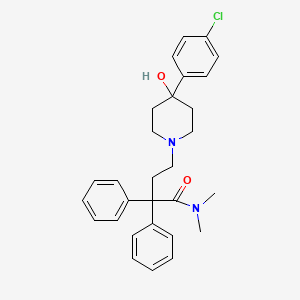
Loperamide
Description
This compound is synthetic opioid that primarily affects opiate receptors in the intestine and is used to treat diarrhea. This compound has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury.
This compound, also known as imodium or diamide, belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. This compound is a drug which is used for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease or gastroenteritis. also used for reducing the volume of discharge from ileostomies. This compound is considered to be a practically insoluble (in water) and relatively neutral molecule. This compound has been primarily detected in blood. Within the cell, this compound is primarily located in the membrane (predicted from logP).
Properties
IUPAC Name |
4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-N,N-dimethyl-2,2-diphenylbutanamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RDOIQAHITMMDAJ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)C(=O)C(CCN1CCC(CC1)(C2=CC=C(C=C2)Cl)O)(C3=CC=CC=C3)C4=CC=CC=C4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H33ClN2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
34552-83-5 (mono-hydrochloride) | |
| Record name | Loperamide [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0053179116 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID6045165 | |
| Record name | Loperamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6045165 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
477.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Loperamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0004999 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
53179-11-6 | |
| Record name | Loperamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=53179-11-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Loperamide [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0053179116 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Loperamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00836 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Loperamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6045165 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Loperamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.053.088 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LOPERAMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/6X9OC3H4II | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Loperamide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8344 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Loperamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0004999 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
220-228 | |
| Record name | Loperamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00836 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Pharmacological Mechanisms of Action of Loperamide
Opioid Receptor Agonism
Loperamide functions as an agonist at opioid receptors, with a particular affinity for mu-opioid receptors (MORs) located within the gastrointestinal tract. drugbank.comfrontiersin.orgwikipedia.orgpatsnap.com
This compound selectively binds to mu (μ)-opioid receptors present on both the circular and longitudinal intestinal muscles. drugbank.comfrontiersin.orgwikipedia.orgpatsnap.com This binding initiates a series of intracellular events, including the recruitment of G-protein receptor kinases and the activation of downstream molecular cascades, which collectively inhibit enteric nerve activity. drugbank.com
The agonism of MORs by this compound leads to a significant decrease in the activity of the myenteric plexus, a crucial component of the enteric nervous system responsible for coordinating gastrointestinal motility. frontiersin.orgwikipedia.orgpatsnap.comresearchgate.neted.ac.ukkoreamed.orgfrontiersin.org This modulation of myenteric plexus activity subsequently reduces the tone of both the longitudinal and circular smooth muscles of the intestine, thereby inhibiting propulsive peristaltic movements. frontiersin.orgwikipedia.orgpatsnap.comresearchgate.neted.ac.ukkoreamed.orgfrontiersin.org
A key consequence of this compound's action on the myenteric plexus is the inhibition of neurotransmitter release. Specifically, this compound significantly reduces the release of acetylcholine and prostaglandins within the gastrointestinal tract. drugbank.compatsnap.comresearchgate.netresearchgate.netdrugs.comhres.cadroracle.aihres.ca By diminishing the availability of these excitatory neurotransmitters, this compound effectively reduces propulsive peristalsis and consequently increases intestinal transit time. drugbank.comdrugs.comhres.cadroracle.aihres.ca Studies have shown that the inhibited acetylcholine release can be reversed by naloxone, suggesting an interaction with opiate receptor sites in the myenteric plexus. researchgate.net The inhibition of prostaglandin release may also involve the prevention of prostaglandin biosynthesis from arachidonic acid in the intestine. researchgate.net
Despite being a potent opioid receptor agonist, this compound exhibits remarkable peripheral selectivity, meaning its primary therapeutic effects are confined to the gastrointestinal tract with limited penetration into the central nervous system (CNS) at recommended therapeutic doses. drugbank.comwikipedia.orgpatsnap.comed.ac.ukkoreamed.orgresearchgate.netnih.govwikipedia.orgnih.govpharmacytimes.comnih.gov This peripheral restriction is a critical pharmacological feature that minimizes the typical CNS-related side effects associated with other opioid analgesics, such as euphoria or respiratory depression. drugbank.compatsnap.comed.ac.ukwikipedia.orgnih.govpharmacytimes.com This limited CNS penetration is attributed to several factors, including its low oral bioavailability and extensive first-pass metabolism. drugbank.comwikipedia.orgdrugs.comhres.canih.govnih.govpharmacytimes.com
A significant contributor to this compound's peripheral selectivity is its interaction with the P-glycoprotein (P-gp) efflux pump. drugbank.comwikipedia.orgresearchgate.netnih.govwikipedia.orgpharmacytimes.comsnmjournals.orgnih.govresearchgate.net P-gp is an ATP-binding cassette-dependent efflux transporter widely expressed in various tissues, including the endothelial cells of the blood-brain barrier. researchgate.netwikipedia.orgsnmjournals.orgresearchgate.net this compound is an avid substrate for P-gp, meaning the pump actively transports this compound out of the CNS, effectively preventing its accumulation in the brain. drugbank.comwikipedia.orgresearchgate.netwikipedia.orgpharmacytimes.comsnmjournals.orgnih.govresearchgate.net This active efflux mechanism is instrumental in limiting this compound's CNS exposure and contributes to its favorable safety profile at therapeutic concentrations. drugbank.comresearchgate.netwikipedia.orgpharmacytimes.comsnmjournals.orgnih.gov
