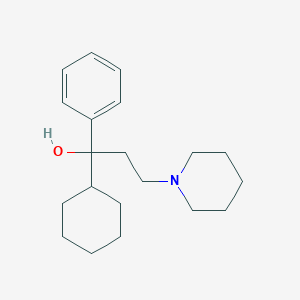
Trihexyphenidyl
Description
Historical Context of Trihexyphenidyl Discovery and Initial Clinical Applications
The emergence of this compound is rooted in mid-20th-century pharmacological research aimed at identifying new therapeutic agents for involuntary muscle movements.
The discovery of this compound was first published in 1949 following a study focused on identifying compounds with antispasmodic activity. drugbank.comdrugbank.comtasmedlab.comnih.govallstrongservices.ca As an antispasmodic agent, it was developed to treat stiffness, tremors, spasms, and poor muscle control. wikipedia.org Its primary mechanism involves acting as a non-selective antagonist of muscarinic acetylcholine receptors, which blocks efferent impulses in parasympathetically innervated structures like smooth muscles. wikipedia.org This anticholinergic action helps to alleviate the motor symptoms associated with Parkinsonism and other movement disorders. patsnap.com
This compound was granted its initial FDA approval on May 13, 1949. drugbank.comtasmedlab.comnih.govfda.gov This approval marked its entry into clinical practice as an adjunct treatment for all forms of parkinsonism. drugbank.comfda.gov Decades later, on June 25, 2003, it was specifically approved for managing idiopathic, postencephalitic, and arteriosclerotic Parkinsonism. wikipedia.orgnih.gov
Over time, the therapeutic role of this compound has been significantly re-evaluated. It is now rarely used as a first-line treatment for Parkinson's disease, having been largely superseded by more effective medications such as Levodopa. drugbank.comdrugbank.comtasmedlab.com It is, however, still used as an adjunctive therapy in Parkinson's disease and to control extrapyramidal symptoms caused by certain central nervous system drugs, such as antipsychotics. drugbank.comnih.gov
| Milestone | Date | Significance |
| Discovery Published | 1949 | First report on the antispasmodic activity of this compound. drugbank.comnih.gov |
| Initial FDA Approval | May 13, 1949 | Granted approval as an adjunct treatment for parkinsonism. drugbank.comtasmedlab.comfda.gov |
| Expanded FDA Approval | June 25, 2003 | Approved for idiopathic, postencephalitic, and arteriosclerotic Parkinsonism. wikipedia.orgnih.gov |
Early Research on Antispasmodic Activity
Current Academic Significance and Research Gaps
Despite its long history, this compound continues to be a subject of academic research, with several identified gaps in the current body of knowledge. One significant area of research is its use in pediatric populations, particularly for treating dystonia. mdpi.comresearchgate.net Studies are focused on developing new formulations, such as orodispersible minitablets, to address the therapeutic needs of children, for whom suitable formulations are often lacking. mdpi.com
Another critical research area involves the long-term effects of this compound, especially on cognitive function in older adults. nih.gov Clinical studies have suggested a potential association between long-term use of anticholinergic drugs and the pathogenesis of neurodegenerative disorders like Alzheimer's disease, but the exact mechanisms remain unclear. nih.gov
Furthermore, there is a recognized gap in understanding the effects of this compound withdrawal. nih.gov Recent studies are exploring the complex interplay between withdrawal from the drug, the gut microbiome, and oxidative stress, highlighting a modern systems-biology approach to understanding its effects. nih.gov The complete mechanism of action of this compound is still not fully elucidated, presenting a fundamental area for ongoing pharmacological investigation. drugbank.com Lastly, emerging research is exploring the novel therapeutic potential of this compound in other areas, such as oncology, specifically for its potential to inhibit the growth of certain types of glioblastoma. frontiersin.org
| Research Area | Identified Gap |
| Pediatric Therapeutics | Lack of suitable formulations for children with dystonia. mdpi.com |
| Neurodegeneration | Unclear long-term cognitive impact and association with Alzheimer's disease. nih.gov |
| Pharmacology | Incomplete understanding of withdrawal effects and interplay with the gut microbiome. nih.gov |
| Mechanism of Action | The precise molecular mechanism in parkinsonian syndromes is not fully understood. drugbank.com |
| Novel Applications | Exploring potential efficacy in new therapeutic areas, such as glioma treatment. frontiersin.org |
Scope and Objectives of the Research Outline
The scope of this article is to present a concise, academic overview of the chemical compound this compound. It focuses exclusively on its historical development, regulatory journey, and current areas of scientific research. The primary objective is to deliver a scientifically accurate and authoritative summary based on credible research findings, while strictly excluding clinical information related to dosage, administration, and safety profiles. This outline serves to structure the available academic knowledge on this compound, highlighting both what is known and the research gaps that remain.
Properties
IUPAC Name |
1-cyclohexyl-1-phenyl-3-piperidin-1-ylpropan-1-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H31NO/c22-20(18-10-4-1-5-11-18,19-12-6-2-7-13-19)14-17-21-15-8-3-9-16-21/h1,4-5,10-11,19,22H,2-3,6-9,12-17H2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HWHLPVGTWGOCJO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CCC(CC1)C(CCN2CCCCC2)(C3=CC=CC=C3)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H31NO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
52-49-3 (hydrochloride) | |
| Record name | Trihexyphenidyl [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000144116 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4023705 | |
| Record name | Trihexyphenidyl | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4023705 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
301.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Trihexyphenidyl | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014520 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Crystals; decomp at 258.5 °C; pH of 1% aq soln 5.5-6.0; solubility (g/100 mL): water at 25 °C 1.0, alcohol 6, chloroform 5; more soluble in methanol; very slightly soluble in ether, benzene /Hydrochloride/, 3.14e-03 g/L | |
| Record name | TRIHEXYPHENIDYL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3196 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Trihexyphenidyl | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014520 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Impurities |
1-phenyl-3-(piperidine-1-yl)propan-1-one | |
| Record name | TRIHEXYPHENIDYL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3196 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
144-11-6 | |
| Record name | (±)-Trihexyphenidyl | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=144-11-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Trihexyphenidyl [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000144116 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Trihexyphenidyl | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00376 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | trihexyphenidyl | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=12268 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Trihexyphenidyl | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4023705 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Trihexyphenidyl | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.005.105 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRIHEXYPHENIDYL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/6RC5V8B7PO | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | TRIHEXYPHENIDYL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3196 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Trihexyphenidyl | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014520 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
114 °C, 258.5 °C | |
| Record name | Trihexyphenidyl | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00376 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | TRIHEXYPHENIDYL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3196 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Trihexyphenidyl | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014520 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Neuropharmacological Mechanisms of Trihexyphenidyl
Acetylcholinergic System Modulation
Trihexyphenidyl's principal effects are mediated through its interaction with the acetylcholinergic system, where it functions as an antagonist at muscarinic acetylcholine receptors. patsnap.compatsnap.com
Non-selective Muscarinic Acetylcholine Receptor Antagonism
This compound acts as a non-selective antagonist of all five subtypes of muscarinic acetylcholine receptors (M1-M5). drugbank.comnih.gov This broad antagonism means that it blocks the action of the neurotransmitter acetylcholine at these receptors throughout the central and peripheral nervous systems. wikipedia.orgpatsnap.com The binding affinities of this compound to these receptor subtypes vary, with research indicating low nanomolar affinity across all five. nih.gov
Selective Antagonism at M1 and M4 Muscarinic Receptors
Despite its non-selective nature, this compound demonstrates a higher antagonistic activity at the M1 and M4 muscarinic acetylcholine receptors. wikipedia.orgdrugbank.com This has led to its description as being selective for these particular subtypes. wikipedia.orgmedchemexpress.com The affinity for M1 receptors, which are abundant in the cerebral cortex and striatum, is particularly high. patsnap.comdrugbank.comnih.gov Studies have shown that the (R)-enantiomer of this compound displays high affinities for both m1 and m4 receptors. researchgate.netnih.gov This selectivity for M1 and M4 receptors is considered a key aspect of its therapeutic efficacy in movement disorders. patsnap.com
Table 1: Binding Affinities of this compound for Muscarinic Receptor Subtypes
| Receptor Subtype | Binding Affinity (nM) |
|---|---|
| M1 | 1.6 |
| M2 | 7 |
| M3 | 6.4 |
| M4 | 2.6 |
| M5 | 15.9 |
Data sourced from a study by Dorje et al. (1991) and Bolden et al. (1992) as cited in a 2019 study. nih.gov
Inhibition of Efferent Impulses in Parasympathetically Innervated Structures
This compound exerts a direct inhibitory effect on the parasympathetic nervous system. medlink.comnih.govmedicines.org.ukpediatriconcall.com This action blocks the efferent impulses in structures that are innervated by parasympathetic nerves. wikipedia.org The result is a relaxation of smooth muscle tissue, which is achieved both through a direct action on the muscle itself and indirectly via the inhibition of the parasympathetic nervous system. medlink.comnih.govpediatriconcall.com
Dopaminergic System Interactions
Beyond its primary role as a muscarinic antagonist, this compound also interacts with the dopaminergic system, which is crucial for motor control.
