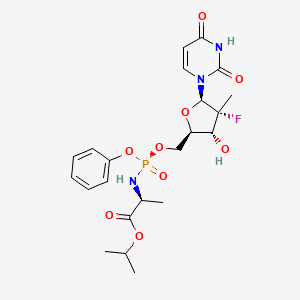
Sofosbuvir
Description
Historical Context of Nucleoside Analog Antivirals in Drug Discovery
Nucleoside analogs have been a cornerstone of antiviral therapy for several decades, proving effective against a range of viruses including HIV, hepatitis B and C, and herpes simplex. researchgate.netnumberanalytics.comresearchgate.net These compounds are synthetic molecules that mimic natural nucleosides, the building blocks of DNA and RNA. researchgate.netnih.govmdpi.com When incorporated into the viral genetic material during replication, they act as "chain terminators," preventing the further elongation of the DNA or RNA strand and thereby inhibiting viral replication. numberanalytics.comnih.gov
The journey of nucleoside analogs in drug discovery began with early, relatively simple modifications to the nucleoside structure. nih.gov Over time, more complex alterations were introduced, leading to the development of potent antiviral drugs like acyclovir and tenofovir. xiahepublishing.comnih.gov Despite their success, the development of nucleoside analogs has faced challenges, including toxicity and the emergence of drug-resistant viral strains. nih.govresearchgate.net
Evolution of Hepatitis C Virus (HCV) Direct-Acting Antiviral (DAA) Discovery
The discovery of the hepatitis C virus in 1989 paved the way for the development of targeted antiviral therapies. nih.govoup.com Initially, treatment for HCV infection relied on interferon-based therapies, often in combination with ribavirin. elsevier.esnih.gov These regimens had limited success rates and were associated with significant side effects. wikipedia.orgelsevier.es
A deeper understanding of the HCV life cycle led to the identification of key viral proteins that could be targeted by drugs. This knowledge spurred the development of direct-acting antivirals (DAAs), which directly interfere with specific steps in the HCV replication process. The first DAAs, the protease inhibitors telaprevir and boceprevir, were approved in 2011 and represented a major step forward in HCV treatment. nih.govelsevier.eskarger.com However, these first-generation DAAs still needed to be used in combination with interferon and ribavirin. xiahepublishing.com
The ultimate goal of HCV research was to develop all-oral, interferon-free treatment regimens. xiahepublishing.com This led to the exploration of other viral targets, including the NS5B polymerase, an essential enzyme for HCV RNA replication. nih.govdrugbank.com
Genesis of Sofosbuvir: A Paradigm Shift in HCV Therapeutic Agents
This compound, a nucleotide analog inhibitor of the HCV NS5B polymerase, emerged as a groundbreaking development in the quest for an ideal HCV treatment. xiahepublishing.comnih.govgastroenterologyandhepatology.net Discovered in 2007 by Michael J. Sofia at Pharmasset, the compound was initially named PSI-7977. wikipedia.orgacs.orghepcoalition.org
The design of this compound incorporated a unique "ProTide" technology. This approach masks the phosphate group of the nucleotide analog, allowing it to be more easily absorbed and delivered to the liver, the primary site of HCV replication. wikipedia.org Once inside the liver cells, this compound is converted to its active triphosphate form, GS-461203. wikipedia.orgdrugbank.compatsnap.com This active metabolite is then incorporated into the growing HCV RNA chain by the NS5B polymerase, acting as a chain terminator and halting viral replication. drugbank.compatsnap.comresearchgate.net
A key advantage of this compound is its high barrier to resistance, a significant improvement over earlier DAAs. drugbank.comgastroenterologyandhepatology.net Clinical trials demonstrated that this compound, in combination with other DAAs, could achieve cure rates of over 90%, even in difficult-to-treat patient populations. xiahepublishing.comtandfonline.com The approval of this compound by the U.S. Food and Drug Administration (FDA) in 2013 ushered in a new era of highly effective, safe, and simple all-oral therapies for hepatitis C. wikipedia.orgnih.govacs.org
Properties
Key on ui mechanism of action |
Sofosbuvir is a direct-acting antiviral agent (pan-genotypic polymerase inhibitor) against the hepatitis C virus. HCV RNA replication is mediated by a membrane-associated multiprotein replication complex. The HCV polymerase (NS5B protein) is an RNA-dependent RNA polymerase (RdRp). It is the essential initiating and catalytic subunit of this replication complex and is critical for the viral replication cycle. There is no human homolog for HCV NS5B RdRp. Sofosbuvir is a monophosphorylated pyrimidine nucleotide prodrug that undergoes intracellular metabolism to form the pharmacologically active uridine analog triphosphate (GS-461203). GS-461203 competes with natural nucleotides for incorporation (by HCV NS5B) into the nascent RNA strand during replication of the viral genome. GS-461203 differs from endogenous pyrimidine nucleotides in that it has been modified at the 2' position with the addition of a methyl and a fluoro functional group. Incorporation of GS-461203 into nascent RNA strongly reduces the efficiency of further RNA elongation by RdRp, resulting in premature termination of RNA synthesis. The stopping of viral replication leads to a rapid decline of HCV viral load and clearing of HCV levels in the body. |
|---|---|
CAS No. |
1190307-88-0 |
Molecular Formula |
C22H29FN3O9P |
Molecular Weight |
529.5 g/mol |
IUPAC Name |
propan-2-yl 2-[[[(4R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate |
InChI |
InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14?,16?,18?,20?,22-,36+/m1/s1 |
InChI Key |
TTZHDVOVKQGIBA-IECBXEDQSA-N |
SMILES |
CC(C)OC(=O)C(C)NP(=O)(OCC1C(C(C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 |
Isomeric SMILES |
CC(C)OC(=O)C(C)N[P@](=O)(OCC1C([C@@](C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 |
Canonical SMILES |
CC(C)OC(=O)C(C)NP(=O)(OCC1C(C(C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 |
Appearance |
white solid powder |
Color/Form |
White to off-white crystalline solid |
Purity |
>98% (or refer to the Certificate of Analysis) |
shelf_life |
>2 years if stored properly |
solubility |
Slightly soluble in water |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
PSI-7977; PSI 7977; PSI7977; GS7977; GS-7977; GS 7977; Sofosbuvir; Sovaldi; Virunon; Vosevi; Hepcinat; Hepcvir; Resof; |
Origin of Product |
United States |
Medicinal Chemistry and Rational Drug Design of Sofosbuvir
Structure-Activity Relationship (SAR) Investigations of Sofosbuvir and its Analogs
The development of this compound was built upon extensive structure-activity relationship (SAR) studies of nucleoside analogs as inhibitors of the HCV NS5B RNA-dependent RNA polymerase, an enzyme critical for viral replication. nih.govnih.gov The core of this compound is a uridine nucleoside modified at the 2'-position of the ribose sugar.
