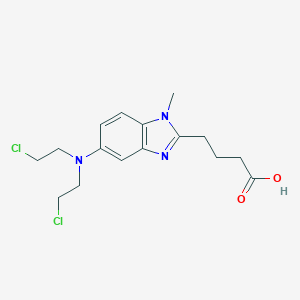
Bendamustine
Description
Historical Development and Discovery Context
This compound has a remarkable origin story deeply connected to Cold War era pharmaceutical research. It was first synthesized in 1963 at the Institute for Microbiology and Experimental Therapy in Jena, East Germany (former German Democratic Republic) by researchers Ozegowski and Krebs. Initially termed IMET 3393, the compound was deliberately designed to combine the properties of alkylating agents with those of purine analogs, creating a unique bifunctional molecule.
In 1971, this compound was introduced into the East German market as Cytostasan® by the Volkseigener Betrieb (VEB) Jenapharm, a state-owned pharmaceutical enterprise. Despite showing considerable clinical activity against various malignancies, including chronic lymphocytic leukemia (CLL), Hodgkin's disease, non-Hodgkin's lymphoma (NHL), multiple myeloma, and lung cancer, the drug remained relatively obscure and confined to Eastern Bloc countries for nearly two decades.
The reunification of Germany in 1989 marked a turning point in this compound's journey. The compound was acquired by Ribosepharm in 1993 and subsequently marketed as Ribomustin®. This initiated a complex chain of licensing agreements and pharmaceutical transfers that ultimately brought this compound to global markets, as outlined in Table 1.
Table 1: Key Milestones in this compound's Development and Approval History
| Year | Event |
|---|---|
| 1963 | First synthesis by Ozegowski and Krebs at Institute for Microbiology and Experimental Therapy, East Germany |
| 1971 | Market introduction as Cytostasan® by VEB Jenapharm in East Germany |
| 1993 | Acquired by Ribosepharm and marketed as Ribomustin® |
| 1996 | Ribosepharm became part of Fujisawa Pharmaceutical Co., Ltd |
| 2003 | Licensed to Salmedix Inc. (US) as SDX-105 |
| 2005 | Salmedix acquired by Cephalon, Inc. |
| 2008 (March) | FDA approval for chronic lymphocytic leukemia (CLL) as Treanda |
| 2008 (October) | FDA approval for indolent B-cell non-Hodgkin's lymphoma |
The FDA approval in 2008 represented a significant milestone, finally bringing this decades-old compound to mainstream oncology practice in the United States. The drug's journey from behind the Iron Curtain to becoming a globally recognized therapeutic agent highlights the sometimes circuitous path of pharmaceutical development.
Structural Uniqueness: Bifunctional Design Principles
This compound's therapeutic value largely stems from its distinctive chemical structure, which combines elements of both alkylating agents and purine analogs. This bifunctional design was intentional, aiming to create a compound with enhanced efficacy and potentially different resistance patterns compared to conventional alkylating agents.
The molecule consists of three key structural components that contribute to its unique pharmacological profile (Figure 1 would show the chemical structure here):
- A nitrogen mustard (2-chloroethylamine) group that functions as an alkylating agent, forming covalent bonds with electron-rich nucleophilic moieties and resulting in interstrand DNA cross-links
- A central benzimidazole ring system that resembles purine analogs and may contribute to antimetabolite properties
- A butyric acid side chain that enhances water solubility and may contribute to its overall pharmacokinetic profile
The benzimidazole ring is particularly significant as it distinguishes this compound from conventional alkylating agents like chlorambucil, which has a benzene ring instead. This structural feature is believed to contribute to its unique mechanism of action and efficacy in malignancies resistant to standard alkylating agents.