Pharmacodynamics of Loperamide in Gastrointestinal Physiology
Effects on Colonic Motility
Decrease in Colonic Mass Movements
Loperamide significantly impacts colonic motility by acting on μ-opioid receptors located in the myenteric plexus of the large intestine. wikipedia.orgdrugbank.comfrontiersin.orgncats.io This interaction leads to a decrease in the activity of the myenteric plexus, which in turn reduces the tone of both the longitudinal and circular smooth muscles of the intestinal wall. wikipedia.orgfrontiersin.orgncats.ioresearchgate.net The reduction in muscle tone inhibits peristaltic activity, thereby increasing the time that material remains in the intestine and facilitating greater water absorption from fecal matter. wikipedia.orgdrugbank.comdrugs.com
Research findings indicate that this compound dose-dependently inhibits colonic motor complexes (CMCs), which are propulsive movements in the colon. frontiersin.orgnih.gov Studies using high-resolution video imaging on isolated mouse colon have shown regional differences in the sensitivity of CMCs to this compound. While CMCs in the mid and distal colon were abolished at relatively low concentrations (e.g., 100 nM), CMCs in the proximal colon persisted at the same concentration, albeit at a significantly slower frequency. frontiersin.org this compound at 100 nM significantly reduced CMC frequency and velocity, an effect that was reversed by naloxone, a μ-opioid receptor antagonist. frontiersin.org
The following table summarizes the impact of this compound on colonic motor complex (CMC) frequency and velocity:
| Parameter | Control (Mean ± SEM) | This compound 100 nM (Mean ± SEM) | P-value |
| CMC Frequency (min⁻¹) | 0.69 ± 0.04 frontiersin.org | 0.36 ± 0.03 frontiersin.org | 0.0001 frontiersin.org |
| CMC Velocity (mm s⁻¹) | 2.39 ± 0.27 frontiersin.org | 1.16 ± 0.16 frontiersin.org | 0.0174 frontiersin.org |
These findings suggest that this compound's most potent effect in retarding colonic transit occurs between the proximal and mid/distal regions of the colon, primarily by suppressing cholinergic neurotransmission in the myenteric plexus. frontiersin.org this compound also inhibits the release of acetylcholine and prostaglandins, further contributing to the reduction in propulsive peristalsis and an increase in intestinal transit time. drugbank.compediatriconcall.comncats.io
Impact on Anal Sphincter Tone and Fecal Continence
A significant pharmacodynamic effect of this compound is its ability to increase the tone of the anal sphincter. nih.govdrugbank.combapen.org.ukpediatriconcall.comncats.ioresearchgate.netjst.go.jpmarvistavet.comresearchgate.netmedscape.com This action plays a crucial role in improving fecal continence and reducing urgency, particularly in patients experiencing chronic diarrhea with fecal incontinence. drugbank.compediatriconcall.comresearchgate.netjst.go.jpresearchgate.netmedscape.comnih.gov
Research has demonstrated that this compound can significantly improve continence to a standard volume of rectally infused saline. nih.gov This improvement is associated with an increase in the maximum basal sphincter pressure. nih.gov Furthermore, this compound can increase the rectal volume required to abolish the recovery of the rectoanal inhibitory reflex and lead to a reduction in rectal compliance. nih.gov These effects collectively suggest a specific action of this compound on the anal sphincter, which aids in maintaining continence. marvistavet.comnih.gov
Pharmacokinetics and Metabolism of Loperamide
Absorption and Bioavailability
Loperamide is well absorbed from the gastrointestinal tract following oral administration. drugbank.com However, despite good absorption, its systemic bioavailability is remarkably low due to significant first-pass metabolism. drugbank.comnih.govmedsafe.govt.nzhres.camedicines.org.ukvettimes.com
Low Oral Absorption
While absorbed from the gut, the amount of this compound that reaches systemic circulation is minimal. drugbank.comresearchgate.net This low oral absorption is a crucial characteristic that contributes to its peripheral action, minimizing central nervous system (CNS) effects at therapeutic doses. vettimes.comresearchgate.net
First-Pass Metabolism Effects
The primary reason for this compound's low bioavailability (less than 1%, often cited as approximately 0.3% to 0.5%) is extensive first-pass metabolism in the liver. drugbank.comnih.govmedsafe.govt.nzhres.camedicines.org.ukvettimes.com This process ensures that a substantial portion of the drug is metabolized before reaching the systemic circulation. drugbank.commedsafe.govt.nzhres.ca Following oral administration, peak plasma concentrations of unchanged this compound typically remain below 2 ng/mL for a 2 mg capsule. drugbank.comfda.gov Plasma concentrations are generally highest around 4 to 5 hours after administration of a capsule formulation and approximately 2.5 hours for a liquid formulation. drugbank.comnih.govvettimes.comfda.gov
Distribution and Protein Binding
This compound exhibits a large volume of distribution and high plasma protein binding. drugbank.comnih.govfda.gov Approximately 95% to 97% of this compound in the blood is bound to plasma proteins, primarily albumin. drugbank.commedsafe.govt.nzhres.camedicines.org.ukvettimes.comfda.govwikipedia.org Despite its lipophilic nature, this compound generally acts peripherally and does not readily cross the blood-brain barrier at therapeutic doses. drugbank.comresearchgate.netwikipedia.orgnih.gov This is largely attributed to its being a substrate for P-glycoprotein (P-gp), an efflux transporter located in the intestinal wall and at the blood-brain barrier, which actively pumps the drug out of cells and limits its systemic and CNS penetration. drugbank.commedsafe.govt.nzhres.camedicines.org.ukvettimes.comwikipedia.orgnih.govfrontiersin.orgdrugs.com
Biotransformation
