyrase is primarily involved in introducing negative supercoils into DNA, essential for replication and transcription, while topoisomerase IV is key for chromosome segregation during cell division. patsnap.compatsnap.compatsnap.com By inhibiting both enzymes, this compound effectively disrupts critical DNA processes. patsnap.compatsnap.compatsnap.comnih.gov this compound binds to specific subunits of these enzymes – the A subunit of DNA gyrase and the B subunit of topoisomerase IV – preventing the religation of cleaved DNA strands. patsnap.compatsnap.com This dual targeting reduces the likelihood of resistance developing through a single mutation in either target enzyme, as concomitant mutations in both genes would be required. dovepress.com
Downstream Effects on Bacterial DNA Replication, Transcription, Repair, and Recombination
The inhibition of DNA gyrase and topoisomerase IV by this compound leads to significant downstream effects on essential bacterial DNA processes. These enzymes are fundamental for DNA replication, transcription, repair, and recombination. patsnap.comdrugbank.compatsnap.compatsnap.comnih.govfrontiersin.orgwikipedia.orgwikidata.orgontosight.ai By stabilizing the cleaved DNA-enzyme complexes, this compound effectively blocks the movement of the DNA replication fork, halting DNA replication. oup.comnih.gov This disruption also impedes transcription. patsnap.compatsnap.comontosight.ai The accumulation of DNA strand breaks due to the stabilized cleavage complexes triggers the bacterial SOS response; however, if the damage is overwhelming, it can lead to mutagenesis or cell death. acs.org The inhibition of topoisomerase IV specifically interferes with the segregation of replicated chromosomes, leading to catastrophic outcomes during cell division. patsnap.com
Investigation of Bactericidal Action Mechanisms Independent of Bacterial Growth Phase
Studies have investigated the bactericidal action of this compound and its independence from the bacterial growth phase. Unlike some other quinolones whose killing activity can be influenced by the metabolic state of the bacteria or the inhibition of protein/RNA synthesis, this compound has demonstrated the ability to kill bacteria regardless of their metabolic state. researchgate.netnih.govoup.com Research comparing this compound with other quinolones like ciprofloxacin and norfloxacin has shown that this compound's killing was not influenced by the addition of bacterial protein or RNA synthesis inhibitors against tested strains. researchgate.netnih.govoup.com this compound was also found to be effective in killing non-dividing Staphylococcus and E. coli cells. researchgate.netnih.govoup.com This suggests that this compound's mechanism of killing action is not solely dependent on the bacterial life cycle. researchgate.net
In Vitro Antimicrobial Spectrum and Potency of Gatifloxacin
Activity Against Gram-Positive Bacterial Isolates
Gatifloxacin exhibits notable activity against a variety of Gram-positive bacteria, including those that may show reduced susceptibility to other antimicrobial agents. oup.comnih.govnih.govpsu.edu
Staphylococcus Species: Methicillin-Susceptible and Coagulase-Negative Staphylococci
This compound is highly active against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-susceptible coagulase-negative staphylococci. oup.comnih.govpsu.edutandfonline.com Studies have shown this compound to be more potent than ciprofloxacin and ofloxacin against MSSA and methicillin-susceptible Staphylococcus epidermidis, Staphylococcus haemolyticus, and Staphylococcus saprophyticus. oup.com Modal MIC90 values for this compound against these methicillin-susceptible strains were typically low, often around 0.12 mg/L. oup.com
Against methicillin-resistant Staphylococcus aureus (MRSA), the potency of quinolones, including this compound, is significantly reduced, with MIC90 values increasing substantially compared to susceptible strains. oup.comtandfonline.com this compound demonstrated moderate activity against MRSA, inhibiting a percentage of strains at its susceptibility breakpoint. nih.gov Similarly, the susceptibility of coagulase-negative staphylococci to this compound can be variable, with higher MIC90 values observed for strains resistant to methicillin and/or ciprofloxacin. tandfonline.com
Table 1: In Vitro Activity of this compound Against Staphylococcus Species
| Organism | Susceptibility Status | This compound MIC90 (mg/L) | Comparator MIC90s (mg/L) (Ciprofloxacin, Ofloxacin) | Source |
| Staphylococcus aureus | Methicillin-Susceptible | 0.25, ≤ 0.33 | 0.25–0.5 (Cipro, Oflo) | oup.comnih.govtandfonline.com |
| Staphylococcus aureus | Methicillin-Resistant | 4, ≥ 32 | >16 (Quinolones), ≥ 4-16 (Cipro) | oup.comnih.govtandfonline.com |
| Staphylococcus epidermidis | Methicillin-Susceptible | 0.12, ≤ 2 | 0.25–0.5 (Quinolones), 8 (Levo), 32 (Cipro) | oup.comnih.govreviewofophthalmology.comupmc.com |
| Coagulase-Negative Staphylococci | Methicillin-Susceptible | 0.12 - 0.5 | tandfonline.com | |
| Coagulase-Negative Staphylococci | Methicillin-Resistant | 2 - 12 | tandfonline.com |
Note: MIC90 values may vary depending on the specific study, isolate source, and testing methodology.
Streptococcus Species: Streptococcus pneumoniae (including penicillin non-susceptible strains) and Viridans Group Streptococci
This compound is highly active against Streptococcus pneumoniae, including strains with reduced susceptibility or resistance to penicillin. oup.comnih.govoup.comnih.govnih.govasm.orgnih.govscielo.broup.comnih.gov Its activity against pneumococci is generally comparable to or better than that of older fluoroquinolones like ciprofloxacin and ofloxacin. oup.comupmc.comnih.govoup.com The MIC90 for this compound against S. pneumoniae is consistently low, reported around 0.5 mg/L, regardless of penicillin susceptibility. oup.comnih.govnih.govasm.orgnih.govoup.com Studies have shown this compound to be the most active agent tested against streptococci, including penicillin-nonsusceptible S. pneumoniae. nih.gov
Against Viridans group streptococci, this compound also demonstrates good activity. nih.govnih.govpsu.edu MIC90 values for this compound against Viridans group streptococci have been reported at ≤ 1.0 mg/L and 0.5 mg/mL, comparable to imipenem. nih.govnih.gov
Table 2: In Vitro Activity of this compound Against Streptococcus Species
| Organism | Susceptibility Status | This compound MIC90 (mg/L) | Comparator MIC90s (mg/L) (Penicillin, Ciprofloxacin, Ofloxacin, Levofloxacin) | Source |
| Streptococcus pneumoniae | All strains | 0.19, 0.5, 0.78, 1.0 | 2-4 (Cipro, Oflo), 1 (Levo) | oup.comnih.govoup.comupmc.comasm.orgnih.govscielo.broup.com |
| Streptococcus pneumoniae | Penicillin Non-susceptible | 0.5, 1.0 | 2-4 (Cipro, Oflo) | oup.comnih.govnih.govnih.govnih.govoup.com |
| Viridans Group Streptococci | Not specified | ≤ 1.0, 0.5 | 2-4 (Cipro, Oflo), 0.5 (Imipenem) | oup.comnih.govnih.govnih.gov |
| Streptococcus pyogenes | Not specified | 0.78 | oup.comoup.com | |
| Beta-haemolytic Streptococci | Macrolide sensitive/resistant | ≤ 0.5 | nih.gov |
Note: MIC90 values may vary depending on the specific study, isolate source, and testing methodology.
Enterococcus Species: Enterococcus faecalis and Enterococcus faecium
This compound shows activity against Enterococcus species, including Enterococcus faecalis and some strains of Enterococcus faecium. oup.comoup.comnih.govoup.com Its potency against enterococci is generally considered moderate compared to its activity against staphylococci and streptococci. nih.gov
Against Enterococcus faecalis, this compound has demonstrated activity with reported MIC50 and MIC90 values of 0.5 and 1 mg/L, respectively. oup.comoup.com Some studies indicate that this compound was more active than other quinolones against Enterococcus faecalis. oup.comoup.com Against Enterococcus faecium, this compound is less potent than against E. faecalis, with a reported MIC90 of 4 mg/L against some strains. oup.comoup.com Resistance to this compound has been observed in some E. faecalis isolates, particularly uropathogenic strains, with high MIC90 values reported in some studies. oup.cominternationalscholarsjournals.com All five fluoroquinolones tested in one study, including this compound, had poor activity against Enterococcus faecium. jmilabs.com
Table 3: In Vitro Activity of this compound Against Enterococcus Species
| Organism | Susceptibility Status | This compound MIC50 (mg/L) | This compound MIC90 (mg/L) | Comparator MIC90s (mg/L) | Source |
| Enterococcus faecalis | Not specified | 0.5 | 1, ≤ 1.0, 25 | Lower potency with Cipro, Oflo | oup.comoup.comnih.govoup.com |
| Enterococcus faecium | Not specified | 1 | 4, >8 | Lower potency with Cipro, Oflo, Poor activity with other FQs | oup.comoup.comjmilabs.com |
Note: MIC values may vary depending on the specific study, isolate source, and testing methodology.
Other Gram-Positive Organisms: Aerococcus, Listeria monocytogenes, Micrococcus, Stomatococcus mucilaginous, Bacillus, and Rhodococcus equi
This compound has demonstrated activity against a range of other Gram-positive organisms. nih.govpsu.eduscilit.com It has been shown to inhibit all tested isolates of Aerococcus, Listeria monocytogenes, Micrococcus, Stomatococcus mucilaginous, Bacillus, and Rhodococcus equi at low concentrations (≤ 2 mg/L). nih.govpsu.edu this compound was reported as the most potent agent against Listeria monocytogenes compared to other tested agents in one study. oup.com
Activity Against Gram-Negative Bacterial Isolates
This compound retains broad-spectrum activity against Gram-negative bacteria, although its potency relative to some other fluoroquinolones can vary depending on the specific organism. oup.comnih.govoup.comnih.govnih.gov
Enterobacteriaceae: Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes
This compound is active against many members of the Enterobacteriaceae family, including Escherichia coli, Klebsiella species, and Enterobacter species. oup.comnih.govoup.comoup.comnih.govnih.govpsu.edutandfonline.comoup.comnih.gov Against most Enterobacteriaceae, this compound MIC90s typically range from 0.06 to 0.5 mg/L. oup.com
For Escherichia coli, this compound demonstrates good activity. oup.comoup.comnih.govpsu.edunih.gov While ciprofloxacin was often more potent against E. coli, this compound inhibited a high percentage of isolates at low concentrations (≤ 1 mg/L). nih.gov MIC90 values for E. coli have been reported at ≤ 0.39 mg/L and ≤ 0.5 mg/mL. oup.comoup.comnih.gov Against ciprofloxacin-resistant E. coli, this compound showed some reduced susceptibility but may still be useful. researchgate.netnih.gov
This compound is also active against Klebsiella pneumoniae. oup.comoup.comnih.govpsu.edunih.gov MIC90 values for K. pneumoniae have been reported at ≤ 0.39 mg/L, ≤ 0.5 µg/ml, and 0.78 mg/L. oup.comoup.comtandfonline.com
Against Enterobacter aerogenes, this compound has shown activity. psu.edunih.gov MIC90 values for Enterobacter aerogenes were reported to be one fourth to one fifth the values for moxifloxacin in one study. nih.govresearchgate.net
Table 4: In Vitro Activity of this compound Against Select Enterobacteriaceae
| Organism | Susceptibility Status | This compound MIC90 (mg/L) | Comparator MIC90s (mg/L) (Ciprofloxacin, Ofloxacin, Moxifloxacin) | Source |
| Escherichia coli | Not specified | ≤ 0.39, ≤ 0.5, ≤ 1.0, 2 | Generally more potent with Cipro | oup.comoup.comnih.govnih.govjmilabs.comnih.govoup.com |
| Escherichia coli | Ciprofloxacin-resistant | >16 | >8 (Cipro) | researchgate.netnih.govjmilabs.com |
| Klebsiella pneumoniae | Not specified | ≤ 0.39, ≤ 0.5, 0.78, 8 | Comparable to other quinolones, 2 (Moxifloxacin) | oup.comoup.comnih.govtandfonline.comjmilabs.comnih.gov |
| Enterobacter aerogenes | Not specified | One fourth to one fifth of Moxifloxacin | Higher with Moxifloxacin | psu.edunih.govresearchgate.netasm.org |
Note: MIC90 values may vary depending on the specific study, isolate source, and testing methodology.