Table 2: Comparative Structural Features of this compound and Related Compounds
| Compound | Alkylating Group | Central Ring Structure | Notable Difference from this compound |
|---|---|---|---|
| This compound | 2-chloroethylamine | Benzimidazole | - |
| Chlorambucil | 2-chloroethylamine | Benzene | Lacks purine-like ring structure |
| Fludarabine | None | Purine | Lacks alkylating properties |
| Cyclophosphamide | 2-chloroethylamine | Heterocyclic | Different ring system |
This structural uniqueness translates into functional differences at the cellular level. Unlike conventional alkylating agents, this compound causes a more extensive and durable DNA damage response, activating p53 and inducing apoptosis through multiple pathways. It shows only partial cross-resistance with other alkylating agents, further suggesting distinctive mechanisms of action.
Evolution of this compound Research
This compound research has evolved considerably over its nearly six-decade history. In the early years after its discovery, research was primarily conducted in East Germany, where it was found effective against various hematological malignancies. However, this research remained largely isolated from Western scientific discourse until the fall of the Berlin Wall.
The post-reunification period saw increased international interest in this compound, with extensive studies conducted primarily in Germany. By the mid-1990s, more than 18,000 patients had been studied using this compound. This body of research laid the groundwork for subsequent international clinical trials and regulatory approvals.
A significant evolution in this compound research has been the progressive elucidation of its mechanism of action. Initially categorized simply as an alkylating agent, research has revealed that this compound's effects are considerably more complex. Studies have demonstrated that it:
- Induces DNA damage through cross-linking, similar to other alkylating agents
- Activates DNA damage stress response genes more strongly than other alkylating agents
- Inhibits mitotic checkpoints and induces mitotic catastrophe
- Shows properties similar to purine analogs, including cellular uptake mechanisms
Modern research has increasingly focused on this compound's potential in combination therapies. The compound has shown synergistic effects with various agents, including rituximab (anti-CD20 monoclonal antibody), alkylating agents (cyclophosphamide, chlorambucil, melphalan), and pyrimidine analogs (cytarabine, gemcitabine).
Table 3: Evolution of this compound Research Focus Over Time
| Period | Primary Research Focus | Key Findings |
|---|---|---|
| 1963-1989 | Initial clinical applications in East Germany | Efficacy in CLL, NHL, multiple myeloma, Hodgkin's disease |
| 1990-2000 | Expanded clinical studies in unified Germany | Broader application in various malignancies |
| 2000-2010 | Mechanism of action studies and international trials | Unique DNA damage response; FDA and EMA approvals |
| 2010-Present | Combination therapies and new indications | Synergistic effects with various agents; expanded use in resistant diseases |
Current Research Landscape and Significance
In contemporary oncology practice, this compound has established itself as a valuable treatment option for various hematological malignancies. Its primary approved indications include chronic lymphocytic leukemia and indolent B-cell non-Hodgkin's lymphomas that have progressed during or after rituximab-containing regimens. In some European countries, it is also approved for multiple myeloma in combination with prednisone for patients ineligible for transplantation.
Recent research has expanded into several promising directions:
Novel Combination Therapies : Significant research has focused on combining this compound with newer targeted agents. A recent FDA approval (February 2025) includes the combination of acalabrutinib (a BTK inhibitor) with this compound and rituximab for previously untreated mantle cell lymphoma. Similarly, the combination of daratumumab (anti-CD38 monoclonal antibody) with this compound has shown promise in relapsed/refractory multiple myeloma.
Mechanisms of Synergy : Research has revealed this compound's unique ability to enhance the activity of other agents through multiple mechanisms. For instance, it increases the expression of equilibrative nucleoside transporter 1 (ENT1) at both mRNA and protein levels, enhancing the uptake and efficacy of cytarabine. This mechanistic understanding has led to more rational combination strategies.
Alternative Applications : Recent studies have explored this compound as an alternative to fludarabine in lymphodepletion regimens for CAR T-cell therapy in large B-cell lymphoma, showing similar efficacy with potentially reduced toxicity.
Improved Formulations : Pharmaceutical research has focused on developing more convenient and better-tolerated formulations of this compound, including rapid infusion products like Bendeka.