This compound undergoes extensive biotransformation, with almost complete extraction by the liver where it is predominantly metabolized, conjugated, and subsequently excreted via the bile. medsafe.govt.nzhres.camedicines.org.uk The main metabolic pathway for this compound is oxidative N-demethylation. drugbank.commedsafe.govt.nzhres.camedicines.org.ukfda.govdrugs.com This process leads to the formation of metabolites such as N-demethyl this compound (also known as desmethyl-loperamide), which are generally considered pharmacologically inactive. drugbank.commedsafe.govt.nz
Cytochrome P450 (CYP) System Metabolism
The metabolism of this compound is primarily mediated by the cytochrome P450 (CYP) enzyme system in the liver. drugbank.comnih.govmedsafe.govt.nzmedicines.org.ukresearchgate.netfda.govdrugs.commedcentral.comdrugbank.com
The two major cytochrome P450 isoenzymes responsible for the oxidative N-demethylation of this compound are CYP3A4 and CYP2C8. drugbank.comnih.govmedsafe.govt.nzmedicines.org.ukfda.govfrontiersin.orgdrugs.commedcentral.comdrugbank.comnih.govontosight.ai In in vitro studies, inhibitors of these enzymes significantly impact this compound metabolism. For instance, ketoconazole, a CYP3A4 inhibitor, has been shown to inhibit N-demethylation by 90%, while quercetin, a CYP2C8 inhibitor, inhibited the process by 40%. fda.govdrugs.comnih.gov Other CYP enzymes, such as CYP2B6 and CYP2D6, may also play a minor role in this compound N-demethylation. drugbank.comfda.govdrugs.comnih.gov The substantial involvement of CYP3A4 and CYP2C8 in this compound's metabolism means that concomitant administration of inhibitors or inducers of these enzymes can significantly affect this compound's plasma levels, potentially altering its therapeutic effects. frontiersin.orgdrugs.comdrugbank.comontosight.ai
Pharmacokinetic Parameters of this compound
| Parameter | Value | Source Indices |
| Bioavailability (Oral) | <1% (approximately 0.3% - 0.5%) | drugbank.comnih.govmedsafe.govt.nzhres.camedicines.org.ukvettimes.com |
| Peak Plasma Time (Capsule) | 4-5 hours (mean 5.2 ± 0.3 hours) | nih.govvettimes.comfda.gov |
| Peak Plasma Time (Liquid/Syrup) | 2.5 hours (mean 2.4 ± 0.7 hours) | drugbank.comvettimes.comfda.gov |
| Plasma Protein Binding | 95-97% (mainly to albumin) | drugbank.commedsafe.govt.nzhres.camedicines.org.ukvettimes.comfda.govwikipedia.org |
| Elimination Half-life | 9.1-14.4 hours (average 10.8-11 hours) | drugbank.comnih.govmedsafe.govt.nzhres.camedicines.org.ukfda.govdrugs.commedcentral.com |
Metabolic Pathways of this compound
| Metabolic Pathway | Primary Enzymes Involved | Metabolite(s) Formed |
| Oxidative N-demethylation | CYP3A4, CYP2C8 | N-demethyl this compound |
| Minor Pathways | CYP2B6, CYP2D6 |
Minor Role of CYP2B6 and CYP2D6
While the primary metabolic pathway for this compound involves the cytochrome P450 (CYP450) isoenzymes CYP2C8 and CYP3A4, other enzymes also contribute to its metabolism. Specifically, CYP2B6 and CYP2D6 have been identified as playing a minor role in the N-demethylation process of this compound. citeab.comnih.govwikipedia.orgguidetopharmacology.orgfishersci.canih.govnih.gov
N-demethylation Process
The predominant metabolic pathway for this compound is oxidative N-demethylation. citeab.comnih.govuni.luwikipedia.orgguidetopharmacology.orgnih.govfishersci.caresearchgate.net This crucial biotransformation is primarily mediated by the cytochrome P450 (CYP450) isozymes CYP2C8 and CYP3A4. citeab.comnih.govuni.luwikipedia.orgguidetopharmacology.orgfishersci.canih.govnih.govuni.luuni.lu The N-demethylation process converts this compound into its main metabolite, N-demethyl this compound (also known as desmethyl-loperamide or DLOP). citeab.comnih.govwikipedia.orgguidetopharmacology.orgnih.govnih.govuni.lufishersci.ca
In vitro studies have provided detailed insights into the roles of these enzymes. For instance, the N-demethylation process of this compound was significantly inhibited by specific CYP450 inhibitors: quercetin, a known CYP2C8 inhibitor, reduced the process by 40%, while ketoconazole, a potent CYP3A4 inhibitor, caused a 90% inhibition. wikipedia.orgguidetopharmacology.orgnih.govnih.gov It is important to note that the metabolites formed during this compound's metabolism are pharmacologically inactive. citeab.comnih.gov
Table 1: Key Enzymes and Inhibitors in this compound N-demethylation
| Enzyme Involved | Primary/Minor Role | Inhibitor | Inhibition Percentage (in vitro) |
| CYP2C8 | Primary | Quercetin | 40% wikipedia.orgguidetopharmacology.orgnih.govnih.gov |
| CYP3A4 | Primary | Ketoconazole | 90% wikipedia.orgguidetopharmacology.orgnih.govnih.gov |
| CYP2B6 | Minor | N/A | N/A |
| CYP2D6 | Minor | N/A | N/A |
Elimination and Excretion
The elimination of this compound and its metabolites from the body is a well-characterized process. citeab.comnih.govuni.luwikipedia.orgguidetopharmacology.orgfishersci.canih.govfishersci.caresearchgate.netfishersci.ca
Predominant Fecal Excretion of this compound and Metabolites
The primary route of excretion for both unchanged this compound and its metabolites is through the feces. citeab.comnih.govuni.luwikipedia.orgguidetopharmacology.orgfishersci.canih.govfishersci.caresearchgate.netfishersci.canih.gov A very small fraction of the absorbed dose, typically around 1% to 2%, is excreted unchanged in the urine. citeab.comnih.govfishersci.canih.gov this compound and its metabolites that enter the systemic circulation undergo biliary excretion, contributing to their predominant fecal elimination. citeab.comnih.gov
Elimination Half-Life
The apparent elimination half-life of this compound in humans ranges from 9.1 to 14.4 hours, with an average of approximately 10.8 hours. citeab.comnih.govwikipedia.orgguidetopharmacology.orgfishersci.canih.govfishersci.cafishersci.ca
Table 2: this compound Elimination Half-Life
| Parameter | Value |
| Average Half-Life | 10.8 hours citeab.comnih.govwikipedia.orgguidetopharmacology.orgfishersci.canih.govfishersci.cafishersci.ca |
| Reported Range | 9.1 to 14.4 hours citeab.comnih.govwikipedia.orgguidetopharmacology.orgfishersci.canih.govfishersci.cafishersci.ca |
Mechanistic Studies of Loperamide's Cardiovascular Toxicity
Electrophysiological Alterations
Conduction Slowing