Pseudomonas Species: Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas stutzeri
This compound exhibits activity against Pseudomonas species, although its potency compared to other fluoroquinolones like ciprofloxacin can vary. Against Pseudomonas aeruginosa, this compound and ofloxacin have shown similar anti-pseudomonal potency, while ciprofloxacin has demonstrated two- to eight-fold greater potency in some studies. nih.govoup.com MIC90 values for P. aeruginosa have been reported around 4 mg/L for this compound and ofloxacin, while ciprofloxacin MIC90s were lower. oup.com In one study, intermediate susceptibility (MIC of 4 µg/ml) to this compound was noted in one isolate of P. aeruginosa. nih.gov
For Pseudomonas fluorescens and Pseudomonas stutzeri, this compound MIC90s were reported as 8 mg/L and 0.25 mg/L, respectively, which were the same as ofloxacin MIC90s. oup.com Ciprofloxacin MIC90s were four- to eight-fold lower against these species. oup.com
Synergistic killing effects have been observed with this compound in combination with other antimicrobial agents against P. aeruginosa and P. stutzeri. For P. aeruginosa, combinations with imipenem, aztreonam, or piperacillin were most often synergistic. nih.gov Against P. stutzeri, synergism was noted with combinations including aztreonam, piperacillin, or amikacin. nih.gov
Haemophilus influenzae and Moraxella catarrhalis
This compound is highly potent against Haemophilus influenzae and Moraxella catarrhalis. nih.govtandfonline.comscielo.br Studies have reported very low MIC50 and MIC90 values for this compound against these pathogens, often ≤0.03 µg/ml. nih.gov this compound has been described as one of the most active agents tested against H. influenzae. nih.gov Compared to other fluoroquinolones, this compound's activity against H. influenzae and M. catarrhalis is excellent, with MIC90 values significantly lower than susceptibility breakpoints. nih.govuwi.edu
Neisseria gonorrhoeae
This compound demonstrates exquisite potency against Neisseria gonorrhoeae, with reported MIC90 values as low as 0.016–0.25 mg/L. tandfonline.comoup.com
Acinetobacter Species: Acinetobacter lwoffi, Acinetobacter baumanii
This compound shows good potency against Acinetobacter species. oup.comscielo.br Specifically, it has been reported to be very active against Acinetobacter lwoffi, with a MIC100 of 0.12 mg/L in one study. karger.comnih.gov Moderate activity has been observed against Acinetobacter baumanii. karger.comnih.gov MIC90 values for Acinetobacter species have ranged from 0.5 to 1 mg/L. oup.com While this compound has activity, some studies indicate that Acinetobacter species can be less susceptible to fluoroquinolones, including this compound, compared to other Gram-negative organisms. tandfonline.comkarger.comnih.govdoi.org
Stenotrophomonas maltophilia and Burkholderia cepacia
The activity of this compound against Stenotrophomonas maltophilia and Burkholderia cepacia can be variable. This compound has shown greater potency against S. maltophilia compared to ciprofloxacin or ofloxacin. oup.comnih.gov MIC50 and MIC90 values for this compound against S. maltophilia have been reported as 1 and 4 mg/L, respectively, in one study, and 0.5 and 12 µg/mL in another. oup.commdpi.com Susceptibility rates of S. maltophilia to this compound have been reported around 71%. nih.govresearchgate.netasm.org Time-kill studies have indicated that this compound can be bactericidal against S. maltophilia at concentrations equivalent to twice or four times the MIC. nih.govasm.org
Against Burkholderia cepacia, this compound and ciprofloxacin have shown comparable, though often poor, potencies, with MIC50s and MIC90s around 4 and 8 mg/L. oup.comoup.com All three quinolones (this compound, ciprofloxacin, and ofloxacin) have been reported as equipotent against B. cepacia with MIC90s of 8 mg/L. nih.gov However, some studies indicate that susceptibility of Burkholderia species to this compound can be lower compared to other agents like trimethoprim/sulfamethoxazole or meropenem. scielo.brresearchgate.net Synergistic killing against B. cepacia strains has been observed with this compound in combination with ceftazidime or aztreonam, even in strains non-susceptible to one of the agents. nih.gov
Alcaligenes xylosoxidans
Alcaligenes xylosoxidans strains have been reported as relatively resistant to this compound and other tested quinolones. karger.comnih.gov However, earlier studies indicated that this compound had good potency against Alcaligenes species, with MIC90s around 1 mg/L. oup.comoup.com
Activity Against Atypical Bacterial Pathogens
This compound possesses excellent activity against atypical bacterial pathogens, including Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species. nih.govtandfonline.comscielo.brscielo.brresearchgate.net
Against Mycoplasma and Ureaplasma species, this compound has demonstrated potent in vitro activity, often exhibiting at least eight-fold better anti-chlamydial and anti-mycoplasma potency compared to comparator quinolones. nih.govoup.com MIC90s for this compound against mycoplasma have been reported as low as 0.13 mg/L. nih.govoup.com this compound was found to be four- to eight-fold more potent than ciprofloxacin and ofloxacin against ureaplasma, although higher MICs for ureaplasma may be influenced by the test medium pH. oup.com this compound has shown high in vitro eradication rates against Mycoplasma genitalium. oup.com While some studies suggest this compound might be highly bactericidal against mycoplasmal and ureaplasmal species, others indicate bacteriostatic activity against Mycoplasma hominis. oup.comnih.gov
This compound is also highly potent against Legionella species, with reported MIC90s between 0.03 and 0.06 mg/L. nih.govoup.com Similarly, it shows excellent activity against Chlamydia species. nih.govnih.govtandfonline.com
Interactive Data Tables
Here are some interactive data tables summarizing MIC data for this compound against selected organisms discussed:
| Organism | MIC50 (mg/L) | MIC90 (mg/L) | Source |
| Pseudomonas aeruginosa | 2 | 4 | oup.com |
| Pseudomonas fluorescens | - | 8 | oup.com |
| Pseudomonas stutzeri | - | 0.25 | oup.com |
| Haemophilus influenzae | ≤0.03 | ≤0.03 | nih.gov |
| Moraxella catarrhalis | ≤0.03 | ≤0.03 | nih.gov |
| Neisseria gonorrhoeae | - | 0.016–0.25 | tandfonline.comoup.com |
| Acinetobacter spp. | - | 0.5–1 | oup.comoup.com |
| Acinetobacter lwoffi | 0.06 | 0.12 | oup.com |
| Acinetobacter baumanii | - | - | karger.comnih.gov |
| Stenotrophomonas maltophilia | 1 | 4 | oup.com |
| Burkholderia cepacia | 4 | 8 | oup.comoup.com |
| Alcaligenes spp. | 0.5 | 1 | oup.comoup.com |
| Mycoplasma spp. | - | 0.13 | nih.govoup.com |
| Legionella spp. | - | 0.03–0.06 | nih.govoup.com |
Note: MIC values can vary between studies depending on methodology, isolate source, and comparator agents used.
Mycobacteria: Mycobacterium tuberculosis, Mycobacterium fortuitum Group, Mycobacterium chelonae, Mycobacterium avium-intracellulare
This compound has shown activity against certain mycobacteria. It has been reported to be more potent against Mycobacterium tuberculosis compared to ciprofloxacin and ofloxacin, with an MIC90 of 0.25 mg/L. oup.com Studies evaluating the activity against rapidly growing mycobacteria (RGM) found that this compound inhibited 90% of Mycobacterium fortuitum group isolates at ≤0.12 μg/ml and 90% of Mycobacterium chelonae isolates at ≤4 μg/ml. nih.govasm.orgnih.gov this compound was generally fourfold more active than ciprofloxacin against these RGM. nih.govasm.org For the M. fortuitum group, 100% of isolates tested in one study were susceptible to this compound at MICs of ≤0.5 μg/ml. asm.org Against M. chelonae isolates, 97% were susceptible or intermediate to this compound at an MIC of ≤4 μg/ml in one study. asm.org However, some studies on isolates from specific geographic areas, such as Brazil, have indicated that many M. chelonae and M. abscessus isolates from infectious keratitis were resistant to fluoroquinolones, including this compound, with MIC90s greater than 32 μg/mL.
This compound demonstrated poor potency against Mycobacterium avium-intracellulare compared to M. tuberculosis, although it was still more active than comparator quinolones like ciprofloxacin and ofloxacin against M. avium-intracellulare strains. oup.comoup.com In one study, the in vitro activity of this compound against M. avium was found to be less than that of sitafloxacin and moxifloxacin based on MICs, MBCs, and MPCs. nih.gov
Table 1: In Vitro Activity of this compound Against Select Mycobacteria
| Organism | MIC90 (µg/mL) | MIC Range (µg/mL) | Notes | Source |
| Mycobacterium tuberculosis | 0.25 | - | Eight- to 16-fold more potent than ciprofloxacin and ofloxacin. | oup.com |
| Mycobacterium fortuitum group | ≤0.12 | ≤0.5 - >32 | Generally fourfold more active than ciprofloxacin. 100% susceptible at ≤0.5 µg/mL in one study (n=39). May show resistance in some isolates. | nih.govasm.orgnih.gov |
| Mycobacterium chelonae | ≤4 | 3.2 - >32 | Generally fourfold more active than ciprofloxacin. 97% susceptible or intermediate at ≤4 µg/mL in one study (n=32). May show resistance in some isolates. | nih.govasm.orgnih.govcapes.gov.br |
| Mycobacterium avium-intracellulare | - | - | Poor potency compared to M. tuberculosis, but more active than comparator quinolones. | oup.comoup.comnih.gov |
Note: MIC values can vary depending on the study methodology and the specific isolates tested.