Key SAR findings that led to the discovery of this compound include:
2'-C-Methyl Substitution: The introduction of a methyl group at the 2'-C position of the ribose was found to be crucial for potent anti-HCV activity. researchgate.net This modification, particularly in the β-configuration, enhances the inhibitory activity against the NS5B polymerase. nih.gov Analogs with changes to this methyl group, such as increasing its size or altering its stereochemistry, resulted in a loss of inhibitory activity. researchgate.net
2'-α-Fluoro Substitution: The addition of a fluorine atom at the 2'-α position was another critical modification. This substitution, in combination with the 2'-β-C-methyl group, resulted in potent inhibitors of the HCV NS5B polymerase. nih.govnih.gov
Base Moiety: While modifications to the ribose were key, the identity of the nucleobase also played a role. While analogs with the natural purine bases adenine and guanine showed potency, this compound, a uridine analog, proved to be highly effective. researchgate.net Swapping the nucleoside and prodrug moieties of PSI-7851 (the diastereomeric mixture that contains this compound) and another antiviral, INX-08189, demonstrated that the toxicity of INX-08189 was primarily linked to its nucleoside component, highlighting the importance of the specific nucleoside scaffold in this compound. nih.gov
A variety of β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methylribose nucleosides demonstrated potent inhibition of the HCV NS5B polymerase in clinical studies. nih.gov Ultimately, second-generation compounds were favored for their improved potency and pharmacokinetic profiles, which allowed for once-daily dosing. nih.gov
Table 1: SAR of this compound Analogs
| Analog | Modification | Impact on Activity |
|---|---|---|
| 2'-C-Methyladenosine | 2'-C-methyl group on adenosine | Potent inhibitor of HCV RNA replication researchgate.net |
| 2'-C-Methylguanosine | 2'-C-methyl group on guanosine | Potent inhibitor of HCV RNA replication researchgate.net |
| Increased Steric Bulk at 2'-C | Larger group than methyl at 2'-C position | Loss of inhibitory activity researchgate.net |
| Altered Stereochemistry at 2'-C | Change in the stereo- or regiochemistry of the 2'-methyl substituent | Loss of inhibitory activity researchgate.net |
| INX-08189 | Different nucleoside and prodrug moiety | Showed cell-based toxicity mainly due to the nucleoside component nih.gov |
Prodrug Design Principles: The ProTide Approach and Phosphoramidate Strategy
A significant hurdle in developing nucleoside-based antivirals is their poor cell permeability and the often inefficient initial phosphorylation step required for their activation to the triphosphate form. csic.esresearchgate.net To overcome this, this compound was designed as a phosphoramidate prodrug using the ProTide (Pro-nucleotide) approach. csic.esnih.govwikipedia.org
The ProTide strategy masks the two negative charges of the nucleoside monophosphate with an amino acid ester and an aryloxy group. csic.es This lipophilic modification enhances the molecule's ability to cross cell membranes. acs.orgeg.net Once inside the target cell, these masking groups are enzymatically cleaved to release the 5'-monophosphate, which is then further phosphorylated to the active triphosphate form. researchgate.netwikipedia.org
The activation of this compound involves the following key steps:
Hydrolysis of the carboxyl ester by human cathepsin A (CatA) or carboxylesterase 1 (CES1). researchgate.net
Cleavage of the phosphoramidate bond by histidine triad nucleotide-binding protein 1 (HINT1). wikipedia.org
Subsequent phosphorylations by cellular kinases to form the active triphosphate metabolite, GS-461203. nih.govwikipedia.org
This targeted delivery to the liver, where the necessary metabolic enzymes are abundant, is a key feature of this compound's design. researchgate.net
Stereochemical Considerations in this compound Development
The synthesis of the phosphoramidate prodrug of this compound results in a mixture of two diastereomers at the phosphorus center, designated as Sp and Rp. nih.govacs.org During development, it was discovered that these diastereomers have different biological activities.
PSI-7851 is the diastereomeric mixture of PSI-7976 (the Rp-isomer) and PSI-7977 (the Sp-isomer, which is this compound). researchgate.net Separation of these isomers revealed that PSI-7977 (this compound) was approximately tenfold more active as an inhibitor of HCV RNA replication than PSI-7976. researchgate.netacs.org
The determination of the absolute stereochemistry of the more active diastereomer was crucial. X-ray crystallography of a single crystal of PSI-7977 established its phosphoramidate stereochemistry as Sp. nih.govresearchgate.net This was a significant finding, as it correlated the stereochemistry of a phosphoramidate prodrug with its biological activity for the first time. nih.govresearchgate.net Consequently, the single Sp-diastereomer, this compound, was selected for clinical development. researchgate.net
Computational Chemistry and Molecular Modeling in this compound Design
Computational chemistry and molecular modeling played a significant role in understanding the mechanism of action of this compound and in the broader context of designing HCV inhibitors. anl.govcu.edu.eg These techniques provide insights into the binding of drugs to their target enzymes and can help predict their activity. cu.edu.egresearchgate.net
Molecular docking studies have been used to investigate the interaction of this compound's active triphosphate form (GS-461203) with the HCV NS5B polymerase. drugbank.com These studies help to visualize how the drug fits into the active site of the enzyme and interacts with key amino acid residues, leading to the inhibition of viral RNA synthesis. drugbank.com
Quantitative structure-activity relationship (QSAR) models have also been employed to evaluate the performance of this compound against different HCV genotypes. nih.gov For instance, one study suggested that this compound would have better activity against genotypes 1a and 3b compared to 2b and 4a based on QSAR values. nih.gov
Furthermore, molecular dynamics simulations have been used to explore the stability of this compound within the active site of its target protein. tbzmed.ac.ir These computational approaches are valuable tools in rational drug design, aiding in the optimization of lead compounds and the prediction of their biological properties. cu.edu.eg
Table 2: Chemical Compounds Mentioned
| Compound Name | |
|---|---|
| This compound | |
| PSI-6130 | |
| PSI-7851 | |
| PSI-7976 | |
| PSI-7977 | |
| GS-461203 | |
| INX-08189 | |
| R1479 | |
| Balapiravir | |
| Adenosine | |
| Guanosine | |
| Uridine | |
| Ledipasvir | |
| Daclatasvir | |
| Simeprevir | |
| Velpatasvir | |
| Ribavirin | |
| Amiodarone | |
| Tenofovir alafenamide | |
| Remdesivir | |
| IDX-184 | |
| R7128 | |
| NITD-008 | |
| T-705 (favipiravir) | |
| BMS-986094 | |
| 4'-azidocytidine | |
| 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) | |
| Chloranilic acid (CA) | |
| Ombitasvir | |
| Paritaprevir | |
| Ritonavir | |
| Dasabuvir | |
| Glecaprevir | |
| Asunaprevir | |
| Salicylic acid | |
| Methenamine | |
| Acetylsalicylic acid | |
| Prontosil | |
| Isoniazid | |
| Sulfanilamide | |
| Tafluprost | |
| Isoprinosine | |
| 1-methylimidazole-2-thiol | |
| 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose | |
| N,O-bis(trimethylsilyl)acetamide (BTSA) |
Synthetic Methodologies for Sofosbuvir and Its Precursors
Total Synthesis Routes of Sofosbuvir
One common approach begins with a protected 2'-deoxy-2'-fluoro-2'-C-methyluridine nucleoside. This key intermediate is then coupled with a chiral phosphoramidate reagent. A significant challenge in this step is controlling the stereochemistry at the phosphorus center, as only the (S)-diastereomer of the phosphoramidate is therapeutically active. nih.gov
Another strategy involves the use of Grignard reagents in the formation of key intermediates. While effective, the exothermic nature of Grignard reactions requires careful management, often through dosing-controlled semi-batch processes on an industrial scale. researchgate.net
Researchers have also explored alternative coupling methods. One such method involves the condensation of a protected fluorinated sugar with a uracil derivative, followed by hydrolysis and deprotection to yield the core nucleoside. This nucleoside is then reacted with a phosphorochloridate derivative to form the final phosphoramidate linkage. google.com
The table below outlines a selection of reported total synthesis routes, highlighting the diversity of approaches taken.
