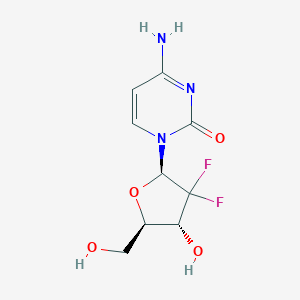
Gemcitabine
Description
Nucleoside Transporter-Mediated Cellular Uptake Dynamics
Gemcitabine’s hydrophilic nature necessitates specialized transmembrane transport proteins for cellular entry. Two families of nucleoside transporters mediate this process: equilibrative nucleoside transporters (ENTs) and concentrative nucleoside transporters (CNTs) .
- ENT1 (SLC29A1) and ENT2 (SLC29A2) facilitate sodium-independent bidirectional transport, equilibrating this compound concentrations across membranes. ENT1 exhibits high affinity for this compound (Km ≈ 10–50 µM), whereas ENT2 operates at millimolar affinity.
- CNT1 (SLC28A1) and CNT3 (SLC28A3) utilize sodium or proton gradients to actively accumulate this compound intracellularly, with CNT3 showing the highest transport capacity.
In pancreatic cancer, ENT1 accounts for >80% of this compound uptake under physiological conditions. Downregulation of ENT1, observed in chemoresistant tumors, reduces intracellular drug accumulation and clinical response. Conversely, CNT3 overexpression in ENT1-deficient cells restores this compound sensitivity by enabling active transport.
Metabolic Activation Pathways and Rate-Limiting Phosphorylation Steps
Intracellular activation of this compound involves sequential phosphorylation to its mono- (dFdCMP), di- (dFdCDP), and triphosphate (dFdCTP) forms:
- Deoxycytidine kinase (dCK) catalyzes the rate-limiting initial phosphorylation, converting this compound to dFdCMP. dCK activity varies widely across tumors, with low expression linked to resistance.
- UMP-CMP kinase and nucleoside diphosphate kinase (NDPK) further phosphorylate dFdCMP to dFdCDP and dFdCTP, respectively.
- Inactivation pathways :
Regulatory factors :
- HuR, an RNA-binding protein, stabilizes dCK mRNA, enhancing phosphorylation capacity.
- dFdCTP provides negative feedback inhibition on dCK, modulating activation kinetics.
DNA Incorporation Mechanisms and Masked Chain Termination Phenomena
dFdCTP competes with endogenous dCTP for incorporation into elongating DNA strands during replication. Key mechanistic features include:
- Misincorporation : DNA polymerases incorporate dFdCTP opposite deoxyguanosine, causing minimal distortion due to its structural similarity to dCTP.
- Masked chain termination : After dFdCTP incorporation, one additional deoxynucleotide is added, shielding the this compound residue from 3'→5' exonuclease proofreading. This results in irreversible replication fork stalling.
- DNA damage response : Stalled forks activate ATM/ATR signaling, leading to S-phase arrest and apoptosis via caspase-3/7 activation.
Single-strand DNA breaks accumulate within 4–6 hours post-treatment, progressing to double-strand breaks and chromatin fragmentation.
Ribonucleotide Reductase Inhibition and dNTP Pool Modulation
dFdCDP, the diphosphate metabolite, inhibits ribonucleotide reductase (RNR) , which catalyzes the conversion of ribonucleotides to deoxyribonucleotides (dNTPs):
- Targets the RNR large subunit (RRM1) and small subunit (RRM2), disrupting allosteric regulation.
- Depletes dCTP pools by >90% within 2 hours, creating a "dNTP sink" that enhances dFdCTP incorporation.
| dNTP | Concentration (µM) Pre-Gemcitabine | Post-Gemcitabine (2 hr) |
|---|---|---|
| dCTP | 25 | 2.3 |
| dATP | 150 | 145 |
| dGTP | 35 | 30 |
| dTTP | 50 | 48 |
This selective dCTP depletion amplifies this compound’s DNA-incorporation efficiency by 8–10 fold.
Self-Potentiation Effects in Intracellular Metabolic Processing
This compound uniquely enhances its own activation through two synergistic mechanisms:
- RNR inhibition : By reducing dCTP levels, dFdCDP alleviates competition for dCK, increasing dFdCTP synthesis.
- Prolonged retention : dFdCTP exhibits a half-life >20 hours in tumor cells, compared to 2–4 hours for endogenous dNTPs.
Mathematically, these effects follow cooperative kinetics :
$$
\text{dFdCTP accumulation} \propto \frac{[dCK] \cdot [\text{this compound}]}{K_m + [dCTP]}
$$
Where RNR inhibition reduces [dCTP], lowering the denominator and amplifying activation.
This self-potentiation underlies this compound’s schedule-dependent efficacy, with prolonged infusions (e.g., 24-hour continuous) yielding 3–5× higher dFdCTP levels than bolus dosing.