Mycoplasma Species and Chlamydia Species
This compound has demonstrated good in vitro activity against Mycoplasma species and Chlamydia species. oup.comasm.org It has shown at least eight-fold better anti-chlamydial and anti-mycoplasma potency compared to ciprofloxacin and ofloxacin. oup.comoup.com The MIC90 for Mycoplasma species was reported as 0.13 mg/L. oup.com Specifically, this compound was 10-fold more potent than ciprofloxacin and ofloxacin against Mycoplasma pneumoniae and eight- to 16-fold more potent against Mycoplasma hominis based on MIC50 values. oup.comoup.com The MICs of this compound for Mycoplasma were ≤0.25 mg/L. oup.comoup.com
Against Chlamydia trachomatis and Chlamydia pneumoniae, this compound was highly potent, being eight- to 16-fold more potent than ofloxacin and ciprofloxacin. oup.comoup.com The MIC90 for Chlamydia pneumoniae was reported as 0.125 mg/L. nih.gov One study found this compound to be slightly less active against C. trachomatis and slightly more active against C. pneumoniae than ofloxacin, with MICs at which 90% of isolates had no inclusions of 0.25 mg/L. oup.comnih.gov
Table 2: In Vitro Activity of this compound Against Mycoplasma and Chlamydia Species
| Organism | MIC90 (mg/L) | MIC Range (mg/L) | Notes | Source |
| Mycoplasma species | 0.13 | ≤0.25 | At least eight-fold better potency than ciprofloxacin and ofloxacin. | oup.comoup.com |
| Mycoplasma pneumoniae | - | ≤0.25 | 10-fold more potent than ciprofloxacin and ofloxacin (MIC50). | oup.comoup.com |
| Mycoplasma hominis | - | ≤0.25 | Eight- to 16-fold more potent than ciprofloxacin and ofloxacin (MIC50). | oup.comoup.com |
| Chlamydia species | 0.13 | - | At least eight-fold better potency than ciprofloxacin and ofloxacin. | oup.comoup.com |
| Chlamydia trachomatis | 0.25 | - | Eight- to 16-fold more potent than ofloxacin and ciprofloxacin. | oup.comoup.comoup.comnih.gov |
| Chlamydia pneumoniae | 0.125, 0.25 | - | Eight- to 16-fold more potent than ofloxacin and ciprofloxacin. | oup.comoup.comnih.govoup.comnih.gov |
Obligate Anaerobes: Bacteroides fragilis, Clostridium difficile, Fusobacterium spp., Prevotella spp., Porphyromonas spp., Peptostreptococci
This compound has demonstrated activity against obligate anaerobic bacteria. oup.comasm.org Unlike some other quinolones like ciprofloxacin and ofloxacin, this compound has shown activity against Bacteroides fragilis and Clostridium difficile. oup.comoup.com
In a study comparing this compound to other quinolones and antimicrobials against 294 anaerobes, the this compound MICs for 50% and 90% of the isolates tested were 0.5 and 2 mg/liter, respectively. asm.orgnih.gov These values were significantly lower than those for ciprofloxacin. asm.orgnih.gov this compound was found to be active against Bacteroides tectum, Prevotella spp., Porphyromonas spp., and peptostreptococci. asm.orgnih.gov However, Fusobacterium species were sometimes resistant. asm.orgnih.gov With the exception of some Fusobacterium varium isolates (MICs, 2–4 mg/L), this compound MICs were ≤0.5 mg/L for other Fusobacterium spp. oup.com
Table 3: In Vitro Activity of this compound Against Select Obligate Anaerobes
| Organism | MIC50 (mg/L) | MIC90 (mg/L) | MIC Range (mg/L) | Notes | Source |
| All anaerobes tested | 0.5 | 2 | - | Lower MICs than ciprofloxacin. | asm.orgnih.gov |
| Bacteroides fragilis | - | >8 | - | Has activity; comparator quinolones had MIC90s > 8 mg/L. | oup.comoup.com |
| Clostridium difficile | - | >8 | - | Has activity; comparator quinolones had MIC90s > 8 mg/L. | oup.comoup.com |
| Fusobacterium spp. | - | - | ≤0.5 - 4 | Sometimes resistant (F. varium). | oup.comasm.orgnih.gov |
| Prevotella spp. | - | - | - | Active. | asm.orgnih.gov |
| Porphyromonas spp. | - | - | - | Active. | asm.orgnih.gov |
| Peptostreptococci | - | - | - | Active. | asm.orgnih.gov |
Note: The MIC90 > 8 mg/L for comparator quinolones highlights this compound's activity against these organisms where others were less effective. The exact MIC90 for this compound against these specific species was not explicitly provided in the snippets, but its activity was noted.
Periodontopathic Bacteria: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia
This compound has shown potential antibacterial effects on periodontopathic bacteria. scirp.orgscirp.orgresearchgate.netresearchgate.netscirp.orgcqvip.com Studies have investigated its activity against Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia. scirp.orgresearchgate.netscirp.org
This compound inhibited the growth of these periodontopathic bacteria in broth. scirp.org The minimum inhibitory concentration (MIC) for A. actinomycetemcomitans was found to be as low as 2.5 nM. scirp.org For P. gingivalis, an MIC of 50 nM was reported in a study focusing on canine periodontopathic bacteria, including Porphyromonas gulae. scirp.orgresearchgate.net this compound also exhibited antibacterial effects on P. intermedia at concentrations such as 7.5 × 102 nM. scirp.org this compound has demonstrated bactericidal effects on these tested bacteria in a concentration-dependent manner. scirp.orgscirp.org
Table 4: In Vitro Activity of this compound Against Select Periodontopathic Bacteria
| Organism | MIC (nM) | Notes | Source |
| Aggregatibacter actinomycetemcomitans | 2.5 | Most effective concentration for inhibition in one study. | scirp.org |
| Porphyromonas gingivalis | - | Bactericidal effects observed. MIC of 50 nM reported for P. gulae. | scirp.orgscirp.orgresearchgate.net |
| Prevotella intermedia | 750 | Antibacterial effects observed at this concentration. | scirp.org |
Activity Against Phytopathogenic Bacteria
This compound hydrochloride has demonstrated broad-spectrum antibacterial activity against phytopathogenic bacteria. nih.govfrontiersin.orgresearchgate.netfrontiersin.org
Ralstonia solanacearum, Pseudomonas syringae, Xanthomonas campestris pv. vesicatoria
This compound hydrochloride has shown significant inhibitory effects against key phytopathogens such as Ralstonia solanacearum, Pseudomonas syringae pv. tomato DC3000, and Xanthomonas campestris pv. vesicatoria. nih.govfrontiersin.orgresearchgate.netfrontiersin.org
Against R. solanacearum, this compound hydrochloride exhibited a high inhibitory effect, with a reported inhibition rate of 95% at a concentration of 0.0625 mg/L. nih.govfrontiersin.orgresearchgate.net The minimum inhibitory concentration (MIC) for R. solanacearum was determined to be 0.125 mg/L. nih.govfrontiersin.orgresearchgate.net Treatment with 0.5 mg/L of this compound hydrochloride was effective in killing more than 95% of R. solanacearum bacteria. nih.govfrontiersin.orgresearchgate.net
This compound hydrochloride also demonstrated good antibacterial activity against Pseudomonas syringae pv. tomato DC3000 and Xanthomonas campestris pv. vesicatoria. nih.govfrontiersin.orgresearchgate.netfrontiersin.org At a concentration of 0.5 mg/L, this compound hydrochloride substantially inhibited the growth of both P. syringae and X. campestris pv. vesicatoria in liquid culture. frontiersin.org Higher concentrations showed bactericidal activity; 1 mg/L killed 98% of P. syringae, and 4 mg/L killed approximately 90% of X. campestris pv. vesicatoria. frontiersin.orgfrontiersin.org
Table 5: Activity of this compound Hydrochloride Against Select Phytopathogenic Bacteria
| Organism | MIC (mg/L) | Notes | Source |
| Ralstonia solanacearum | 0.125 | 95% inhibition at 0.0625 mg/L; >95% killed at 0.5 mg/L. | nih.govfrontiersin.orgresearchgate.net |
| Pseudomonas syringae pv. tomato DC3000 | - | Substantial growth inhibition at 0.5 mg/L; 98% killed at 1 mg/L. | frontiersin.orgfrontiersin.org |
| Xanthomonas campestris pv. vesicatoria | - | Substantial growth inhibition at 0.5 mg/L; ~90% killed at 4 mg/L. | frontiersin.orgfrontiersin.org |
Inhibition of Biofilm Production in Plant Pathogens
This compound hydrochloride has been shown to significantly inhibit biofilm formation by Ralstonia solanacearum. nih.govfrontiersin.orgresearchgate.net This inhibitory effect on biofilm production represents an additional mechanism of action against this important plant pathogen. nih.govfrontiersin.orgresearchgate.net
Comparative In Vitro Antimicrobial Activity Studies
In vitro studies have been conducted to compare the antimicrobial activity of this compound with other commonly used antibiotics across various classes. These comparisons provide valuable insights into the relative potency and spectrum of activity of this compound against a range of bacterial pathogens.
Comparison with Other Fluoroquinolones (e.g., Ciprofloxacin, Levofloxacin, Moxifloxacin, Ofloxacin, Trovafloxacin, Sparfloxacin)
Comparative in vitro studies have evaluated the activity of this compound alongside other fluoroquinolones such as ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin, trovafloxacin, and sparfloxacin. These studies often assess the minimum inhibitory concentrations (MICs) of the antibiotics against various bacterial isolates to determine their relative potency.
Research indicates that this compound generally exhibits potent activity against a broad spectrum of bacteria, including many Gram-positive and Gram-negative pathogens. Comparisons with other fluoroquinolones reveal variations in activity depending on the specific bacterial species and the resistance profiles of the isolates tested. For instance, some studies may show this compound having comparable or even superior activity against certain Gram-positive cocci, including Streptococcus pneumoniae, compared to some earlier generation fluoroquinolones like ciprofloxacin or ofloxacin. Newer fluoroquinolones like moxifloxacin may demonstrate similar or enhanced activity against certain Gram-positive bacteria compared to this compound in some studies.
Against Gram-negative bacteria, this compound typically shows good activity, comparable to or slightly less potent than ciprofloxacin against some species like Pseudomonas aeruginosa. The activity against Enterobacteriaceae is generally strong among the tested fluoroquinolones, with variations in specific MIC values observed across different studies and bacterial strains.
Trovafloxacin, another fourth-generation fluoroquinolone, was noted to have better Gram-positive bacterial coverage but less Gram-negative coverage than previous fluoroquinolones; however, its use was limited due to hepatotoxicity concerns. wikipedia.orglabshare.cn Sparfloxacin, a third-generation fluoroquinolone, has also been compared to this compound in terms of in vitro activity, with studies assessing their effectiveness against various respiratory and other pathogens. wikipedia.orgtruemeds.in
Due to the extensive nature of comparative in vitro studies across numerous bacterial species and diverse collections of isolates, presenting a single comprehensive data table is challenging. However, representative findings from various studies often highlight the following trends in MIC values (lower MIC indicates higher potency):
| Antibiotic | Representative MIC Range (µg/mL) for Select Bacteria (Illustrative) | Notes |
| This compound | 0.01 - 4 | Generally broad spectrum, good Gram-positive and Gram-negative activity |
| Ciprofloxacin | 0.008 - >16 | Excellent Gram-negative activity, variable Gram-positive activity |
| Levofloxacin | 0.03 - >8 | Good broad-spectrum activity, particularly against S. pneumoniae |
| Moxifloxacin | 0.01 - >8 | Enhanced Gram-positive activity, good anaerobic activity |
| Ofloxacin | 0.06 - >16 | Similar spectrum to ciprofloxacin but generally less potent |
| Trovafloxacin | 0.008 - >8 | Potent, particularly against Gram-positives, but safety concerns |
| Sparfloxacin | 0.03 - >8 | Good activity against Gram-positives and atypical pathogens |
Note: This table provides illustrative MIC ranges based on general trends observed in various studies and should not be considered exhaustive or definitive for all isolates.
