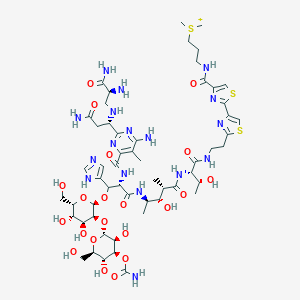
Bleomycin
Description
Historical and Evolutionary Significance of Bleomycin
Discovery and Isolation from Streptomyces verticillus
This compound was first isolated in 1962 by Japanese microbiologist Hamao Umezawa from the soil bacterium Streptomyces verticillus. Umezawa’s team at the Institute of Microbial Chemistry in Tokyo identified the compound through fermentation studies, observing its unique ability to inhibit bacterial growth and tumor cells. Early challenges in isolating this compound arose from the bacterium’s resistance to genetic manipulation. S. verticillus exhibited poor sporulation and lysozyme resistance, complicating plasmid DNA transfer and protoplast regeneration. Despite these hurdles, intergeneric conjugation with Escherichia coli eventually enabled genetic analysis of the this compound biosynthetic cluster, confirming its hybrid nonribosomal peptide synthetase (NRPS)-polyketide synthase (PKS) origin.
The initial isolation process yielded a mixture of this compound analogs, with This compound A2 and B2 emerging as the most clinically relevant due to their DNA-cleaving activity. Structural analysis revealed a complex glycopeptide backbone comprising a bithiazole ring, a metal-binding domain, and a disaccharide moiety, which collectively facilitate DNA intercalation and radical-mediated strand scission.
Evolution as a Glycopeptide Antibiotic of Research Interest
This compound’s classification as a glycopeptide antibiotic places it alongside vancomycin and teicoplanin, though its mechanism diverges significantly. While traditional glycopeptides target bacterial cell wall synthesis, this compound’s primary action involves DNA damage via oxidative free radicals . This dual functionality—antibiotic and antitumor—has driven sustained scientific interest.
Structural and Functional Innovations
- Bithiazole Moiety : Enhances DNA binding affinity, enabling sequence-specific intercalation at guanine-cytosine-rich regions.
- Metal-Binding Domain : Chelates iron (Fe²⁺) to form a reactive complex that generates hydroxyl radicals, causing single- and double-strand DNA breaks.
- Carbohydrate Side Chains : Modulate solubility and cellular uptake, influencing pharmacokinetics and tissue specificity.
The evolutionary significance of this compound lies in its biosynthetic pathway. The gene cluster (blm) in S. verticillus spans 30 open reading frames, encoding NRPS, PKS, and tailoring enzymes that assemble the peptide-polyketide hybrid scaffold. This modular architecture allows for natural analog diversity, inspiring synthetic biology efforts to engineer novel derivatives with reduced toxicity.
Historical Milestones in this compound Research Development
The trajectory of this compound research reflects its growing clinical and scientific impact:
Key Advances:
- Clinical Expansion : Beyond lymphoma, this compound became a cornerstone in treating testicular cancer, ovarian cancer, and malignant pleural effusions.
- Toxicity Mitigation : Cumulative dose limits (≤400 units) and pulmonary function monitoring reduced life-threatening fibrosis.
- Mechanistic Insights : Studies by JoAnne Stubbe elucidated the role of iron-oxygen complexes in DNA strand scission, informing targeted therapies.