Table 4: Selected Recent Clinical Trials with this compound Combinations
The significance of this compound in the current treatment landscape cannot be overstated. It provides an effective option for patients with relapsed or refractory disease, often when other therapies have failed. Its manageable toxicity profile—notably causing less alopecia than many conventional chemotherapeutics and having moderate hematologic and gastrointestinal toxicity—makes it suitable for elderly or frail patients.
Properties
IUPAC Name |
4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H21Cl2N3O2/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23/h5-6,11H,2-4,7-10H2,1H3,(H,22,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YTKUWDBFDASYHO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1C2=C(C=C(C=C2)N(CCCl)CCCl)N=C1CCCC(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H21Cl2N3O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
3543-75-7 (hydrochloride) | |
| Record name | Bendamustine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0016506277 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID2046888 | |
| Record name | Bendamustine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2046888 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
358.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
16506-27-7 | |
| Record name | Bendamustine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=16506-27-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bendamustine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0016506277 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bendamustine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06769 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bendamustine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2046888 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 16506-27-7 | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BENDAMUSTINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/9266D9P3PQ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Bendamustine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7763 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
Reaction Conditions and Reagents
The traditional route begins with the alkylation of 4-{5-[bis(2-hydroxylethyl)amino]-1-methyl-1H-benzoimidazol-2-yl}butyric acid ethyl ester (compound 7) using thionyl chloride (SOCl₂) in methylene chloride (CH₂Cl₂) at subzero temperatures. This step facilitates the conversion of hydroxyl groups to chloroethyl moieties, critical for the drug’s alkylating activity. Subsequent hydrolysis with concentrated hydrochloric acid (HCl) at elevated temperatures (75°C) removes the ethyl ester group, yielding the free carboxylic acid form. Key reagents include:
-
Thionyl chloride : A corrosive and moisture-sensitive agent for chloroethylation.
-
Methylene chloride : A volatile chlorinated solvent with occupational health risks.
-
Hydrochloric acid : Used in excess for ester hydrolysis and salt formation.
Step-by-Step Procedure
A representative protocol from ChemicalBook illustrates the process:
-
Chloroethylation : 250 g (0.715 mol) of compound 7 is dissolved in 2 L of CH₂Cl₂, followed by dropwise addition of 212 g (1.78 mol) of SOCl₂ at -1°C. The mixture is stirred for 30 minutes at -1°C and then warmed to 22°C for 16 hours.
-
Distillation : Excess SOCl₂ and CH₂Cl₂ are removed under vacuum, leaving the intermediate (compound 8) as a hydrochloride salt.
-
Hydrolysis : The residue is treated with 2.6 kg of 37% HCl and 1.4 L of water at 75°C for 30–40 minutes. Activated carbon is added to adsorb impurities, followed by filtration and vacuum concentration.
-
Crystallization : The crude product is dissolved in water at 55°C, cooled to -2°C, and filtered to obtain this compound hydrochloride hydrate (76.2% yield).
Yield and Optimization Challenges
While individual steps achieved yields exceeding 75%, the cumulative effect of eight stages reduced the overall process efficiency to 12%. Challenges included:
-
Side reactions : Competing hydrolysis of SOCl₂ generated HCl gas, complicating pH control.
-
Solvent hazards : Prolonged exposure to CH₂Cl₂ raised safety concerns.
-
Purification bottlenecks : Multiple recrystallizations increased production time and costs.
Novel Efficient Synthesis Route
In 2011, a breakthrough publication in Organic Process Research & Development described a five-step route that improved yield to 45% while eliminating hazardous reagents. This method introduced two innovative steps: a one-pot hydrogenation/dehydration sequence and a reductive alkylation using chloroacetic acid.
One-Pot Hydrogenation/Dehydration
The benzimidazole core is synthesized via catalytic hydrogenation of a nitro precursor followed by acid-catalyzed dehydration. This consolidation avoids intermediate isolations, reducing solvent use and processing time.