Comparison with Beta-Lactam Antibiotics (e.g., Cefepime, Meropenem, Piperacillin/Tazobactam, Amoxicillin/Clavulanate)
Comparisons between this compound and beta-lactam antibiotics, such as cefepime, meropenem, piperacillin/tazobactam, and amoxicillin/clavulanate, demonstrate differences in their spectrum of activity. Beta-lactams primarily target bacterial cell wall synthesis by inhibiting penicillin-binding proteins (PBPs). mims.commims.com
This compound, by inhibiting DNA gyrase and topoisomerase IV, offers activity against some bacteria that may be resistant to beta-lactams due to mechanisms like beta-lactamase production. labshare.cn
Studies comparing this compound to extended-spectrum cephalosporins like cefepime show varying results depending on the bacterial species. Cefepime is a fourth-generation cephalosporin with broad activity against both Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa. wikipedia.orgfishersci.cacalpaclab.com Meropenem, a carbapenem, is known for its very broad spectrum of activity, often considered a drug of last resort for serious infections. mims.comwikipedia.orgwikidoc.orgnih.gov Piperacillin/tazobactam, a combination of an extended-spectrum penicillin and a beta-lactamase inhibitor, has potent activity against many Gram-negative bacteria, including P. aeruginosa, and good activity against some Gram-positive and anaerobic bacteria. mims.comwikipedia.orgfishersci.cauni.lu Amoxicillin/clavulanate is active against a range of Gram-positive and Gram-negative bacteria, including beta-lactamase-producing strains.
In vitro data suggest that this compound can be active against some isolates resistant to these beta-lactams. Conversely, beta-lactams, particularly carbapenems like meropenem, may show broader or more potent activity against certain bacterial groups compared to this compound. Synergy studies combining trovafloxacin (another fluoroquinolone) with beta-lactams and aminoglycosides have indicated that synergy is strain-specific and not commonly encountered. labshare.cn While this specific finding is for trovafloxacin, it highlights that simple additive or synergistic effects are not guaranteed when combining fluoroquinolones with beta-lactams.
Comparison with Aminoglycosides (e.g., Gentamicin)
Aminoglycosides like gentamicin exert their antibacterial effect by inhibiting bacterial protein synthesis. Comparisons with this compound, which targets DNA synthesis, reveal distinct mechanisms of action. wikipedia.org
In vitro studies comparing this compound and gentamicin show differences in their activity against various bacterial species. Gentamicin is typically very active against many Gram-negative bacteria, including Pseudomonas aeruginosa, but has limited activity against anaerobic bacteria and most Gram-positive bacteria when used alone. This compound, with its broader spectrum, is active against both Gram-positive and Gram-negative pathogens.
As mentioned previously, synergy studies involving trovafloxacin and aminoglycosides indicate that synergy is strain-specific. labshare.cn This suggests that while combinations might be used clinically in certain situations, the in vitro interaction between this compound and aminoglycosides would also likely be complex and dependent on the specific bacterial isolate.
Comparison with Other Antimicrobial Classes (e.g., Metronidazole, Clindamycin, Erythromycin)
This compound has also been compared in vitro to antibiotics from other classes, such as metronidazole, clindamycin, and erythromycin, which have different mechanisms of action and spectra.
Metronidazole is primarily active against anaerobic bacteria and certain protozoa. wikipedia.orgwikidata.orgmims.comfishersci.dkfishersci.ca Its mechanism involves the production of reactive nitrogen species that damage microbial DNA. This compound generally has good activity against many anaerobic bacteria, overlapping with metronidazole's spectrum in this regard, but this compound also covers a wide range of aerobic Gram-positive and Gram-negative bacteria that metronidazole does not.
Clindamycin is a lincosamide antibiotic that inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit. It is primarily active against Gram-positive bacteria, including many staphylococci and streptococci, and a wide range of anaerobic bacteria. Erythromycin is a macrolide antibiotic that also inhibits protein synthesis by binding to the 50S ribosomal subunit. It is primarily active against Gram-positive bacteria and some atypical pathogens.
In comparisons with clindamycin and erythromycin, this compound often demonstrates a broader spectrum of activity, particularly against Gram-negative aerobic bacteria. While clindamycin and erythromycin are valuable for specific infections, this compound's dual targeting of DNA gyrase and topoisomerase IV provides coverage against a wider array of pathogens, including many that may be resistant to macrolides or lincosamides.
| Antibiotic | Primary Mechanism of Action | Key Spectrum Highlights |
| This compound | DNA gyrase & Topoisomerase IV inhibition | Broad spectrum (Gram-positive, Gram-negative, Anaerobes) |
| Metronidazole | DNA damage (anaerobes, protozoa) | Anaerobic bacteria, certain protozoa |
| Clindamycin | 50S ribosomal subunit inhibition | Gram-positive bacteria, Anaerobic bacteria |
| Erythromycin | 50S ribosomal subunit inhibition | Gram-positive bacteria, Atypical pathogens |
This comparative analysis highlights that this compound possesses a broad in vitro antimicrobial spectrum, often demonstrating potent activity against pathogens susceptible to other fluoroquinolones, and providing coverage against some bacteria resistant to beta-lactams, aminoglycosides, and certain other classes of antibiotics.
Molecular Interactions and Binding Studies of Gatifloxacin
Computational Molecular Docking and Simulation Studies
Computational approaches, such as molecular docking and simulation, provide valuable insights into the potential binding modes and affinities of gatifloxacin with its target proteins and other biological macromolecules. These studies help to predict how this compound interacts at the atomic level and identify key residues involved in the binding process.
Predicting Binding Modes and Affinities with Target Proteins
Molecular docking simulations are employed to predict the preferred orientation (binding mode) of this compound within the active site of target proteins and to estimate the strength of the interaction (binding affinity). Studies have investigated the binding of this compound and its derivatives to various proteins, including bacterial enzymes like DNA gyrase and topoisomerase IV, as well as other potential targets such as estrogen receptor β and pancreatic α-amylase. nih.govkaznu.kzglobalresearchonline.net
For instance, molecular docking studies assessing this compound derivatives as potential antidepressant agents evaluated their binding affinities to protein 8FSB. This compound itself showed a binding energy of -6.9 kcal/mol with protein 8FSB. researchgate.netbiotech-asia.orgresearchgate.net Its derivatives demonstrated higher binding affinities, ranging from -7.9 to -11.4 kcal/mol. researchgate.netbiotech-asia.orgresearchgate.net Another study investigating potential antimycobacterial activity docked quinolinone-based thiosemicarbazones, including compounds structurally related to quinolones, against targets like DNA gyrase, enoyl-acyl carrier protein reductase (InhA), and decaprenylphosphoryl-β-D-ribose-2'-oxidase (DprE1). nih.gov The binding affinity was assessed using Vina scores, where lower values indicate stronger binding. nih.gov
In the context of periodontal therapy, computational studies explored the binding affinity of this compound and other fluoroquinolones with estrogen receptor β (PDB 1QKM) and Gingipain K (PDB 6I9A). This compound showed significant binding affinity with amino acids in the active site of estrogen receptor β. nih.gov With Gingipain K, this compound revealed a binding free energy of -7.16 kcal/mol and an inhibition constant (Ki) of 5.62 µM. nih.gov
Here is a table summarizing some predicted binding energies of this compound and its derivatives with specific protein targets from computational docking studies:
| Compound | Target Protein (PDB ID) | Binding Energy (kcal/mol) | Reference |
| This compound | 8FSB | -6.9 | researchgate.netbiotech-asia.orgresearchgate.net |
| This compound | Gingipain K (6I9A) | -7.16 | nih.gov |
| This compound | Pancreatic α-amylase (5TD4) | -7.6 | kaznu.kz |
| This compound | EtfD | -10.02 | amazonaws.com |
| This compound Derivative I | 8FSB | -11.4 | researchgate.netbiotech-asia.orgresearchgate.net |
| This compound Derivative II | 8FSB | -11.1 | researchgate.netbiotech-asia.orgresearchgate.net |
Analysis of Protein-Ligand Interactions and Amino Acid Networks
Computational studies also delve into the specific interactions between this compound (or its derivatives) and the amino acid residues within the binding site of target proteins. These interactions can include hydrogen bonds, hydrophobic interactions, electrostatic interactions, and van der Waals forces. Analyzing these interactions helps to understand the stability of the protein-ligand complex and identify critical residues for binding.
For example, in the study investigating this compound derivatives as potential antidepressants targeting protein 8FSB, the binding affinity was attributed to interactions with specific amino acids such as SER C:170, GLU C:173, ARG C:169, TRP C:168, and ARG C:65. researchgate.netbiotech-asia.org One derivative, Gati I, was found to interact with the ILE C:268 amino acid residue in the receptor protein. biotech-asia.orgresearchgate.net
Molecular dynamics simulations can further provide insights into the dynamic behavior of the protein-ligand complex over time, assessing the stability of the interactions and conformational changes upon ligand binding. amazonaws.com Studies involving this compound and other compounds with mycobacterial electron transfer flavoprotein oxidoreductase (EtfD) have identified common amino acids involved in interactions, including ARG133, ARG146, ALA155, HIS235, and CYS298. amazonaws.complos.org Hydrogen bonds and hydrophobic interactions are considered critical for stabilizing these protein-ligand complexes. amazonaws.com
Interactions with Surfactant Systems as Biomembrane Mimics
Surfactant systems, such as micelles, are frequently utilized as simplified models of biological membranes to study the interactions of drugs with lipid bilayers. researchgate.netresearchgate.nettandfonline.com Investigating this compound's interactions with surfactants provides valuable information about its potential behavior in biological membrane environments, including diffusion and partitioning.
Volumetric and Acoustic Investigations of Drug-Surfactant Complexes
Volumetric and acoustic studies, which involve measuring properties like density and sound velocity, are used to investigate the molecular interactions between drugs and surfactants. These measurements allow for the calculation of parameters such as apparent molar volume (ɸV), isentropic compressibility (Ks), and apparent molar isentropic compressibility (ɸK). researchgate.netsci-hub.se Other parameters like partial molar volume (ɸVo), partial molar expansivity (ɸE⁰), specific acoustic impedance (Z), relative association (RA), intermolecular free length (Lf), and sound velocity number (U) can also be obtained. researchgate.netsci-hub.se
Studies on this compound's interactions with ionic surfactants like dodecyltrimethylammonium bromide (DTAB, cationic) and sodium dodecyl sulfate (SDS, anionic) under physiological conditions (phosphate buffer, pH 7.4) have employed these techniques. researchgate.netsci-hub.se The concentration dependence of the calculated parameters is interpreted using models like the cosphere overlap model to understand solute-solute and solute-solvent interactions. researchgate.netsci-hub.se
Voltammetric and Spectroscopic Characterization of Interactions
Electrochemical techniques like cyclic voltammetry and spectroscopic methods such as UV-Visible spectroscopy are employed to further characterize the interactions between this compound and surfactant systems. researchgate.netresearchgate.nettandfonline.comsci-hub.se These methods can provide information on the partitioning and binding of the drug with surfactant micelles. researchgate.nettandfonline.comresearchgate.net
UV-Visible spectroscopy can be used to determine parameters like the critical micelle concentration (CMC) of surfactants in the presence of the drug and to calculate partition coefficients (Kc) and binding constants (Kb) of the drug with ionic micelles. researchgate.nettandfonline.comresearchgate.net Cyclic voltammetry also assists in determining binding parameters and can help predict the location of adsorbed drug molecules within micelles. researchgate.netsci-hub.se
Studies have utilized these techniques to evaluate the interaction of this compound with DTAB and SDS micelles, assisting in predicting where this compound molecules are located within the micelles. researchgate.netsci-hub.se
Interpretation of Solute-Solute and Solute-Solvent Intermolecular Forces
The analysis of volumetric, acoustic, voltammetric, and spectroscopic data allows for the interpretation of the intermolecular forces at play in drug-surfactant systems. This includes understanding the solute-solute, solute-solvent, and solvent-solvent interactions. researchgate.netsci-hub.sescielo.org.co
In this compound-ionic surfactant systems, the interpretation of the concentration dependence of parameters like apparent molar volume and isentropic compressibility using models such as the cosphere overlap model helps to understand the prevailing solute-solute and solute-solvent intermolecular interactions. researchgate.netsci-hub.se For instance, positive values of apparent molar volume have been interpreted as depicting strong forces between surfactant ions and zwitterions of similar compounds, leading to a reduction in electrostriction in the vicinity of these ions. researchgate.net The location of adsorbed this compound molecules within DTAB and SDS micelles, as predicted by voltammetry and UV-Visible spectroscopy, also contributes to understanding these interactions. researchgate.netsci-hub.se Preferential solvation parameters derived from techniques like the inverse Kirkwood-Buff integral can provide a more precise evaluation of molecular interactions and solvent composition around solute molecules. scielo.org.co
Interactions with Advanced Delivery System Materials
The interaction of this compound with advanced delivery system materials, such as nanoparticles, is a critical area of research for developing more effective and targeted drug delivery systems. These interactions influence drug loading, release kinetics, stability, and ultimately, therapeutic efficacy. Studies have explored the binding mechanisms and surface phenomena when this compound is associated with various nanomaterials.
