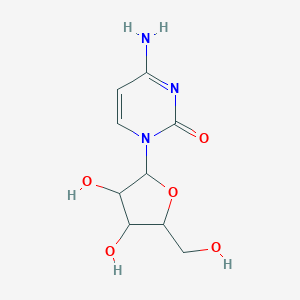
Cytarabine
Description
Historical Development and Evolution of Cytarabine Research
Discovery and Early Clinical Adoption
This compound was first isolated in 1959 from the Caribbean sponge Cryptotethia crypta. Its antileukemic properties were identified through screening programs at the University of California, leading to FDA approval in 1969 for AML treatment. The pivotal 1973 study establishing the "7+3" regimen (7 days of this compound infusion + 3 days of daunorubicin) achieved complete remission (CR) rates of 60-70% in younger AML patients, setting the standard for induction therapy.
Key Historical Milestones:
| Year | Development | Impact |
|---|---|---|
| 1960 | This compound patent filed | Enabled large-scale production |
| 1969 | FDA approval for AML | First targeted antimetabolite for leukemia |
| 1999 | Liposomal formulation (DepoCyt®) approved | Reduced neurotoxicity in CNS lymphoma |
| 2017 | CPX-351 (Vyxeos®) approval | Improved survival in secondary AML |
Optimization of Dosing Strategies
The Swedish AML Registry study (2024) demonstrated that intensifying this compound from 100 mg/m²/day to 1 g/m² twice daily improved median overall survival (OS) from 6.4 to 10.7 months in older patients with secondary/high-risk AML. This regimen maintained tolerable toxicity, with 60% achieving CR versus 36% on standard dosing.
Properties
IUPAC Name |
4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6-,7+,8-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UHDGCWIWMRVCDJ-CCXZUQQUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=CN(C(=O)N=C1N)[C@H]2[C@H]([C@@H]([C@H](O2)CO)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C9H13N3O5 | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
69-74-9 (hydrochloride) | |
| Record name | Cytarabine [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000147944 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID3022877 | |
| Record name | Cytarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022877 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
243.22 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Cytarabine appears as colorless crystals. Used as an antiviral agent., Solid | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>36.5 [ug/mL] (The mean of the results at pH 7.4), Freely soluble, 1 G IN ABOUT 5 ML WATER & 500 ML ALC; 1 G IN ABOUT 1000 ML CHLOROFORM & 300 ML METHANOL, Soluble in water, 4.38e+01 g/L | |
| Record name | SID47193873 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Prisms from 50% ethanol, WHITE TO OFF-WHITE CRYSTALLINE POWDER | |
CAS No. |
147-94-4 | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Cytarabine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=147-94-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cytarabine [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000147944 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cytarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022877 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Cytarabine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.005.188 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CYTARABINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/04079A1RDZ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
414 to 415 °F (NTP, 1992), 186-188, 212-213 °C, 212 - 213 °C | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Preparation Methods
Synthetic Routes and Reaction Conditions
Cytarabine is synthesized from cytidine through a series of chemical reactions. The traditional preparation method involves the use of polyphosphoric acid to synthesize this compound from cytidine. The reaction conditions typically include heating and the use of solvents such as water and alcohol. The yield of this process ranges from 29% to 40% .
Industrial Production Methods
In industrial settings, this compound is produced in large quantities using similar synthetic routes but with optimized reaction conditions to maximize yield and purity. The process involves the use of advanced purification techniques to ensure the final product meets pharmaceutical standards .
Chemical Reactions Analysis
Hydrolysis and Deamination
Cytarabine undergoes pH-dependent hydrolysis and enzymatic deamination to form 1-β-D-arabinofuranosyluracil (ara-U) , its primary inactive metabolite.
Key Reactions:
- Deamination by cytidine deaminase :
This compound → ara-U (via NH₃ elimination) - Acid-catalyzed hydrolysis :
Degrades via cleavage of the glycosidic bond at pH < 3 . - Base-catalyzed degradation :
Forms uracil arabinoside under alkaline conditions (pH > 9) .