| Starting Material | Key Steps | Overall Yield | Reference |
| 2-deoxy-2-fluoro-2-methyl-pentonic acid γ-lactone | Benzyl protection, dynamic kinetic resolution | 40% (8 steps) | researchgate.net |
| Protected fluorinated sugar and uracil derivative | Condensation, hydrolysis, deprotection, phosphoramidation | Not specified | google.com |
| 2'-deoxy-2'-fluoro-2'-C-methyluridine | Coupling with a chiral phosphoramidate reagent | Not specified | chemicalbook.com |
Synthesis of Key Intermediates and Chiral Building Blocks
The efficient synthesis of this compound relies heavily on the preparation of key intermediates and chiral building blocks. researchgate.net The core nucleoside, 2'-deoxy-2'-fluoro-2'-C-methyluridine, and the chiral phosphoramidate side chain are the two primary components. researchgate.netnih.gov
Synthesis of the Core Nucleoside:
A crucial intermediate in many synthetic routes is 2'-deoxy-2'-fluoro-2-methyl-D-ribose-gamma-lactone. google.com One approach to this intermediate starts with (2S,3R)-ethyl-3-((R)-2,2-dimethyl-1,3-dioxocyclopent-4-yl)-2,3-dihydroxy-2-methyl propionate. This starting material undergoes cyclization, oxidation, fluorination, hydrolysis, and benzoylation to yield the target lactone. patsnap.com
Another strategy for accessing the core nucleoside involves a de novo approach. This method features an enantioselective α-oxidation reaction followed by a Mukaiyama aldol coupling to construct the pentose skeleton. acs.orgnih.gov This strategy provides a flexible platform for creating various C-nucleosides and fluorinated pentoses, including the precursor to PSI-6130, the nucleoside core of this compound. acs.orgnih.gov
Synthesis of the Chiral Phosphoramidate:
The chiral phosphoramidate portion of this compound is essential for its biological activity. nih.gov The synthesis of this component, typically isopropyl (S)-2-((chloro(phenoxy)phosphoryl)amino)propanoate, presents its own stereochemical challenges. One method to achieve the desired stereoisomer is through the kinetic resolution of a racemic mixture. nih.gov
Enzymatic methods have proven effective in this resolution. For example, an engineered phosphotriesterase from Pseudomonas diminuta can be used for the kinetic resolution of the phosphoramidate intermediate. rsc.org This enzyme selectively hydrolyzes the undesired (R)-diastereomer, leaving the desired (S)-diastereomer in high purity. nih.govrsc.org
Development of Novel and Efficient Synthetic Approaches
Continuous efforts are being made to develop more efficient, cost-effective, and scalable synthetic routes for this compound. researchgate.net These novel approaches often focus on improving stereoselectivity, reducing the number of steps, and minimizing waste.
Another area of development is the use of novel catalysts. For instance, a dimeric catalyst has been developed for the stereoselective direct coupling of nucleosides to chlorophosphoramidates. epa.gov This catalyst system has been shown to be highly effective in the synthesis of uprifosbuvir, a related ProTide drug, and holds potential for greening the synthesis of other ProTides like this compound. epa.gov
The exploration of alternative reaction pathways, such as those involving Mitsunobu reactions, has also been investigated to circumvent the formation of isomers and improve yields. patsnap.com Furthermore, processes utilizing intermediates like crystalline N-Benzoyl this compound have been developed to streamline the synthesis and purification process. google.comgoogle.com
The table below summarizes some novel synthetic approaches and their key features.
| Approach | Key Feature | Advantage | Reference |
| Dynamic Kinetic Resolution | Control of stereochemistry during phosphorylation | High stereoselectivity without resolution | researchgate.net |
| Dimeric Catalyst | Stereoselective direct coupling | High purity in fewer steps | epa.gov |
| Mitsunobu Reaction | Avoidance of isomer formation | Improved yield | patsnap.com |
| Crystalline Intermediates | Use of N-Benzoyl this compound | Streamlined synthesis and purification | google.comgoogle.com |
Green Chemistry Principles Applied to this compound Synthesis
A significant advancement in this area is the development of a catalytic process for the synthesis of ProTide drugs, including those structurally similar to this compound. Merck received the 2020 Greener Reaction Conditions Award from the U.S. Environmental Protection Agency for developing a multifunctional catalyst that stereoselectively assembles ProDrugs. epa.gov This new process improved the manufacturing efficiency of an important antiviral by over 85%, significantly reducing waste and hazards. epa.gov
Key aspects of this greener approach include:
Catalyst Development: The identification and optimization of a dimeric catalyst for the direct and stereoselective coupling of nucleosides with chlorophosphoramidates. This eliminates the need for chiral reagents and reduces the number of reaction steps. epa.gov
Solvent Replacement: The replacement of hazardous solvents like dichloromethane with more environmentally benign alternatives such as 1,3-dioxolane. epa.gov
Furthermore, biocatalysis has emerged as a valuable tool for promoting greener manufacturing processes in the pharmaceutical industry. nih.gov The use of enzymes, such as engineered phosphotriesterases for the kinetic resolution of phosphoramidate intermediates, offers advantages in terms of specificity, efficiency, and environmental friendliness compared to traditional chemical methods. nih.govrsc.org These enzymatic processes often operate under milder conditions and can significantly reduce the generation of chemical waste.
These developments highlight a clear trend towards incorporating green chemistry principles into the synthesis of complex pharmaceuticals like this compound, aiming for more sustainable and cost-effective production methods.
Molecular and Biochemical Mechanisms of Sofosbuvir Action
Inhibition of HCV NS5B RNA-Dependent RNA Polymerase (RdRp)
The primary mechanism of sofosbuvir's antiviral activity is the inhibition of the hepatitis C virus non-structural protein 5B (NS5B) RNA-dependent RNA polymerase (RdRp). drugbank.comdroracle.ai This enzyme is essential for the replication of the viral RNA genome. patsnap.com this compound, a nucleotide analog prodrug, is designed to interfere with this process, ultimately suppressing viral propagation. drugbank.comnih.gov
This compound is administered as a prodrug, meaning it is inactive upon administration and must be converted into its pharmacologically active form within the host's cells. droracle.aiwikipedia.org Following oral administration, this compound is absorbed and undergoes extensive metabolism within hepatocytes, the primary site of HCV replication. patsnap.comwikipedia.org
This multi-step intracellular activation pathway begins with the hydrolysis of the carboxyl ester moiety by human cathepsin A (CatA) or carboxylesterase 1 (CES1). wikipedia.orgtga.gov.au This is followed by the cleavage of the phosphoramidate portion by histidine triad nucleotide-binding protein 1 (HINT1). wikipedia.orgtga.gov.au The resulting molecule then undergoes a series of phosphorylations by cellular kinases, specifically uridine monophosphate-cytidine monophosphate (UMP-CMP) kinase and nucleoside diphosphate (NDP) kinase. drugbank.com This enzymatic cascade culminates in the formation of the active uridine analog triphosphate, GS-461203 (also known as PSI-7409). drugbank.comnih.govnih.gov This active metabolite mimics the natural uridine triphosphate that the viral polymerase uses to build new RNA strands. patsnap.com
The active triphosphate metabolite of this compound, GS-461203, acts as a competitive inhibitor of the HCV NS5B polymerase. wjgnet.com The NS5B polymerase has a highly conserved active site, characterized by a "GDD" (glycine-aspartate-aspartate) motif, which is critical for its catalytic function. cu.edu.egasm.org This motif, along with two magnesium ions (Mg2+), facilitates the nucleotidyl transfer reaction required for RNA synthesis. drugbank.comcu.edu.eg GS-461203 specifically binds to this active site, effectively competing with the natural nucleotide substrates. drugbank.commedex.com.bd
Table 1: Key Molecular Interactions of this compound's Active Metabolite
| Interacting Molecule | Binding Site | Key Features of Interaction |
| GS-461203 | HCV NS5B Polymerase Active Site | Binds to the GDD motif and two Mg2+ ions. drugbank.comcu.edu.eg |
| GS-461203 | HCV NS5B Polymerase Active Site | Competes with natural uridine triphosphate for binding. wjgnet.com |