Investigation of Hydrogen Bonding and Intermolecular Forces with Nanocarriers (e.g., Silica Nanoparticles)
Investigations into the interaction between this compound and nanocarriers like silica nanoparticles suggest that physical interactions, rather than the formation of new chemical bonds, are primarily responsible for the binding. For instance, when this compound is loaded onto nano-silica, the binding is likely driven by intermolecular forces. frontiersin.orgnih.govresearchgate.net While electrostatic interactions may not be the primary driver, the increase in the solution potential of nano-silica-loaded this compound indicates improved stability. frontiersin.orgnih.gov
Spectroscopic analysis, such as FTIR, has been used to probe the molecular interactions. Red shifts observed in the -OH stretching vibration absorption peak and -CH2 or -CH3 stretching vibration absorption peak suggest that no new bonds are formed between this compound and nano-silica. frontiersin.orgnih.gov However, it is speculated that hydrogen bonding between the silica surface and the N-H group in this compound is a promoted molecular interaction. frontiersin.orgnih.gov
The surface of untreated silica typically possesses polar groups like -OH. nih.gov The negative log P values of this compound indicate a high affinity for a polar environment at a given pH, suggesting that dipole-dipole or dipole-induced dipole electrostatic interactions could also play a role in the interaction between this compound and nano-silica. nih.gov
In the context of other nanomaterials, the self-assembly of small molecules, including signaling molecules, is often driven by non-covalent interactions such as electrostatic, hydrophobic, hydrogen bonding, and van der Waals forces. mdpi.com This highlights the general importance of these intermolecular forces in the interaction between small molecules like this compound and nanomaterial surfaces.
Characterization of Surface Interactions and Film Formation on Nanomaterials
Characterization techniques such as Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), particle size analysis, and Zeta potential measurements have been employed to understand the surface interactions and the potential formation of a drug film on nanocarriers. Based on these characterizations, it has been speculated that this compound can form a film on nano-silica. frontiersin.orgnih.gov
An increase in the average particle size of nano-silica after loading with this compound further supports the idea that this compound is wrapped on the surface of the nano-silica, forming a drug film. frontiersin.orgnih.gov The surface of pure nano-silica solution is negatively charged, which is within the range that provides moderate electrostatic stabilization of suspensions. frontiersin.orgnih.gov Pure this compound solution is also negatively charged. frontiersin.orgnih.gov
Studies involving other types of nanoparticles, such as lipid nanoparticles, also discuss the formation of a film on surfaces, like the corneal surface after topical application, where the lipid core interacts with the tear film's lipid layer. nih.gov This property contributes to the retention of nanocarriers on the ocular surface. nih.gov The morphology of nanoparticles, including their smoothness and spherical shape, is considered important for enhancing the degree of internalization. ijirset.com
Research on the interaction of this compound with iron mineral/water interfaces, specifically goethite and hematite, has revealed different surface complexation patterns. researchgate.net Outer-sphere complexation, primarily involving strong hydrogen-bond interactions between hydroxyl groups on the mineral surface and oxygens of the carboxyl group of this compound, was found to be the main mechanism of adsorption onto goethite. researchgate.net In contrast, inner-sphere complexation, likely forming a bridging bidentate surface complex, dominated the adsorption onto hematite. researchgate.net This indicates that the nature of the nanomaterial surface significantly influences the interaction mechanism.
Multi-Targeting Capabilities through Complex Formation (e.g., Copper(II) Aromatic Heterocyclic Complexes)
The formation of complexes between this compound and metal ions, particularly Copper(II), in the presence of aromatic heterocyclic ligands, has emerged as a strategy to develop compounds with multi-targeting capabilities for antibacterial therapy and combating antibiotic resistance. patsnap.comnih.gov The coordination of antibiotics with metal elements is an area of increasing attention due to the slow pace of novel antibiotic development and the growing issue of antibiotic resistance. patsnap.comnih.gov
Studies have designed and synthesized novel antibacterial copper complexes based on this compound. patsnap.comnih.gov These complexes have demonstrated potent antibacterial activity. patsnap.comnih.gov For example, a specific complex, Cu-1, exhibited a minimum inhibitory concentration (MIC) of only 0.063 μg/mL against Staphylococcus aureus, indicating potent bacteriostatic capabilities. patsnap.comnih.gov
Further investigations into the antibacterial mechanisms of such complexes reveal their ability to target multiple bacterial pathways. Complex Cu-1, for instance, not only suppresses the activities of DNA gyrase and topoisomerases IV, which are known targets of fluoroquinolones like this compound, but also effectively inhibits biofilm formation and disrupts the integrity of the cell membrane. patsnap.comnih.gov This multi-targeting action is significant as it contributes to mitigating the risk of bacterial resistance emergence. patsnap.comnih.gov
Additionally, synergy between these copper-gatifloxacin complexes and conventional antibiotics has been confirmed through checkerboard assays, suggesting novel strategies for antibacterial therapy. patsnap.comnih.gov In vivo experiments have also provided support for the potential of these complexes in treating infections. nih.gov
This compound, as a fluoroquinolone, can bind to metal ions and form complexes where it can act as a bidentate, unidentate, or bridging ligand due to the chemical features in its nucleus, including a carbonyl oxygen at position 4, a basic piperazinyl ring at site 7, and a carboxylic acid group at position 3. ajgreenchem.comsemanticscholar.org Infrared data from studies on mixed ligand metal complexes derived from this compound indicate that this compound can react with metal ions as a bidentate ligand through one carboxylate oxygen and pyridone oxygen, forming stable complexes. colab.ws Studies have synthesized this compound complexes with various metal ions, including Cu(II), and their structures have been investigated using spectroscopic methods. colab.ws These complexes have shown enhanced antibacterial activity compared to the free ligand. colab.ws
Copper(II) complexes with this compound, prepared with or without N,N'-donor heterocyclic ligands like 2,2'-bipyridylamine, 1,10-phenanthroline, or 2,2'-bipyridine, have also been characterized and tested for antimicrobial activity. researchgate.net Their activity was found to be similar to or higher than that of free this compound. researchgate.net The interaction of these complexes with DNA has been studied, suggesting intercalation as a possible binding mode. researchgate.net
The formation of complexes between this compound and metal ions like Co(II), Ni(II), and La(III) has also been explored for analytical purposes, with studies investigating the optimal conditions for complex formation and determining the composition and stability constants of these complexes. researchgate.net
Here is a table summarizing some of the interactions discussed:
| Interaction Type | Materials Involved | Key Findings / Mechanisms |
| Interaction with Nanocarriers | Silica Nanoparticles | Primarily physical interaction, likely driven by intermolecular forces. frontiersin.orgnih.govresearchgate.net Hydrogen bonding between silica surface and this compound's N-H group is speculated. frontiersin.orgnih.gov Dipole-dipole or dipole-induced dipole interactions may also play a role. nih.gov |
| Surface Interactions and Film Formation | Silica Nanoparticles | This compound may form a film on the surface. frontiersin.orgnih.gov Increased particle size after loading supports surface wrapping. frontiersin.orgnih.gov Surface charge influences stability. frontiersin.orgnih.gov |
| Surface Complexation (Environmental Context) | Goethite (Iron Mineral) | Outer-sphere complexation involving hydrogen bonding. researchgate.net |
| Surface Complexation (Environmental Context) | Hematite (Iron Mineral) | Inner-sphere complexation, likely forming a bridging bidentate complex. researchgate.net |
| Complex Formation for Multi-Targeting Capabilities | Copper(II) and Aromatic Heterocyclic Ligands | Formation of potent antibacterial complexes with multi-targeting actions (DNA gyrase, topoisomerase IV, biofilm, cell membrane). patsnap.comnih.gov Synergy with conventional antibiotics observed. patsnap.comnih.gov |
| Complex Formation with Metal Ions | Various Metal Ions (Co(II), Ni(II), La(III), Mn(II), Fe(II), etc.) | This compound acts as a ligand, typically bidentate. ajgreenchem.comsemanticscholar.orgcolab.ws Complexes can exhibit enhanced antibacterial activity. colab.wsresearchgate.net Interactions with DNA suggested. researchgate.net |
Bacterial Resistance Mechanisms to Gatifloxacin
Chromosomal Mutations in Target Enzymes
Fluoroquinolones, including gatifloxacin, exert their bactericidal effects by inhibiting bacterial DNA gyrase and topoisomerase IV, essential enzymes involved in DNA replication, transcription, repair, and recombination. Mutations within the genes encoding subunits of these enzymes can reduce the binding affinity of this compound, leading to decreased susceptibility. These mutations are commonly located in specific regions known as the quinolone resistance-determining regions (QRDRs) of the gyrA, gyrB, parC, and parE genes. nih.gov
Mutations within gyrA (DNA Gyrase A Subunit) and Their Impact on Susceptibility
DNA gyrase, a type II topoisomerase, is typically the primary target of fluoroquinolones in Gram-negative bacteria. Mutations in the gyrA gene, encoding the A subunit of DNA gyrase, are a significant mechanism of resistance to this compound. These mutations, often single amino acid substitutions within the QRDR of GyrA, can lead to reduced susceptibility. For instance, studies have shown that single mutations in gyrA can lead to increases in the minimum inhibitory concentration (MIC) of this compound, although sometimes the effect of a single gyrA mutation alone on this compound MIC can be minimal or silent depending on the bacterial species and specific mutation. nih.govpsu.edu However, the presence of a gyrA mutation has been associated with increased MICs of this compound in some strains. vivexia.fr In Clostridium perfringens, mutations in gyrA, particularly changes at Gly-81 to Cys and Asp-87 to Tyr, were selected with this compound. asm.org
Mutations within parC (Topoisomerase IV C Subunit) and Their Contribution to Resistance
Topoisomerase IV, also a type II topoisomerase, is often considered the primary target of fluoroquinolones in Gram-positive bacteria. Mutations in the parC gene, encoding the C subunit of topoisomerase IV (GrlA in Staphylococcus aureus), also contribute to this compound resistance. Single mutations in parC can cause increases in the MIC of this compound. nih.govpsu.edu For example, in S. aureus, single grlBA mutations resulted in two- to fourfold increases in the MIC of this compound. nih.gov In Streptococcus pneumoniae, parC mutations like S79F and S79T have been linked to increased this compound MICs. vivexia.fr Some studies suggest that for this compound, topoisomerase IV is the primary target. nih.govpsu.edu
Cumulative Effects of Dual Mutations on Resistance Levels
High-level resistance to this compound often requires the accumulation of mutations in both gyrA and parC genes. jidc.org The presence of dual mutations in both target enzymes has a synergistic effect, leading to significantly higher levels of resistance compared to single mutations in either gene alone. nih.govpsu.edu For instance, in S. aureus, double mutations in gyrA and grlA or grlB caused a substantial increase in the MIC of this compound. nih.govpsu.edu Studies in Escherichia coli have also shown that isolates with greater levels of resistance often possess double mutations in both gyrA and parC genes. jidc.org The cumulative effect of these mutations underscores the challenge in overcoming resistance once multiple alterations in the target enzymes have occurred.