Conduction slowing, characterized by prolongation of the QRS duration and PQ interval on an electrocardiogram (ECG), is a prominent feature of loperamide-induced cardiotoxicity. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca This effect is primarily mediated by this compound's inhibition of voltage-gated sodium channels (Nav1.5) in cardiac cells. wikipedia.orgfishersci.cauni.luguidetopharmacology.org The fast sodium current (INa), responsible for the rapid depolarization phase (phase 0) of the cardiac action potential, is crucial for efficient impulse conduction. When this compound blocks these channels, it impairs electrical conduction, leading to a widened QRS complex and, in some cases, atrioventricular (AV) block. fishersci.cauni.luguidetopharmacology.org
Preclinical studies have demonstrated that this compound can slow conduction (QRS-duration) at concentrations significantly higher than therapeutic levels. For instance, in an isolated rabbit ventricular-wedge model, conduction slowing was observed starting at 0.3 µM, approximately 1200-fold its human Free Therapeutic Plasma Concentration (FTPC). wikipedia.orguni.luguidetopharmacology.orgfishersci.ca In anesthetized guinea pigs, this compound also slowed conduction time (QRS-duration) and increased the PQ-interval at overdose exposures. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca
Table 1: this compound's Inhibitory Effects on Cardiac Ion Channels (IC50 Values)
| Ion Channel | Current | IC50 Value (µM) | Fold over Human FTPC | Reference |
| hERG | IKr | 0.033 - 0.089, 0.390 | >1560 | wikipedia.orguni.luguidetopharmacology.orgfishersci.ca |
| Nav1.5 | INa | 0.239 - 2.9, 0.526 | >1560 | wikipedia.orguni.luguidetopharmacology.orgfishersci.ca |
| Cav1.2 | ICa | 4.091 | >1560 | wikipedia.orguni.luguidetopharmacology.orgfishersci.ca |
Note: IC50 values can vary depending on experimental conditions and measurement protocols. wikipedia.orguni.lu
Associated Arrhythmias and Cardiac Events
High concentrations of this compound can lead to a combination of conduction slowing and alterations in repolarization time, resulting in cardiac proarrhythmia and life-threatening ventricular arrhythmias. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca These include, but are not limited to, Torsades de Pointes (TdP), ventricular tachycardia, ventricular fibrillation, atrioventricular block, Brugada syndrome ECG features, cardiac arrest, and sudden death. wikipedia.orgfishersci.cauni.luguidetopharmacology.org The exact mechanisms are complex but are primarily linked to this compound's inhibitory effects on cardiac ion channels. wikipedia.orguni.lu
Torsades de Pointes (TdP)
Torsades de Pointes (TdP) is a polymorphic ventricular tachycardia often associated with prolongation of the corrected QT (QTc) interval. wikipedia.orgfishersci.ca this compound's potent inhibition of the human ether-à-go-go-related gene (hERG) potassium channel, which underlies the rapid delayed rectifier potassium current (IKr), is the most common mechanism for drug-induced QT prolongation and, consequently, TdP. wikipedia.orgfishersci.cauni.luguidetopharmacology.orgfishersci.ca Inhibition of hERG channels delays ventricular repolarization, making cardiac cells vulnerable to early afterdepolarizations (EADs) and triggering TdP. This compound has been shown to block hERG currents with IC50 values ranging from 33 to 390 nM, indicating a high affinity for this channel. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca
Ventricular Tachycardia and Fibrillation
This compound overdose can lead to various forms of ventricular tachyarrhythmias, including monomorphic and polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF). wikipedia.orgfishersci.cauni.luguidetopharmacology.org These arrhythmias are often associated with significant QRS prolongation, indicating a contribution from sodium channel blockade in addition to QT prolongation. wikipedia.orgfishersci.cauni.luguidetopharmacology.org The inhibition of both hERG-mediated IKr and the fast sodium current (INa) are considered the most likely basis for this cardiac electrophysiological toxicity at overdose exposures. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca In an isolated rabbit ventricular-wedge model, this compound caused cardiac arrhythmias, including ventricular tachycardia-like activity, at concentrations of 3 µM, approximately 12,000-fold its human FTPC. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca
Atrioventricular (AV) Block
Atrioventricular (AV) block, a disturbance in the electrical conduction between the atria and ventricles, has been reported in cases of this compound toxicity. wikipedia.orgfishersci.cauni.luguidetopharmacology.org This conduction abnormality is primarily attributed to the inhibitory effects of this compound on cardiac sodium channels (Nav1.5), which are essential for the rapid propagation of electrical impulses through the AV node and His-Purkinje system. wikipedia.orgfishersci.cauni.luguidetopharmacology.org In anesthetized guinea pigs, this compound was observed to elicit Type II/III AV block at overdose exposures. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca
Brugada Syndrome ECG Features
This compound toxicity has been associated with the unmasking or induction of Brugada syndrome-like ECG features. wikipedia.orgfishersci.cauni.luguidetopharmacology.org Brugada syndrome is a cardiac channelopathy characterized by specific ST segment elevation patterns in the right precordial leads (V1-V3) of the ECG, often linked to mutations in sodium channel genes. This compound's potent inhibition of cardiac sodium channels (Nav1.5) can mimic the effects of genetic sodium channel dysfunction, leading to the characteristic coved-type ST segment elevation and an increased risk of ventricular arrhythmias. fishersci.cauni.lu
Cardiac Arrest and Sudden Death
The most severe outcomes of this compound's cardiovascular toxicity are cardiac arrest and sudden death. wikipedia.orgfishersci.cauni.luguidetopharmacology.org These catastrophic events are the culmination of the profound electrophysiological disturbances caused by high concentrations of this compound, including marked QT prolongation, significant QRS widening, and the precipitation of malignant ventricular arrhythmias such as TdP and ventricular fibrillation. wikipedia.orgfishersci.cauni.luguidetopharmacology.org The inhibition of both hERG potassium channels and sodium channels plays a critical role in increasing the risk of these fatal cardiac events. wikipedia.orguni.luguidetopharmacology.orgfishersci.ca
Clinical Efficacy and Comparative Studies of Loperamide
Acute Diarrhea Management
Loperamide is a primary treatment option for acute, non-specific diarrhea, including traveler's diarrhea. medcentral.comaafp.org Its effectiveness in this context is well-documented through clinical studies.