Once GS-461203 is incorporated into the elongating viral RNA strand by the NS5B polymerase, it functions as a chain terminator. patsnap.comdrugbank.comdroracle.ai Although it possesses a 3'-hydroxyl group, which is typically required for the addition of the next nucleotide, the presence of a methyl group at the 2' position of the ribose sugar creates a steric clash with the incoming nucleotide. wikipedia.orgresearchgate.net This steric hindrance prevents the formation of the subsequent phosphodiester bond, thereby halting further elongation of the RNA chain. researchgate.net The premature termination of viral RNA synthesis effectively disrupts the production of new, viable viral genomes. patsnap.com
Specific Binding Interaction with the NS5B Active Site (GDD Motif)
Molecular Basis of Pan-Genotypic Antiviral Activity
Hepatitis C virus is classified into several distinct genotypes. drugbank.com A significant advantage of this compound is its pan-genotypic activity, meaning it is effective against multiple HCV genotypes. wjgnet.comnih.gov This broad-spectrum efficacy is attributed to the high degree of conservation of the NS5B polymerase active site across different HCV genotypes. wjgnet.comresearchgate.net The critical residues within the active site, including the GDD motif, are largely identical, ensuring that the active metabolite of this compound can effectively bind to and inhibit the polymerase from various genotypes. asm.org In vitro studies have demonstrated this compound's activity against genotypes 1, 2, 3, 4, 5, and 6. drugbank.comnih.gov
Table 2: In Vitro Activity of this compound Across HCV Genotypes
| HCV Genotype | Activity | Reference |
| Genotype 1 | Effective | drugbank.comnih.gov |
| Genotype 2 | Effective | drugbank.comnih.gov |
| Genotype 3 | Effective | drugbank.comnih.gov |
| Genotype 4 | Effective | drugbank.comnih.gov |
| Genotype 5 | Effective | drugbank.com |
| Genotype 6 | Effective | drugbank.com |
Selectivity Towards Viral Polymerases Versus Host Cellular Polymerases
An essential characteristic of an effective antiviral agent is its selectivity for viral targets over host cellular machinery, which minimizes toxicity. patsnap.com this compound exhibits a high degree of selectivity for the HCV NS5B polymerase over human DNA and RNA polymerases. tga.gov.aunih.gov The active triphosphate metabolite, GS-461203, does not significantly inhibit human DNA polymerases α and β, nor mitochondrial RNA polymerase. asm.org This selectivity is crucial as RNA-dependent RNA polymerization is not a catalytic activity found in human polymerases, thus reducing the potential for off-target effects and contributing to this compound's favorable safety profile. patsnap.comresearchgate.net
Pharmacology of Sofosbuvir at the Molecular and Cellular Level
Intracellular Metabolism and Activation Pathways
The activation of sofosbuvir is a multi-step process that occurs within hepatocytes, the primary target cells for HCV. europa.eutaylorandfrancis.com The parent drug, this compound, accounts for only about 4% of the drug-related material systemic exposure. europa.eu The metabolic activation pathway is characterized by low affinity and high capacity hydrolase and nucleotide phosphorylation pathways. who.int
Role of Hydrolases (Cathepsin A and Carboxylesterase 1)
The initial step in this compound's activation involves the hydrolysis of its carboxyl ester moiety. tga.gov.au This reaction is catalyzed by two key human hydrolases: Cathepsin A (CatA) and Carboxylesterase 1 (CES1). drugbank.comamazonaws.com Both enzymes are responsible for cleaving the ester bond, a critical step for the subsequent metabolic events. tga.gov.auwikiwand.com In vitro studies have shown that both CatA and CES1 efficiently hydrolyze this compound. drugbank.com While CES1 is considered a major enzyme for this hydrolysis due to its high abundance in the liver, CatA also plays a significant role. semanticscholar.org In fact, in vitro data suggest that CatA preferentially hydrolyzes the S-diastereomer of this compound, which is the active component, whereas CES1 does not show such stereoselectivity. drugbank.com
Involvement of Phosphoramidase (Histidine Triad Nucleotide-Binding Protein 1 - HINT1)
Following the initial hydrolysis, the next crucial step is the cleavage of the phosphoramidate bond. tga.gov.augoogle.com This reaction is carried out by the enzyme Histidine Triad Nucleotide-Binding Protein 1 (HINT1), which acts as a phosphoramidase. nih.govosti.gov HINT1 is essential for removing the amino acid portion of the prodrug, which then yields the nucleoside monophosphate. nih.govresearchgate.net The involvement of HINT1 is a critical activation step, and studies where HINT1 was knocked down have shown a reduction in the intracellular formation of the corresponding nucleoside monophosphate from this compound. nih.gov
Sequential Phosphorylation by Nucleotide Kinases (UMP-CMP Kinase, NDP Kinase)
The final stage of this compound's activation is a two-step sequential phosphorylation process. europa.eudrugbank.com After the formation of the nucleoside monophosphate by HINT1, it is first phosphorylated to the diphosphate form by UMP-CMP kinase (UMP-CMPK). drugbank.comsemanticscholar.org Subsequently, the diphosphate is converted to the active triphosphate metabolite, GS-461203, by nucleoside diphosphate kinase (NDPK). drugbank.comsemanticscholar.org This active triphosphate is what ultimately gets incorporated into the replicating HCV RNA chain, leading to chain termination and inhibition of viral replication. europa.eu
Characterization of Inactive Metabolites (e.g., GS-331077)
During the metabolic processing of this compound, not all of the parent drug is converted to the active triphosphate form. Dephosphorylation of the various nucleotide intermediates results in the formation of the nucleoside metabolite GS-331077. tga.gov.au This metabolite is considered inactive as it cannot be efficiently rephosphorylated to the active triphosphate form and lacks anti-HCV activity in vitro. tga.gov.autga.gov.au GS-331007 is the predominant circulating metabolite, accounting for over 90% of the total systemic drug-related material exposure following oral administration of this compound. europa.euwho.int The peak plasma concentration of GS-331007 is typically observed between 2 to 4 hours post-dose. europa.eu While this compound has a relatively short half-life of about 0.4 hours, its inactive metabolite GS-331077 has a much longer half-life of 27 hours. wikiwand.commagicinepharma.com
Membrane Transport Mechanisms: P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP) Substrate Interactions
This compound itself is a substrate for the efflux transporters P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP). europa.eunih.gov These transporters are present in the intestines and can pump this compound back into the gut, potentially reducing its absorption. wikiwand.com Therefore, potent inducers of intestinal P-gp, such as rifampicin and St. John's wort, can significantly decrease the plasma concentration of this compound, leading to a reduced therapeutic effect. europa.eutga.gov.au Conversely, co-administration with drugs that inhibit P-gp and/or BCRP may increase this compound plasma concentrations. europa.eu However, the major circulating inactive metabolite, GS-331077, is not a substrate for P-gp or BCRP. europa.eugilead.com Neither this compound nor GS-331007 act as inhibitors of P-gp and BCRP. europa.eu
Hepatic Metabolism and Endogenous Enzyme Involvement (Excluding Cytochrome P450)
The metabolism of this compound is primarily a hepatic process, but it notably does not involve the cytochrome P450 (CYP450) enzyme system. amazonaws.comnih.gov this compound is not metabolized by CYP450 isoenzymes, and it does not induce or inhibit these enzymes. nih.govoup.com This characteristic minimizes the potential for drug-drug interactions with medications that are metabolized by the CYP450 pathway. semanticscholar.orghep-druginteractions.org The key endogenous enzymes involved in its hepatic metabolism are the hydrolases (Cathepsin A and Carboxylesterase 1) and the phosphoramidase (HINT1), followed by the endogenous nucleotide kinases (UMP-CMPK and NDPK) that are part of the pyrimidine nucleotide biosynthesis pathway. tga.gov.autaylorandfrancis.com The entire metabolic activation process, from the parent drug to the active triphosphate, occurs within the hepatocytes. europa.eutaylorandfrancis.com
Plasma Protein Binding Characteristics
The interaction of this compound and its metabolites with plasma proteins is a critical factor in its pharmacokinetic profile, influencing its distribution and availability to target tissues.