Table 1: Thermodynamic Parameters of this compound Degradation
| Parameter | Value (Citric Acid) | Value (Saline) | Source |
|---|---|---|---|
| ΔH (kJ/mol) | -5.42 | -20.40 | |
| Half-life (min) | 45.12 | 62.45 | |
| Activation Energy (kJ/mol) | 28.24 | 23.21 |
Phosphorylation and DNA Incorporation
This compound requires intracellular activation via phosphorylation to exert cytotoxic effects:
Metabolic Pathway:
- Phosphorylation by deoxycytidine kinase → This compound monophosphate (ara-CMP)
- Subsequent phosphorylation → This compound triphosphate (ara-CTP)
- Incorporation into DNA :
Table 2: Kinetic Effects of this compound on Model Okazaki Fragments
| Parameter | Control (OKA) | This compound-Substituted (ARAC) |
|---|---|---|
| Melting Temp (°C) | 46.8 | 42.4 |
| ΔG (kcal/mol at 37°C) | -4.1 | -2.9 |
| DNA Duplex Destabilization | None | Observed via ¹H NMR |
Interaction with Enzymatic Targets
This compound’s reactivity is governed by its inhibition of key enzymes:
Enzymatic Inhibition:
- DNA polymerase α/β : Direct competitive inhibition (Kᵢ = 0.1–1.0 μM)
- Ribonucleotide reductase : Reduces dCTP pools, enhancing ara-CTP incorporation
- Cytidine deaminase : Primary catabolic enzyme (Km = 0.2 mM)
Table 3: Stability in Clinical Formulations
| Condition | Degradation Rate (%) | Half-life |
|---|---|---|
| pH 7.4, 25°C | <5% over 7 days | 7 days |
| pH 5.0, 40°C | 15% over 24 hours | 6.8 hours |
| Light exposure | 10% over 48 hours | 48 hours |
Photodegradation and Oxidative Pathways
Advanced oxidation processes (AOPs) degrade this compound in wastewater:
- UV/H₂O₂ systems : Achieve >95% degradation via hydroxyl radical (·OH) attack
- Fenton-like reactions : Fe³⁺/UV systems yield 80% degradation in 60 min
- Primary degradation products : Arabinofuranosyluracil, cytosine, and arabinose
Solid-State Reactivity
This compound exhibits distinct hydrogen-bonding patterns influencing stability:
- Intramolecular H-bonds : O2’–H···O5’ stabilizes arabinose conformation
- Crystal packing : NH···O interactions with Gln97/Asp133 in dCK complexes
Compatibility with Excipients
This compound demonstrates variable stability in parenteral admixtures:
| Excipient | Compatibility | Observations |
|---|---|---|
| 0.9% NaCl | Stable | No precipitation over 7 days |
| 5% Dextrose | Compatible | pH-dependent degradation |
| Citrate buffers | Reactive | Accelerates deamination |
Scientific Research Applications
Pharmacokinetics
Cytarabine is poorly absorbed when taken orally due to extensive first-pass metabolism; therefore, it is typically administered via intravenous or subcutaneous routes. Its pharmacokinetic properties include:
- Bioavailability: High when administered intravenously.
- Volume of Distribution: High due to low plasma protein binding.
- Metabolism: Primarily occurs in the liver with renal excretion of metabolites .
Clinical Applications
This compound has a wide range of applications in oncology, particularly for treating different types of leukemia. Below are some key indications:
Liposomal Formulations
Recent advancements have led to the development of liposomal formulations of this compound, which improve its pharmacokinetic profile and reduce systemic toxicity. Liposomal this compound has shown efficacy in treating CNS relapses in ALL and has been compared favorably to traditional therapies .
Efficacy Studies
A study evaluating liposomal this compound for CNS relapse in ALL demonstrated comparable safety and efficacy profiles to methotrexate and traditional this compound formulations. The European Working Group for Adult ALL initiated trials to further explore this application .
Case Studies
-
Case Study: Liposomal this compound in CNS Relapse
- Patient Profile: Adult patient with recurrent ALL.
- Treatment Regimen: Administered liposomal this compound.
- Outcome: Achieved significant CNS response after one cycle; tolerated well without severe adverse effects.
-
Case Study: Differentiation Induction
- Patient Profile: AML patient receiving low-dose this compound.
- Treatment Regimen: Low-dose this compound combined with differentiation agents.
- Outcome: Induced maturation of leukemic cells without significant toxicity, suggesting potential as a differentiation therapy.