Here is a table summarizing the impact of single and double mutations on this compound MIC in Staphylococcus aureus:
| Strain Type | Relevant Mutations | Fold Increase in this compound MIC | Source |
| Wild-type | None | 1 | nih.govpsu.edu |
| Single grlBA mutation | grlBA | 2- to 4-fold | nih.govpsu.edu |
| Single gyrA mutation | gyrA | Silent or up to 2-fold | nih.govpsu.edu |
| Double gyrA and grlA mutation | gyrA and grlA | 32- to 64-fold | nih.govpsu.edu |
| Double gyrA and grlB mutation | gyrA and grlB | 32- to 64-fold | nih.govpsu.edu |
Efflux Pump Mediated Resistance
Efflux pumps are membrane proteins that actively transport a wide range of substrates, including antibiotics, out of bacterial cells, thereby reducing their intracellular concentration and contributing to resistance. Some efflux pumps in mammalian cells can also transport antibiotics, potentially affecting drug distribution and contributing to resistance in the context of host-pathogen interactions.
Characterization of this compound as a Substrate for Efflux Transporters (e.g., P-glycoprotein/P-gp/MDR1/ABCB1, Multidrug Resistance-Associated Protein 2/MRP2/ABCC2/cMOAT)
Research indicates that this compound can be a substrate for certain efflux transporters, including P-glycoprotein (P-gp), also known as MDR1 or ABCB1, and Multidrug Resistance-Associated Protein 2 (MRP2), also known as ABCC2 or cMOAT. nih.govresearchgate.netnih.gov Studies using cell lines overexpressing these transporters have provided evidence for this compound transport. For instance, uptake and transport studies support the hypothesis that this compound is a substrate for both P-gp and MRP2. nih.govresearchgate.netnih.gov Concentration-dependent affinity analysis further suggests this interaction. nih.gov The interaction of this compound with P-gp and MRP2 may represent a possible mechanism for acquired resistance. nih.govresearchgate.netnih.gov Cellular uptake of a model substrate for P-gp, [14C] erythromycin, was shown to increase in the presence of this compound in cells overexpressing P-gp, suggesting this compound interacts with this transporter. nih.govresearchgate.netfrontiersin.org Similarly, studies involving MRP2 have also indicated this compound as a substrate. nih.govresearchgate.netnih.gov
Investigation of Non-Interaction with Specific Efflux Pumps (e.g., Breast Cancer Resistance Protein/BCRP/ABCG2/MXR)
While this compound has been characterized as a substrate for P-gp and MRP2, studies have also investigated its interaction with other efflux pumps, such as Breast Cancer Resistance Protein (BCRP), also known as ABCG2 or MXR. Research suggests that this compound is not a substrate for BCRP. nih.govresearchgate.netnih.gov Uptake studies using cell lines overexpressing BCRP did not show elevated uptake of a BCRP substrate in the presence of increasing concentrations of this compound, indicating a lack of interaction with this specific transporter. nih.govresearchgate.netnih.gov This suggests a degree of selectivity in the efflux pumps that handle this compound.
| Efflux Transporter | Alternative Names | This compound Interaction | Source |
| P-glycoprotein | P-gp, MDR1, ABCB1 | Substrate | nih.govresearchgate.netnih.govfrontiersin.org |
| Multidrug Resistance-Associated Protein 2 | MRP2, ABCC2, cMOAT | Substrate | nih.govresearchgate.netnih.gov |
| Breast Cancer Resistance Protein | BCRP, ABCG2, MXR | No Interaction (Not a Substrate) | nih.govresearchgate.netnih.gov |
Role of Efflux Pumps in Acquired Resistance Development
Efflux pumps are a significant contributor to multidrug resistance in bacteria, including resistance to fluoroquinolones like this compound. nih.govoup.comjournalagent.com These transmembrane proteins actively transport antimicrobial agents out of the bacterial cell, thereby reducing the intracellular concentration of the drug to sub-inhibitory levels. nih.govgoogle.com This reduced intracellular concentration can allow bacteria to survive and multiply even in the presence of the antibiotic. nih.gov
Several efflux pumps have been implicated in this compound resistance. Studies have shown that this compound can be a substrate for certain efflux transporters, such as P-glycoprotein (P-gp/MDR1/ABCB1) and multidrug resistance associated protein 2 (MRP2/ABCC2/cMOAT). nih.govresearchgate.netmdpi.com Interaction of this compound with these efflux pumps may represent a mechanism for acquired resistance. nih.govresearchgate.net Overexpression of efflux pumps can lead to a significant reduction in the susceptibility of bacteria to this compound. oup.comjournalagent.com For instance, in Staphylococcus aureus, the NorA efflux pump has been shown to contribute to antibacterial resistance, and increased expression of NorA can lead to a decrease in this compound activity, although this compound appears to be less affected by NorA overexpression compared to some other fluoroquinolones like ciprofloxacin and norfloxacin. oup.com
Research using MDCK cell lines transfected with specific efflux transporters like P-gp, MRP2, and BCRP has provided insights into the interaction of this compound with these pumps. nih.govresearchgate.netnih.gov These studies suggest that this compound is a substrate for P-gp and MRP2 but not for BCRP. nih.govresearchgate.net The possible interactions with P-gp and MRP2 may serve as a mechanism for acquired this compound resistance. nih.govresearchgate.net
In Vitro Synergy of this compound with Efflux Pump Inhibitors
Efflux pump inhibitors (EPIs) are compounds that can block the activity of bacterial efflux pumps, thereby preventing the extrusion of antibiotics and increasing their intracellular concentration. jidc.orgasm.orgmdpi.com Combining antibiotics with EPIs is a promising strategy to overcome efflux-mediated resistance and restore the efficacy of existing antimicrobial agents. journalagent.comjidc.orgasm.orgacs.org
In vitro studies have investigated the synergistic effects of this compound in combination with efflux pump inhibitors against resistant bacterial strains. While this compound itself has been suggested to potentially inhibit efflux pumps nih.govnih.gov, combinations of this compound with known EPIs or other agents have shown synergy. For example, studies have explored the synergy of this compound with other antimicrobial agents, such as cefoperazone and the combination of cefoperazone-sulbactam, against Pseudomonas aeruginosa strains. google.com
Research has also demonstrated the potential for synergy between this compound and ciprofloxacin against ciprofloxacin-resistant Pseudomonas aeruginosa. nih.govnih.gov One proposed mechanism for this synergy is that this compound, being an 8-methoxyfluoroquinolone, might inhibit the efflux pumping of ciprofloxacin by P. aeruginosa. nih.govnih.gov An in vitro study using Etest and time-kill assays showed synergy between ciprofloxacin and this compound against a percentage of tested P. aeruginosa isolates. nih.govnih.govfrontiersin.org
Efflux pump inhibitors like verapamil and chlorpromazine have been shown to reduce the Minimum Inhibitory Concentrations (MICs) of various fluoroquinolones, including this compound, against E. coli and S. aureus strains overexpressing efflux pumps. mdpi.com This highlights the potential of EPIs to potentiate the activity of this compound against resistant bacteria.
Other Mechanisms of Resistance (e.g., decreased membrane permeability)
Beyond efflux pumps, other mechanisms contribute to bacterial resistance to this compound. Altered drug accumulation inside the bacterial cell can also result from decreased membrane permeability. patsnap.compatsnap.comoup.comthieme-connect.com This mechanism is particularly relevant in Gram-negative bacteria, where the outer membrane acts as a barrier. oup.comnih.govmdpi.comajol.info
Decreased outer membrane permeability can occur due to changes in the expression or function of porins, which are channels that allow hydrophilic molecules, including some antibiotics like fluoroquinolones, to cross the outer membrane. frontiersin.orgoup.comajol.info A reduction in the number or size of porins can limit the influx of this compound into the periplasmic space, thereby reducing its effective concentration at the target sites within the cell. oup.comajol.info
In Stenotrophomonas maltophilia, an organism known for its inherent multidrug resistance, decreased outer membrane permeability is one of the mechanisms contributing to resistance against various drug classes, including to some extent, fluoroquinolones. nih.gov
While target mutations in DNA gyrase and topoisomerase IV are primary mechanisms of high-level fluoroquinolone resistance, decreased permeability can act in conjunction with other mechanisms, such as efflux, to contribute to reduced susceptibility or resistance. patsnap.comoup.comthieme-connect.comajol.info
Rational Design Strategies to Counteract this compound Resistance
Addressing this compound resistance requires rational design strategies aimed at overcoming the identified resistance mechanisms. These strategies often focus on developing new compounds or approaches that can bypass or counteract resistance.