Detailed Research Findings
This compound itself is moderately bound to human plasma proteins, with reported binding percentages ranging from 61% to 65%. nih.govtga.gov.auwikipedia.orgdrugbank.comtga.gov.autga.gov.aunih.govnih.gov This binding is independent of the drug concentration within the range of 1 to 20 µg/mL. nih.govtga.gov.autga.gov.autga.gov.aunih.gov In contrast, its major circulating metabolite, GS-331007, exhibits minimal binding to plasma proteins. nih.govtga.gov.auwikipedia.orgtga.gov.autga.gov.aunih.gov This low level of protein binding for GS-331007 means that a larger fraction of the metabolite is free in the plasma and available for distribution and elimination. nih.govtga.gov.auwikipedia.org
Another metabolite, GS-566500, has a plasma concentration-time profile similar to that of the parent drug, this compound. tga.gov.aufda.gov While detailed binding characteristics for GS-566500 are less extensively documented, its presence in plasma alongside this compound and the primary metabolite GS-331007 has been confirmed in pharmacokinetic studies. tga.gov.aufda.gov
The binding of this compound is primarily to human plasma proteins, although specific protein interactions, such as with albumin and alpha-1-acid glycoprotein, are not as extensively detailed in the provided literature as for some other antiviral agents. drugbank.com For context, other direct-acting antivirals used in hepatitis C therapy, such as simeprevir, are highly bound to plasma proteins, primarily albumin and to a lesser extent, alpha-1-acid glycoprotein. drugbank.com
The following table summarizes the plasma protein binding characteristics of this compound and its primary metabolite:
| Compound | Plasma Protein Binding (%) | Concentration Dependency |
| This compound | 61-65% nih.govtga.gov.auwikipedia.orgdrugbank.comtga.gov.autga.gov.aunih.govnih.gov | Independent (1-20 µg/mL) nih.govtga.gov.autga.gov.autga.gov.aunih.gov |
| GS-331007 | Minimal nih.govtga.gov.auwikipedia.orgtga.gov.autga.gov.aunih.gov | Not applicable |
Mechanisms of Resistance to Sofosbuvir at the Molecular Level
Molecular Basis of NS5B Mutations Conferring Reduced Susceptibility (e.g., S282T)
The primary resistance-associated substitution (RAS) that confers reduced susceptibility to sofosbuvir is the S282T mutation in the NS5B RNA-dependent RNA polymerase. oup.comresearchgate.net This substitution involves the change of a serine (S) residue to a threonine (T) at position 282 of the NS5B protein. oup.com The S282 residue is located within the highly conserved catalytic site of the polymerase, the area responsible for viral RNA replication. researchgate.netnih.gov
The molecular mechanism of resistance is based on steric hindrance. researchgate.net The active form of this compound, a uridine nucleotide analog triphosphate, features a methyl group at the 2' position of its ribose sugar. The substitution of serine with the bulkier threonine at position 282 introduces a methyl group on the amino acid's side chain. researchgate.netnih.gov Structural analyses and modeling suggest that the methyl group of the T282 side chain sterically clashes with the 2'-C-methyl group of the incoming this compound analog. researchgate.netnih.gov This conflict is believed to hinder the proper positioning and subsequent incorporation of the drug into the nascent viral RNA chain, thereby reducing the drug's inhibitory effect. researchgate.net
Recent biochemical studies have further elucidated this mechanism, highlighting a complex network of hydrogen bonds. nih.gov The S282 residue normally forms a hydrogen bond with the 2'-hydroxyl of the incoming nucleotide substrate. The S282T mutation disrupts this crucial interaction network, which also involves the adjacent G283 residue and the template RNA strand, ultimately affecting the binding and incorporation of this compound. nih.gov
While the S282T mutation is the signature RAS for this compound, it confers only a modest level of resistance, typically causing a 2- to 19-fold decrease in susceptibility in vitro. researchgate.netmdpi.com Crucially, this mutation significantly impairs the replication fitness of the virus, making it less viable. nih.gov This high fitness cost is a primary reason why the S282T mutation is rarely detected in patients who experience treatment failure in clinical trials. nih.govnih.govmdpi.com
Structural and Molecular Simulation Studies of Resistance-Associated Mutations in NS5B
Structural biology and computational simulations provide atomic-level insights into the mechanisms of drug resistance. X-ray crystallography studies of the HCV NS5B polymerase have been instrumental in visualizing the enzyme's active site. These studies confirm that the S282 residue is positioned strategically to interact with the incoming nucleotide substrate. researchgate.netnih.gov The structural models clearly show how the substitution of serine with the bulkier threonine at this position would create a steric conflict with the 2'-methyl group of the this compound molecule, providing a physical basis for the observed resistance. researchgate.net
Molecular dynamics (MD) simulations, which model the movements and interactions of atoms over time, have further corroborated and expanded upon these structural findings. semanticscholar.orgresearchgate.net These computational studies have demonstrated that:
The S282T mutation can destabilize the binding of the active this compound triphosphate within the polymerase's catalytic site. semanticscholar.orgresearchgate.net
Free energy calculations based on MD simulations have shown that a greater amount of energy is required for the this compound triphosphate to move through the nucleotide entry tunnel of the polymerase when certain mutations, like A218S, are present. researchgate.net
Simulations comparing the NS5B polymerase from this compound-responder and resistant clinical samples have revealed differences in the conformational stability of the drug-enzyme complex, as measured by the Root Mean Square Deviation (RMSD), further highlighting the impact of mutations on drug binding. semanticscholar.orgresearchgate.net
These simulation studies also reinforce the importance of the intricate network of hydrogen bonds between the polymerase (at residues S282 and the adjacent G283), the incoming nucleotide, and the RNA template strand. nih.gov The S282T mutation disrupts this delicate balance, which is essential for both efficient polymerase activity and susceptibility to this compound. nih.gov
Analytical Chemistry of Sofosbuvir in Research and Quality Control
Quantitative Determination Methods for Sofosbuvir and its Metabolites in Research Matrices
Accurate quantification of this compound and its metabolites in complex biological matrices is essential for pharmacokinetic and bioequivalence studies. A range of analytical techniques has been developed and validated for this purpose.
Chromatographic methods are the cornerstone for the analysis of this compound and its metabolites due to their high resolving power and sensitivity.