Reduction in Diarrhea Duration and Frequency
A study comparing this compound to placebo in children with acute diarrhea found that those receiving this compound were 34% less likely to have diarrhea 24 hours after treatment and experienced a 16% reduction in diarrheal stools within the first 24 hours. nih.gov Another study indicated that after a single 4 mg dose of this compound, the recurrence of the first liquid or unformed stools was delayed by 24 hours or more, suggesting a restoration of normal intestinal peristalsis and transit time. hres.ca
Comparison with Other Antidiarrheal Agents (e.g., Diphenoxylate, Bismuth Subsalicylate)
This compound has been compared to other antidiarrheal agents, demonstrating favorable efficacy profiles.
This compound vs. Diphenoxylate: Double-blind clinical studies have indicated that this compound is at least as effective as diphenoxylate for controlling acute diarrhea and may be more effective in reducing daily stool frequency and improving fecal consistency. medcentral.com In single-dose studies, 4 mg of this compound exhibited a significantly longer duration of effect than 5 mg of diphenoxylate. researchgate.net While both medications effectively resolve diarrhea, some research suggests this compound is more effective and better tolerated, with fewer reported side effects compared to diphenoxylate. singlecare.com
Comparative Efficacy of this compound vs. Other Antidiarrheal Agents
| Agent Compared | Outcome (this compound vs. Agent) | Research Finding | Source |
|---|---|---|---|
| Diphenoxylate | Acute Diarrhea Control | At least as effective, potentially more effective in reducing stool frequency and improving consistency. Longer duration of effect in single doses. medcentral.comresearchgate.net | medcentral.comresearchgate.netsinglecare.com |
| Bismuth Subsalicylate | Acute Diarrhea Control | Faster and more effective relief, significantly reduced unformed bowel movements. nih.govnih.gov | nih.govnih.gov |
Adjunctive Therapy in Traveler's Diarrhea with Antibiotics
For traveler's diarrhea, this compound can be used as an adjunctive therapy with antibiotics, particularly in moderate to severe cases. medcentral.comaafp.org Studies have shown that combining this compound with antibiotic therapy offers an advantage over antibiotics alone by decreasing illness duration and increasing the probability of early clinical cure. nih.govresearchgate.netnih.govoup.com A meta-analysis of six paired studies found that the odds of clinical cure at 24 hours and 48 hours favored combination therapy, with summary odds ratios of 2.6 and 2.2 respectively. nih.govoup.com This suggests that adjunctive this compound can accelerate the resolution of symptoms in traveler's diarrhea. medwave.cl
Chronic Diarrhea Management
This compound is also effective in controlling and providing symptomatic relief for chronic diarrhea, including that associated with inflammatory bowel disease and post-gastrointestinal surgery. medcentral.comnih.gov
Inflammatory Bowel Disease (IBD)-Associated Diarrhea
This compound is utilized for the symptomatic control of chronic diarrhea linked to inflammatory bowel disease (IBD), including Crohn's disease and chronic ulcerative colitis. medcentral.comhres.canih.govhealthline.commayoclinic.org In patients with milder Crohn's disease, this compound can help manage diarrheal symptoms. healthline.com While it is not a primary treatment for the underlying inflammation in IBD, it can be effective for managing severe diarrhea associated with the condition. mayoclinic.orgdroracle.ai One study involving patients with chronic diarrhea due to Crohn's disease, ulcerative colitis, and other causes found that this compound effectively relieved symptoms in a significant majority of patients, with the average number of stools decreasing substantially after one month of treatment. nih.gov It is important to note that this compound is generally not recommended for acute ulcerative colitis or during an IBD flare-up without medical advice, as it does not address the underlying inflammation and may pose risks. droracle.aicrohnsandcolitis.ca
Post-Gastrointestinal Surgery Diarrhea (e.g., Ileostomy Output Reduction)
This compound is effective in controlling chronic diarrhea resulting from bowel resection or organic lesions, and in reducing intestinal peristalsis and transit time in patients with ileostomies and other intestinal resections. medcentral.comhres.ca For patients with a high-output ileostomy, this compound is often considered a first-line treatment to reduce stoma losses. leicestershospitals.nhs.ukdroracle.ai It can decrease ileostomy output by 20-30% by reducing intestinal motility. leicestershospitals.nhs.uk Studies have shown that this compound significantly reduces stoma output and aids in fluid regulation in patients with newly formed ileostomies. researchgate.net It is preferred over opiate drugs for this purpose due to its non-addictive and non-sedative properties. droracle.ai
Effect of this compound on Ileostomy Output
| Study/Finding | Effect on Ileostomy Output | Additional Benefits | Source |
|---|---|---|---|
| Kristensen et al. (2017) | Reduces ileostomy output by 20-30% | Preferred over codeine phosphate (non-sedative, non-addictive, no fat malabsorption) | leicestershospitals.nhs.uk |
| Randomized Controlled Trial (Newly formed ileostomy) | Significantly reduced stoma output | Helped in fluid regulation; no statistical difference in electrolyte levels compared to placebo | researchgate.net |
| Clinical Nutrition (2021) | Effective in reducing fecal wet weight and sodium excretion in short bowel syndrome with ostomy | First-line treatment for high ileostomy output | droracle.ai |
Chemotherapy-Induced Diarrhea (CID)
Chemotherapy-induced diarrhea (CID) represents a significant and often dose-limiting toxicity in cancer treatment. This compound is widely recognized as a primary first-line therapy for the management of CID biotech-asia.orgjhoponline.comeviq.org.au. It is specifically recommended for managing Grade 2 CID, characterized by more severe diarrhea that may necessitate medical intervention biotech-asia.org. The mechanism involves its agonistic effects on opioid receptors in the gastrointestinal (GI) tract, leading to decreased peristalsis and increased fluid reabsorption jhoponline.com. While generally effective, standard or high-dose this compound therapy may not be successful in all cases, with 9% to 30% of aggressive CID cases proving refractory jhoponline.com. In instances where diarrhea persists beyond 24 to 48 hours despite high-dose this compound, alternative treatments such as subcutaneous octreotide or oral budesonide may be considered jhoponline.comengland.nhs.uk. It is crucial to note that antidiarrheal agents like this compound are contraindicated if immune-related colitis is suspected eviq.org.au.