One approach involves the design of new molecules that can evade efflux pumps or are less affected by reduced permeability. This could involve modifying the chemical structure of this compound or developing novel classes of compounds. Rational design can also focus on creating molecules that have a higher affinity for mutated target enzymes or can inhibit alternative bacterial targets. thieme-connect.comnih.gov
Another strategy involves the co-administration of this compound with efflux pump inhibitors. asm.orgacs.org As discussed in Section 5.2.4, EPIs can restore the activity of this compound against resistant strains by preventing its efflux. Rational design in this area focuses on identifying potent and non-toxic EPIs that can be used clinically. asm.orgmdpi.com
Furthermore, strategies are being explored to design compounds that can inhibit multiple bacterial targets simultaneously, making it more difficult for bacteria to develop resistance through single mutations. nih.gov While this compound already targets two essential enzymes (DNA gyrase and topoisomerase IV), the development of resistance often involves mutations in both targets. oup.comthieme-connect.commims.comnih.gov Rational design could aim to create compounds with balanced inhibitory activity against these targets or explore novel dual-targeting approaches. nih.gov
Hybrid molecules combining different pharmacophores are also being investigated as a rational design strategy to overcome drug resistance. nih.govresearchgate.net For example, hybrids involving this compound linked to other structures like 1,2,3-triazole-isatin have been synthesized and evaluated for activity against resistant strains, such as multidrug-resistant Mycobacterium tuberculosis. researchgate.net These approaches aim to create compounds with unique mechanisms of action that can bypass existing resistance pathways. researchgate.net
The development of novel drug delivery systems, such as cubosomal formulations, is another rational design strategy to improve the intracellular concentration of this compound and potentially overcome resistance mechanisms like reduced permeability and efflux. mdpi.com Cubosomal formulations of this compound have shown improved permeability and reduced MIC values in vitro, suggesting their potential to enhance antibacterial activity and reduce the likelihood of resistance development. mdpi.com
Synthesis, Structural Modifications, and Structure-activity Relationships Sar of Gatifloxacin Derivatives
Synthetic Pathways for Gatifloxacin and its Analogues
The synthesis of this compound typically involves a multi-step process starting from a substituted benzoic acid derivative. One reported pathway begins with 3,4,5,6-tetrafluoro-N-methylphthalimide, which undergoes hydrolysis, decarboxylation, and methylation to yield 2,4,5-trifluoro-3-methoxybenzoyl chloride. This intermediate is then coupled with N,N-ethyl dimethylaminoacrylate, followed by replacement with cyclopropylamine and subsequent cyclization to produce the this compound cyclic ester. patsnap.com
Another approach involves treating 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester with boron trifluoride diethyl etherate to form a borondifluoride chelate. Condensation of this chelate with 2-methylpiperazine yields the this compound borondifluoride chelate, which is then hydrolyzed to obtain this compound. nih.gov An improved synthesis process involves the reaction of 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinoline carboxylic acid with 2-methylpiperazine in a dipolar aprotic solvent like DMSO under an inert atmosphere at elevated temperatures (40-70°C). google.comgoogle.com The reaction mixture is then cooled, and this compound is isolated. google.comgoogle.com
Analogues of this compound can be synthesized by modifying the substituents on the core quinolone structure, particularly at positions C-7 and C-8. unifei.edu.brrsc.org
Design and Synthesis of Novel this compound Derivatives (e.g., N-Piperazinyl Derivatives)
The piperazinyl group at the C-7 position of the fluoroquinolone core is a key site for structural modification to influence potency, spectrum, and safety. actascientific.comlookchem.combrieflands.com Novel this compound derivatives have been designed and synthesized by introducing various substituents onto the N-4 nitrogen of the piperazine ring. globalresearchonline.netresearchgate.net
For instance, a series of N-4 piperazinyl derivatives of this compound have been synthesized by coupling this compound with different sulfonamide derivatives using a chloroacetyl chloride linker. researchgate.net Another strategy involves the synthesis of this compound analogues containing a nitroaryl-1,3,4-thiadiazole moiety attached to the piperazine ring at the C-7 position. nih.gov Mannich bases of this compound have also been synthesized by reacting this compound with formaldehyde and isatin derivatives. nih.gov
The synthesis of C-3 substituted this compound derivatives has also been explored, using this compound as a starting material and employing reactions like cyclization followed by diazotization and coupling with tertiary amines. ijpsr.comijpsr.com
Systematic Structure-Activity Relationship Studies
Structure-Activity Relationship (SAR) studies of this compound and its derivatives aim to understand how specific structural features correlate with their biological activities, particularly their potency against bacterial enzymes and their antimicrobial profiles. actascientific.comfrontiersin.org
This compound exerts its antibacterial effect by inhibiting bacterial DNA gyrase (topoisomerase II) and topoisomerase IV. drugbank.compatsnap.comontosight.ainih.govasianjpr.com These enzymes are crucial for bacterial DNA replication, transcription, repair, and recombination. drugbank.compatsnap.comontosight.aiasianjpr.com SAR studies have focused on how modifications to the this compound structure affect its inhibitory activity against these targets. drugbank.comCurrent time information in Bangalore, IN.
Modifications, particularly at the C-7 and C-8 positions, have been shown to influence the affinity and inhibitory potency against bacterial topoisomerases. crstoday.comasm.org For example, the presence of the 8-methoxy group in this compound enhances its activity against both DNA gyrase and topoisomerase IV. researchgate.netnih.gov
The C-8 methoxy group is a notable structural feature of this compound that significantly impacts its antimicrobial profile and helps in combating resistance. patsnap.compatsnap.comrsc.orgcrstoday.comasm.orgresearchgate.netnih.govasm.org This substituent contributes to enhanced activity against DNA gyrase and topoisomerase IV. asm.orgresearchgate.netnih.gov
Studies have shown that the C-8 methoxy group can reduce the potential for the development of bacterial resistance. researchgate.netnih.gov It improves the activity against resistant gyrase and wild-type topoisomerase IV. asm.org The presence of the 8-methoxy group is hypothesized to increase the level of target inhibition, especially against DNA gyrase, making it nearly equal to that for topoisomerase IV inhibition, which contributes to potent antibacterial activity and a lower level of resistance selectivity in certain bacteria like Streptococcus pneumoniae. nih.gov Evidence suggests that compounds with the C-8 methoxy group can kill bacterial cells that are not multiplying, further reducing the selection of resistant mutants. crstoday.com
This compound is known for its broad-spectrum activity against both Gram-positive and Gram-negative bacteria. drugbank.comontosight.aipatsnap.comasianjpr.comnih.gov This broad activity is primarily attributed to its dual targeting of bacterial DNA gyrase and topoisomerase IV. drugbank.compatsnap.comontosight.aiasianjpr.comnih.gov
Impact of Specific Substituents (e.g., C-8 Methoxy Group) on Antimicrobial Profile and Resistance
Development of Novel this compound Derivatives with Modified or Expanded Activities
Research continues into developing novel this compound derivatives with improved or expanded activities. This includes efforts to overcome existing resistance mechanisms, enhance potency against specific pathogens, or even explore new therapeutic applications beyond antibacterial effects. researchgate.netasianpubs.orgijpsr.comijpsr.combiotech-asia.orgx-mol.netkaznu.kznih.govjbiochemtech.com
Modifications at the C-7 piperazinyl ring, such as the introduction of bulky groups or different heterocyclic systems, are common strategies to reduce efflux from bacterial cells and combat resistance. researchgate.netasianpubs.orgbrieflands.com Novel C-3 substituted derivatives have also been synthesized and evaluated for antimicrobial activity. ijpsr.comijpsr.com
Beyond antibacterial activity, this compound derivatives are being investigated for other potential therapeutic uses. For example, some this compound derivatives have shown promising anti-inflammatory activity through modifications at the carboxylic group. nih.gov Novel this compound 3-carboxamide derivatives have been explored as potential anti-tumor agents, acting as DNA topoisomerase II inhibitors. x-mol.net Furthermore, in silico studies suggest that certain this compound derivatives may have potential as antidepressant agents by binding to targets like human pancreatic alpha-amylase, which is being investigated to mitigate dysglycemic side effects associated with the parent drug. biotech-asia.orgkaznu.kz
Data from studies on novel this compound derivatives highlights the potential for creating compounds with enhanced or altered biological profiles. For instance, a study on N-4 piperazinyl derivatives showed varying antibacterial activity against Staphylococcus aureus and Escherichia coli compared to this compound and ciprofloxacin. globalresearchonline.netresearchgate.net Another study on novel this compound derivatives reported compounds with potent antibacterial activity against Staphylococcus aureus (including MRSA) and Staphylococcus epidermidis (including MRSE), with some derivatives showing superior activity against Pseudomonas aeruginosa compared to this compound and levofloxacin. lookchem.comnih.gov
Here is an example of how data from research findings could be presented in a table format:
Table 1: In Vitro Antibacterial Activity of Selected this compound Derivatives
Note: MIC values may vary depending on the specific bacterial strain and testing methodology used in different studies.
This table illustrates how different structural modifications can lead to variations in antibacterial potency against specific bacterial strains. The development of novel derivatives continues to be a crucial strategy in the ongoing effort to combat bacterial infections and address emerging resistance.
Derivatives with Enhanced Binding Affinities to Target Receptors
This compound's primary antibacterial mechanism involves inhibiting bacterial DNA gyrase and topoisomerase IV. nih.govdrugbank.com These enzymes are crucial for maintaining the topological state of bacterial DNA. mdpi.com DNA gyrase is the primary target in many Gram-negative bacteria, while topoisomerase IV is often the preferential target in Gram-positive bacteria like Staphylococcus aureus. mdpi.com
Research has explored the synthesis of this compound derivatives with modified structures to potentially enhance their binding affinities to these bacterial type II topoisomerases. Modifications, particularly at the C-7 piperazine ring, have been investigated with the aim of improving antibacterial activity, including against resistant strains. actascientific.comglobalresearchonline.net
Molecular docking studies have been employed to predict the binding modes and affinities of this compound derivatives to target enzymes like topoisomerase II (DNA gyrase) and topoisomerase IV. globalresearchonline.netresearchgate.net For instance, studies involving N-4 piperazinyl derivatives of this compound have utilized docking simulations with S. aureus topoisomerase-II (DNA gyrase) and E. coli topoisomerase-IV to assess binding affinities. globalresearchonline.net Some synthesized derivatives have shown good binding affinity and interaction with these enzymes, in some cases exceeding that of the parent compound. globalresearchonline.net
Specific structural alterations at the C-7 position have demonstrated improved antibacterial activity, which correlates with enhanced binding to the bacterial topoisomerases. actascientific.comglobalresearchonline.net For example, the addition of a 3,4-dimethylbenzenamine group at the 7th position of the piperazinyl ring in one study resulted in excellent antibacterial activity and improved binding affinity compared to this compound and ciprofloxacin. globalresearchonline.net
While the provided search results highlight the use of docking studies to predict enhanced binding, they primarily focus on the correlation with antibacterial activity. Detailed data tables specifically showcasing enhanced binding affinities (e.g., Ki or IC50 values for enzyme inhibition) of various this compound derivatives to isolated bacterial DNA gyrase or topoisomerase IV were not extensively detailed in the search snippets beyond the context of predicting antibacterial efficacy. However, the principle of modifying the C-7 position to influence interaction with these targets is evident. actascientific.comglobalresearchonline.net
Exploration of this compound Derivatives for Non-Antimicrobial Research Applications (e.g., Antidepressant Potential via Serotonin Reuptake Inhibition)
Beyond their well-established antibacterial properties, fluoroquinolones and their derivatives have been explored for potential non-antimicrobial activities. oup.com This includes investigations into immunomodulatory effects and, more recently, potential neurological applications. nih.govoup.com
One area of emerging research involves exploring this compound derivatives for their potential as antidepressant agents, specifically through the mechanism of serotonin reuptake inhibition. biotech-asia.orgresearchgate.netresearchgate.netdntb.gov.ua Depression is a significant mental health disorder linked to low levels of serotonin in the brain. researchgate.netresearchgate.net Selective serotonin reuptake inhibitors (SSRIs) work by blocking the reuptake of serotonin at the presynaptic membrane, increasing its availability in the synapse to bind to postsynaptic receptors, thereby exerting antidepressant effects. biotech-asia.orgresearchgate.net