Indirect Enhancement of Dopamine Release in the Striatum via Nicotinic Acetylcholine Receptor Modification
Research suggests that this compound can indirectly enhance the release of dopamine in the striatum. drugbank.com This effect is thought to be mediated through the modification of nicotinic acetylcholine receptor neurotransmission. drugbank.comnih.gov Studies in mouse models have shown that this compound increases striatal dopamine release and that this action is dependent on functional nicotinic receptors. nih.govnih.gov This indirect dopaminergic activity may contribute to its therapeutic effects in conditions characterized by dopamine deficiency, such as Parkinson's disease. patsnap.com
Potential Binding to Dopamine Receptors
There is also evidence to suggest that this compound may directly bind to dopamine receptors. wikipedia.orgmdpi.comresearchgate.netijiapp.com At higher concentrations, its affinity for dopaminergic receptors is thought to be responsible for some of its neuropsychiatric effects. mdpi.comresearchgate.net This potential interaction with dopamine receptors adds another layer of complexity to its neuropharmacological profile. ijiapp.com
Table 2: Summary of this compound's Neuropharmacological Actions
| System | Receptor/Mechanism | Effect |
|---|---|---|
| Acetylcholinergic | Non-selective Muscarinic Receptor Antagonist | Blocks acetylcholine at M1-M5 receptors |
| Selective M1/M4 Antagonist | Higher affinity for M1 and M4 subtypes | |
| Parasympathetic Inhibition | Blocks efferent impulses, relaxes smooth muscle | |
| Dopaminergic | Nicotinic Receptor Modification | Indirectly enhances dopamine release in the striatum |
| Dopamine Receptor Binding | Potential direct binding to dopamine receptors |
Psychostimulant-like Effects and Dopamine Signaling
Recent studies suggest that this compound exhibits psychostimulant-like properties that may be mediated through its influence on dopamine signaling. oaji.netresearchgate.net Research in animal models has demonstrated that this compound can increase locomotor activity, a behavioral marker often associated with psychostimulants that enhance dopamine transmission. oaji.net This effect was dose-dependent and could be prevented by pretreatment with olanzapine, an antagonist of dopamine receptors, further implicating the dopaminergic system in this compound's stimulant-like actions. oaji.netresearchgate.net
Furthermore, in the forced swim test, a model used to assess antidepressant-like effects, this compound was found to decrease immobility time in mice. oaji.netresearchgate.net This outcome is consistent with the actions of many antidepressant medications that enhance monoamine signaling, particularly dopamine. oaji.net While the primary therapeutic action of this compound is attributed to its anticholinergic properties, these findings suggest a potential indirect or direct modulation of dopamine neurotransmission. oaji.netresearchgate.net Some evidence points towards the possibility that by blocking inhibitory muscarinic M4 receptors on midbrain dopamine neurons, this compound could indirectly increase their activity and subsequent dopamine release. oaji.net Other studies propose that this compound may also modify nicotinic acetylcholine receptor neurotransmission, which in turn could lead to an indirect enhancement of dopamine release in the striatum. drugbank.comnih.govnih.gov
Glutamatergic and GABAergic System Influence
This compound demonstrates a notable influence on both the glutamatergic and GABAergic systems, the primary excitatory and inhibitory neurotransmitter systems in the brain, respectively.
Inhibition of Hippocampal Glutamatergic Synaptic Transmissions
Research has shown that this compound can inhibit action potential-dependent glutamatergic synaptic transmission between hippocampal neurons. nih.gov Specifically, it has been observed to suppress the frequency of excitatory postsynaptic currents (EPSCs) in primary hippocampal cultures. nih.govresearchgate.netnih.gov This inhibitory effect on glutamatergic transmission is significant as overstimulation of this system is a key factor in the sustenance of seizures. researchgate.net The concentration-response relationship for this suppression yielded an IC50 value of approximately 6.3 µM. nih.govresearchgate.netresearchgate.net Interestingly, this inhibition of synaptic transmission by this compound appears to occur through a mechanism that is independent of N-methyl-D-aspartate (NMDA) receptor, muscarinic acetylcholine receptor (mAChR), and α7 nicotinic acetylcholine receptor (nAChR) inhibition. nih.govresearchgate.netnih.gov
Inhibition of Hippocampal GABAergic Synaptic Transmissions
Concurrently with its effects on the glutamatergic system, this compound also inhibits action potential-dependent GABAergic synaptic transmissions in the hippocampus. nih.gov It reduces the frequency of inhibitory postsynaptic currents (IPSCs) recorded from cultured hippocampal neurons. nih.govresearchgate.netnih.gov The suppressive effect on both glutamatergic and GABAergic transmissions is likely mediated by a single mechanism of action, as the concentration-response relationships for both are overlapping and can be described by a single sigmoidal function. nih.gov The IC50 for the reduction in both EPSC and IPSC frequencies was found to be approximately 6.3 µM. nih.govresearchgate.netresearchgate.net Importantly, this compound did not affect the probability of transmitter release, as it had no effect on the frequency of miniature IPSCs and EPSCs, nor did it alter the activity of postsynaptic GABAA and glutamate receptors. researchgate.netnih.govresearchgate.net
Role in Organophosphorus-Induced Acetylcholinesterase Inhibition and Seizures
The inhibitory effects of this compound on glutamatergic and GABAergic systems are particularly relevant in the context of organophosphorus (OP)-induced neurotoxicity. Acute exposure to OP compounds leads to the inhibition of acetylcholinesterase (AChE), causing an accumulation of acetylcholine and subsequent cholinergic crisis, which includes severe and prolonged seizures. researchgate.netnih.govcdnsciencepub.com While these seizures are initiated by muscarinic receptor hyperstimulation, they are sustained by glutamatergic hyperexcitation. researchgate.net
Preclinical studies have indicated that this compound is more effective than atropine in counteracting the seizures and neuropathology triggered by OP-induced AChE inhibition. researchgate.netnih.gov This enhanced efficacy has been attributed to its ability to block both mAChRs and NMDA-type glutamatergic receptors. researchgate.netnih.gov By inhibiting the overstimulated glutamatergic synaptic transmission, this compound helps to control the seizures that are a devastating consequence of OP poisoning. researchgate.net Animal studies have suggested that antimuscarinics with additional antagonistic activity at NMDA receptors, such as this compound, are effective in blocking OP-induced seizure activity. researchgate.net
Central Inhibition of Cerebral Motor Centers
A key aspect of this compound's neuropharmacological profile is its ability to exert a direct central inhibition of cerebral motor centers. wikipedia.orgnih.govdrugs.com While its peripheral anticholinergic effects contribute to its therapeutic action, particularly in reducing muscle rigidity and spasms, its central actions are crucial. wikipedia.orgdrugs.com The precise mechanism is not fully understood, but it is thought to involve the blockade of efferent impulses in parasympathetically innervated structures. wikipedia.orgdrugs.com At higher doses, this direct central inhibition becomes more pronounced. wikipedia.orgnih.gov This central activity is believed to contribute to its effectiveness in managing the motor symptoms of Parkinson's disease. nih.gov
Receptor Binding Affinities and Selectivity Studies
This compound is a non-selective muscarinic acetylcholine receptor antagonist, meaning it binds to all five subtypes (M1, M2, M3, M4, and M5). drugbank.comnih.gov However, it displays a higher affinity for the M1 and M4 receptor subtypes. drugbank.comresearchgate.net Studies have demonstrated that this compound has a greater affinity for central muscarinic receptors in the cerebral cortex compared to those located peripherally. drugbank.com
Binding studies have revealed the stereoselectivity of this compound, with the (R)-enantiomer showing significantly higher affinity for muscarinic receptors than the (S)-enantiomer. researchgate.netualberta.ca For instance, the (R)-enantiomer of this compound has up to a 427-fold greater affinity for rat striatal muscarinic receptors and a 69 to 525-fold greater binding affinity for the five cloned human muscarinic receptor subtypes compared to its (S)-isomer. ualberta.ca
The selectivity profile of this compound has been compared to other muscarinic antagonists like atropine and pirenzepine. capes.gov.br While atropine shows little selectivity among muscarinic receptor subtypes, this compound, along with dicyclomine and pirenzepine, demonstrates a higher affinity for the neuronal M1 muscarinic receptor subtype. capes.gov.br
Table 1: Receptor Binding Affinities of this compound for Muscarinic Acetylcholine Receptor Subtypes This table is interactive. You can sort the data by clicking on the column headers.