Mechanism of Action
Cytarabine exerts its effects by inhibiting DNA polymerase, an enzyme crucial for DNA synthesis. It is incorporated into DNA during the S-phase of the cell cycle, leading to the termination of DNA chain elongation and ultimately causing cell death. This mechanism makes this compound particularly effective against rapidly dividing cancer cells .
Comparison with Similar Compounds
Sodium Nitrite vs. Sodium Nitrate (NaNO₃)
Key Findings :
- Sodium nitrite is more effective in immediate pathogen inhibition but poses higher toxicity and carcinogenic risks .
- Sodium nitrate’s slower nitrite release reduces NOC formation but requires microbial conversion for preservative effects .
Key Findings :
Sodium Nitrite vs. Organic Nitrite Donors
Key Findings :
- Organic donors like allylic nitro compounds provide sustained nitric oxide release, reducing off-target toxicity .
Toxicity Profile
- Acute Toxicity : Sodium nitrite ingestion >1 g causes methemoglobinemia, hypotension, and death .
- Carcinogenicity: Forms NOCs in processed meats, linked to colorectal cancer . Mitigation strategies include adding antioxidants (e.g., vitamin C) .
- Chronic Exposure : Rodent studies show forestomach hyperplasia and splenic toxicity at 3,000–5,000 ppm .
Biological Activity
Cytarabine, also known as cytosine β-D-arabinofuranoside, is a pyrimidine nucleoside analog primarily used in the treatment of acute myeloid leukemia (AML) and other hematological malignancies. Its biological activity is characterized by its ability to inhibit DNA synthesis, thereby exerting cytotoxic effects on rapidly dividing cells. This article explores the mechanisms of action, pharmacokinetics, clinical efficacy, and recent advancements in drug formulations related to this compound.
This compound's primary mechanism involves its conversion into this compound triphosphate (Ara-CTP) within the cell. This active form competes with deoxycytidine triphosphate (dCTP) for incorporation into DNA during replication. The incorporation of Ara-CTP results in the termination of DNA chain elongation, leading to cell cycle arrest at the S-phase and subsequent apoptosis of malignant cells.
- Key Enzymes Involved :
- Deoxycytidine kinase (DCK) activates this compound by phosphorylating it to Ara-CTP.
- Cytidine deaminase (CDA) is responsible for the catabolism of this compound, influencing its bioavailability and efficacy.
Pharmacokinetics
This compound has a short plasma half-life, typically around 1 to 3 hours, necessitating continuous infusion or frequent dosing to maintain therapeutic levels. Its pharmacokinetic profile can be affected by various factors including patient genetics and concurrent medications.
| Parameter | Value |
|---|---|
| Half-life | 1-3 hours |
| Peak plasma levels | ~0.5 hours post-infusion |
| Bioavailability | Variable (due to CDA activity) |
Clinical Efficacy
This compound is a cornerstone in the treatment regimens for AML and is often used in combination with other agents such as anthracyclines. The efficacy varies among patients due to genetic polymorphisms affecting drug metabolism.
Case Studies
- Ewing Sarcoma : A Phase II study demonstrated that intermediate doses of this compound significantly reduced EWS/FLI1 protein levels in Ewing sarcoma cells both in vitro and in vivo, indicating potential utility beyond traditional leukemias .
- Acute Myeloid Leukemia : Clinical trials have shown that high-dose this compound regimens improve remission rates in patients with AML, particularly those with favorable cytogenetics .
Recent Advancements
Recent research has focused on enhancing the delivery and efficacy of this compound through novel drug formulations:
- Cholic Acid-Cytarabine Conjugates : These liver-targeting conjugates have shown improved absorption and specificity for hepatic tissues, potentially reducing systemic toxicity while maintaining antitumor activity .
- Pharmacogenomic Biomarkers : Studies are exploring genetic markers that predict patient response to this compound therapy, aiming to tailor treatments based on individual metabolic profiles .
Q & A
Basic Research Questions
Q. What experimental methodologies are recommended for elucidating cytarabine’s mechanism of action in acute myeloid leukemia (AML) cells?