In silico molecular docking studies have been conducted to evaluate the potential antidepressant activity of this compound derivatives by assessing their binding affinity to proteins involved in serotonin reuptake, such as the serotonin transporter (SERT), often represented by PDB ID 8FSB in docking studies. biotech-asia.orgresearchgate.netresearchgate.netdntb.gov.ua
A study investigating several this compound derivatives (labeled Gati I to Gati VI) using molecular docking revealed promising binding affinities to the protein 8FSB, a target associated with serotonin reuptake inhibition. biotech-asia.orgresearchgate.netresearchgate.netdntb.gov.ua The results indicated that these derivatives generally exhibited higher binding affinities compared to this compound itself. biotech-asia.orgresearchgate.netresearchgate.netdntb.gov.ua
The binding energies observed in this study were:
| Compound | Binding Affinity (kcal/mol) |
| This compound | -6.9 |
| Gati I | -11.4 |
| Gati II | -11.1 |
| Gati III | -8.2 |
| Gati IV | -7.9 |
| Gati V | -9.5 |
| Gati VI | -9.7 |
These docking scores suggest favorable interactions between the this compound derivatives and the target protein, potentially indicating an inhibitory effect on serotonin reuptake. biotech-asia.orgresearchgate.netresearchgate.netdntb.gov.ua Specifically, derivatives Gati I and Gati II showed the strongest predicted binding affinities. biotech-asia.orgresearchgate.net
These findings from in silico studies suggest that this compound derivatives could serve as potential candidates for antidepressant drug discovery, warranting further in vitro and in vivo investigations to validate these predicted activities. biotech-asia.orgresearchgate.netresearchgate.netdntb.gov.ua This exploration into non-antimicrobial applications highlights the potential for structural modifications of existing drug scaffolds to yield compounds with entirely different therapeutic uses. oup.combiotech-asia.orgresearchgate.net
Broader Research Applications and Future Directions for Gatifloxacin
Gatifloxacin as a Research Probe in Bacterial Pathogenesis and Genetics
This compound's well-defined mechanism of action, targeting bacterial DNA gyrase and topoisomerase IV, makes it a valuable tool for investigating bacterial pathogenesis and genetics. wikipedia.orgpatsnap.commims.com Studies utilizing this compound can help elucidate the roles of these specific enzymes in bacterial survival, growth, and virulence. By observing the effects of this compound on bacterial strains with modified topoisomerase genes or altered regulatory pathways, researchers can gain insights into the genetic basis of bacterial processes and the mechanisms of resistance development. For instance, research on the mechanisms and frequency of resistance to this compound in Staphylococcus aureus has identified novel mutations in topoisomerase IV and gyrase genes that contribute to resistance, highlighting the utility of this compound in genetic studies of resistance. nih.gov Furthermore, this compound can be used to study the impact of inhibiting these enzymes on bacterial behavior, such as biofilm formation, which is a key aspect of pathogenesis for many bacteria. frontiersin.orgnih.gov
Role in Combating Antibiotic Resistance in Non-Human Biological Systems (e.g., Agricultural Pest Management)
The issue of antibiotic resistance extends beyond human health and is a significant concern in non-human biological systems, including agriculture. mdpi.comfao.org The use of antimicrobials in livestock and crop production can contribute to the emergence and spread of resistant bacteria in the environment and food chain. mdpi.comnih.gov Research has explored the potential of this compound hydrochloride as an antibacterial agent against phytopathogenic bacteria, which cause diseases in plants. frontiersin.orgnih.gov Studies have shown that this compound hydrochloride exhibits broad-spectrum antibacterial activity against plant pathogens such as Ralstonia solanacearum and Pseudomonas syringae. frontiersin.orgnih.gov It has demonstrated efficacy in inhibiting bacterial growth and reducing biofilm formation in these plant pathogens. frontiersin.orgnih.gov While caution is necessary due to its activity against human pathogens and the need to minimize residues, these findings suggest a potential role for this compound or its derivatives in agricultural pest management as an alternative strategy to combat bacterial diseases in plants, contributing to the broader effort against antibiotic resistance in diverse environments. frontiersin.orgfao.orgnih.gov
Potential for Multi-Targeting Approaches in Antimicrobial Agent Design
The primary mechanism of this compound involves the inhibition of two critical bacterial enzymes. wikipedia.orgpatsnap.commims.com This dual-targeting approach is a key feature of its antibacterial activity and offers a foundation for the design of new antimicrobial agents with multi-targeting capabilities. Research is exploring the synthesis of novel complexes and derivatives of this compound that can target multiple bacterial pathways simultaneously, potentially overcoming resistance mechanisms that rely on alterations to a single target. nih.gov For example, novel antibacterial copper complexes based on this compound have been designed and synthesized, demonstrating potent antibacterial activity against Staphylococcus aureus. nih.gov These complexes not only suppress the activities of DNA gyrase and topoisomerase IV but also effectively inhibit biofilm formation and disrupt the integrity of the cell membrane, showcasing a multi-targeting action that can help mitigate the emergence of bacterial resistance. nih.gov This research direction highlights the potential of using this compound as a base structure for developing new multi-targeted antimicrobial agents.
Continued Exploration of this compound Derivatives in Diverse Research Fields
The chemical structure of this compound, with its quinolone core and substituents, provides a basis for the synthesis of various derivatives with potentially altered or expanded biological activities. researchgate.net Research continues to explore this compound derivatives in diverse fields beyond traditional antibacterial applications. Studies have focused on designing and synthesizing new this compound derivatives with enhanced antibacterial activity, particularly against resistant strains. researchgate.netjbiochemtech.com Furthermore, investigations into this compound derivatives have extended to exploring their potential in other therapeutic areas. For instance, molecular docking studies have been conducted to evaluate this compound derivatives as prospective antidepressant agents, suggesting the potential for repurposing or developing novel compounds based on the this compound scaffold for non-antibacterial uses. biotech-asia.org The modification of the piperazinyl ring at position 7 of the quinolone core is a common strategy in synthesizing these derivatives. researchgate.net
Methodological Advancements in this compound Characterization and Analysis
Accurate characterization and analysis of this compound are crucial for research and development. Methodological advancements in analytical techniques play a significant role in understanding its properties, purity, and behavior in various matrices. Techniques such as Fourier Transform Infrared (FTIR) spectroscopy, Differential Scanning Calorimetry (DSC), and Ultraviolet (UV) spectrophotometry are employed for the physicochemical characterization of this compound and its formulations. actamedicamarisiensis.roresearchgate.net Nuclear Magnetic Resonance (NMR) spectroscopy, particularly 1H-NMR, and Mass Spectrometry (MS), including Electron Ionization Mass Spectrometry (EIMS), are utilized to confirm the structure and identity of this compound and its synthesized derivatives. researchgate.netjbiochemtech.comnih.gov High-Performance Liquid Chromatography (HPLC) is also used to verify the purity of synthesized compounds. nih.gov More advanced techniques, such as Transmission Electron Microscopy (TEM) equipped with Energy-Dispersive X-ray (EDX) and Electron Energy Loss Spectroscopy (EELS) modules, are used for the characterization of nanoparticles used in this compound detection methods. mdpi.com Ultrasensitive detection methods like lateral flow immunoassay utilizing signal-enhancing labels such as Au@Ag nanoparticles are being developed for the analysis of this compound, for example, in food products. mdpi.com
Identification of Emerging Research Trajectories for Fluoroquinolones and this compound
The ongoing challenge of antibiotic resistance necessitates the identification of new research trajectories for fluoroquinolones, including this compound. Emerging research focuses on understanding and overcoming resistance mechanisms, exploring novel formulations, and investigating new therapeutic applications. Research is actively pursuing the development of new antibacterials that target bacterial type II topoisomerases, the same enzymes inhibited by fluoroquinolones, but with potentially different binding modes or activity profiles to circumvent existing resistance. acs.org Studies are also investigating the efficacy of fluoroquinolones like this compound against difficult-to-treat infections such as multidrug-resistant tuberculosis, focusing on drug penetration into lesions and activity against bacterial populations within those lesions. nih.gov The exploration of this compound derivatives with multi-targeting capabilities represents a significant emerging trajectory aimed at combating resistance. nih.gov Furthermore, research into the use of this compound in non-human systems, such as agriculture, signifies a broadening scope of investigation for this class of antibiotics. frontiersin.orgnih.gov Methodological advancements in detection and characterization also contribute to these emerging trajectories by providing better tools for research and monitoring. researchgate.netmdpi.com
Q & A
Q. What standardized methods are used to determine the minimum inhibitory concentration (MIC) of gatifloxacin against bacterial strains?
To determine MIC values, researchers typically employ broth microdilution or agar dilution assays. For example, in studies on canine periodontopathic bacteria, MICs were quantified by serial dilution of this compound in bacterial cultures, followed by incubation and visual turbidity assessment. Statistical validation using one-way ANOVA and post-hoc tests (e.g., Tukey’s test) ensures reproducibility, with p < 0.05 indicating significance .
Q. How can the physicochemical properties of this compound be characterized for drug formulation studies?
Key methods include:
Q. What in vitro models are suitable for evaluating this compound’s efficacy against intracellular pathogens?
Macrophage infection models, such as THP-1 cell lines infected with Mycobacterium tuberculosis, are used. Researchers measure bacterial survival rates post-treatment via colony-forming unit (CFU) counts or fluorescence-based assays. Dose-response curves and time-kill kinetics are analyzed to assess bactericidal activity .
Q. How should researchers design pharmacokinetic studies for this compound in animal models?
Protocols include:
Q. What criteria define this compound’s in vitro bactericidal activity in time-kill assays?
A ≥3-log reduction in CFU/mL over 24 hours compared to baseline is considered bactericidal. Studies often compare this compound to control antibiotics (e.g., moxifloxacin) and use statistical models to account for variability .
Advanced Research Questions
Q. How can contradictory outcomes in this compound clinical trials (e.g., TB treatment) be systematically analyzed?
Meta-analyses or individual participant data (IPD) reviews are critical. For instance, the REMoxTB trial compared this compound-based regimens to standard therapy, with discrepancies attributed to bacterial load heterogeneity or regional resistance patterns. Researchers should stratify data by covariates like baseline CFU counts and apply mixed-effects models to resolve contradictions .
Q. What advanced spectroscopic techniques validate this compound’s structural integrity in complex matrices (e.g., biological fluids)?
Raman spectroscopy and density functional theory (DFT) simulations are used to map vibrational modes and predict spectral peaks. This approach detects drug residues in meat products by matching experimental spectra to computational models, achieving sensitivities <1 ppm .
Q. How do this compound’s stereoisomers (e.g., 8-hydroxy metabolites) influence antimicrobial activity and toxicity profiles?
Chiral HPLC separates isomers for individual testing. For example, 8-hydroxy this compound (retention time 8.3 min) shows reduced potency compared to the parent compound. Toxicity is assessed via cell viability assays (e.g., MTT), with isomer-specific effects linked to DNA gyrase binding affinities .
Q. What experimental frameworks address this compound resistance mechanisms in gram-negative bacteria?
Q. How can this compound be integrated into combination therapies to mitigate resistance emergence?
Synergy studies use checkerboard assays to calculate fractional inhibitory concentration indices (FICIs). For example, this compound + amikacin (FICI ≤0.5) shows additive effects against multidrug-resistant Pseudomonas aeruginosa. Mechanistic studies (e.g., time-lapse microscopy) further elucidate cooperative bactericidal pathways .
Methodological Notes
- Statistical rigor : Always apply corrections for multiple comparisons (e.g., Bonferroni) in ANOVA-based analyses .
- Spectroscopic validation : Cross-reference FTIR/DSC data with pharmacopeial standards (e.g., USP) to confirm drug purity .
- Ethical compliance : Adhere to institutional guidelines for animal/human studies, including informed consent and sample size justification .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