| Receptor Subtype | Binding Affinity (nM) | Reference |
|---|---|---|
| M1 | 1.6 | nih.gov |
| M2 | 7 | nih.gov |
| M3 | 6.4 | nih.gov |
| M4 | 2.6 | nih.gov |
| M5 | 15.9 | nih.gov |
Pharmacokinetic and Pharmacodynamic Profiling in Research
Absorption and Distribution Dynamics
Trihexyphenidyl is readily absorbed from the gastrointestinal tract following oral administration. wikipedia.orgmedicines.org.ukdrugfuture.com The onset of its pharmacological action typically occurs within one hour of an oral dose, with peak effects observed between two to three hours. wikipedia.orgpdr.net The duration of action for a single dose is dose-dependent and generally lasts from 6 to 12 hours. wikipedia.orgpdr.net
Studies have reported varying peak plasma concentrations (Cmax) depending on the administered dose. For instance, single oral doses of 2 mg, 4 mg, and 15 mg of this compound HCl have resulted in Cmax values of approximately 10 ng/mL, 7.15 ± 2.58 ng/mL, and 50 ng/mL, respectively. ualberta.ca One study noted a Cmax of 7.2 ng/mL with a time to peak concentration (Tmax) of 1.3 hours. drugbank.com It is generally well-tolerated when taken with food. nih.gov
Table 1: Pharmacokinetic Parameters of this compound
| Parameter | Value | Reference |
|---|---|---|
| Onset of Action | ~1 hour | wikipedia.orgpdr.net |
| Peak Effects | 2-3 hours | wikipedia.orgpdr.net |
| Duration of Action | 6-12 hours | wikipedia.orgpdr.net |
This compound effectively crosses the blood-brain barrier, a crucial characteristic for its action on the central nervous system. pdr.netnih.govpatsnap.com Its ability to penetrate the brain is attributed to its lipophilic nature. cambridge.org Research indicates that high concentrations of the drug are achieved in the brain. nih.gov This high tissue uptake is facilitated by a process known as intralysosomal uptake. ualberta.canih.govcambridge.org This mechanism involves the accumulation of the drug within lysosomes, which are acidic organelles within cells. ualberta.cacambridge.org The ability of this compound to cross the blood-brain barrier allows it to exert its effects on central motor centers. wikipedia.org
Once in the bloodstream, this compound binds to plasma proteins. Specific data on the extent of this binding is not extensively detailed in all sources, but it is known to bind to albumin. drugbank.com One study, under controlled laboratory conditions using a dialysis bag, determined that this compound is 36.13% to 41.92% bound to albumin. drugbank.comnih.gov The binding of drugs to plasma proteins can influence their distribution and availability to target tissues.
The volume of distribution (Vd) for this compound is described as being relatively large. ualberta.cacambridge.org A large Vd suggests that the drug distributes extensively into tissues outside of the plasma. ualberta.ca This is consistent with its lipophilic properties and its ability to accumulate in tissues like the brain. ualberta.cacambridge.org However, specific quantitative values for the volume of distribution are not consistently available across the reviewed literature. drugbank.com
Plasma Protein Binding Characteristics
Biotransformation Pathways
The primary metabolic pathway for this compound involves the hydroxylation of its alicyclic groups. ualberta.canih.govmims.com This biotransformation process occurs in the liver. cambridge.org Studies investigating the metabolism of similar antiparkinsonian drugs have shown a tendency for hydroxylation to occur preferentially on the alicyclic ring system when present. nih.gov Research indicates that this compound is excreted in the urine, potentially as an unchanged drug, as well as in the bile. wikipedia.orgcambridge.orgmims.com More detailed information on the specific enzymes involved, such as cytochrome P450 isoenzymes, is an area of ongoing research. eventscribe.net One study suggests that CYP2D6, CYP2C19, and CYP3A4 are primarily involved in its metabolism. eventscribe.net
Table 2: Compound Names Mentioned
| Compound Name |
|---|
| This compound |
| This compound Hydrochloride |
| Albumin |
| Levodopa |
| Atropine |
| Scopolamine butyl bromide |
| Dopamine |
| Acetylcholine |
| Biperiden |
| Procyclidine |
Role of Enterohepatic Circulation
Enterohepatic circulation is a process where substances are metabolized in the liver, excreted into the bile, and subsequently reabsorbed from the small intestine back into the portal circulation. slideshare.net For this compound, research indicates that its involvement in enterohepatic circulation is minimal. nih.govsmolecule.com This suggests that once excreted into the bile, the compound is not significantly reabsorbed into the bloodstream for further circulation.
Elimination and Excretion Mechanisms
The elimination of this compound from the body occurs through metabolic processes and subsequent excretion. The primary route of excretion is through the urine. cambridge.orgwikipedia.orgdrugs.com Some sources also indicate that a smaller portion is eliminated through bile. cambridge.orgmedscape.commims.com
Metabolically, this compound undergoes hydroxylation of its alicyclic groups. nih.govmims.com This process transforms the compound into more water-soluble metabolites, specifically isomeric hydroxylated forms identified as 1-(hydroxycyclohexyl)-1-phenyl-3-piperidinopropan-1-ols, which can then be more readily excreted by the kidneys. nih.gov A significant portion of the drug is likely excreted unchanged in the urine. cambridge.orgwikipedia.orgdrugs.comnih.gov
Half-life Variability and Clinical Implications
The elimination half-life of a drug, which is the time it takes for the plasma concentration of the compound to reduce by half, is a critical pharmacokinetic parameter. For this compound, there is notable variability in its reported half-life. Some studies report a mean elimination half-life of approximately 3.2 to 4.1 hours. wikipedia.orgdrugbank.comdrugs.com Other sources suggest a wider range of 5 to 10 hours. nih.govsmolecule.com More extensive studies indicate a much longer elimination half-life of about 33 hours. nih.govcambridge.orgmedscape.commims.com
This variability has significant clinical implications in a research setting. A shorter half-life might necessitate more frequent administration to maintain steady-state concentrations, while a longer half-life allows for less frequent dosing. researchgate.net One study observed that in previously untreated patients, the serum concentration-time plot was biphasic, showing a rapid initial distribution phase followed by a slower elimination phase. nih.gov However, in patients on long-term therapy, only the slower elimination phase was apparent. nih.gov The elimination kinetics were found to be first-order, with a rapid half-life of 3.7 ± 0.4 hours, and interestingly, no correlation was found between the half-life and the patient's age, duration of therapy, or the specifics of their condition. nih.gov The lack of a clear relationship between serum levels and therapeutic response in dystonia suggests a complex pharmacodynamic effect that is not directly correlated with plasma concentrations. nih.gov
Interactive Data Table: Reported Half-life of this compound
| Reported Half-Life (Hours) | Source(s) |
|---|---|
| 3.2 ± 0.3 | drugbank.com |
| 3.3 - 4.1 | wikipedia.orgdrugs.com |
| 3.7 ± 0.4 | nih.gov |
| 5 - 10 | nih.gov |
| ~33 | nih.govcambridge.orgmedscape.commims.com |
Comparative Pharmacokinetics with Other Anticholinergic Agents
When comparing the pharmacokinetics of this compound to other anticholinergic agents used for similar indications, such as benztropine, biperiden, and orphenadrine, several differences and similarities emerge. cambridge.org As a class, these drugs are generally absorbed rapidly after oral administration. nih.gov
The oral bioavailability of these agents varies, ranging from 30% to over 70%. nih.gov They all tend to have a large volume of distribution, indicating rapid distribution into tissues. nih.gov Their clearance is relatively low compared to hepatic blood flow, and they are extensively metabolized, primarily through N-dealkylation and hydroxylation. nih.gov Excretion of the parent drug and its metabolites occurs via both urine and bile. nih.gov
In terms of receptor binding affinity, which can influence the required dose, this compound shows different potency compared to other anticholinergics. For instance, in rat brain muscarinic receptors, the IC50 values (a measure of inhibitory concentration) were found to be 0.026 µM for this compound, compared to 0.018 µM for benztropine and 0.37 µM for orphenadrine. ualberta.ca
Pharmacodynamic Response Trajectories
The pharmacodynamic response to this compound describes the time course of its effects on the body. Following oral administration, the onset of action is typically within one hour. nih.govwikipedia.orgmedscape.com The peak of its activity is observed approximately 2 to 3 hours after ingestion. nih.govwikipedia.org The duration of action for a single dose is dose-dependent and generally lasts between 6 and 12 hours. wikipedia.orgmedscape.com
Research has shown that while acute side effects tend to parallel the rise and fall of serum anticholinergic levels, the therapeutic response, for instance in dystonia, does not follow the same direct correlation. nih.gov In studies assessing motor symptom improvement in Parkinson's disease, effects began to appear at 30 minutes, with maximum improvement noted at 90 minutes. researchgate.net This suggests that the ultimate clinical effect may be related to more complex downstream neurochemical adaptations rather than simply the immediate presence of the drug in the plasma.
