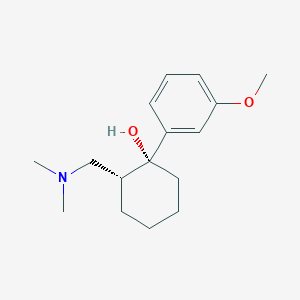
Tramadol
Description
Dual Opioid and Monoaminergic Receptor Interactions
Tramadol exhibits a complex pharmacological profile characterized by both opioid receptor activation and monoaminergic effects. This dual mechanism contributes to its analgesic efficacy while potentially reducing some adverse effects typically associated with classical opioids.
The opioid component of this compound's action involves binding to μ-opioid receptors, though with significantly lower affinity compared to traditional opioids. This compound's affinity for the μ-opioid receptor is approximately 6,000 times less than morphine and about 100-fold less than dextropropoxyphene. However, this compound undergoes extensive hepatic metabolism, with O-desmethylthis compound (M1) being the most pharmacologically significant metabolite. M1 exhibits approximately 200 times greater μ-opioid binding affinity than the parent compound, making it a crucial contributor to the analgesic effect.
The monoaminergic component involves inhibition of serotonin (5-HT) and norepinephrine (NA) reuptake in the central nervous system. (+)-Tramadol predominantly inhibits serotonin reuptake and stimulates 5-HT release, while (-)-tramadol primarily inhibits norepinephrine reuptake. This inhibition enhances descending inhibitory pain pathways, modulating pain perception at the spinal level.
This dual mechanism explains why this compound may be effective for various pain conditions, including neuropathic pain that often responds poorly to conventional opioids. As noted in clinical literature, "the complementary and synergistic actions of the two enantiomers improve the analgesic efficacy and tolerability profile of the racemate".
| Mechanism | Primary Component | Function |
|---|---|---|
| Opioid | (+)-Tramadol & (+)-M1 metabolite | μ-opioid receptor agonism |
| Serotonergic | (+)-Tramadol | Inhibition of serotonin reuptake & stimulation of 5-HT release |
| Noradrenergic | (-)-Tramadol | Inhibition of norepinephrine reuptake |
Stereoselectivity in Enantiomer-Specific Target Modulation
This compound is administered as a racemic mixture of two enantiomers, each contributing uniquely to its overall pharmacological profile. The stereochemistry of this compound is central to understanding its complex mechanism of action.
Structurally, this compound [2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol] possesses two stereogenic centers at the cyclohexane ring, theoretically resulting in four possible configurational forms: (1R,2R), (1S,2S), (1R,2S), and (1S,2R) isomers. However, commercially available this compound is specifically a racemate of the hydrochlorides of the (1R,2R)-(+)- and (1S,2S)-(−)-enantiomers.
The stereoselective pharmacological actions of this compound's enantiomers can be summarized as follows:
The (+)-enantiomer demonstrates stronger binding to μ-opioid receptors and predominantly inhibits serotonin reuptake, with this effect being approximately four times more potent than the (-)-enantiomer.
The (-)-enantiomer primarily inhibits noradrenaline reuptake, enhancing noradrenergic neurotransmission in pain modulation pathways.
The metabolite (+)-M1 shows the highest affinity for μ-opioid receptors (Ki=3.4 nM), far exceeding the parent compound's affinity.
Studies investigating the intrinsic efficacy at the human μ-opioid receptor have established a rank order of: (+)-M1 > (+/-)-M5 > (-)-M1. This stereoselectivity extends to this compound's metabolism as well, with both O- and N-demethylation processes and renal elimination demonstrating enantioselective characteristics.
| Compound | Affinity for μ-opioid receptor (Ki) | Relative Binding Strength |
|---|---|---|
| (+)-M1 | 3.4 nM | Highest |
| (+/-)-M5 | 100 nM | Moderate |
| (-)-M1 | 240 nM | Lower |
| (+/-)-Tramadol | 2.4 μM | Lowest |
| Morphine (reference) | ~6000× stronger than this compound | Very high |
The use of the racemic mixture in clinical practice, rather than individual enantiomers, is supported by evidence that "the racemate seems to have superior efficacy and safety when compared to either enantiomer, (+) or (-)". This suggests that the combined actions of both enantiomers provide optimal therapeutic benefit.