Properties
IUPAC Name |
3-[[2-[2-[2-[[(2S,3R)-2-[[(2S,3S,4R)-4-[[(2S,3R)-2-[[6-amino-2-[(1S)-3-amino-1-[[(2S)-2,3-diamino-3-oxopropyl]amino]-3-oxopropyl]-5-methylpyrimidine-4-carbonyl]amino]-3-[3-[4-carbamoyloxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(1H-imidazol-5-yl)propanoyl]amino]-3-hydroxy-2-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]ethyl]-1,3-thiazol-4-yl]-1,3-thiazole-4-carbonyl]amino]propyl-dimethylsulfanium | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C55H83N17O21S3/c1-20-33(69-46(72-44(20)58)25(12-31(57)76)64-13-24(56)45(59)82)50(86)71-35(41(26-14-61-19-65-26)91-54-43(39(80)37(78)29(15-73)90-54)92-53-40(81)42(93-55(60)88)38(79)30(16-74)89-53)51(87)66-22(3)36(77)21(2)47(83)70-34(23(4)75)49(85)63-10-8-32-67-28(18-94-32)52-68-27(17-95-52)48(84)62-9-7-11-96(5)6/h14,17-19,21-25,29-30,34-43,53-54,64,73-75,77-81H,7-13,15-16,56H2,1-6H3,(H13-,57,58,59,60,61,62,63,65,66,69,70,71,72,76,82,83,84,85,86,87,88)/p+1/t21-,22+,23+,24-,25-,29?,30?,34-,35-,36-,37?,38?,39?,40?,41-,42?,43?,53?,54?/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OYVAGSVQBOHSSS-WXFSZRTFSA-O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(N=C(N=C1N)C(CC(=O)N)NCC(C(=O)N)N)C(=O)NC(C(C2=CN=CN2)OC3C(C(C(C(O3)CO)O)O)OC4C(C(C(C(O4)CO)O)OC(=O)N)O)C(=O)NC(C)C(C(C)C(=O)NC(C(C)O)C(=O)NCCC5=NC(=CS5)C6=NC(=CS6)C(=O)NCCC[S+](C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=C(N=C(N=C1N)[C@H](CC(=O)N)NC[C@@H](C(=O)N)N)C(=O)N[C@@H]([C@H](C2=CN=CN2)OC3C(C(C(C(O3)CO)O)O)OC4C(C(C(C(O4)CO)O)OC(=O)N)O)C(=O)N[C@H](C)[C@H]([C@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCCC5=NC(=CS5)C6=NC(=CS6)C(=O)NCCC[S+](C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C55H84N17O21S3+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
9041-93-4 (sulfate (salt)) | |
| Record name | Bleomycin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0011056067 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
Molecular Weight |
1415.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Bleomycin appears as colorless or yellowish powder. Possible bluish color depending on copper content. (NTP, 1992), Solid | |
| Record name | BLEOMYCIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/19884 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Bleomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014435 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Soluble (NTP, 1992), Soluble, Colorless or yellowish powder which becomes bluish depending on copper content. Very sol in water, methanol; practically insol in acetone, ethyl acetate, butyl acetate, ether; slightly sol in ethanol. /Bleomycins/, HIGHLY SOL IN WATER & METHANOL; SPARINGLY SOL IN ALC; INSOL IN ACETONE & ETHYL ACETATE; CREAM-COLORED POWDER OR SOFT, FLUFFY LUMPS. /SULFATE SALT/, Freely soluble in water., Sol in water and methanol but insol in acetone and ether., 2.82e-02 g/L | |
| Record name | BLEOMYCIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/19884 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Bleomycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00290 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | BLEOMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3208 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Bleomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014435 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Colorless to yellow powder | |
CAS No. |
11056-06-7 | |
| Record name | BLEOMYCIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/19884 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Bleomycin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0011056067 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bleomycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00290 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | BLEOMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3208 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Bleomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014435 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
71 °C | |
| Record name | Bleomycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00290 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bleomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014435 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Preparation Methods
Glycosylation and Disaccharide Assembly
The carbohydrate moiety, critical for DNA interaction, was constructed via stereoselective glycosylation. 1,6-Di-O-acetyl-3,4-di-O-benzyl-2-O-[2,4,6-tri-O-acetyl-3-O-(N-acetylcarbamoyl)-α-D-mannopyranosyl]-β-L-gulopyranose (3 ) was converted to its glycosyl chloride (4 ), which underwent coupling with N<sup>α</sup>,N<sup>im</sup>-bis(t-Boc)-(S)-erythro-β-hydroxyhistidine (7 ) to yield α-O-glycosidated product 16 . Trichloroacetimidate activation improved coupling yields to >80%, addressing historical challenges in glycosidic bond formation.