Interactive Data Table: Pharmacodynamic Timeline of this compound
| Pharmacodynamic Parameter | Time | Source(s) |
|---|---|---|
| Onset of Action | ~1 hour | nih.govwikipedia.orgmedscape.com |
| Peak Activity | 2 - 3 hours | nih.govwikipedia.org |
| Duration of Action | 6 - 12 hours | wikipedia.orgmedscape.com |
| Peak Motor Improvement | ~1.5 hours | researchgate.net |
Therapeutic Applications and Efficacy Research
Parkinsonism Management
Trihexyphenidyl is indicated for the symptomatic treatment of all forms of parkinsonism, including idiopathic, postencephalitic, and arteriosclerotic types. rxlist.commedscape.comnih.gov It has been in clinical use for Parkinson's disease since 1949 and received FDA approval for this indication in 2003. nih.govwikipedia.org The medication works by blocking the action of acetylcholine, a neurotransmitter, which helps to relax muscles and reduce symptoms like stiffness, tremors, and poor muscle control. medlineplus.gov
Idiopathic Parkinson's Disease
In the management of idiopathic Parkinson's disease, this compound can be used as an initial monotherapy, particularly in younger patients where tremor is the predominant symptom. drugs.com It is also frequently used as an adjunctive therapy to levodopa. rxlist.commedscape.comnih.gov The drug has been shown to provide a substantial alleviation of symptoms, although it does not cure the disease. wikipedia.org
Postencephalitic Parkinsonism
This compound is effective in treating postencephalitic parkinsonism. medscape.comcambridge.orgnih.gov Patients in this subgroup may sometimes require and tolerate higher daily doses of the medication to achieve optimal symptom control compared to those with other forms of parkinsonism. rxlist.comcambridge.org
Arteriosclerotic Parkinsonism
The use of this compound is also indicated for arteriosclerotic parkinsonism. medscape.comcambridge.orgnih.gov As with other forms of parkinsonism, it helps in managing the characteristic motor deficits.
Adjunctive Therapy with Levodopa and Dopamine Agonists
This compound is often used as an adjuvant therapy in combination with levodopa, the standard treatment for Parkinson's disease. rxlist.commedscape.comnih.gov This combination can be beneficial as it provides an alternative mechanism of action by suppressing cholinergic activity. researchgate.net When used with levodopa, the dosages of both drugs may need to be adjusted to optimize symptom control and manage side effects. cambridge.orgdrugs.com
Combination therapy may also include dopamine agonists, such as pramipexole and ropinirole, as part of a multi-faceted approach to treatment. medscape.comwikipedia.org Research in animal models has shown that this compound can have differential interactions with various dopamine receptor agonists. nih.gov For instance, one study observed that this compound potentiated the effects of a D1 agonist while reducing the effects of a D2 agonist. nih.gov
Symptomatic Improvement Rates and Predictors of Response
Clinical experience suggests that a significant percentage of patients with Parkinson's disease respond positively to this compound. It is estimated that 50-75% of individuals experience a 20-30% improvement in their symptoms. wikipedia.org The medication is particularly noted for its effect on tremor, though it also helps with rigidity and bradykinesia. cambridge.orgnih.gov
A study comparing the effectiveness of this compound and levodopa on motor symptoms in Parkinson's disease patients found that both drugs led to significant improvements. researchgate.net The most substantial improvement with this compound was seen in the tremor sub-score, which was comparable to the improvement seen with levodopa. researchgate.netresearchgate.net
Table 1: Comparison of Symptom Improvement with this compound vs. Levodopa
| Symptom Category (UPDRS-III Sub-score) | Mean Improvement with this compound (%) | Mean Improvement with Levodopa (%) |
|---|---|---|
| Total Motor Score | 27.0 ± 14.7 | 61.3 ± 14.4 |
| Tremor | 53.8 ± 22.8 | 67.1 ± 22.9 |
| Bradykinesia | 22.2 ± 27.2 | 67.9 ± 32.1 |
| Rigidity | 29.5 ± 28.0 | 65.3 ± 25.5 |
| Axial Symptoms | 8.1 ± 13.3 | 50.7 ± 16.0 |
Data sourced from a comparative study on motor symptom improvement. researchgate.net
Predictors for a better response to this compound for tremor include having a milder baseline tremor severity. researchgate.netresearchgate.net Conversely, factors that may predict a higher risk of cognitive side effects, suggesting a need for caution with anticholinergic medications like this compound, include advanced age (over 70), male sex, history of falls or freezing, bilateral disease onset, and the presence of mild cognitive impairment. nih.gov
Management of Drug-Induced Extrapyramidal Symptoms (EPS)
This compound is also indicated for the control of extrapyramidal symptoms (EPS) that are induced by certain central nervous system drugs. drugbank.comrxlist.com These drugs include antipsychotics like phenothiazines, thioxanthenes, and butyrophenones. rxlist.comnih.govmedicinenet.com EPS can manifest as parkinsonian-like symptoms, akathisia (restlessness), and dystonia. cpravikumar.comrad-ar.or.jp
This compound helps to restore the balance between acetylcholine and dopamine in the brain, which is disrupted by these medications. drugs.com However, it is important to note that this compound does not alleviate the symptoms of tardive dyskinesia and may even worsen them in some cases. rxlist.commedicinenet.com Therefore, its use is not recommended for patients with tardive dyskinesia unless they have a co-occurring Parkinson's disease. rxlist.com
Antipsychotic-Induced Parkinsonism and EPS
This compound is frequently utilized to manage extrapyramidal side effects (EPS) that arise from treatment with antipsychotic medications, particularly first-generation agents like haloperidol, fluphenazine, and chlorpromazine. nih.gov These drug-induced movement disorders manifest as symptoms resembling Parkinson's disease, including tremors, rigidity, and bradykinesia (slowness of movement). medicinenet.compatsnap.com The therapeutic action of this compound in this context is attributed to its ability to counteract the relative excess of acetylcholine that occurs due to the dopamine blockade caused by antipsychotics. patsnap.com
Research indicates that this compound can provide significant symptomatic relief for patients experiencing these side effects. wikipedia.org It is often used as an adjunctive therapy to manage muscle stiffness, spasms, and poor muscle control. nih.govpatsnap.com However, prophylactic use of this compound to prevent drug-induced parkinsonism during neuroleptic therapy is not recommended. nih.gov Studies have shown that while effective, this compound is not a first-line treatment for idiopathic Parkinson's disease, having been largely replaced by medications like levodopa. drugbank.com
In comparative studies, this compound's efficacy has been weighed against other agents. For instance, a double-blind, crossover study comparing it with amantadine for neuroleptic-induced parkinsonism found both drugs to be equivalently effective. dovepress.compsychiatryonline.org Another study noted little difference in efficacy between this compound and benztropine for this indication. mdpi.com
Specificity for Dystonia, Akathisia, and Oculogyric Crises
This compound has demonstrated utility in treating specific and often distressing extrapyramidal symptoms:
Dystonia: This condition involves sustained or intermittent involuntary muscle contractions causing twisting and repetitive movements or abnormal postures. cochranelibrary.com this compound is used to manage acute dystonic reactions, which can appear shortly after initiating antipsychotic treatment. psychopharmacologyinstitute.com It is believed to work by reducing acetylcholine's effect in the basal ganglia, a key area for motor control. cochranelibrary.com
Akathisia: Characterized by a subjective feeling of inner restlessness and a compelling urge to move, akathisia is a common side effect of antipsychotic drugs. arizona.edu While beta-blockers are often considered first-line treatment, anticholinergic agents like this compound may be considered, particularly if parkinsonian symptoms are also present. cambridge.orgnih.gov However, its use in older adults for akathisia is generally not recommended due to potential side effects. arizona.edu
Oculogyric Crises: These are a form of acute dystonia characterized by a sustained, involuntary upward deviation of the eyes. bioline.org.br Centrally acting anticholinergics, including this compound, are a mainstay in the management of these episodes. nih.gov However, in some cases of olanzapine-induced oculogyric crisis, this compound has been reported to be ineffective, necessitating a change in the antipsychotic medication. bioline.org.br
Other Neurological Indications
Beyond drug-induced movement disorders, research has explored the utility of this compound in a range of other neurological conditions.