High-Performance Liquid Chromatography (HPLC) and Ultra-Performance Liquid Chromatography (UPLC):
Reverse-phase high-performance liquid chromatography (RP-HPLC) is a widely used technique for the determination of this compound. These methods are often validated according to the International Conference on Harmonisation (ICH) guidelines. nih.govsciensage.infoijpsnonline.comjcdronline.orgijbpas.com A typical RP-HPLC method might utilize a C18 column and a mobile phase consisting of a mixture of an aqueous buffer (like phosphate or ammonium acetate) and an organic solvent (such as acetonitrile or methanol). nih.govjcdronline.org For instance, one validated RP-HPLC method for the simultaneous estimation of this compound and velpatasvir employed a Promosil C18 column with a mobile phase of ammonium acetate buffer (pH 7.0), acetonitrile, and methanol in a 20:40:40 v/v/v ratio, with UV detection at 260 nm. nih.gov
Ultra-Performance Liquid Chromatography (UPLC), which uses smaller particle size columns, offers faster analysis times and improved resolution compared to traditional HPLC. UPLC methods have been successfully developed for the simultaneous determination of this compound and its primary metabolite, GS-331007, in human plasma. outbreak.info
Liquid Chromatography-Mass Spectrometry (LC-MS):
Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) is a highly sensitive and selective technique for the quantification of this compound and its metabolites in biological fluids. outbreak.info These methods often employ electrospray ionization (ESI) in the positive ion mode and multiple reaction monitoring (MRM) for detection. For example, a UPLC-ESI-MS/MS method was developed for the simultaneous estimation of this compound and its metabolite GS-331007 in human plasma, achieving high sensitivity and a short run time. outbreak.info The structure of this compound has been unambiguously confirmed by various spectroscopic methods including mass spectrometry. europa.eu
Interactive Table: Chromatographic Conditions for this compound Analysis
| Technique | Column | Mobile Phase | Flow Rate | Detection | Analyte(s) | Retention Time(s) (min) |
| RP-HPLC | Promosil C18 | Ammonium acetate buffer (pH 7.0), acetonitrile, methanol (20:40:40, v/v/v) | 1.0 mL/min | UV at 260 nm | This compound, Velpatasvir | 3.72 (this compound), 7.09 (Velpatasvir) nih.gov |
| RP-HPLC | Agilent Eclipse® XDB-C18 (4.6×250 mm, 5µm) | Gradient with 0.03M KH2PO4 (pH 2.5) | 1 mL/min | UV at 260 nm | This compound | 3.166 ijpsnonline.com |
| RP-HPLC | Phenomenex Luna® LC C18 (150×4.6mm, 5µm) | Acetonitrile: Methanol: Water (50:30:20 v/v/v) | 1 mL/min | UV at 260 nm | This compound | 2.1 sciensage.info |
UV-Visible spectrophotometry offers a simpler and more cost-effective alternative for the quantification of this compound in bulk and pharmaceutical dosage forms. ijbpas.com The method is based on the measurement of the absorbance of this compound at its wavelength of maximum absorption (λmax), which is typically around 260 nm. sciensage.infoijbpas.com These methods are validated for linearity, accuracy, and precision as per ICH guidelines. ijbpas.com For instance, a developed UV spectrophotometric method for this compound estimation used a mixture of acetonitrile and methanol (30:70) as a solvent and demonstrated linearity in the concentration range of 5-25 µg/ml. ijbpas.com
Interactive Table: UV-Vis Spectrophotometric Methods for this compound
| Method | Solvent | λmax (nm) | Linearity Range (µg/ml) | Correlation Coefficient (r²) |
| UV Spectrophotometry | Acetonitrile: Methanol (30:70) | 260 | 5 - 25 | 0.9991 ijbpas.com |
| UV Spectrophotometry | Not specified | 260 | 40 - 240 | Not specified ijpsnonline.com |
Electro-analytical techniques, particularly voltammetric methods, have emerged as sensitive and rapid alternatives for the determination of this compound. tandfonline.comresearchgate.net These methods are based on the electrochemical oxidation or reduction of the analyte at the surface of a modified electrode.
Several studies have reported the development of electrochemical sensors for this compound quantification. tandfonline.comrsc.org One such sensor utilized a glassy carbon electrode modified with multi-walled carbon nanotubes, reduced graphene oxide, and ortho-aminophenol, employing differential pulse voltammetry (DPV) for analysis. tandfonline.com Another approach involved the development of a molecularly imprinted polymer (MIP) based sensor on a pencil graphite electrode, which indirectly detects this compound by measuring the change in the signal of a redox probe. rsc.orgrsc.orgresearchgate.net These sensors have demonstrated high sensitivity with low detection limits and have been successfully applied to determine this compound in pharmaceutical formulations and biological samples like urine. tandfonline.comrsc.orgresearchgate.net
Interactive Table: Electro-analytical Methods for this compound Determination
| Method/Sensor | Electrode | Technique | Linearity Range | Limit of Detection (LOD) |
| Biomimetic electrochemical sensor | Modified glassy carbon electrode (MWCNT, RGO, OAP) | DPV | 0.53–74.13 ng/mL | 0.05 ng/mL tandfonline.com |
| Molecularly Imprinted Polymer (MIP) Sensor | Modified pencil graphite electrode | DPV (indirect) | 1.0 × 10⁻¹³ M to 1.0 × 10⁻¹¹ M | 3.1 × 10⁻¹⁴ M rsc.orgresearchgate.net |
Spectrophotometric Methods (UV-Vis)
Purity Profiling and Identification of Related Substances/Impurities
Purity profiling is a crucial aspect of quality control for active pharmaceutical ingredients (APIs) and finished drug products. It involves the identification and quantification of impurities, which can originate from the manufacturing process or degradation. Regulatory bodies like the EMA have specifications for impurities in this compound. europa.eu
Several process-related impurities of this compound have been identified. One such impurity is this compound impurity A, which is a diastereoisomer of the parent drug. vulcanchem.com Another identified process-related impurity is a phosphoryl impurity. RP-HPLC methods have been developed to separate and quantify these impurities from the main compound. For instance, a method using an Agilent Eclipse XDB-C18 column with a mobile phase of 0.1% trifluoroacetic acid in water and acetonitrile (50:50) was able to separate this compound from its phosphoryl impurity. vulcanchem.com The structures of these impurities are often confirmed using techniques like NMR and mass spectrometry. europa.euscirp.org
Interactive Table: Known Impurities of this compound
| Impurity Name | Type | Molecular Formula | Molecular Weight ( g/mol ) |
| This compound Impurity A | Diastereoisomer | C₂₂H₂₉FN₃O₉P | 529.45 vulcanchem.com |
| This compound Impurity B | Process-related | C₁₆H₁₈FN₂O₈P | 416.08 |
| Phosphoryl Impurity | Process-related | Not specified | Not specified |
Forced Degradation Studies and Chemical Stability Assessment
Forced degradation studies are essential to understand the chemical stability of a drug substance and to develop stability-indicating analytical methods. ijper.org These studies involve subjecting the drug to various stress conditions as per ICH guidelines, such as acid and base hydrolysis, oxidation, heat, and light. ijper.org
This compound has been shown to be susceptible to degradation under acidic, basic, and oxidative conditions, while it remains relatively stable under thermal and photolytic stress. scirp.orgijper.org
Acid Hydrolysis: Degradation of this compound is observed in the presence of acid (e.g., 1N HCl at 80°C), leading to the formation of specific degradation products. scirp.org One identified acid degradation product has a molecular weight of 416.08. scirp.org
Base Hydrolysis: this compound shows significant degradation under basic conditions (e.g., 0.5N NaOH at 60°C). scirp.org Two base degradation impurities, Impurity-A (MW 453.13) and Impurity-B (MW 411.08), have been isolated and characterized. scirp.org
Oxidative Degradation: Treatment with oxidizing agents like hydrogen peroxide also results in the degradation of this compound. scirp.orgijper.org An oxidative degradation product with a molecular weight of 527.15 has been identified. scirp.org
The degradation products are typically separated using HPLC and characterized by mass spectrometry (MS) to elucidate their structures and establish the degradation pathways. scirp.orgijper.orgresearchgate.net
Interactive Table: Summary of Forced Degradation Studies of this compound
| Stress Condition | Reagent/Condition | Observed Degradation | Identified Degradation Products (Molecular Weight) |
| Acid Hydrolysis | 1N HCl, 80°C, 10 h | 8.66% scirp.org | 416.08 scirp.org |
| Base Hydrolysis | 0.5N NaOH, 60°C, 24 h | 45.97% scirp.org | Impurity-A (453.13), Impurity-B (411.08) scirp.org |
| Oxidative Degradation | 30% H₂O₂, 80°C, 2 days | 0.79% scirp.org | 527.15 scirp.org |
| Thermal Degradation | Dry heat | Stable scirp.org | - |
| Photolytic Degradation | UV light | Stable scirp.org | - |
Validation of Analytical Methods According to Academic and Regulatory Guidelines
The validation of analytical methods is a mandatory requirement by regulatory authorities like the ICH and FDA to ensure that the methods are reliable, reproducible, and suitable for their intended purpose. nih.govsciensage.infoijpsnonline.comjcdronline.orgijbpas.com The validation process for analytical methods used for this compound encompasses several key parameters:
Specificity: The ability of the method to accurately measure the analyte in the presence of other components such as impurities, degradation products, and matrix components. sciensage.infojcdronline.org
Linearity: The method's ability to elicit test results that are directly proportional to the concentration of the analyte within a given range. sciensage.infoijpsnonline.comijbpas.com
Accuracy: The closeness of the test results obtained by the method to the true value. It is often determined by recovery studies. sciensage.infoijbpas.com
Precision: The degree of agreement among individual test results when the method is applied repeatedly to multiple samplings of a homogeneous sample. This includes repeatability (intra-day precision) and intermediate precision (inter-day precision). sciensage.infoijbpas.com
Limit of Detection (LOD): The lowest amount of analyte in a sample that can be detected but not necessarily quantitated as an exact value. sciensage.info
Limit of Quantitation (LOQ): The lowest amount of analyte in a sample that can be quantitatively determined with suitable precision and accuracy. sciensage.info
Robustness: A measure of the method's capacity to remain unaffected by small, but deliberate variations in method parameters, providing an indication of its reliability during normal usage. sciensage.info
Numerous studies on the analytical methods for this compound consistently report successful validation of these parameters according to ICH guidelines, demonstrating the reliability of the developed methods for quality control and research purposes. nih.govsciensage.infoijpsnonline.comjcdronline.orgijbpas.com
Interactive Table: Validation Parameters for a Typical RP-HPLC Method for this compound
| Validation Parameter | This compound |
| Linearity Range (µg/mL) | 10-50 sciensage.info |
| Correlation Coefficient (r²) | 0.999 sciensage.info |
| Accuracy (% Recovery) | 98.8-100.5% sciensage.info |
| Precision (%RSD) | < 2% sciensage.info |
| LOD (µg/mL) | 1.87 sciensage.info |
| LOQ (µg/mL) | 5.65 sciensage.info |
Preclinical Development and Analog Exploration of Sofosbuvir
In Vitro Antiviral Efficacy Models (e.g., HCV Replicon Assays) for Lead Characterization
The preclinical evaluation of sofosbuvir's antiviral activity heavily relied on in vitro models, particularly the hepatitis C virus (HCV) replicon system. researchgate.net These systems are instrumental in determining a compound's effectiveness in inhibiting viral replication within a cellular context.