Sequential Coupling of Modular Units
The aglycon core was assembled through stepwise coupling:
-
Valerate attachment : Benzyl valerate (8 ) was coupled to 16 under Mitsunobu conditions.
-
Bithiazole incorporation : Threonylbithiazole (9 ) introduced the DNA-intercalating domain.
-
Pyrimidoblamic acid addition : N<sup>α</sup>-t-Boc-pyrimidoblamic acid (10 ) completed the metal-binding region.
Final methylation of this compound demethyl A₂ (2 ) yielded this compound A₂, demonstrating identical DNA cleavage selectivity and potency to natural isolates in plasmid relaxation assays.
Table 1: Key Intermediates in this compound A₂ Synthesis
| Intermediate | Role | Yield (%) | Reference |
|---|---|---|---|
| 3 | Carbohydrate precursor | 92 | |
| 4 | Glycosyl chloride activator | 95 | |
| 16 | Glycosylated histidine derivative | 78 | |
| 2 | Demethylated precursor | 64 |
Microbial Fermentation and Strain Optimization
Industrial-scale this compound production relies on optimized fermentation of Streptomyces verticillus. The CN104004804A patent details a high-yield process using engineered Streptomyces sp. SB9026.
Strain Development
SB9026, deposited as CCTCC M 2011292, was derived through UV mutagenesis and selection for 6′-dehydroxy-BLM S overproduction. Comparative genomics revealed upregulation of blm cluster transporters and resistance genes.
Fermentation Media Optimization
Response surface methodology (RSM) identified critical media components:
Table 2: Optimized Fermentation Media Composition
| Component | Seed Medium (g/L) | Production Medium (g/L) |
|---|---|---|
| Glucose | - | 20 |
| Zulkovsky starch | - | 15 |
| Soybean meal | 15 | 20 |
| Yeast extract | 5 | 5 |
| CuSO₄·5H₂O | - | 0.1 |
| ZnSO₄·7H₂O | - | 0.5 |
Copper and zinc supplementation increased titers 3-fold by stabilizing metalloenzymes in the blm pathway.
Bioreactor Scale-Up
A 15-L fed-batch process achieved 30 mg/L 6′-dehydroxy-BLM S:
-
Temperature gradient : 32°C → 30°C (24 h) → 28°C (60 h)
-
Dissolved oxygen : Maintained at 40–60% via cascade agitation (210–400 rpm)
-
Glucose feeding : Automated to keep residual sugars at 1 g/L, preventing catabolite repression.
Biosynthetic Pathway Engineering
The 54-kb blm gene cluster (GenBank AF527415) encodes a hybrid nonribosomal peptide synthetase (NRPS)-polyketide synthase (PKS) system.
Modular Organization of Blm Megasynthetase
Critical Post-Translational Modifications
Table 3: Key Enzymes in this compound Biosynthesis
| Enzyme | Function | Domain Architecture |
|---|---|---|
| BlmI | Initiates peptide chain | A-PCP-C |
| BlmVII | Hybrid PKS/NRPS interface | KS-AT-ACP-C-A-PCP |
| BlmX | Carrier protein activation | PPTase |
| BlmIV | Bithiazole cyclization | P450 monooxygenase |
Comparative Analysis of Production Methods
Table 4: Synthesis vs. Fermentation Performance
| Parameter | Chemical Synthesis | Microbial Fermentation |
|---|---|---|
| Purity | >98% | 85–90% |
| Yield | 64 mg (0.02%) | 30 mg/L |
| Cycle Time | 6–8 months | 10 days |
| Scalability | Milligram scale | Multi-kilogram scale |
| Cost per gram | $12,000 | $450 |
Chemical synthesis enables analog development but remains impractical for bulk production. Fermentation balances yield and cost but requires extensive downstream processing .