Essential Tremor Research
While the primary focus of this compound has been on parkinsonism and dystonia, its anticholinergic properties suggest a potential role in managing other types of tremors. Essential tremor is a common movement disorder, but specific research on the efficacy of this compound for this condition is less extensive compared to its other indications. Anticholinergics, in general, may help reduce tremor, but their use is often limited by side effects. parkinsons.org.uk
Dystonia in Cerebral Palsy: Efficacy and Limitations
This compound is often used off-label to treat dystonia in individuals with cerebral palsy (CP), a condition characterized by motor problems and movement disorders. cochranelibrary.compediatriconcall.com The rationale is to reduce the involuntary muscle contractions and improve function. cochranelibrary.com
However, the evidence for its effectiveness in this population is mixed and requires careful consideration. A Cochrane review concluded that there is currently insufficient evidence to definitively say whether this compound is an effective treatment for dystonia in people with CP. cochranelibrary.comnih.gov The review found no clear evidence that it reduces dystonia or improves upper limb function. cochranelibrary.com Some studies suggest that any beneficial effects may take several months to become apparent and might not occur in all types of dystonia associated with CP. virginia.edu
A retrospective study of 101 children with CP treated with this compound for dystonia and/or sialorrhea found that the majority tolerated the medication well and showed improvements. nih.gov Conversely, another systematic review suggested that this compound results in little to no difference in dystonia and motor function in children with CP. frontierspartnerships.org
| Study Type | Key Findings | Limitations |
|---|---|---|
| Cochrane Review | Insufficient evidence to confirm efficacy for dystonia in CP. cochranelibrary.comnih.gov No significant improvement in dystonia or upper limb function noted. cochranelibrary.com | Based on a small number of studies, highlighting the need for larger, high-quality trials. cochranelibrary.comnih.gov |
| Retrospective Study (n=101) | Majority of children tolerated this compound well with reported improvements in dystonia and sialorrhea. nih.gov | Retrospective design, potential for bias. |
| Systematic Review | Suggests little to no difference in dystonia and motor function for children with CP. frontierspartnerships.org | Included various study designs, making direct comparisons difficult. |
Sialorrhea (Drooling) in Pediatric and Adult Populations
This compound's anticholinergic effects lead to a decrease in saliva production, making it a treatment option for sialorrhea (excessive drooling). parkinsons.org.ukparkinson.org This can be a significant issue for both children and adults with neurological conditions such as cerebral palsy and Parkinson's disease. pediatriconcall.comparkinson.org
In pediatric populations, particularly those with cerebral palsy, this compound has been used off-label to manage drooling. pediatriconcall.com A systematic review of anticholinergic treatments for sialorrhea in children found that this compound, among other agents, has shown efficacy. nih.gov One study reported improvement in drooling in 33% to 85% of children treated. nih.gov
For adults with Parkinson's disease, where drooling can be a troublesome symptom, anticholinergic medications like this compound can help by reducing saliva production. parkinsons.org.ukparkinson.org
Potential in Huntington's Chorea and Spasmodic Torticollis
Huntington's Chorea: Research into the use of this compound for Huntington's chorea is limited. While the core pathology of Huntington's involves different neurotransmitter systems than those primarily targeted by this compound, the complex nature of movement disorders sometimes leads to empirical trials of various agents.
Spasmodic Torticollis (Cervical Dystonia): This is a focal dystonia affecting the neck muscles. researchgate.net this compound has been shown to be an efficacious treatment for this condition. researchgate.net However, studies comparing it to botulinum toxin injections suggest that botulinum toxin provides more reliable symptomatic relief with fewer adverse effects. researchgate.net Anticholinergic drugs like this compound are often used orally in the management of spasmodic torticollis. cambridge.org
Experimental and Novel Therapeutic Areas
Beyond its established applications, this compound is the subject of ongoing research for novel therapeutic uses. These investigations explore its potential in treating conditions unrelated to its primary indications, leveraging its unique pharmacological properties. Experimental studies are examining its efficacy in acute medical emergencies and in the context of complex diseases like cancer.
Organophosphate Poisoning and Cholinergic Crisis
This compound has been investigated as a countermeasure for poisoning by organophosphorus (OP) compounds, which are found in pesticides and chemical nerve agents. researchgate.net These agents inhibit the enzyme acetylcholinesterase (AChE), leading to an excess of the neurotransmitter acetylcholine and resulting in a life-threatening cholinergic crisis. researchgate.net
Preclinical studies have indicated that this compound may be more effective than atropine, a standard antidote, in counteracting the severe effects of OP-induced acetylcholinesterase inhibition, such as cholinergic crisis and seizures. researchgate.netnih.govnih.gov The enhanced efficacy of this compound was initially thought to stem from its ability to cross the blood-brain barrier and block not only muscarinic acetylcholine receptors (mAChRs) but also N-methyl-D-aspartate (NMDA) receptors, which are involved in the seizure activity that follows OP exposure. nih.govnih.gov Seizures following OP exposure are initiated by muscarinic receptor overstimulation but are sustained by glutamatergic hyperexcitation, which is a primary cause of brain damage. researchgate.net
Further research has revealed a more complex mechanism. Studies have demonstrated that this compound can suppress synaptic transmission through a pathway independent of its action on mAChRs, NMDA receptors, and α7 nicotinic receptors (nAChRs). nih.govnih.gov This suggests that its ability to inhibit action potential-dependent synaptic transmission may be a key factor in its effectiveness against OP intoxication. nih.gov In addition to these actions, the anticonvulsant effects of this compound in the context of poisoning by the nerve agent soman may also be partially linked to its influence on the striatal dopaminergic system. nih.gov Case reports have documented the use of this compound in treating neurological complications, such as extrapyramidal syndrome, that can arise during the intermediate phase of recovery from organophosphate poisoning. mums.ac.irmums.ac.ir
Table 1: Mechanistic Profile of this compound in Organophosphate Poisoning
| Feature | This compound (THP) | Atropine |
|---|---|---|
| Primary Mechanism | Muscarinic Acetylcholine Receptor (mAChR) Antagonist researchgate.netnih.gov | Muscarinic Acetylcholine Receptor (mAChR) Antagonist researchgate.net |
| Blood-Brain Barrier | High permeability nih.govnih.gov | Crosses the blood-brain barrier |
| Additional Receptor Targets | N-methyl-D-aspartate (NMDA) Receptor Antagonist nih.govnih.govα7 Nicotinic Receptor (nAChR) Inhibitor nih.govnih.gov | Primarily targets mAChRs |
| Other Proposed Mechanisms | Inhibition of voltage-gated Na+ channels nih.govModulation of the striatal dopaminergic system nih.gov | Limited to mAChR antagonism |
| Reported Efficacy | More effective than atropine in countering OP-induced seizures and neuropathology in preclinical studies researchgate.netnih.govnih.gov | Standard treatment, but may be less effective against CNS effects nih.gov |
Influence on Glioblastoma Growth
Glioblastoma (GBM) is the most common and aggressive primary brain tumor, known for its rapid growth and resistance to therapy. nih.govresearchgate.netnih.gov Recent preclinical studies have explored the repurposing of existing drugs, including this compound, as a potential therapeutic strategy for this challenging cancer. nih.govfrontiersin.org A key advantage of this compound is its high permeability across the blood-brain barrier, allowing it to reach tumors within the brain. nih.govfrontiersin.org
Research has shown that this compound can target and inhibit the proliferation and survival of glioblastoma stem cells (GSCs), which are thought to drive tumor growth and recurrence. researchgate.netmdpi.comtandfonline.com In vitro studies have confirmed the therapeutic potential of this compound, demonstrating its ability to effectively suppress the growth, proliferation, and survival of mesenchymal-subtype glioblastoma (MES-GBM) cells, which are particularly aggressive and therapy-resistant. nih.govresearchgate.netnih.gov These effects were observed with limited impact on non-cancerous cells. nih.govresearchgate.netnih.gov
Table 2: Research Findings on this compound's Effect on Glioblastoma Cells
| Parameter | Finding | Cell Line | Source |
|---|---|---|---|
| IC50 | ~20 μM | GBM1 | tandfonline.com |
| Apoptosis | 19% increase compared to control after 48 hours | GBM1 | tandfonline.com |
| Cell Proliferation | 8% reduction after 48 hours | GBM1 | tandfonline.com |
| Cell Cycle | 7% increase in G0/G1 phase (indicating arrest) | GBM1 | tandfonline.com |
| Cell Viability | Significant, dose-dependent reduction in tumor stem cell viability | Multiple GBM models | nih.govfrontiersin.org |
| General Effect | Suppresses growth, proliferation, and survival of GBM stem cells | Classical and Mesenchymal Subtypes | nih.govresearchgate.netnih.gov |
Additive Anticholinergic Effects with Other Agents
The co-administration of this compound with other drugs possessing anticholinergic properties can lead to a potentiation of these effects, resulting in a range of adverse events. medicines.org.uk This synergistic action is a critical consideration in clinical practice, particularly in patient populations already susceptible to the side effects of anticholinergic medications. cambridge.org
Common classes of drugs that exhibit anticholinergic activity and may interact with this compound include:
Antihistamines: Many first-generation antihistamines, such as Diphenhydramine, possess significant anticholinergic properties. thekingsleyclinic.com When taken with this compound, there is an increased risk of side effects like drowsiness, confusion, dry mouth, and constipation. thekingsleyclinic.com
Tricyclic Antidepressants (TCAs): TCAs, such as Amitriptyline and Imipramine, are known for their anticholinergic effects. thekingsleyclinic.commedscape.com The concurrent use with this compound can intensify these effects, potentially leading to more severe complications. medicines.org.uk
Monoamine Oxidase Inhibitors (MAOIs): MAOIs can interact with anticholinergic agents like this compound, leading to classic anticholinergic symptoms such as dry mouth, blurred vision, and urinary retention. pediatriconcall.commedicines.org.uk
Spasmolytics: Other antispasmodic agents, which often have anticholinergic mechanisms, can have their effects amplified when used with this compound. wikipedia.org
The potentiation of anticholinergic effects can also occur with other medications, including certain phenothiazines, clozapine, disopyramide, nefopam, and amantadine. mims.commedicines.org.uk The resulting symptoms can range from mild discomfort to severe complications, including paralytic ileus, hyperthermia, and anticholinergic intoxication syndrome, which is characterized by central nervous system effects like confusion, hallucinations, and delirium. drugs.com
Interactions with Dopaminergic Medications
The interplay between this compound and dopaminergic medications, particularly Levodopa, is complex, involving both pharmacokinetic and pharmacodynamic interactions. These interactions can influence the therapeutic efficacy and side effect profile of both drugs.