Synergistic Pathways in Nociceptive Signal Inhibition
The analgesic efficacy of this compound results from synergistic interactions between its multiple mechanisms of action, providing a more comprehensive approach to pain modulation than single-mechanism analgesics.
When both the opioid and monoaminergic systems are simultaneously activated, "a supra-additive inhibitory action results from the simultaneous activation of the two receptors". This means the degree of pain relief is greater than the sum of the individual components of this compound's action. The uptake inhibition in both non-opioid and opioid systems occurs in the same concentration range (0.5-50 μM), facilitating this synergistic effect.
At the molecular level, this compound enhances inhibitory pain pathways through multiple complementary mechanisms:
Activation of μ-opioid receptors, primarily by the M1 metabolite, inhibits the release of excitatory neurotransmitters from primary afferent terminals.
Inhibition of serotonin reuptake enhances serotonergic neurotransmission in descending pain inhibitory pathways.
Inhibition of norepinephrine reuptake strengthens noradrenergic modulation of pain signals.
Stimulation of serotonin release further augments descending inhibitory control of pain transmission.
This multi-modal approach to pain modulation explains why this compound may be effective for a wider range of painful conditions than classical opioids, including neuropathic pain states that typically respond poorly to conventional analgesics. As noted in clinical literature, "the monoaminergic component possibly allows this compound's efficacy to stretch over a wider range of painful pathologies than other opioids".
The synergistic action also contributes to this compound's improved side effect profile compared to traditional opioids. For instance, the monoaminergic component may counterbalance some of the opioid-mediated adverse effects, resulting in less respiratory depression and constipation at equianalgesic doses.
Comparative Efficacy Relative to Classical Opioid Agonists
When comparing this compound to classical opioid analgesics, several distinguishing features emerge regarding both efficacy and safety profile.
In terms of analgesic potency, this compound is considerably less potent than morphine. Following parenteral administration, this compound's analgesic potency is approximately 10% of that of morphine. On a dose-by-dose basis, this compound has about one-tenth the potency of morphine but is practically equally potent when compared with pethidine and codeine.
| Characteristic | This compound | Classical Opioids (e.g., Morphine) |
|---|---|---|
| Analgesic Potency | ~10% of morphine | Higher potency |
| Mechanism of Action | Dual opioid and monoaminergic | Primarily μ-opioid receptor agonism |
| Respiratory Depression | Less pronounced | More pronounced |
| Constipation | Reduced incidence | Higher incidence |
| Abuse Potential | Lower (Schedule IV) | Higher (Schedule II for many) |
| Efficacy for Neuropathic Pain | Effective due to monoaminergic action | Generally less effective |
| Serotonin Syndrome Risk | Present | Lower risk |
Despite its lower potency, this compound provides clinically meaningful pain relief. Studies have shown that this compound provides postoperative pain relief comparable to pethidine, and its analgesic efficacy can be further improved by combination with non-opioid analgesics.
A key advantage of this compound over classical opioids lies in its improved safety profile:
Respiratory depression : this compound produces less pronounced respiratory depression compared to equianalgesic doses of morphine. In large clinical and post-marketing studies including over 21,000 patients, no clinically relevant respiratory depression was reported with this compound use at therapeutic doses.
Constipation : Another opioid side effect that is reduced with this compound use is constipation. This proves to be a significant advantage with long-term therapy and could benefit patients by preventing postoperative ileus.
Dependence and abuse potential : The dual mechanism of action "may reduce the risk of opioid-associated adverse reactions and the potential for tolerance, dependence or abuse," although these risks remain present.
However, this compound carries unique risks not typically associated with classical opioids. Its serotonergic effects create potential for serotonin syndrome, particularly when combined with other serotonergic medications, as illustrated in the following table:
| Opioids | SSRIs, SNRIs, TCAs, St John's wort, lithium | MAOIs |
|---|---|---|
| Morphine, codeine, buprenorphine, oxycodone | Should be safe | Possible rare interaction |
| Fentanyl, tapentadol, methadone | Possible rare interaction | Increased risk of serotonin syndrome |
| This compound, pethidine, dextromethorphan | Increased risk of serotonin syndrome | Contraindicated |
This compound has been found particularly useful in specific patient populations: those with risk of poor cardiopulmonary function, after thoracic or upper abdominal surgery, and when non-opioid analgesics are contraindicated. It is also effective for managing chronic pain of both malignant and nonmalignant origin, particularly neuropathic pain.