| Treatment Group | Diarrhea Controlled within 24 hours (%) | Tablets Required (within 72 hours) |
|---|---|---|
| This compound | 47 | 4.37 |
| Diphenoxylate + Atropine | 37 | 5.75 |
| Treatment Group | Complete Resolution of Diarrhea (%) | Mean Duration of Antidiarrheal Therapy to Remission (days) |
|---|---|---|
| Octreotide | 80 | 3.4 |
| This compound | 30 | 6.1 |
Specific Research on Irinotecan-Induced Diarrhea
Irinotecan-induced diarrhea (IID), a common and often severe side effect of irinotecan chemotherapy, poses a significant challenge in patient management clinicsinoncology.commdpi.com. This compound is considered the initial drug of choice for IID, demonstrating a notable reduction in its incidence in several studies jhoponline.comclinicsinoncology.com. High-dose this compound regimens have been shown to be moderately effective in controlling IID eviq.org.aunih.gov. Early intervention with this compound is emphasized for effective management of IID clinicsinoncology.com. For patients experiencing this compound-refractory IID, octreotide has proven to be an effective alternative, yielding high response rates nih.gov. A study investigating the pathophysiology of CPT-11 (irinotecan)-induced delayed-onset diarrhea and the efficacy of combined antidiarrheal medication found that while acetorphan alone resulted in a 36% response rate (4 of 11 patients), the combination of acetorphan and this compound significantly improved the response rate to 90% (9 of 10 patients) (P < .02) ascopubs.org.
| Treatment Group | Patients Responded (%) |
|---|---|
| Acetorphan Alone | 36 |
| Acetorphan + this compound | 90 |
Fecal Incontinence Management
This compound is a preferred pharmacological agent for the management of fecal incontinence, particularly when the condition is associated with liquid stool gastroenterologyandhepatology.netmedscape.comjst.go.jp. Its mechanism of action in this context involves increasing gut transit time, which facilitates increased water absorption from the stool, leading to a firmer, more manageable consistency medscape.com. Additionally, this compound may enhance the tone of the internal anal sphincter (IAS), further contributing to improved continence gastroenterologyandhepatology.netmedscape.comjst.go.jp. Research has explored this compound's efficacy in managing fecal incontinence, sometimes in conjunction with other conservative therapies such as fiber supplements or biofeedback gastroenterologyandhepatology.netucc-today.comdiva-portal.orgresearchgate.netpelvicfloordisordersnetwork.org. A prospective controlled study involving women with fecal incontinence found that treatment with methylcellulose and this compound resulted in a significantly higher cure rate (46%) compared to expectant management (0%) (p<0.01) researchgate.net. While effective, some reviews indicate that the evidence base for this compound's use in fecal incontinence can be limited, and potential side effects should be considered ucc-today.com.
| Treatment Group | Cure Rate (%) |
|---|---|
| Methylcellulose + this compound | 46 |
| Expectant Management (Controls) | 0 |
Combination Therapies (e.g., with Simethicone)
This compound is frequently combined with simethicone to provide symptomatic relief for acute diarrhea that is accompanied by gas-related abdominal discomfort, including bloating, cramping, and flatulence bmj.comimodium.comalberta.cagoodrx.commedex.com.bdclinicaltrials.eunih.gov. In this combination, this compound works to slow down intestinal movement and reduce the water content of stool, while simethicone functions as an anti-foaming agent, aiding in the breakdown and passage of gas bubbles in the gut alberta.cagoodrx.commedex.com.bd. Randomized controlled trials have demonstrated that the co-administration of this compound and simethicone is clinically more effective than either drug administered alone in alleviating the duration and symptoms of acute diarrhea, including a faster resolution of gas-related symptoms bmj.comacpjournals.orgresearchreview.co.nz. One study reported that the combination product achieved a significantly higher patient-assessed rating at the end of the study compared to individual components or placebo (p<0.001) researchreview.co.nz. A completed Phase 3 trial further investigated the efficacy and safety of a this compound hydrochloride/simethicone chewable tablet formulation for the treatment of acute diarrhea with associated abdominal discomfort and flatulence drugbank.comfda.gov.
Research on Loperamide's Abuse Potential and Dependence
Historical Reassessment of Abuse Potential
Initially synthesized in 1969 and medically used since 1976, loperamide was once categorized as a Schedule V drug by the Federal Drug Administration (FDA) due to its opioid-like misuse potential, before becoming available without a prescription in 1988 nih.gov. For a long time, it was believed to have low abuse potential pedemmorsels.comresearchgate.netpowerpak.comwikipedia.org. However, over the past decade, there has been a concerning rise in documented cases of this compound misuse and abuse addictioncenter.compedemmorsels.compowerpak.comwikipedia.org.
Epidemiological trends from the National Poison Data System (NPDS) revealed a 71% increase in calls related to intentional this compound exposure between 2011 and 2014 upstate.edupowerpak.com. Similarly, web-forum postings discussing oral this compound abuse increased tenfold between 2010 and 2011 addictioncenter.comupstate.edunih.gov. This surge in abuse aligns with the broader opioid epidemic, as individuals seek alternative, more accessible, and cheaper substances to manage opioid withdrawal or achieve euphoric effects addictioncenter.comupstate.edu. The FDA issued a Drug Safety Communication in June 2016, highlighting serious and fatal cardiac events associated with high-dose this compound use, and later, in January 2018, approved new packaging with fewer doses to deter abuse powerpak.comfda.gov.
Motivations for Supratherapeutic Use
Individuals engage in supratherapeutic use of this compound primarily for two reasons: to self-treat opioid withdrawal symptoms and to induce euphoria and analgesia drugbank.comaddictioncenter.comnih.govpedemmorsels.comupstate.edupowerpak.comfda.govnjpies.org.