Levodopa Absorption and Efficacy
This compound's anticholinergic action can delay gastric emptying. drugs.com This delay can lead to increased gastrointestinal degradation of Levodopa, thereby decreasing its absorption and reducing its peak serum concentrations. drugs.comnih.gov Studies have indicated that this interaction can diminish the therapeutic efficacy of Levodopa in some patients. nih.gov Research in both rats and humans has shown that the concurrent administration of this compound can decrease plasma levels of orally administered Levodopa. nih.gov One study reported a 16% to 20% reduction in Levodopa's peak serum concentrations when co-administered with this compound. drugs.com This reduction in absorption may necessitate adjustments in Levodopa dosage to maintain optimal therapeutic outcomes. medscape.comdrugs.com
Potentiation of Levodopa's Dopaminergic Effects
Despite the potential for reduced absorption, some clinical observations suggest that this compound can enhance the therapeutic effects of Levodopa in certain contexts. medscape.com This potentiation may be particularly beneficial for controlling tremor, a symptom that can be resistant to Levodopa monotherapy. cambridge.orgresearchgate.net However, the mechanisms underlying this potentiation are not fully understood and may involve complex interactions within the basal ganglia circuitry. A study in hemiparkinsonian monkeys demonstrated that the combination of this compound and a Levodopa ester initially produced a potentiated effect, although this effect diminished over time. nih.gov
Exacerbation of Dyskinesia with Levodopa
A significant concern with the concomitant use of this compound and Levodopa is the potential to increase drug-induced involuntary movements, or dyskinesias. drugs.comrxlist.com While Levodopa itself is a primary cause of dyskinesias in long-term Parkinson's disease treatment, the addition of an anticholinergic agent can exacerbate these movements. medscape.comtg.org.au This may require a reduction in the dosage of either or both drugs to manage these motor complications effectively. drugs.com
Interactions with Antipsychotics
The use of this compound in patients receiving antipsychotic medications presents a unique set of challenges, primarily related to the risk of tardive dyskinesia.
Synthesis and Structural-activity Relationship Sar Studies
Synthetic Methodologies
The construction of the trihexyphenidyl molecule can be accomplished through distinct synthetic strategies, primarily categorized as linear and convergent pathways. iiab.mewikipedia.orgscribd.com These methods are crucial for the efficient production of the compound.
Linear Synthesis
The linear synthesis of this compound is a sequential process that builds the molecule step-by-step. iiab.mewikipedia.org A common approach begins with the aminomethylation of acetophenone. iiab.mewikipedia.orgneu.edu.tr This initial step involves the reaction of acetophenone with paraformaldehyde and piperidine, which undergoes a Mannich reaction to form the intermediate, 2-(1-piperidino)propiophenone. iiab.mewikipedia.orggpatindia.com Subsequently, this intermediate is subjected to a Grignard reaction with cyclohexylmagnesium bromide to yield the final this compound molecule. iiab.mewikipedia.orgneu.edu.tr
Mannich Reaction and Grignard Reaction in Synthesis
The Mannich and Grignard reactions are cornerstone transformations in the synthesis of this compound. iiab.mewikipedia.org The Mannich reaction is a three-component condensation that forms a β-amino carbonyl compound, which is a key structural motif in many pharmaceuticals, including this compound. derpharmachemica.comipinnovative.comnih.govhumanjournals.com Specifically, it is used to introduce the piperidine-containing side chain onto the propiophenone backbone. neu.edu.trgpatindia.com
The subsequent Grignard reaction is a powerful carbon-carbon bond-forming reaction. In the synthesis of this compound, a Grignard reagent, cyclohexylmagnesium bromide, is used to introduce the cyclohexyl group to the carbonyl carbon of the previously formed Mannich base. iiab.mewikipedia.orgneu.edu.tr This reaction is critical for creating the tertiary alcohol that is a key feature of the final molecule. Recent advancements have explored the use of solvents like methyl tert-butyl ether (MTBE) to improve the safety and stability of the Grignard reaction by allowing for milder reaction temperatures.
Structure-Activity Relationship Analysis
The biological activity of this compound and its analogs is intricately linked to their molecular structure. The relationship between specific structural features and the resulting pharmacological effects is elucidated through structure-activity relationship (SAR) studies. gpatindia.comgpatindia.com
Importance of Heterocyclic or Carbocyclic R1/R2 Groups
For anticholinergic activity in this class of compounds, the presence of either heterocyclic or carbocyclic groups at the R1 and R2 positions is crucial. gpatindia.comgpatindia.com In this compound, these positions are occupied by a phenyl group and a cyclohexyl group. iucr.org Studies on analogs have shown that modifications to these rings can significantly impact activity. For instance, replacing the cyclohexyl ring with another benzene ring tends to decrease the affinity for the dopamine transporter. lookchem.comresearchgate.netnih.gov The nature of these bulky lipophilic groups is a key determinant of the compound's interaction with its target receptors.
Role of X (Oxygen or Absence)
A critical feature of the this compound molecular structure is the presence of a hydroxyl (-OH) group, where oxygen (X) is a key component. This hydroxyl group is considered a typical stereochemical feature essential for its pharmacological activity. iucr.org The oxygen atom, being electron-donating, plays a significant role in the molecule's ability to interact with its receptor. iucr.org
Impact of N-Substituent (Quaternary Ammonium Salt, Tertiary Amine, Alkyl Groups)
The nitrogen atom within the piperidine ring of this compound is another key pharmacophoric element. Modifications to this site, known as the N-substituent, have a profound impact on the compound's activity. This compound itself is a tertiary amine.
General SAR principles for this class of compounds show that the N-substituent can be a tertiary amine or can be converted into a quaternary ammonium salt. gpatindia.comgpatindia.com Quaternization of the nitrogen, for example by creating a methiodide derivative, involves adding an additional alkyl group (like a methyl group) to the nitrogen, giving it a permanent positive charge. This modification often affects the compound's potency and its ability to cross the blood-brain barrier.
Studies on this compound methiodide, a quaternary ammonium salt, have been instrumental in understanding receptor interactions. For instance, research has shown high stereoselectivity for both the tertiary amine (this compound) and its quaternary salt at various muscarinic receptor subtypes. nih.gov The size of the alkyl groups on the nitrogen also influences receptor selectivity. Increasing the bulk of the N-substituent can alter the affinity for different receptor subtypes. For example, in adrenergic agents, increasing the bulk of the nitrogen substituent tends to decrease α-receptor activity while increasing β-receptor activity. While a different receptor system, this illustrates the general principle of how N-substituent size can modulate receptor selectivity.