Properties
IUPAC Name |
(1R,2R)-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexan-1-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H25NO2/c1-17(2)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)19-3/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3/t14-,16+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
TVYLLZQTGLZFBW-ZBFHGGJFSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CC1CCCCC1(C2=CC(=CC=C2)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN(C)C[C@H]1CCCC[C@@]1(C2=CC(=CC=C2)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H25NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90858931, DTXSID401167150 | |
| Record name | Tramadol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90858931 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1R,2R)-2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID401167150 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
263.37 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Tramadol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014339 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>39.5 [ug/mL] (The mean of the results at pH 7.4), Soluble, 7.50e-01 g/L | |
| Record name | SID26663897 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Tramadol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00193 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Tramadol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014339 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
123154-38-1, 27203-92-5 | |
| Record name | (1R,2R)-2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=123154-38-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Tramadol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=27203-92-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Tramadol [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0027203925 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (+)-trans-Tramadol free base | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0123154381 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Tramadol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00193 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Tramadol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90858931 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1R,2R)-2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID401167150 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Tramadol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.043.912 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRAMADOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/39J1LGJ30J | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | TRAMADOL, (+)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0NG5TTM63P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | TRAMADOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7047 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Tramadol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014339 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
178-181 °C, 180 - 181 °C | |
| Record name | Tramadol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00193 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Tramadol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014339 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Preparation Methods
Reaction Mechanism and Conditions
The patent EP1346978A1 introduces a novel one-pot process that eliminates distillation:
-
Acidification : A cis/trans this compound base mixture reacts with anhydrous HCl gas in isopropyl alcohol.
-
Hydration : Water (3–5% v/v) is added to precipitate trans-tramadol hydrochloride selectively.
Optimized Parameters :
| Parameter | Range | Impact on Purity/Yield |
|---|---|---|
| Temperature | 40–50°C | Higher temps reduce cis solubility |
| Water content | 3–5% | Maximizes trans precipitation |
| Solvent | Isopropyl alcohol | Enhances cis isomer solubility |
This method achieves 93–97% trans purity with yields exceeding 85%.
Monohydrate Intermediate Formation
An improved monohydrate intermediate step further enhances purity:
-
Adjust the this compound base mixture to pH 7.5–8.5 using acetic acid.
-
Crystallize trans-tramadol monohydrate at 40–50°C.
Results :
Biomimetic Synthesis Approaches
Recent advances mimic enzymatic catalysis to improve stereoselectivity. A biomimetic method using borane tribromide achieves demethylation of 3-methoxyphenyl intermediates at −50°C, yielding this compound with 89% enantiomeric excess.
Key Steps :
-
Demethylation : Borane tribromide selectively removes methyl groups without racemization.
-
Cyclization : Acid-catalyzed ring closure forms the cyclohexanol backbone.
This method reduces byproducts like (3-((1R,2R)-2-((dimethylamino)methyl)-1-hydroxyclohexyl)phenol (Impurity 9) to <0.5%.
Stereochemical Considerations and Isomer Separation
pH-Dependent Crystallization
Adjusting pH to 7–10 preferentially crystallizes the trans isomer due to its lower solubility in weakly alkaline conditions. For example:
Solvent Polarity Effects
Polar solvents like ethanol increase cis isomer solubility, enabling selective trans precipitation:
| Solvent | Trans Solubility (mg/mL) | Cis Solubility (mg/mL) |
|---|---|---|
| Ethanol | 15 | 62 |
| Isopropanol | 9 | 41 |
Purification and Quality Control
Impurity Profiling
LC-MS analyses identify four major this compound impurities:
-
Impurity 6 : Dehydration product (m/z 264 [M+H]⁺).
-
Impurity 7 : Cyclohexene derivative (m/z 264 [M+H]⁺).
-
Impurity 9 : Demethylated phenol (m/z 250 [M+H]⁺).
-
Impurity 11 : Oxidative dimer (m/z 526 [M+H]⁺).