- Methodological Answer : Use in vitro assays to measure this compound’s incorporation into DNA via competitive inhibition of DNA polymerase. Employ metabolomic profiling (e.g., LC-MS) to track the activation of this compound by deoxycytidine kinase and its deactivation by cytidine deaminase . Combine flow cytometry with Annexin V/PI staining to quantify apoptosis in AML cell lines (e.g., HL-60) after this compound exposure. For in vivo models, utilize patient-derived xenografts (PDX) to correlate drug efficacy with tumor regression .
Q. How can researchers standardize cytotoxicity assays to ensure reproducibility in this compound studies?
- Methodological Answer : Adopt the MTT or CellTiter-Glo assays for viability measurements, with strict controls for cell density, incubation time, and this compound concentration gradients (e.g., 0.1–10 μM). Include positive controls (e.g., daunorubicin) and negative controls (untreated cells). Validate results across multiple cell lines (e.g., THP-1, MOLM-13) and replicate experiments ≥3 times. Document all protocols using FAIR data principles .
Q. What analytical techniques are most effective for quantifying intracellular this compound metabolites?
- Methodological Answer : Use high-performance liquid chromatography (HPLC) with UV detection or liquid chromatography-tandem mass spectrometry (LC-MS/MS) to measure this compound triphosphate (Ara-CTP) levels. Optimize sample preparation to prevent metabolite degradation (e.g., rapid freezing in liquid nitrogen). Cross-validate results with enzymatic assays targeting deoxycytidine kinase activity .
Advanced Research Questions
Q. How do genetic mutations (e.g., TP53, FLT3-ITD) influence this compound resistance in AML, and what experimental models best recapitulate this?
- Methodological Answer : Perform whole-exome sequencing on this compound-resistant AML samples to identify co-occurring mutations. Use CRISPR-Cas9 to introduce mutations (e.g., TP53 KO) into isogenic cell lines and assess IC50 shifts. Validate findings in PDX models treated with this compound monotherapy. Analyze RNA-seq data to identify dysregulated pathways (e.g., DNA repair, nucleoside transport) .
Q. What strategies optimize this compound’s pharmacokinetic profile in preclinical models to enhance blood-brain barrier penetration?
- Methodological Answer : Develop liposomal or nanoparticle formulations to improve this compound’s stability and bioavailability. Use pharmacokinetic modeling (e.g., non-compartmental analysis) to calculate AUC and half-life in murine models. Validate CNS delivery via cerebrospinal fluid (CSF) sampling and compare tumor burden in brain xenografts vs. systemic models .
Q. How can researchers resolve contradictory data on this compound’s synergy with anthracyclines (e.g., daunorubicin) across different AML subtypes?
- Methodological Answer : Conduct dose-matrix experiments (e.g., Chou-Talalay synergy assays) to calculate combination indices (CI) in genetically annotated AML cell lines. Stratify results by molecular subgroups (e.g., NPM1 mutant vs. wild-type). Use RNA interference to knock down putative resistance genes (e.g., ABCG2) and reassess synergy .
Q. What epigenetic biomarkers (e.g., DNA methylation, miRNA) predict this compound response, and how can they be validated clinically?
- Methodological Answer : Perform reduced-representation bisulfite sequencing (RRBS) or methylome arrays on pretreatment AML biopsies. Correlate methylation patterns (e.g., CDKN2B hypermethylation) with complete remission rates. Validate candidates in prospective cohorts using droplet digital PCR (ddPCR) for targeted methylation analysis .
Q. How should researchers design studies to evaluate this compound’s off-target effects on normal hematopoietic stem cells (HSCs)?
- Methodological Answer : Use colony-forming unit (CFU) assays to compare this compound’s impact on CD34+ HSCs vs. AML blasts. Employ single-cell RNA-seq to identify HSC subpopulations with differential sensitivity. Validate findings in ex vivo cultures of healthy donor-derived HSCs .
Guidance for Addressing Methodological Challenges
- Data Contradictions : Apply Bradford Hill criteria to assess causality in conflicting studies. For example, if this compound shows efficacy in in vitro models but fails in PDX, evaluate differences in tumor microenvironment (e.g., stromal cell interactions) using spatial transcriptomics .
- Translational Gaps : Use patient-derived organoids (PDOs) to bridge in vitro and clinical data. Test this compound combinations in PDOs and correlate results with matched patient outcomes .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