Analog Development for Specific Receptor Modulation (e.g., Dopamine Transporter)
Beyond its primary anticholinergic activity, this compound and its analogs have been investigated for their effects on other targets, such as the dopamine transporter (DAT). nih.gov This research is partly driven by the search for potential treatments for cocaine dependence, aiming to find a compound that can block cocaine binding to DAT without inhibiting dopamine uptake. nih.govresearchgate.net
A series of this compound analogs were synthesized and evaluated to understand the structural requirements for DAT interaction. nih.gov Several key structure-activity relationships emerged from these studies:
Phenyl Ring Modification : Introducing methyl or halogen substituents to this compound's phenyl ring was found to enhance the compound's ability to block the binding of a cocaine analog to DAT, more so than its ability to inhibit dopamine uptake. nih.govresearchgate.net
Cyclohexyl Ring Replacement : Replacing the cyclohexyl ring with another benzene ring generally resulted in compounds with lower affinities for the dopamine transporter. nih.govlookchem.com
Piperidine Ring Modification : Altering the piperidine ring tended to increase the affinity for the dopamine transporter. nih.govresearchgate.net
One particular analog, designated as 5f , which featured a modified piperidine ring, demonstrated low muscarinic activity, suggesting a reduced potential for common anticholinergic side effects. nih.gov Although this specific analog did not prove to be an effective cocaine antagonist in in-vivo studies, the research provided valuable insights into the structural modifications needed to shift the activity of the this compound scaffold towards the dopamine transporter. nih.gov
Table 1: SAR of this compound Analogs at the Dopamine Transporter (DAT) This table is a representative summary based on published findings. Specific values are not provided in the source material.
| Modification to this compound Structure | Effect on DAT Affinity | Effect on Muscarinic Receptor Affinity |
|---|---|---|
| Methylation/Halogenation of Phenyl Ring | Enhanced blockade of cocaine analog binding | Variable |
| Replacement of Cyclohexyl with Benzene Ring | Decreased | Variable |
| Modification of Piperidine Ring | Increased | Potentially Decreased (e.g., analog 5f) |
Stereochemistry Research
This compound has a chiral center at the carbon atom bonded to the hydroxyl, phenyl, and cyclohexyl groups. iucr.org This means it exists as a pair of enantiomers, (R)-Trihexyphenidyl and (S)-Trihexyphenidyl. The drug is typically used as a racemic mixture, meaning it contains equal amounts of both enantiomers. iucr.org
Research has demonstrated significant stereoselectivity in the pharmacological activity of this compound, with the two enantiomers exhibiting different affinities for muscarinic receptors. nih.govnih.gov
The (R)-(-)-enantiomer is substantially more potent than the (S)-enantiomer. Studies have shown that the (R)-form can have up to 1700-fold higher affinity for certain muscarinic receptor subtypes. nih.gov
The (R)-enantiomer is a potent and selective antagonist for the M1 muscarinic receptor subtype. nih.gov It has shown a 91-fold selectivity for M1 over M2α receptors. nih.gov
The stereochemical demands are most stringent at M1 receptors, indicating a very specific fit is required for high-affinity binding. nih.gov
This high degree of stereoselectivity is also observed in the quaternary ammonium salt derivative, this compound methiodide. nih.gov The difference in affinity between the enantiomers is less pronounced at M2 receptors compared to M1 and M3 receptors, suggesting that the hydrophobic phenyl and cyclohexyl groups interact more weakly with the M2 subtype. ulb.ac.be
Table 2: Stereoselectivity of this compound Enantiomers at Muscarinic Receptors Data derived from pA2 values reported in pharmacological studies.
| Enantiomer | Receptor Subtype | Relative Affinity/Potency |
|---|---|---|
| (R)-Trihexyphenidyl | M1 Muscarinic | Very High (pA2 ≈ 8.8 - 10.1) nih.govnih.gov |
| (S)-Trihexyphenidyl | M1 Muscarinic | Very Low (pA2 ≈ 6.1) nih.gov |
| (R)-Trihexyphenidyl | M2 Muscarinic (cardiac) | Moderate (pA2 ≈ 7.6) nih.gov |
| (S)-Trihexyphenidyl | M2 Muscarinic (cardiac) | Low (pA2 ≈ 5.7) nih.gov |
Analytical Methodologies for Trihexyphenidyl Research
Spectrophotometric Methods (e.g., Ion Pair Extraction)
Spectrophotometric methods offer a simple and cost-effective approach for the determination of Trihexyphenidyl. One such method involves ion pair extraction. ajrconline.orgresearchgate.net This technique is based on the formation of an ion pair complex between the positively charged this compound molecule and a suitable anionic dye, such as bromocresol green. ajrconline.orgresearchgate.net The resulting colored complex can be extracted into an organic solvent and quantified by measuring its absorbance at a specific wavelength. ajrconline.orgresearchgate.net
For instance, a developed and validated ion pair extraction method demonstrated linearity in the concentration range of 2 µg/mL to 18 µg/mL, with the absorbance measured at 410 nm. ajrconline.orgresearchgate.net This method has been successfully applied for the estimation of this compound in tablet dosage forms. ajrconline.orgresearchgate.net Another spectrophotometric method, based on the principle of additivity of absorbance, has been used for the simultaneous estimation of this compound and Haloperidol in a combined tablet formulation. nih.gov The absorption maxima for this compound and Haloperidol were found to be 206.0 nm and 245.0 nm, respectively, in a mixture of methanol and 0.1N HCl (90:10). nih.gov The method was linear over a concentration range of 1.0-5.0 µg/ml for this compound. nih.gov
Similarly, a simultaneous equation method has been developed for the estimation of Trifluoperazine hydrochloride and this compound hydrochloride. tsijournals.com This method utilizes the absorbance maxima of 257.5 nm for Trifluoperazine hydrochloride and 210 nm for this compound hydrochloride in distilled water. tsijournals.com Linearity was observed in the concentration range of 4-35 µg/mL for this compound hydrochloride. tsijournals.com
Table 1: Spectrophotometric Methods for this compound Analysis
| Method | Principle | Reagent(s) | Wavelength (λmax) | Linearity Range | Reference |
|---|---|---|---|---|---|
| Ion Pair Extraction | Formation of a colored complex | Bromocresol green | 410 nm | 2-18 µg/mL | ajrconline.orgresearchgate.net |
| Simultaneous Equation | Additivity of absorbance | Methanol and 0.1N HCl (90:10) | 206.0 nm | 1.0-5.0 µg/mL | nih.gov |
| Simultaneous Equation | Additivity of absorbance | Distilled water | 210 nm | 4-35 µg/mL | tsijournals.com |
High-Performance Liquid Chromatography (HPLC-UV)
High-Performance Liquid Chromatography (HPLC) coupled with Ultraviolet (UV) detection is a widely used and robust technique for the analysis of this compound. ajpaonline.comglobalresearchonline.netimpactfactor.org Reversed-phase HPLC (RP-HPLC) is the most common mode of separation. ijprems.comscholarsresearchlibrary.com These methods offer high specificity, accuracy, and the ability to simultaneously quantify this compound with other drugs in combination formulations. ajpaonline.comijprems.comscholarsresearchlibrary.com
Several RP-HPLC methods have been developed and validated for the simultaneous estimation of this compound with other active pharmaceutical ingredients such as Risperidone, Haloperidol, Trifluoperazine, and Chlorpromazine. ajpaonline.comglobalresearchonline.netijprems.comscholarsresearchlibrary.comejpmr.comresearchgate.netresearchgate.netchemconsai.comajpaonline.comsphinxsai.com The choice of stationary phase, mobile phase composition, flow rate, and detection wavelength are critical parameters that are optimized to achieve good resolution and peak symmetry. scholarsresearchlibrary.comresearchgate.net
Commonly used stationary phases include C18 columns. ijprems.comscholarsresearchlibrary.comresearchgate.netresearchgate.netajpaonline.comsphinxsai.com The mobile phase typically consists of a mixture of an organic solvent (e.g., methanol, acetonitrile) and an aqueous buffer (e.g., phosphate buffer, ammonium acetate). globalresearchonline.netijprems.comscholarsresearchlibrary.comejpmr.comresearchgate.netresearchgate.netchemconsai.comajpaonline.com The pH of the buffer is adjusted to ensure the analyte is in a suitable ionic form for retention and separation. ejpmr.comresearchgate.netresearchgate.netchemconsai.com Detection is usually performed at a wavelength where this compound and co-administered drugs exhibit significant absorbance. globalresearchonline.netscholarsresearchlibrary.comejpmr.comresearchgate.netresearchgate.netchemconsai.comsphinxsai.com
Table 2: Examples of HPLC-UV Methods for this compound Analysis
| Co-analyte(s) | Column | Mobile Phase | Flow Rate (mL/min) | Detection Wavelength (nm) | Retention Time (min) | Reference |
|---|---|---|---|---|---|---|
| Risperidone | Altima C18 (4.6 × 150 mm, 5 µm) | Methanol: Acetonitrile (60:30 v/v) | 1.0 | 260 | Not specified | ijprems.com |
| Haloperidol | Zorbax Eclipse XDB C18 (150 mm x 4.6 mm, 5 µm) | 0.05 M Phosphate Buffer (pH 3.75): Acetonitrile (65:35 v/v) with 0.5 mL triethylamine | 1.2 | 210 | Not specified | ajpaonline.comajpaonline.com |
| Trifluoperazine | Kromasil-C18 | Methanol: Sodium Acetate in water (80:20, v/v) | 1.0 | 232 | 3.54 | scholarsresearchlibrary.com |
| Chlorpromazine | Inertsil ODS 3V (250 mm x 4.6 mm; 5µ) | Ammonium acetate buffer: Methanol (15:85 v/v) | 1.2 | Not specified | 5.260 | |