Chemical Reactions Analysis
DNA Cleavage via Metal-Oxygen Complex Formation
Bleomycin forms an activated low-spin ferric-hydroperoxide complex (Fe<sup>III</sup>-OOH, ABLM) in the presence of Fe<sup>II</sup> and O<sub>2</sub>. This intermediate abstracts hydrogen atoms from the C4′ position of deoxyribose in DNA, leading to single- and double-strand breaks . Key steps include:
-
Activation : Fe<sup>II</sup>-BLM + O<sub>2</sub> → Fe<sup>III</sup>-OOH (ABLM) .
-
H Abstraction : ABLM extracts H from DNA’s C4′, generating a radical intermediate and Fe<sup>IV</sup>=O .
-
Strand Scission : The radical reacts with O<sub>2</sub>, forming a peroxyl radical that fragments DNA .
Kinetic and Thermodynamic Parameters of ABLM Reactions
Direct monitoring of ABLM decay and DNA interaction via circular dichroism revealed distinct kinetic profiles:
| Reaction Parameter | ABLM + DNA | ABLM Decay (No DNA) |
|---|---|---|
| Rate (s<sup>−1</sup>) | 0.044 ± 0.02 | 0.018 ± 0.003 |
| k<sub>H</sub>/k<sub>D</sub> | 1.7 ± 0.2 | 3.6 ± 0.9 |
| Activation Energy (kcal/mol) | 4.7 ± 0.9 | 9.3 ± 0.9 |
Data from .
The lower activation energy for DNA-linked reactions (4.7 vs. 9.3 kcal/mol) indicates preferential C–H bond cleavage over N–H abstraction in BLM’s degradation .
Comparative Reactivity with Enzymatic Systems
This compound’s catalytic profile aligns more closely with chloroperoxidase than cytochrome P-450 :
| Reaction Type | This compound | Chloroperoxidase | Cytochrome P-450 |
|---|---|---|---|
| Peroxidation (o-dianisidine) | Yes | Yes | No |
| N-Demethylation | Yes* | Yes | Yes |
| Aliphatic Hydroxylation | No | No | Yes |
| O<sub>2</sub> Evolution | Yes | Yes | No |
*Requires peroxides/iodosobenzene .
this compound catalyzes O<sub>2</sub> evolution from peroxyacids and chlorination via H<sub>2</sub>O<sub>2</sub>, mirroring chloroperoxidase .
Double-Strand DNA Damage Mechanism
This compound induces double-strand breaks (DSBs) at specific sites with high efficiency:
-
Target Sites : 5'-GC-3' and 5'-GT-3' sequences, with C4′ H atoms oriented toward the minor groove .
-
Efficiency : 43% of lesions at cytidine31 in hairpin DNA result in DSBs .
-
Structural Basis : Fe<sup>II</sup>-BLM binds DNA such that the metal center accesses opposing strand H atoms simultaneously .
Oxidative Reactions Beyond DNA Cleavage
This compound participates in diverse redox reactions:
-
N-Demethylation : Oxidizes N,N-dimethylaniline using H<sub>2</sub>O<sub>2</sub> or peroxyacids .
-
Mitochondrial Damage : Disrupts phosphatidylethanolamine synthesis, increasing ROS in fungi .
Role of Metal Ions in Reactivity
-
Iron Dependency : Fe<sup>II</sup> is essential for O<sub>2</sub> activation and ABLM formation .
-
Copper Binding : BLM-Cu complexes are inert but may stabilize the drug prior to Fe substitution .
This compound’s chemical reactivity hinges on its ability to harness Fe and O<sub>2</sub> for targeted DNA damage, with kinetic and structural studies elucidating its preference for H-atom abstraction over alternative mechanisms. Its dual capacity for single- and double-strand cleavage, coupled with oxidative versatility, underpins both therapeutic efficacy and toxicity .
Scientific Research Applications
Clinical Applications
Bleomycin has been widely utilized in the treatment of several malignancies. Its primary indications include:
- Hodgkin's Lymphoma: Often used in combination chemotherapy regimens such as ABVD (Adriamycin, this compound, Vinblastine, Dacarbazine).