Control Strategies :
Analytical Methods
-
HPLC : X-Bridge C18 column (5 µm, 250 × 4.6 mm) with PDA-ELSD detection.
-
IR Spectroscopy : Confirms monohydrate formation via O-H stretch at 3350 cm⁻¹.
Industrial-Scale Manufacturing
| Ingredient | Percentage | Function |
|---|---|---|
| This compound HCl | 60% | Active |
| HPC K100 | 25% | Matrix former |
| Magnesium stearate | 1.5% | Lubricant |
Chemical Reactions Analysis
Types of Reactions
Tramadol undergoes several types of chemical reactions, including:
Reduction: Reduction reactions are less common but can occur under specific conditions.
Substitution: this compound can undergo substitution reactions, particularly in the presence of strong nucleophiles.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Reduction: Reducing agents such as lithium aluminum hydride can be used.
Substitution: Strong nucleophiles like sodium hydride can facilitate substitution reactions.
Major Products
The major products formed from these reactions include O-desmethylthis compound and various substituted derivatives, depending on the reagents and conditions used .
Scientific Research Applications
Pharmacological Profile
Tramadol acts as a mu-opioid receptor agonist and a serotonin-norepinephrine reuptake inhibitor (SNRI) , which contributes to its analgesic effects. It is primarily used for the treatment of moderate to severe pain, including:
- Postoperative pain
- Chronic pain syndromes (e.g., rheumatoid arthritis, fibromyalgia)
- Neuropathic pain
- Labor pain
- Osteoarthritis and cancer-related pain
This compound's analgesic potency is approximately one-tenth that of morphine, making it a valuable option for patients who require effective pain control without the higher risks associated with stronger opioids .
Pain Management
This compound is indicated for:
- Acute Pain: Effective in managing postoperative and injury-related pain.
- Chronic Pain: Often prescribed for conditions like fibromyalgia and chronic back pain, where it serves as a second-line treatment option .
The drug's unique mechanism allows it to address both nociceptive and neuropathic pain pathways, making it suitable for diverse patient populations .
Off-Label Uses
This compound has also been explored for various off-label applications:
- Restless Legs Syndrome (RLS): It is occasionally prescribed for refractory cases where first-line treatments fail .
- Premature Ejaculation: Some studies suggest this compound's efficacy in delaying ejaculation, although this use remains controversial .
- Psychiatric Disorders: There is emerging interest in this compound's potential antidepressant effects, warranting further investigation into its use in psychiatric care .
Pharmacokinetics and Dosing
This compound is rapidly absorbed after oral administration, with peak plasma concentrations occurring within 1.6 to 2 hours. The recommended dosing regimen typically involves:
| Formulation | Dosage Range | Frequency |
|---|---|---|
| Immediate Release | 50–100 mg every 4–6 hours | As needed |
| Extended Release | 200 mg once daily | Daily |
The bioavailability of this compound is approximately 75%, influenced by first-pass metabolism .
Efficacy in Pain Management
A systematic review highlighted this compound's effectiveness across various pain types, noting its role in postoperative settings and chronic pain management. A study indicated that patients with fibromyalgia experienced significant relief when this compound was included in their treatment regimen .
Abuse Potential
Despite its therapeutic benefits, this compound has been associated with abuse potential. Research indicates that while it poses a lower risk of addiction compared to traditional opioids, misuse has been documented, particularly among young adults. This necessitates careful monitoring when prescribing this compound .
Mechanism of Action
Tramadol exerts its effects through multiple mechanisms:
Opioid Receptor Activation: This compound binds to the μ-opioid receptor, although with lower affinity compared to other opioids.
Monoamine Reuptake Inhibition: This compound inhibits the reuptake of serotonin and norepinephrine, enhancing their levels in the synaptic cleft and contributing to its analgesic effects.
Ion Channel Modulation: This compound affects ion channels, particularly voltage-gated sodium channels, which play a role in pain signaling.
Comparison with Similar Compounds
Morphine
Tapentadol
Codeine and Hydrocodone
This compound/Acetaminophen Combination
- Efficacy : Synergistic effect (SPID48 score: 28.3 vs 18.7 for placebo; p < 0.001) in postoperative pain .
- Dosing: 37.5 mg this compound + 325 mg acetaminophen QID reduces opioid exposure while maintaining efficacy .