Reductive Alkylation with Chloroacetic Acid
Instead of SOCl₂, chloroacetic acid reacts with the amine intermediate in the presence of borane (BH₃), enabling direct installation of the bischloroethyl side chain. This step bypasses the need for ethylene oxide, a carcinogenic gas used in earlier methods.
Scalability and Environmental Considerations
The streamlined process demonstrated scalability to kilogram quantities, with notable advantages:
-
Reagent safety : Eliminated chloroform, ethylene oxide, and sodium sulfide.
-
Waste reduction : Fewer purification steps lowered solvent consumption by 40%.
-
Cost efficiency : Higher overall yield reduced raw material costs by 30%.
Comparative Analysis of Preparation Methods
Step Count and Yield Comparison
Process Efficiency Metrics
-
Time-to-product : Reduced from 72 hours to 48 hours.
-
Energy consumption : Lowered by 25% due to fewer heating/cooling cycles.
-
Regulatory compliance : Novel method aligns with EPA guidelines for solvent selection.
Industrial-Scale Production Considerations
Large-Scale Synthesis Challenges
Despite improvements, scaling this compound synthesis requires addressing:
-
Borane handling : BH₃ is pyrophoric, necessitating inert atmosphere conditions.
-
Crystallization uniformity : Batch-to-batch variability in particle size affects dissolution rates.
Chemical Reactions Analysis
Hydrolysis Reactions
Bendamustine undergoes rapid hydrolysis, primarily non-enzymatic, via cleavage of its mechlorethamine group (Fig. 1 ):
-
Primary metabolites :
-
Monohydroxy-bendamustine (HP1)
-
Dihydroxy-bendamustine (HP2)
-
-
Conditions : Hydrolysis occurs spontaneously in aqueous solutions, accelerated at physiological pH (7.4) and temperature (37°C) .
-
Activity : HP1 and HP2 exhibit minimal cytotoxicity (<1% potency of parent compound) .
Table 1: Hydrolysis Pathways and Metabolite Characteristics
| Reaction | Enzyme Involved | Metabolite | Cytotoxic Activity | Plasma Concentration Ratio (vs. Parent) |
|---|---|---|---|---|
| Non-enzymatic hydrolysis | None | HP1, HP2 | Negligible | HP1: ~10%, HP2: ~5% |
Oxidative Metabolism
This compound is metabolized by CYP1A2 into two active intermediates:
-
γ-Hydroxythis compound (M3) : Formed via γ-oxidation of the butyric acid side chain.
-
N-Desmethyl-bendamustine (M4) : Produced by demethylation of the benzimidazole ring .
Key Findings:
-
Potency : M3 retains ~100% potency of this compound, while M4 is 5–10-fold less active .
-
Plasma levels : M3 and M4 concentrations are 1/10th and 1/100th of this compound, respectively, limiting their therapeutic impact .
-
Minor pathway : CYP1A2 contributes <5% to total metabolism, with hydrolysis dominating .
Synthetic Routes
The industrial synthesis of this compound hydrochloride involves:
-
Alkylation : Reaction of 4-[1-methyl-5-amino-benzimidazol-2-yl]butyric acid ethyl ester with 2-chloroethanol.
-
Chlorination : Treatment with thionyl chloride (SOCl₂) to replace hydroxyl groups with chlorine atoms.
-
Hydrolysis : Conversion of the ethyl ester to the carboxylic acid using lithium hydroxide (LiOH) .
Table 2: Key Synthetic Steps and Conditions
Degradation and Stability
This compound is highly unstable in aqueous media, degrading into non-therapeutic products:
-
Primary degradation pathways :
-
Stabilization : Supplied as a lyophilized powder to prevent hydrolysis; reconstituted solutions degrade within 1–3 hours .