Properties
IUPAC Name |
4-amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C9H11F2N3O4.ClH/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17;/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17);1H/t4-,6-,7-;/m1./s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OKKDEIYWILRZIA-OSZBKLCCSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)(F)F.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=CN(C(=O)N=C1N)[C@H]2C([C@@H]([C@H](O2)CO)O)(F)F.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C9H12ClF2N3O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
95058-81-4, 103882-84-4 | |
| Record name | Gemcitabine hydrochloride [USAN:USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0122111039 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID3047849 | |
| Record name | 2'-Deoxy-2',2'-difluorocytidine monohydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3047849 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
299.66 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
122111-03-9 | |
| Record name | Gemcitabine hydrochloride | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=122111-03-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Gemcitabine hydrochloride [USAN:USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0122111039 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | 2'-Deoxy-2',2'-difluorocytidine monohydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3047849 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Cytidine, 2'-deoxy-2',2'-difluoro-, hydrochloride (1:1) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.108.693 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GEMCITABINE HYDROCHLORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/U347PV74IL | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Preparation Methods
Stepwise Synthesis and Optimization
Step 1: Protection of Cytidine
Cytidine undergoes N- and O-protection using benzoyl or toluyl anhydrides in the presence of boron trifluoride etherate. This yields 3',5'-O,N4-tri-benzoyl-2,2'-dehydrocytidine tetrafluoroborate (II).
Step 2: Ring-Opening and Oxidation
Treatment with 2% sodium bicarbonate aqueous solution opens the anhydride ring, forming 3',5'-O,N4-tri-benzoyl-2'-arabinosylcytidine (III). Subsequent oxidation with chromium trioxide/pyridine or dimethyl sulfoxide/trifluoroacetic anhydride generates the 2'-keto intermediate (IV).
Step 3: Deoxidation Fluorination
The critical difluorination step employs XtalFluoro-E or XtalFluoro-M reagents with villiaumite catalysts (e.g., triethylamine hydrofluoride). This converts the 2'-keto group to a difluoro moiety, yielding 3',5'-O,N4-tri-benzoyl-2'-deoxy-2',2'-difluorocytidine (V) with 98% purity.
Step 4: Deprotection and Salt Formation
Ammoniacal methanol removes benzoyl groups, and hydrochloric acid treatment produces this compound hydrochloride. Final crystallization in isopropanol achieves 99.3% purity with a 78% yield.
Table 1: Key Parameters for Cytidine-Based Synthesis
| Step | Reagents/Conditions | Intermediate | Yield (%) | Purity (%) |
|---|---|---|---|---|
| 1 | BF₃·Et₂O, benzoyl anhydride | II | 87 | 98 |
| 2 | CrO₃/pyridine | IV | 73 | 98 |
| 3 | XtalFluoro-E, Et₃N·3HF | V | 82 | 99 |
| 4 | NH₃/MeOH, HCl | This compound·HCl | 78 | 99.3 |
Dibenzoate Protection and Coupling Approach
Ribose Protection and Activation
US8299239B2 describes a method starting with 2-deoxy-D-erythro-2,2-difluoro-ribofuranose-3,5-dibenzoate. The 1-hydroxyl group is converted to a mesylate leaving group using methanesulfonyl chloride, enabling nucleophilic substitution with protected cytosine.
Cytosine Coupling and Deprotection
The mesylated ribose reacts with N4-acetylcytosine in anhydrous tetrahydrofuran, forming protected this compound. Sequential deprotection with sodium methoxide and hydrochloric acid yields the final hydrochloride salt. This method achieves 95% purity but requires rigorous chromatography to remove α-anomers.
Prodrug Synthesis through Amino Acid Conjugation
Rationale for Prodrug Development
To enhance cellular uptake via amino acid transporters, this compound-threonine (Gem-Thr) prodrugs were synthesized. The approach targets pancreatic cancer cells with overexpressed LAT1 transporters.
Synthetic Pathway
Step 1: Amide Bond Formation
this compound reacts with N-Boc-threonine using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and hydroxybenzotriazole (HOBt) in DMF/DMSO. The Boc-protected intermediate (Gem-Thr-Boc) forms with 90% yield.
Step 2: Boc Deprotection
Treatment with 4M HCl in dioxane removes the Boc group, yielding Gem-Thr. NMR confirms the amide bond (δ 10.99 ppm) and absence of epimerization.
Table 2: Pharmacokinetic Comparison of Gem-Thr vs. Free this compound
| Parameter | Gem-Thr (4 mg/kg) | Free this compound (4 mg/kg) |
|---|---|---|
| AUC (μg·min/mL) | 3437.92 ± 1180.56 | 948.38 ± 52.04 |
| Terminal t₁/₂ (min) | 537.23 ± 227.78 | 532.68 ± 177.90 |
| CL (mL/min/kg) | 1.26 ± 0.39 | 4.23 ± 0.23 |
Comparative Analysis of Industrial Viability
Yield and Purity Considerations
Cost and Scalability
XtalFluoro reagents in the cytidine method are cost-prohibitive for large-scale production. Conversely, dibenzoate protection uses cheaper reagents but generates more waste.