- Safety: Hepatotoxicity risk at >4 g/day acetaminophen; lower GI bleeding vs NSAIDs .
Venlafaxine (Structural Isomer)
- Structural Similarity : O-desmethylvenlafaxine shares m/z 264 and chromatographic retention time with this compound, causing assay interference .
- Functional Difference : Venlafaxine is an SNRI without analgesic properties, highlighting this compound’s unique dual mechanism .
Data Tables
Table 1: Pharmacokinetic and Pharmacodynamic Comparison
| Compound | Bioavailability | Half-Life (h) | CYP Dependency | Key Metabolites |
|---|---|---|---|---|
| This compound | 68% | 5.1 (parent) | CYP2D6, 3A4 | M1 (active), M2, M5 |
| Tapentadol | 32% | 4–6 | Glucuronidation | Tapentadol-O-glucuronide |
| Codeine | 90% | 2.5–3.5 | CYP2D6 | Morphine (10–15%) |
Table 2: Adverse Event Incidence in Clinical Trials
| Compound | Nausea (%) | Seizures (%) | Respiratory Depression (%) | Constipation (%) |
|---|---|---|---|---|
| This compound | 26–30 | 1.2–4.5 | <1 | 10–15 |
| Morphine | 30–40 | <1 | 15–20 | 40–50 |
| Tapentadol | 20–25 | <1 | <1 | 15–20 |
Biological Activity
Tramadol is a synthetic opioid analgesic widely used for managing moderate to severe pain. Its biological activity is characterized by a complex mechanism involving multiple pathways, including opioid receptor agonism, serotonin and norepinephrine reuptake inhibition, and modulation of various pain pathways. This article provides a detailed overview of this compound's biological activity, including its pharmacokinetics, pharmacodynamics, and associated clinical findings.
This compound operates primarily through:
- Opioid Receptor Agonism : this compound binds to the μ-opioid receptors (μ-OR), which are crucial for its analgesic effects. The primary active metabolite, O-desmethylthis compound (M1), exhibits significantly higher potency at these receptors—up to 200 times more than this compound itself .
- Serotonin and Norepinephrine Reuptake Inhibition : this compound also functions as a serotonin-norepinephrine reuptake inhibitor (SNRI), enhancing the levels of these neurotransmitters in the synaptic cleft, which contributes to its analgesic properties .
- Interaction with Other Receptors : It influences various other receptor systems, including alpha2-adrenoreceptors and NMDA receptors, which play roles in modulating pain perception .
Pharmacokinetics
This compound is rapidly absorbed after oral administration, with peak plasma concentrations typically reached within 1.6 to 3 hours. The bioavailability is approximately 75%, influenced by first-pass metabolism in the liver . The following table summarizes key pharmacokinetic parameters:
| Parameter | Value |
|---|---|
| Bioavailability | ~75% |
| Peak Plasma Concentration (Cmax) | ~300 μg/L |
| Time to Peak Concentration (Tmax) | 1.6 - 3 hours |
| Elimination Half-life | 5-6 hours |
Genetic Factors Influencing Response
The variability in this compound response can be attributed to genetic polymorphisms in the cytochrome P450 enzyme system, particularly CYP2D6. Individuals with different CYP2D6 genotypes exhibit significant differences in this compound metabolism and efficacy:
- Extensive Metabolizers (EM) : Normal function of CYP2D6 leads to effective conversion to M1.
- Poor Metabolizers (PM) : Reduced or absent CYP2D6 function results in lower M1 levels and potentially less effective pain relief .
Efficacy and Safety
A study involving over 88,000 patients indicated that this compound use was associated with higher all-cause mortality rates compared to naproxen and diclofenac. The hazard ratio for this compound was found to be significantly elevated (HR = 1.71 compared to naproxen) during a one-year follow-up period .
Chronic Use Effects
Research on chronic this compound use has demonstrated adverse histopathological changes in animal models. A study on rats showed significant oxidative stress markers and increased apoptosis in brain and testicular tissues after prolonged this compound administration . The findings suggest potential long-term effects on fertility and psychological health.
Summary of Research Findings
Recent studies have highlighted the multifaceted biological activity of this compound, revealing both therapeutic benefits and risks associated with its use. Key findings include:
- Increased Risk of Dementia : A retrospective cohort study found a dose-response relationship between this compound use and the incidence of all-cause dementia among older adults .