Self-Treatment of Opioid Withdrawal Symptoms
This compound has been anecdotally referred to as the "poor man's methadone" due to its ability to alleviate opioid withdrawal symptoms addictioncenter.compedemmorsels.comupstate.edupowerpak.comnih.govfda.gov. This motivation is prevalent among individuals struggling with opioid dependence, especially as access to prescription opioids becomes more restricted addictioncenter.comupstate.edumissouri.edu. Web-based studies indicate that almost 70% of discussions related to this compound's extra-medical use focus on its role in self-treating opioid withdrawal upstate.edunih.gov. Reported daily doses for this purpose often range from 70 mg to 100 mg, significantly exceeding the typical therapeutic dose of 16 mg per day nih.govdrugs.com.
Induction of Euphoria and Analgesia
At very high doses, this compound can overcome the blood-brain barrier efflux mechanisms, allowing it to exert central opioid effects, including euphoria and, to a lesser extent, analgesia drugbank.comaddictioncenter.comnih.govpedemmorsels.comfrontiersin.orgresearchgate.netmedicaltoxic.comwikipedia.orgdrugs.com. Individuals may ingest anywhere from 50 to 400 pills in a single day to achieve a euphoric sensation similar to that of oxycodone or heroin addictioncenter.com. While the majority of online discussions express skepticism about this compound's euphoric potential, a notable portion (around 25%) still discusses its ability to produce such effects nih.gov.
Pharmacokinetic Manipulation to Enhance Central Effects
To enhance this compound's central effects and overcome its limited blood-brain barrier penetration, users often co-administer it with other substances that alter its pharmacokinetics frontiersin.orgmedicaltoxic.compowerpak.comfda.govdrugs.commedcentral.com. This compound is a substrate for both P-glycoprotein (P-gp) and the cytochrome P450 (CYP) enzymes, specifically CYP3A4 and CYP2C8 drugbank.comnih.govfrontiersin.orgwikipedia.orgmedcentral.comtg.org.aunih.govresearchgate.netmedsafe.govt.nzresearchgate.net.
Co-administration with P-glycoprotein Inhibitors (e.g., Quinidine, Ritonavir)
P-glycoprotein is an efflux transporter that actively pumps this compound out of the brain, limiting its CNS penetration at therapeutic doses frontiersin.orgresearchgate.netwikipedia.orgtg.org.aunih.gov. Co-administration with P-gp inhibitors can increase this compound's systemic absorption and its ability to cross the blood-brain barrier, thereby enhancing its central effects frontiersin.orgpowerpak.comwikipedia.orgmedcentral.comtg.org.aunih.gov.
Table 1: Impact of P-glycoprotein Inhibitors on this compound Plasma Levels
| P-glycoprotein Inhibitor | This compound Dose (single) | Effect on this compound Plasma Levels (Increase Factor) | Associated CNS Effects (Psychomotor Tests) | Reference |
| Quinidine | 16 mg | 2 to 3-fold | Respiratory depression observed nih.gov | wikipedia.orgmedsafe.govt.nznih.gov |
| Ritonavir | 16 mg | 2 to 3-fold | Not specified in this context | medcentral.commedsafe.govt.nzdrugs.com |
| Itraconazole | 4 mg | 3 to 4-fold (also CYP3A4 inhibitor) | Not associated with CNS effects | drugs.commedsafe.govt.nz |
| Ketoconazole | 16 mg | 5-fold (also CYP3A4 inhibitor) | Not associated with CNS effects | medsafe.govt.nz |
Note: While some studies show increased plasma levels with P-gp inhibitors, the direct correlation with CNS effects at therapeutic doses can vary. Respiratory depression with quinidine and this compound was noted in a specific study nih.gov.
Co-administration with CYP3A4 or CYP2C8 Inhibitors (e.g., Cimetidine, Gemfibrozil, Itraconazole)
This compound is extensively metabolized, primarily by the hepatic cytochrome P450 isoenzymes CYP3A4 and CYP2C8, with CYP2B6 and CYP2D6 playing minor roles drugbank.comnih.govmedcentral.comnih.govmedsafe.govt.nzresearchgate.net. Inhibiting these enzymes can decrease this compound's metabolism, leading to increased plasma concentrations and a higher likelihood of CNS effects drugbank.compowerpak.comdrugs.commedcentral.comnih.govresearchgate.netresearchgate.netnih.gov.
Table 2: Impact of CYP Inhibitors on this compound Plasma Levels
| CYP Inhibitor | Primary CYP Inhibition | This compound Dose (single) | Effect on this compound Plasma Levels (Increase Factor) | Reference |
| Cimetidine | CYP3A4 | Varies (supratherapeutic) | Increased serum levels drugbank.compowerpak.commedcentral.comresearchgate.netnih.gov | drugbank.compowerpak.commedcentral.comdrugs.comresearchgate.netnih.gov |
| Gemfibrozil | CYP2C8 | 4 mg | Approximately 2-fold | medcentral.comnih.govresearchgate.netmedsafe.govt.nz |
| Itraconazole | CYP3A4, P-gp | 4 mg | 3 to 4-fold | drugs.commedcentral.comnih.govresearchgate.netmedsafe.govt.nz |
| Itraconazole + Gemfibrozil | CYP3A4, P-gp, CYP2C8 | 4 mg | 4-fold (peak), 13-fold (total exposure) | nih.govmedsafe.govt.nz |
Note: The combined inhibition of CYP3A4 and CYP2C8 can lead to a significant increase in this compound exposure nih.govmedsafe.govt.nz.