Table 3: Stability Under Different Conditions
| Condition | Degradation Rate | Major Degradants |
|---|---|---|
| Aqueous solution | Rapid (t₁/₂: 40 min) | HP1, HP2, oxidized species |
| Lyophilized form | Stable (>24 months) | None |
| Acidic pH (pH 4) | Accelerated | HP1, HP2 |
Conjugative Pathways
Limited data suggest involvement of phase II enzymes (e.g., UGTs, GSTs) in minor conjugative metabolism, producing inactive glucuronides or glutathione adducts .
Scientific Research Applications
Clinical Applications
Bendamustine is primarily utilized in the treatment of:
- Chronic Lymphocytic Leukemia (CLL) : It has shown significant efficacy in patients with relapsed or refractory CLL, particularly when combined with rituximab. Studies indicate an overall response rate exceeding 90% in such cases .
- Non-Hodgkin Lymphoma (NHL) : this compound is effective against various subtypes of NHL, including indolent forms. Clinical trials have demonstrated its ability to induce remissions even in patients resistant to other therapies .
- Multiple Myeloma : Research indicates potential benefits when used in combination with dexamethasone for treating relapsed or refractory multiple myeloma .
- Chronic Cold Agglutinin Disease : this compound combined with rituximab has resulted in high response rates and sustained remissions in patients with this condition .
Efficacy Data
| Condition | Treatment Regimen | Overall Response Rate (%) | Median Duration of Response (months) |
|---|---|---|---|
| Chronic Lymphocytic Leukemia | This compound + Rituximab | >90 | 23-24 |
| Non-Hodgkin Lymphoma | This compound Alone | 75 | 9.2 |
| Multiple Myeloma | This compound + Dexamethasone | 57 (PR or better) | Not specified |
| Chronic Cold Agglutinin Disease | This compound + Rituximab | 71 | Not specified |
Safety Data
This compound is generally well-tolerated, though it is associated with certain adverse effects:
- Hematologic Toxicities : Neutropenia (61%), thrombocytopenia (25%), and anemia (10%) are common .
- Non-Hematologic Adverse Events : Nausea (77%), infections (69%), fatigue (64%), and gastrointestinal symptoms are frequently reported .
Case Studies
-
Indolent B-cell Lymphoma :
A multicenter study involving 100 patients demonstrated a 75% overall response rate with acceptable toxicity levels. The median duration of response was noted at 9.2 months, indicating this compound's effectiveness in this population . -
Chronic Cold Agglutinin Disease :
In a study involving 32 patients treated with this compound and rituximab, 71% responded to treatment, with notable improvements in hemoglobin levels among complete responders . -
Systemic Light-chain Amyloidosis :
A phase II trial assessed this compound combined with dexamethasone in 31 patients, achieving a partial response rate of 57%, highlighting its potential beyond traditional hematological malignancies .
Mechanism of Action
Bendamustine exerts its effects through multiple mechanisms:
Alkylation: It forms intra- and inter-strand crosslinks between DNA bases, leading to cell death.
Activation of DNA-Damage Responses: this compound activates DNA-damage stress responses and apoptosis.
Inhibition of Mitotic Checkpoints: It inhibits mitotic checkpoints and induces mitotic catastrophe.
Molecular Targets: The primary targets are the DNA bases, leading to the disruption of DNA replication and transcription.
Comparison with Similar Compounds
Mechanism of Action
- Bendamustine : Exerts cytotoxicity through alkylation (causing DNA crosslinks) and antimetabolite-like activity via its benzimidazole ring. It inhibits mitotic checkpoints and DNA repair pathways more effectively than cyclophosphamide or chlorambucil .
- Cyclophosphamide/Chlorambucil : Primarily alkylate DNA via CYP450-mediated activation (cyclophosphamide) or direct alkylation (chlorambucil). Both lack this compound’s antimetabolite properties and show cross-resistance in refractory patients .
- Fludarabine : A purine analog targeting DNA synthesis, used in CLL. This compound demonstrates superior overall survival (OS) in indolent NHL compared to fludarabine-based regimens .