| Chlorpromazine, Trifluoperazine | C-18 (250×4.6) mm, 5 μm | Methanol: Ammonium acetate buffer (1% w/v, pH 6.5) (85:15 v/v) | 1.0 | 215 | 5.7 | researchgate.net |
Radioreceptor Assays for Anticholinergic Drugs
Radioreceptor assays provide a highly sensitive method for measuring the total anticholinergic activity of drugs in biological samples. nih.govnih.govresearchgate.netmedlink.comtodaysgeriatricmedicine.com This assay is based on the principle of competitive binding, where the drug of interest in a sample competes with a radiolabeled ligand for binding to specific receptors, in this case, muscarinic cholinergic receptors. nih.govnih.govmedlink.com The amount of radioactivity bound to the receptors is inversely proportional to the concentration of the anticholinergic drug in the sample. nih.gov
Radioimmunoassay (RIA) for Trace Analysis
Radioimmunoassay (RIA) is another highly sensitive and specific technique that has been developed for the trace analysis of this compound. ajrconline.orgresearchgate.netvirginia.edudrugfuture.com This method involves the use of an antibody that is specific to the drug. researchgate.net
A specific and sensitive RIA for this compound was developed using an antiserum produced in rabbits immunized with a this compound-protein conjugate. researchgate.net The assay was capable of quantifying as little as 7.8 pg of this compound in 200 µL of human plasma. researchgate.net The assay demonstrated minimal cross-reactivity with major metabolites and other co-administered drugs. researchgate.net This high sensitivity makes RIA particularly suitable for pharmacokinetic studies where plasma concentrations of the drug can be very low. researchgate.netvirginia.edudrugfuture.com
HPTLC Estimation
High-Performance Thin-Layer Chromatography (HPTLC) is a planar chromatographic technique that offers several advantages, including the ability to analyze multiple samples simultaneously, lower solvent consumption, and simplicity. ajrconline.orgglobalresearchonline.netresearchgate.net
An HPTLC method has been developed for the simultaneous determination of this compound HCl, Trifluoperazine HCl, and Chlorpromazine HCl in a tablet dosage form. researchgate.net The separation was achieved on precoated silica gel plates using a mobile phase consisting of toluene, methanol, acetone, and ammonia. researchgate.net The developed plates were scanned and quantified at 213 nm. researchgate.net The method was found to be linear over a specific concentration range for this compound HCl. researchgate.net
Table 3: HPTLC Method for this compound Analysis
| Co-analytes | Stationary Phase | Mobile Phase | Detection Wavelength (nm) | Linearity Range (µg) | Reference |
|---|---|---|---|---|---|
| Trifluoperazine HCl, Chlorpromazine HCl | Precoated silica gel G 60 F254 on aluminum foil | Toluene: Methanol: Acetone: Ammonia (7:1:2:0.1% v/v) | 213 | 2.0 - 10.0 | researchgate.net |
Validation of Analytical Methods (Accuracy, Precision, Specificity, Sensitivity, Reproducibility)
The validation of analytical methods is a critical requirement to ensure the reliability and quality of the generated data. ijprems.comscholarsresearchlibrary.comsphinxsai.com Validation is performed according to guidelines established by regulatory bodies such as the International Council for Harmonisation (ICH). ajpaonline.comscholarsresearchlibrary.comejpmr.comajpaonline.com The key validation parameters include:
Accuracy: This parameter assesses the closeness of the test results obtained by the method to the true value. It is often determined by recovery studies, where a known amount of the standard drug is added to a sample and the percentage recovery is calculated. ajpaonline.comijprems.comscholarsresearchlibrary.comsphinxsai.com For this compound, recovery values are typically expected to be within a narrow range around 100%. scholarsresearchlibrary.comsphinxsai.com
Precision: Precision refers to the degree of agreement among individual test results when the method is applied repeatedly to multiple samplings of a homogeneous sample. It is usually expressed as the relative standard deviation (RSD) for a series of measurements. ajrconline.orgresearchgate.netijprems.comscholarsresearchlibrary.com Precision is evaluated at different levels: repeatability (intra-day precision) and intermediate precision (inter-day precision). ajrconline.orgresearchgate.netijprems.comscholarsresearchlibrary.com
Specificity: Specificity is the ability of the method to assess the analyte unequivocally in the presence of components that may be expected to be present, such as impurities, degradation products, or matrix components. ajrconline.orgresearchgate.netscholarsresearchlibrary.com In chromatographic methods, this is demonstrated by the resolution of the analyte peak from other peaks. scholarsresearchlibrary.com
Sensitivity: This is determined by the limit of detection (LOD) and the limit of quantitation (LOQ). The LOD is the lowest amount of analyte in a sample that can be detected but not necessarily quantitated as an exact value, while the LOQ is the lowest amount of analyte in a sample that can be quantitatively determined with suitable precision and accuracy. ajrconline.orgresearchgate.netajpaonline.comejpmr.comresearchgate.net
Reproducibility: This assesses the precision between different laboratories and is often evaluated during the method transfer process.
Validated analytical methods for this compound have consistently demonstrated high accuracy, with recovery values typically between 98% and 102%. scholarsresearchlibrary.com Precision is also high, with RSD values for both intra-day and inter-day analyses generally below 2%. ajrconline.orgresearchgate.netijprems.comscholarsresearchlibrary.com Linearity is established by demonstrating a direct proportionality between the concentration of the analyte and the analytical response over a defined range, with correlation coefficients (r²) close to 1. ijprems.comscholarsresearchlibrary.comejpmr.com
Q & A
Basic Research Questions
Q. What methodological approaches are recommended for designing randomized controlled trials (RCTs) to evaluate Trihexyphenidyl’s efficacy in treating drug-induced extrapyramidal symptoms?
- Answer : RCTs should include double-blinding, placebo-controlled arms, and standardized dosing regimens (e.g., 2–15 mg/day for adults). Stratify participants by age, comorbid conditions (e.g., Parkinson’s disease), and concurrent antipsychotic use to control confounding variables. Use validated scales like the Brief Psychiatric Rating Scale (BPRS) for outcome measurement . Data collection should follow protocols for adverse event monitoring, particularly anticholinergic effects (e.g., dry mouth, cognitive impairment). For pediatric populations, adjust dosing based on weight (e.g., 0.2–0.5 mg/kg/day) and include long-term follow-up to assess developmental impacts .
Q. How can researchers address variability in this compound’s pharmacokinetics across demographic subgroups?
- Answer : Conduct pharmacokinetic (PK) studies with stratified sampling:
- Age : Compare clearance rates in pediatric vs. adult cohorts.
- Hepatic function : Use liver enzyme biomarkers (e.g., CYP2D6 activity) to adjust dosing.
- Drug interactions : Test co-administration with antipsychotics (e.g., haloperidol) via in vitro CYP450 inhibition assays .
- Methodological tools : Employ high-performance liquid chromatography (HPLC) for plasma concentration analysis and population PK modeling to identify covariates .
Advanced Research Questions
Q. What strategies resolve contradictions in studies reporting this compound’s cognitive effects in schizophrenia patients?
- Answer :
- Data harmonization : Re-analyze raw datasets using meta-analytic techniques to adjust for confounders (e.g., baseline cognitive scores, concurrent medications).
- Mechanistic studies : Combine fMRI with neuropsychological tests to isolate this compound’s impact on cholinergic pathways vs. dopamine modulation .
- Contradiction table :
| Study | Population | Cognitive Outcome | Proposed Bias Source |
|---|---|---|---|
| A (2019) | Adults (n=30) | Improved memory | Uncontrolled for dose titration |
| B (2021) | Adolescents (n=45) | Worsened executive function | Confounding by antipsychotic polypharmacy |
Q. How can in silico modeling improve this compound’s target specificity to reduce off-target anticholinergic effects?
- Answer :
- Molecular docking : Simulate this compound’s binding affinity to M1/M4 muscarinic receptors vs. peripheral M2/M3 subtypes. Use tools like AutoDock Vina to optimize structural analogs .
- In vitro validation : Test top candidates in transfected HEK293 cells expressing human muscarinic receptors. Measure cAMP inhibition and compare to existing data on receptor subtype selectivity .
- Translational gap : Address species-specific receptor expression differences by cross-referencing rodent and human transcriptomic datasets (e.g., GTEx Portal) .
Methodological Guidelines
- Data collection : Follow secondary data analysis frameworks (e.g., coding, cleaning, structuring) as in Grhasia Hospital’s study on this compound-antipsychotic combinations .
- Ethical considerations : Disclose conflicts of interest (e.g., pharmaceutical funding) and retain raw data for ≥5 years to enable replication .
- Reporting standards : Adhere to Beilstein Journal guidelines: Limit main text to critical experiments; append supplementary materials (e.g., full PK curves, adverse event logs) .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