Chemical Reactions Analysis
Phosphorylation and Activation Pathway
Gemcitabine undergoes sequential phosphorylation to form active metabolites:
This cascade enables "self-potentiation," where dFdCDP-mediated dNTP depletion enhances dFdCTP incorporation into DNA .
Deamination and Metabolic Inactivation
Up to 90% of administered this compound is deaminated by cytidine deaminase (CDA) , producing the inactive metabolite 2',2'-difluorodeoxyuridine (dFdU) . Key factors influencing this reaction:
- Tissue specificity : High CDA activity in the liver and blood .
- Pharmacogenetic variability : Polymorphisms in CDA genes affect inactivation rates .
DNA/RNA Interaction Mechanisms
dFdCTP incorporation into nucleic acids drives cytotoxicity through:
- Masked chain termination : dFdCTP integrates into DNA, allowing one additional nucleotide addition before stalling replication .
- RNA synthesis inhibition : Misincorporation into RNA disrupts processing and function .
- Enzyme inhibition : dFdCDP suppresses RNR, while dFdCTP blocks CTP synthetase, depleting nucleotide reserves .
Prodrug Strategies to Enhance Stability
The This compound-threonine amide (Gem-Thr) prodrug demonstrates improved pharmacokinetics:
| Parameter | Gem-Thr (4 mg/kg) | Free this compound (4 mg/kg) |
|---|---|---|
| AUC (μg·min/mL) | 1739.88 ± 282.00 | 948.38 ± 52.04 |
| Total Clearance (mL/min/kg) | 0.60 ± 0.10 | 4.23 ± 0.23 |
| Metabolic Stability | 1.83-fold increase | Baseline |
Synthesis involves amide bond formation between this compound and N-Boc-L-threonine using HOBt/EDC coupling, followed by Boc deprotection . This modification leverages amino acid transporters (e.g., LAT-1) for targeted uptake in pancreatic cancer cells .
Critical Enzymatic Interactions
Scientific Research Applications
Approved Indications
Gemcitabine is approved for use in several cancer types, often in combination with other agents. The following table summarizes its approved indications:
Drug Delivery Systems
Recent studies have focused on enhancing the delivery mechanisms of this compound to improve its therapeutic efficacy while minimizing side effects. Various innovative drug delivery systems include:
- Polymeric Nanoparticles : These systems enhance the bioavailability of this compound and target tumor cells more effectively.
- Liposomes : Encapsulation of this compound in liposomes has shown improved pharmacokinetics and reduced systemic toxicity .
- Aerosol Delivery : Research has demonstrated the potential of aerosolized this compound to treat pulmonary metastases, particularly in osteosarcoma models, showing significant necrosis in treated tumors .
Case Studies
- Pancreatic Cancer : In clinical trials, this compound monotherapy yielded objective response rates ranging from 5% to 12%, with median survival times between 3.9 to 6.3 months. Combination therapies have shown improved outcomes compared to monotherapy .
- Ovarian Cancer : A Phase II trial reported a response rate of 57.1% in patients treated with this compound, with a median progression-free survival of 13.4 months .
- Colorectal Cancer : Recent analyses indicate that this compound serves as a second-line treatment option, with ongoing research identifying novel molecular targets to enhance its efficacy against colorectal tumors .
Challenges and Future Directions
Despite its effectiveness, the clinical use of this compound faces challenges such as:
- Short Biological Half-Life : Rapid metabolism limits its therapeutic window.
- Drug Resistance : Increased expression of ribonucleotide reductase can lead to diminished drug efficacy.
Future research aims to overcome these barriers through advanced drug delivery systems and combination therapies that can enhance the selectivity and effectiveness of this compound.
Mechanism of Action
Gemcitabine acts as an antimetabolite, mimicking the building blocks of RNA and DNA. It disrupts DNA synthesis by incorporating itself into the DNA strand during replication, leading to chain termination and apoptosis . The molecular targets include ribonucleotide reductase and DNA polymerase . This mechanism is similar to that of cytarabine but with a broader spectrum of antitumor activity .
Comparison with Similar Compounds
Comparison with Similar Chemotherapeutic Agents
Gemcitabine + Cisplatin (GC) vs. This compound + Carboplatin (GCa) in Advanced Urothelial Carcinoma (aUC)
- Survival Outcomes: No significant differences in median progression-free survival (PFS), cancer-specific survival (CSS), or overall survival (OS) were observed among full-dose GC, dose-reduced GC, and GCa in real-world studies (median OS: ~18 months) .