- Testicular Cancer: Effective as part of combination therapy for germ cell tumors.
- Squamous Cell Carcinoma: Approved for use in head and neck cancers.
- Malignant Lymphoma: Utilized in treating both Hodgkin's and non-Hodgkin's lymphoma.
- Pleurodesis: Acts as a sclerosing agent to manage malignant pleural effusions by inducing adhesion of the lung to the chest wall .
Research Applications
In addition to its clinical uses, this compound serves as a critical tool in research, particularly in modeling pulmonary fibrosis:
Pulmonary Fibrosis Model
The administration of this compound to rodents is a standard model for studying idiopathic pulmonary fibrosis (IPF). This model helps researchers understand the disease's pathophysiology and evaluate potential antifibrotic therapies. Key findings from recent studies include:
- Interventional Timing: Studies indicate that timing interventions relative to this compound administration significantly affects outcomes. Early interventions (within 7 days) tend to focus on preventing fibrosis, while later interventions assess therapeutic efficacy .
- Study Characteristics: Between 2008 and 2019, approximately 74.4% of studies using this model investigated interventions aimed at fibrogenesis. A shift towards more therapeutic studies has been observed, reflecting a growing understanding of IPF and its treatment strategies .
Case Studies
Several notable case studies highlight the effectiveness and challenges associated with this compound:
- Case Study: Hodgkin's Lymphoma
- Case Study: Testicular Cancer
- Research Study: Pulmonary Fibrosis
Summary Table of this compound Applications
| Application Area | Specific Uses | Notes |
|---|---|---|
| Clinical Oncology | Hodgkin's lymphoma, testicular cancer | Often combined with other agents for effectiveness |
| Sclerotherapy | Malignant pleural effusions | Induces lung adhesion to prevent fluid accumulation |
| Research Models | Pulmonary fibrosis studies | Helps evaluate antifibrotic therapies |
| Mechanistic Studies | DNA damage response investigations | Provides insights into cancer cell biology |
Mechanism of Action
The primary mechanism of action of bleomycin involves its ability to bind to DNA and induce strand breaks . This compound forms a complex with metal ions, such as iron, creating a metallothis compound complex . This complex generates ROS, which cause oxidative damage to DNA, leading to single- and double-strand breaks . The DNA damage triggers cell cycle arrest and apoptosis, effectively killing cancer cells .
Comparison with Similar Compounds
Pingyangmycin (Bleomycin A5)
- Structural Differences : Pingyangmycin, a component of the this compound complex, shares the core bleomycinic acid structure but differs in the terminal amine moiety .
- Efficacy: Demonstrates high activity against human colon, stomach, and nasopharyngeal cancer xenografts in nude mice .
- Toxicity : Lower pulmonary toxicity in murine models compared to standard this compound, enhancing its therapeutic index .
- Clinical Use : Widely used in China since 1979 for head and neck cancers .
Boanmycin (this compound A6, BAM)
Table 1: Structural and Functional Comparison of this compound Derivatives
Comparison with Functionally Similar Compounds
Rapamycin
- Structural Differences: A macrolide immunosuppressant (vs. glycopeptide for this compound) .
- Clinical Use: Primarily used in immunosuppression and cancer therapy, unlike this compound’s direct DNA cleavage .
Latrunculin and Papuamide B
- Functional Similarity : Both marine sponge-derived compounds mimic this compound’s yeast deletion profile, suggesting overlapping cellular targets .
- Structural Divergence : Latrunculin (actin inhibitor) and Papuamide B (antiviral) differ chemically from this compound .
Table 2: Functional Comparison with Non-Structural Analogs
Key Research Findings
- Formulation Variability : Commercial this compound formulations exhibit compositional differences (e.g., A2/B2 ratios), impacting biological activity and regulatory compliance . For instance, one formulation exceeded pharmacopeial limits for this compound B2 (29.0% vs. 25% max), affecting efficacy .