HCV replicon assays utilize liver cell lines, such as Huh-7 cells, that have been engineered to contain a self-replicating HCV RNA molecule (a replicon). nih.govasm.org These replicons often include a reporter gene, like luciferase, which allows for the quantification of viral replication levels. nih.gov A decrease in reporter gene activity in the presence of a test compound indicates inhibition of HCV replication.
In these assays, this compound demonstrated potent and broad-spectrum anti-HCV activity. The effective concentration (EC50) values, which represent the concentration of the drug that inhibits 50% of viral replication, were determined for various HCV genotypes. For full-length replicons, the EC50 values for this compound were 0.04 µM for genotype 1a, 0.11 µM for genotype 1b, 0.05 µM for genotype 2a, 0.05 µM for genotype 3a, and 0.04 µM for genotype 4a. europa.eu Furthermore, against chimeric 1b replicons that contained the NS5B gene from genotypes 2b, 5a, or 6a, the EC50 values ranged from 0.014 to 0.015 µM. europa.eueuropa.eu The in vitro antiviral activity of this compound against the less common genotypes 4, 5, and 6 was found to be similar to that observed for genotypes 1, 2, and 3. europa.eu
The presence of 40% human serum did not affect the anti-HCV activity of this compound. europa.eueuropa.eu Reduced susceptibility to this compound has been linked to the S282T substitution in the NS5B protein across multiple genotypes in cell culture. europa.eueuropa.euresearchgate.net Site-directed mutagenesis of this S282T substitution in replicons of eight different genotypes resulted in a 2- to 18-fold decrease in susceptibility to this compound. europa.eueuropa.eu
Table 1: In Vitro Antiviral Activity of this compound in HCV Replicon Assays
| HCV Genotype/Replicon | EC50 (µM) |
| Genotype 1a | 0.04 |
| Genotype 1b | 0.11 |
| Genotype 2a | 0.05 |
| Genotype 3a | 0.05 |
| Genotype 4a | 0.04 |
| Chimeric 1b (with NS5B from 2b, 5a, or 6a) | 0.014 - 0.015 |
Design and Synthesis of Novel this compound Analogs for Improved Activity and Profile
Researchers have explored various modifications to the this compound scaffold, including changes to the nucleobase, the sugar moiety, and the phosphoramidate group. acs.orgnih.gov For instance, the synthesis of pyridine and pyrimidine-based thioglycoside phosphoramidates as this compound thio-analogs has been reported. acs.orgnih.gov While these initial thio-analogs showed low to moderate activity against a range of viruses, certain compounds demonstrated notable potential, suggesting that further optimization could lead to improved efficacy. nih.gov
Another area of exploration involves the introduction of different functional groups at various positions of the nucleoside. biorxiv.org Strategies include introducing artificial nucleobases, simplifying the ribose sugar, or adding groups like fluorine or methyl to the sugar core. biorxiv.org For example, the development of 4′-azido-2′-deoxy-2′-fluorocytidine and 4′-azido-2′-dideoxy-2′,2′-difluorocytidine were early explorations in modifying the sugar moiety to enhance anti-HCV activity. nih.gov The goal of these synthetic efforts is to create analogs with increased potency, a higher barrier to resistance, and an improved safety profile. mdpi.com
Table 2: Antiviral Activity of Selected this compound Thio-analogs
| Compound | Target Virus | IC50 (µ g/100 µL) | CC50 (µ g/100 µL) |
| 5b | Coxsackie virus B4 | 4.5 | 17 |
| 11 | Coxsackie virus B4 | 6.0 | 20 |
| 5b | Herpes simplex virus type 1 | 6.3 | 17 |
| 11 | Herpes simplex virus type 1 | 6.6 | 16 |
| 11 | Hepatitis C virus | - | - |
IC50: 50% inhibitory concentration; CC50: 50% cytotoxic concentration. Data from acs.orgnih.gov.
Structure-Based Drug Discovery and Lead Optimization Strategies
Structure-based drug design (SBDD) has been a cornerstone in the development and optimization of antiviral agents like this compound. nih.govnumberanalytics.com This approach utilizes the three-dimensional structure of the viral target, in this case, the HCV NS5B polymerase, to design molecules that can bind with high affinity and specificity. nih.govdomainex.co.uk
The process of SBDD involves several key steps:
Target Identification and Validation: The HCV NS5B RNA-dependent RNA polymerase was identified as a crucial enzyme for viral replication, making it an attractive drug target. nih.govnih.gov
Structural Determination: High-resolution structural information of the NS5B polymerase, often obtained through X-ray crystallography, provides a detailed map of the active site and other potential binding pockets. anl.gov
In Silico Screening and Molecular Docking: Computational techniques are used to screen large libraries of compounds to identify potential inhibitors that can fit into the target's binding site. nih.gov Molecular docking predicts the binding mode and affinity of these compounds. numberanalytics.com
Lead Optimization: Once a lead compound is identified, medicinal chemists synthesize analogs with modifications designed to improve potency, selectivity, and pharmacokinetic properties. numberanalytics.comdomainex.co.uk This iterative process is guided by the structural information of the protein-ligand complex. domainex.co.uk
In the case of this compound, SBDD played a role in understanding its interaction with the NS5B polymerase active site. numberanalytics.com The 2'-methyl group on the sugar moiety is thought to cause a steric clash with an incoming nucleotide, leading to chain termination of the growing viral RNA strand. wikipedia.org This detailed understanding of the mechanism of action at the atomic level allows for the rational design of new analogs with potentially improved characteristics. nih.gov
Repurposing of this compound and its Analogs for Inhibition of Other Viral Targets (e.g., SARS-CoV-2 Main Protease)
The broad-spectrum potential of nucleoside analogs has led to investigations into repurposing this compound and its derivatives against other viral pathogens. asm.org One notable example is the exploration of their activity against SARS-CoV-2, the virus responsible for COVID-19.