- Neurotransmitter Disruption : Chronic exposure has been linked to alterations in neurotransmitter systems, evidenced by metabolomic analyses showing significant biomarker changes related to brain function .
Q & A
Q. What are the primary pharmacological mechanisms of tramadol, and how do they influence experimental design in pain management studies?
this compound exerts dual mechanisms: weak µ-opioid receptor agonism and inhibition of serotonin/noradrenaline reuptake. Researchers must account for both pathways when designing studies, particularly when comparing this compound to pure opioids (e.g., morphine) or non-opioid analgesics. For example, preclinical studies should include assays for opioid receptor binding and neurotransmitter uptake inhibition . Clinical trials should stratify participants based on CYP2D6 polymorphisms, as this compound's active metabolite (O-desmethylthis compound) depends on this enzyme .
Q. How do researchers quantify this compound and its metabolites in biological samples?
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the gold standard for detecting this compound and its 24+ metabolites in urine or plasma. Key parameters include:
Q. What are standard protocols for assessing this compound’s efficacy in cancer pain management?
The Cochrane Collaboration recommends:
- Study design : Randomized controlled trials (RCTs) with active comparators (e.g., morphine, codeine) and placebo controls.
- Outcomes : Pain reduction ≥30% from baseline, patient-reported "much improved" status, and adverse event rates (e.g., nausea, dizziness).
- Dosing : 50–600 mg/day, with titration based on pain severity .
Advanced Research Questions
Q. How can researchers resolve heterogeneity in meta-analyses of this compound for premature ejaculation (PE)?
Heterogeneity arises from variable dosing (25–100 mg on-demand), trial durations (1 day–6 months), and outcome measures (e.g., intravaginal ejaculatory latency time). Mitigation strategies include:
- Subgroup analysis by dose and administration frequency .
- Sensitivity analysis excluding open-label studies .
- Standardized reporting using CONSORT guidelines to reduce bias .
Q. What methodologies address conflicting data on this compound’s association with all-cause mortality in osteoarthritis patients?
A 2019 propensity score-matched cohort study found this compound increased mortality risk vs. NSAIDs (HR: 1.71–2.04) but not vs. codeine. To reconcile contradictions:
Q. How can mixed-methods approaches elucidate this compound abuse drivers in specific populations?
Combining qualitative discourse analysis (e.g., coding media narratives on this compound use in Ghana) with quantitative surveys identifies cultural and socioeconomic factors. For example:
- NVivo software for thematic analysis of 295 newspaper articles .
- Validated questionnaires assessing poly-substance use patterns (e.g., this compound + energy drinks) .
Q. What experimental models validate this compound’s off-label antidepressant effects?
Preclinical models:
- Forced swim test (FST) in rodents to assess serotonin/noradrenaline-mediated antidepressant activity.
- Microdialysis to measure extracellular monoamine levels in the prefrontal cortex . Clinical data mining:
- Analysis of patient-reported outcomes (e.g., 94.6% efficacy in 130 users) via platforms like Drugs.com .
Methodological Challenges
Q. How to optimize LC-MS/MS for this compound metabolite profiling in complex matrices?
- Surfactant-assisted microextraction : Triton X-100 enhances recovery from blood or urine by 391–466× .
- Multivariate optimization : Design-of-experiment (DoE) approaches to balance pH, temperature, and salt content .
Q. What are ethical considerations in studying this compound’s performance-enhancing effects in athletes?
- Blinding protocols : Use placebo-controlled trials to avoid bias in cycling time-trial studies .
- Regulatory alignment : Align with UCI/WADA guidelines, even for non-prohibited substances (e.g., this compound’s 2019 monitoring phase) .
Contradictory Findings and Solutions
Q. Why do animal models show divergent this compound pharmacokinetics vs. humans?
Q. How to interpret this compound’s abuse potential given conflicting DEA and epidemiological data?
- DEA classification : Schedule IV (low abuse risk) based on propoxyphene comparability .
- Field data : African studies report 20–60% nonmedical use rates, driven by lax regulation. Hybrid studies combining urine toxicology (LC-MS/MS) and geospatial analysis are critical .
Tables for Key Data
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