Toxicity :
| Parameter | Full-Dose GC | Dose-Reduced GC | GCa |
|---|---|---|---|
| CR Rate | 22.0% | 7.4% | 16.2% |
| Median OS (Months) | 18.0 | 18.0 | 18.0 |
| Elevated Transaminases | 22.0% | 14.8% | 7.4%* |
| Neutropenia (Grade ≥3) | 36.6% | 29.6% | 32.4% |
This compound + Cisplatin vs. This compound Alone in Biliary Tract Cancer
Efficacy :
Toxicity :
This compound vs. FOLFIRINOX in Metastatic Pancreatic Cancer
- Efficacy: FOLFIRINOX (a combination of oxaliplatin, irinotecan, fluorouracil, and leucovorin) outperformed this compound alone in median OS (11.1 vs. 6.8 months; HR=0.57, P<0.001) and PFS (6.4 vs. 3.3 months; HR=0.47, P<0.001) .
- Toxicity: FOLFIRINOX caused higher rates of febrile neutropenia (5.4% vs. 0%) and fatigue .
Comparison with Targeted Therapies
This compound + Axitinib vs. Other Targeted Combinations in Pancreatic Cancer
Efficacy :
Mechanistic Insights :
| Combination | Rash (All Grades) | Diarrhea (Grade ≥3) |
|---|---|---|
| This compound + Axitinib | 12.1% | 3.2% |
| This compound + Trametinib | 28.9% | 15.8% |
| This compound + Erlotinib | 25.6% | 10.4% |
This compound + Nab-Paclitaxel vs. This compound Alone
- Efficacy :
- Toxicity :
Key Clinical Considerations
- Patient Selection: Cisplatin Eligibility: Full-dose GC is preferred for patients with normal renal function (eGFR ≥60 mL/min), while GCa or dose-reduced GC is reserved for those with renal impairment . Performance Status: FOLFIRINOX is recommended for metastatic pancreatic cancer patients with ECOG PS 0-1 due to higher toxicity .
- Real-World vs.
Biological Activity
Gemcitabine, a nucleoside analog, is a chemotherapeutic agent primarily used in the treatment of various cancers, including pancreatic, breast, and non-small cell lung cancer. Its biological activity is characterized by its mechanism of action, pharmacokinetics, and efficacy in clinical settings. This article provides a comprehensive overview of the biological activity of this compound, highlighting its mechanisms, case studies, and research findings.
This compound exerts its anticancer effects by inhibiting DNA synthesis. It is phosphorylated intracellularly to form this compound triphosphate (dFdCTP), which competes with deoxycytidine triphosphate (dCTP) for incorporation into DNA. This incorporation leads to chain termination during DNA replication. Additionally, this compound inhibits ribonucleotide reductase, reducing the levels of dNTPs necessary for DNA synthesis .
Key Mechanisms:
- Inhibition of DNA Synthesis : this compound is incorporated into DNA, causing chain termination.
- Ribonucleotide Reductase Inhibition : Reduces dNTP levels, further inhibiting DNA synthesis.
Pharmacokinetics
This compound is administered intravenously and has a short half-life (approximately 30 minutes). Its pharmacokinetics are influenced by factors such as dose, administration route, and patient characteristics. The drug is rapidly distributed and metabolized primarily in the liver and kidneys .
Pharmacokinetic Properties:
| Property | Value |
|---|---|
| Half-life | ~30 minutes |
| Bioavailability | Low (due to rapid metabolism) |
| Metabolism | Hepatic (mainly) |
| Excretion | Renal |
Efficacy in Clinical Trials
This compound has been evaluated in numerous clinical trials for various cancers. Below are summaries from significant studies:
Case Study Summaries:
- Pancreatic Cancer :
- Breast Cancer :
- Combination Therapies :
Research Findings
Recent studies have explored novel formulations and conjugates of this compound to enhance its therapeutic efficacy:
- This compound Conjugates : Research on this compound conjugated with cell-penetrating peptides (CPPs) demonstrated improved cellular uptake and cytotoxicity against various cancer cell lines compared to free this compound . For instance, the Gem-Cys-pVEC conjugate showed IC50 values significantly lower than those for unmodified this compound.
- PEGylation : Modifications such as PEGylation have been shown to increase the bioavailability and therapeutic index of this compound. Studies indicated that PEG-gemcitabine had a 21-fold higher bioavailability compared to native this compound after intravenous administration in animal models .
Q & A
Q. How to evaluate synergism in this compound combination therapies using experimental models?
Q. Tables
| Key Variables in this compound Cytotoxicity Assays |
|---|
| Cell Line |
| MIA PaCa-2 |
| PANC-1 |
| Common Biomarkers in this compound Resistance Studies |
|---|
| Biomarker |
| hENT1 |
| dCK |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