- DNA Repair Assessment: this compound’s radiomimetic properties are utilized to measure DNA repair capacity in cancer patients. Tumor patients showed 79.4% repair efficiency vs. 92.4% in controls, highlighting its diagnostic utility .
- Clinical Efficacy in Vascular Tumors : Intralesional this compound achieved 85% cure rates in pediatric facial hemangiomas <2 cm², with minimal adverse effects .
Biological Activity
Bleomycin is a glycopeptide antibiotic derived from Streptomyces verticillus, primarily known for its use in cancer chemotherapy. Its biological activity encompasses a range of mechanisms that affect cellular processes, particularly in cancerous cells, but also extends to other biological systems, including antifungal activity and effects on telomerase activity.
This compound exerts its biological effects through several mechanisms:
- DNA Damage : this compound induces DNA strand breaks through oxidative stress, leading to the formation of free radicals. This action is particularly potent during the G2 phase of the cell cycle, inhibiting cell division and contributing to its antitumor effects .
- Telomerase Activity Modulation : Research indicates that this compound can modify telomerase activity in lung epithelial cells. Initially, this compound treatment results in an increase in telomerase activity, which may protect against apoptosis. However, prolonged exposure leads to a significant reduction in telomerase activity, correlating with increased apoptosis and potential lung fibrosis .
- Antifungal Properties : Recent studies have identified this compound as having significant antifungal activity. It disrupts mitochondrial function in fungal cells, leading to increased reactive oxygen species (ROS) generation and impaired phospholipid biosynthesis, essential for cell viability .
Clinical Applications
This compound is utilized not only in oncology but also in treating various benign conditions:
- Cancer Treatment : It is commonly used in combination chemotherapy regimens for testicular cancer and Hodgkin's lymphoma. The BEP regimen (this compound, Etoposide, and Cisplatin) is particularly effective for germ cell tumors .
- Treatment of Lymphangiomas : A study demonstrated that this compound injections resulted in an excellent response in 55% of pediatric patients with lymphangiomas, showcasing its effectiveness beyond malignancies .
- Wart Treatment : Intralesional this compound has been evaluated for treating common warts, showing a complete cure rate of approximately 84% after treatment sessions .
Case Study 1: this compound-Induced Lung Toxicity
A patient diagnosed with extragonadal non-seminomatous germ cell tumor underwent four cycles of BEP chemotherapy. Post-treatment imaging revealed pulmonary involvement attributed to this compound toxicity, highlighting the need for careful monitoring of pulmonary function during therapy .
Case Study 2: Efficacy in Lymphangiomas
In a cohort of 20 children treated with this compound for lymphangiomas, 55% exhibited an excellent response while 95% showed good response rates. This study underscores the efficacy of this compound in non-cancerous conditions and supports its continued use in clinical practice .
Telomerase Activity
A detailed investigation into the effects of this compound on telomerase activity revealed:
- In Vitro Studies : A significant elevation in mTERT mRNA and a transient increase in telomerase activity were observed within 24 hours post-treatment. However, by 72 hours, telomerase activity decreased significantly below baseline levels .
| Time Point | Telomerase Activity Change (%) |
|---|---|
| 24 hours | +41% |
| 48 hours | +12% |
| 72 hours | -26% |
Antifungal Mechanism
The antifungal mechanism of this compound was elucidated through studies showing its impact on mitochondrial integrity and phospholipid synthesis in yeast models. The compound's ability to induce oxidative stress was linked to its antifungal efficacy .
Q & A
Q. How can CRISPR/Cas9 models elucidate genetic modifiers of this compound resistance in tumors?
- Methodological Answer :
- Genome-wide screens : Perform pooled CRISPR knockout screens in this compound-treated cancer cells; prioritize hits with MAGeCK or BAGEL algorithms .
- Validation : Knock out candidate genes (e.g., RAD51, BRCA1) and measure IC₅₀ shifts and γ-H2AX foci (DNA damage markers) .
- Clinical correlation : Cross-reference hits with TCGA data to assess prognostic significance .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