The SARS-CoV-2 main protease (Mpro or 3CLpro) is an essential enzyme for viral replication, making it a prime target for antiviral drug development. nih.govmdpi.complos.org Several in silico studies, employing molecular docking and virtual screening, have identified this compound as a potential inhibitor of SARS-CoV-2 Mpro. frontiersin.org These computational models predict that this compound can bind to the active site of the protease. frontiersin.org
Experimental studies have provided further evidence for this potential. In vitro assays have shown that this compound can inhibit the enzymatic activity of SARS-CoV-2 Mpro and exhibits antiviral activity against the virus in cell culture. frontiersin.org For instance, this compound was reported to have in vitro antiviral EC50 values of 6.2 and 9.5 μM against SARS-CoV-2. frontiersin.org
Beyond SARS-CoV-2, the antiviral activity of this compound has been investigated against other RNA viruses. As a nucleotide analog, its mechanism of targeting the viral RNA polymerase makes it a candidate for inhibiting other viruses that rely on a similar enzyme for replication. asm.org For example, this compound has been shown to inhibit the replication of flaviviruses like Zika virus, dengue virus, and yellow fever virus, as well as chikungunya virus. asm.org
Formulation Science and Solid-state Characteristics of Sofosbuvir
Polymorphism and Crystalline Forms Research
Polymorphism, the ability of a solid material to exist in multiple crystalline forms, is a critical attribute of active pharmaceutical ingredients (APIs) as different polymorphs can exhibit varied physicochemical properties. iucr2017.orgacs.org Sofosbuvir is known to exhibit extensive polymorphism, with research identifying numerous crystalline and amorphous forms. iucr2017.orgacs.org
Initial reports by Gilead Sciences described eight crystalline polymorphs, including four anhydrous forms (Form 1, Form 6, Form 7, and Form 8) and four unstable solvates. iucr2017.org Subsequent research has expanded this number to over 15 polymorphs, including an amorphous form. iucr2017.orgacs.org The conformational flexibility around the phosphate group in the this compound molecule is a key factor contributing to this rich polymorphism. acs.org
The various polymorphic forms of this compound possess distinct physical properties, such as melting points, which can impact their stability and dissolution characteristics. iucr2017.org For instance, the anhydrous forms have been reported to have melting points ranging from 100 to 140°C. iucr2017.org
Table 1: Reported Polymorphic Forms of this compound and Their Characteristics
| Form | Crystal System | Space Group | Key Characteristics | Melting Point (°C) |
|---|---|---|---|---|
| Form 1 | Monoclinic | P21 | Contains four molecules in the elementary cell. acs.orgnih.gov | 96 acs.orgnih.gov |
| Form 6 | Orthorhombic | P212121 | Most compact and stable at room temperature. nih.gov | 118 acs.orgnih.gov |
| Form 7 | Monoclinic | P21 | Contains two molecules in the elementary cell. acs.orgnih.gov | 125 acs.orgnih.gov |
| Form 8 | Orthorhombic | P212121 | --- | --- |
| Form A | --- | --- | Most thermodynamically stable form at room temperature. acs.orgresearchgate.net | --- |
| Form B | --- | --- | Exhibits higher solubility than Form A. researchgate.net | --- |
| Form α | --- | --- | Characterized by specific XRPD peaks. epo.org | --- |
This table is based on available data and may not be exhaustive.
Research has also explored the thermodynamic relationships between different polymorphs. For example, Forms 6 and 7 are reported to be enantiotropic, while Form 1 is monotropic with respect to Forms 6 and 7. iucr2017.org The manufacturing process for this compound is designed to consistently produce the most thermodynamically stable polymorphic form, although small amounts of a metastable form may be present. europa.eu This control over polymorphism is crucial for ensuring consistent product quality and performance. who.intwho.int
Solubility and Dissolution Rate Studies (Intrinsic Physicochemical Properties)
The solubility and dissolution rate of an API are critical determinants of its oral bioavailability. This compound is characterized as a white to off-white crystalline solid. nih.goveuropa.eu It is classified as a Biopharmaceutical Classification System (BCS) class III drug, indicating high solubility and low permeability. europa.eu
This compound exhibits pH-independent solubility in aqueous media across a pH range of 1.2 to 7.7. ugm.ac.idjcsp.org.pk However, its aqueous solubility is relatively low, which can lead to slow dissolution and potentially variable bioavailability. ugm.ac.idugm.ac.id
Table 2: Solubility of this compound in Various Solvents
| Solvent | Solubility | Reference |
|---|---|---|
| Water | Slightly soluble europa.eu | europa.eu |
| Water (pH 1.2-7.7) | Slightly soluble europa.eu | europa.eu |
| Ethanol | Freely soluble europa.eu | europa.eu |
| Acetone | Freely soluble europa.eu | europa.eu |
| 2-Propanol | Soluble europa.eu | europa.eu |
| Heptane | Insoluble europa.eu | europa.eu |
| Acetonitrile (ACN) | 9.27 mg/mL |
Studies have shown that the solubility of this compound polymorphs increases with temperature. researchgate.net For instance, in binary solvent systems of ethyl acetate + toluene and methyl tert-butyl ether + toluene, the solubility of Form B was found to be higher than that of Form A. researchgate.net
To enhance the dissolution rate of this compound, various formulation strategies have been investigated. Co-crystallization of this compound with hydrophilic sugars like sucralose, xylitol, or mannitol has been shown to significantly increase its dissolution rate. ugm.ac.idugm.ac.id This enhancement is attributed to the formation of new crystalline species with weakened intermolecular bonds. ugm.ac.id For example, co-processing with xylitol resulted in 78.33% of the drug dissolving in the first 5 minutes. ugm.ac.id
Stability in Various Physico-Chemical Environments and Pre-formulation Studies
Pre-formulation studies are essential to understand the stability of a drug substance under various environmental conditions, which informs the development of a stable dosage form. This compound has been subjected to forced degradation studies under conditions of hydrolysis, oxidation, photolysis, and thermal stress as per International Council for Harmonisation (ICH) guidelines. ijper.orgresearchgate.net
These studies have revealed that this compound is susceptible to degradation under acidic, basic, and oxidative conditions. ijper.orgresearchgate.net In acidic and alkaline environments, hydrolysis is a key degradation pathway. ijper.org Specifically, degradation of 23% was observed in 0.1N HCl after 6 hours, and 50% degradation occurred in 0.1N NaOH after 10 hours. ijper.org Oxidative stress, induced by agents like hydrogen peroxide, also leads to the formation of degradation products. ijper.org
Conversely, this compound has demonstrated stability in the solid state and under neutral, thermal, and photolytic stress conditions. europa.euijper.orgresearchgate.net It remains stable in the solid state even at temperatures up to 105°C for one week. europa.eu Furthermore, studies on polymorphs A and B have indicated good solid-state stability under conditions of high temperature, high humidity, and strong light exposure. researchgate.net this compound is also described as a non-hygroscopic crystalline solid. europa.eu
Table 3: Summary of this compound Stability under Stress Conditions
| Stress Condition | Observation |
|---|---|
| Acidic Hydrolysis (0.1N HCl) | Degradation observed (23% after 6 hrs) ijper.org |
| Alkaline Hydrolysis (0.1N NaOH) | Degradation observed (50% after 10 hrs) ijper.org |
| Oxidative (3% H2O2) | Degradation observed (19.02% after 7 days) ijper.org |
| Neutral Hydrolysis | Stable researchgate.net |
| Thermal (Solid State) | Stable up to 105°C for 1 week europa.eu |
| Photolytic | Stable ijper.org |
These stability characteristics are crucial for determining appropriate manufacturing processes, packaging, and storage conditions for this compound-containing products to ensure their quality and shelf-life.
Q & A
Q. What strategies mitigate bias in this compound clinical trials with open-label designs?
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