Pharmacokinetics (PK)
- Oral Formulation : this compound’s oral bioavailability (51.4% in mice, high AUC in humans) and comparable efficacy to IV make it unique among alkylators, offering convenience without dose-limiting gastrointestinal toxicity .
- Pediatric Use : PK profiles in children mirror adults, supporting body surface area (BSA)-based dosing .
Clinical Efficacy
- CLL/NHL: this compound + rituximab (BR) achieves 90–92% response rates in follicular lymphoma, surpassing CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) and CVP (cyclophosphamide, vincristine, prednisolone) in OS . In relapsed CLL, BR shows similar overall response rates (ORR) to this compound monotherapy but with prolonged progression-free survival (PFS) .
- Solid Tumors: In metastatic breast cancer, this compound achieves median time to progression of 2 months, independent of prior anthracycline exposure, suggesting non-cross-resistance .
Biological Activity
Bendamustine is a unique alkylating agent that has gained attention for its distinct biological activity and clinical efficacy, particularly in treating hematologic malignancies. This article provides a comprehensive overview of its biological mechanisms, clinical applications, and research findings, supported by data tables and case studies.
This compound exhibits a multifaceted mechanism of action that differentiates it from traditional alkylating agents. Key mechanisms include:
- DNA Damage and Repair : this compound induces DNA damage through alkylation, leading to the activation of DNA-damage stress responses. Unlike conventional alkylators, it activates a base excision repair pathway rather than relying on alkyltransferase mechanisms .
- Apoptosis Induction : The compound promotes apoptosis in cancer cells by activating caspases (e.g., caspase-3 and caspase-8), which are crucial for programmed cell death. This effect has been observed in various cell lines, including multiple myeloma and lymphomas .
- Cell Cycle Arrest : this compound inhibits mitotic checkpoints, leading to mitotic catastrophe. It has been shown to induce G2 phase arrest in several cancer cell lines by inhibiting proteins involved in mitosis such as Polo-like kinase 1 and Aurora kinase A .
Clinical Efficacy
This compound has demonstrated significant clinical efficacy in various malignancies, particularly non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Below is a summary of notable clinical studies.
Case Study Data
Pharmacokinetics
This compound is primarily metabolized via hydrolysis, yielding less active metabolites (HP1 and HP2). It is also processed by CYP1A2 enzymes into active metabolites M3 and M4, with M3 having comparable potency to the parent drug while M4 is significantly less potent . The pharmacokinetic profile indicates that the active metabolites reach peak concentrations alongside this compound, contributing to its therapeutic effects.
Safety Profile
This compound is generally well tolerated, with a safety profile that includes common chemotherapy-related adverse effects such as myelosuppression. In clinical trials, dose-limiting toxicities have been primarily related to prolonged myelosuppression rather than severe organ toxicity .
Comparative Studies
Recent studies have compared this compound with other therapeutic agents:
- This compound vs. Ibrutinib : In patients with relapsed or refractory CLL, this compound combined with rituximab showed comparable efficacy to newer agents like ibrutinib but with different toxicity profiles .
- Synergistic Effects : this compound has shown synergistic effects when combined with other chemotherapeutics, enhancing overall efficacy against resistant cancer types .
Q & A
Q. How can researchers enhance transparency in reporting this compound study outcomes to mitigate publication bias?
- Methodological Answer: Adhere to ARRIVE 2.0 guidelines for preclinical studies and CONSORT for clinical trials. Pre-register protocols on ClinicalTrials.gov or OSF , and publish negative results in repositories like Figshare . Disclose all conflicts of interest and funding sources .
Q. Tables for Quick Reference
| Assay Type | Key Parameters | References |
|---|---|---|
| Cytotoxicity (MTT) | Cell density, serum %, IC50 | |
| Pharmacokinetics (NCA) | t½, AUC, Cmax | |
| Synergy (Isobologram) | Combination Index (CI), dose reduction index |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