Tolerance and Dependence Mechanisms
At therapeutic doses, this compound functions as an opioid receptor agonist, primarily targeting μ-opioid receptors within the myenteric plexus of the large intestine to reduce gastrointestinal motility. wikipedia.orgmims.comed.ac.uk Its limited central nervous system (CNS) effects at these doses are attributed to its poor oral bioavailability, extensive first-pass metabolism, and active efflux by the P-glycoprotein (P-gp) transporter, which effectively pumps the drug out of the brain. wikipedia.orged.ac.ukpowerpak.comnih.gov
However, when this compound is consumed in significantly higher, supratherapeutic doses (often exceeding 70 mg), it can saturate the P-gp transporter. wikipedia.orgnih.govresearchgate.netnih.gov This saturation allows this compound to bypass the efflux mechanism and penetrate the CNS, where it can then exert central opioid-like effects, including euphoria. wikipedia.orgnih.govresearchgate.netnih.gov Prolonged use at these elevated doses can lead to the development of both tolerance and physical dependence. wikipedia.orgresearchgate.net
Studies in animals have demonstrated that parenteral administration of this compound can induce physical dependence and cross-tolerance to other opioids. drugs.com For instance, in morphine-dependent monkeys, high doses of this compound were shown to prevent the manifestation of morphine withdrawal signs. drugs.commedcentral.com While tolerance to the antidiarrheal effect of this compound has generally not been reported in humans, the drug's opioid agonist activity, particularly when it achieves CNS penetration at high doses, underlies the potential for physical dependence. ed.ac.ukdrugs.commedcentral.com
Withdrawal Symptoms Upon Abrupt Cessation
Abrupt discontinuation of chronic, high-dose this compound use can precipitate a range of withdrawal symptoms that are characteristic of opioid withdrawal syndrome. wikipedia.orgresearchgate.netaddictioncenter.comswissmedic.chnih.gov These symptoms arise from the body's physiological adaptation to the continuous presence of the opioid agonist and the subsequent disruption upon its removal.
Commonly reported withdrawal symptoms include:
Nausea and vomiting addictioncenter.com
Anxiety and irritability addictioncenter.comswissmedic.ch
Depression addictioncenter.com
Abdominal cramps and diarrhea (rebound effect) addictioncenter.comswissmedic.chunitedrecoveryproject.com
Excessive sweating addictioncenter.com
Muscle aches and pains addictioncenter.com
Restlessness swissmedic.chunitedrecoveryproject.com
Intestinal colic swissmedic.ch
While the severity of this compound withdrawal symptoms may vary, they can cause significant discomfort and distress, often compelling individuals to continue using the drug to alleviate these unpleasant sensations. unitedrecoveryproject.com
Epidemiology and Trends of this compound Misuse
This compound misuse has emerged as a growing public health concern, particularly in the United States. nih.govnih.govmedicaltoxic.comnih.govresearchgate.net It has been colloquially termed "the poor man's methadone" due to its increasing use as an accessible and inexpensive alternative for managing opioid withdrawal symptoms or, less frequently, for achieving euphoric effects. wikipedia.orgnih.govresearchgate.netnih.gov
Data from the National Poison Data System (NPDS) in the United States reveal a significant escalation in intentional this compound exposures. Between 2010 and 2015, there was a 91% increase in reported cases. powerpak.comnih.govmedicaltoxic.comnih.govmdpi.comresearchgate.net This trend indicated an approximate increase of 38 new cases annually during that period. nih.govmedicaltoxic.com
The demographic analysis of this compound misuse indicates that a substantial proportion of cases, roughly one-third, involve teenagers and young adults. powerpak.commedicaltoxic.comnih.govmdpi.com Furthermore, male individuals are more frequently implicated in intentional this compound abuse, accounting for 77% of 179 cases reported to the NPDS between 2008 and 2016, with a median age of 26 years. powerpak.comnih.govmedicaltoxic.commdpi.com Conversely, female subjects were more often involved in reported suicidal attempts using this compound. powerpak.commdpi.com A critical risk factor identified in these trends is a history of previous substance use, particularly opioid abuse; in a review of New York cases, 68% of individuals reported prior opioid abuse. medicaltoxic.com
The rise in this compound misuse has also been mirrored by increased online interest. Google Trends analyses show a pronounced increase in searches for terms such as "this compound high" and "this compound withdrawal" starting around 2010-2011, reflecting growing public awareness and discussion of its illicit uses. powerpak.comnih.gov The widespread availability and low cost of this compound are significant contributing factors to its appeal for misuse. nih.govnih.govmdpi.com
However, regulatory interventions have shown a positive impact. Following warnings issued by the U.S. Food and Drug Administration (FDA) in 2016 regarding the risks associated with high-dose this compound use, coupled with labeling requirements and packaging restrictions, a trend reversal has been observed. researchgate.netresearchgate.net Reports of intentional misuse, abuse, and suspected suicide related to this compound to US poison centers have shown a decrease after 2017, suggesting the potential effectiveness of such public health measures. researchgate.net
Table: Trends in Intentional this compound Exposures (US National Poison Data System, 2010-2015) powerpak.comnih.gov
| Year | Total Intentional this compound Exposures | Annual Increase (Approx.) |
| 2010 | 201 | - |
| 2011 | - | - |
| 2012 | - | - |
| 2013 | - | - |
| 2014 | - | - |
| 2015 | 383 | 38 cases/year |
| Overall Increase (2010-2015) | 91% |
Table: Reasons for Intentional this compound Exposure (US National Poison Data System, 2010-2015) powerpak.com
| Reason for Exposure | Percentage of Cases |
| Suspected Suicide | 48.8% |
| Intentional Misuse | 32.8% |
| Intentional Abuse | 13.1% |
| Other | 5.2% |
Preclinical and in Vitro Research Models
Animal Models for Gastrointestinal Motility Studies
Loperamide is frequently employed in animal models to induce a state of reduced gastrointestinal motility, mimicking conditions such as constipation. These models are valuable for studying the underlying mechanisms of GI dysmotility and for evaluating potential therapeutic interventions.
This compound is a commonly utilized agent to induce constipation in various animal species, including rats and mice. This induction allows researchers to investigate the pathophysiology of constipation and to assess the efficacy of novel laxative agents or prokinetic compounds scielo.brmdpi.comresearchgate.netfrontiersin.orgnih.govmdpi.comscielo.br. Administration of this compound leads to a notable reduction in defecation frequency, decreased fecal water content, and diminished fecal quantity mdpi.comfrontiersin.orgmdpi.comfrontiersin.orgscielo.br. Furthermore, it significantly prolongs intestinal transit time, a key indicator of constipation mdpi.comresearchgate.netmdpi.comscielo.brfrontiersin.orgscielo.brresearchgate.netnih.govcambridge.orgjmb.or.kr.
Doses of this compound used to induce constipation vary depending on the animal model and study design. In mice and rats, doses such as 5 mg/kg have been administered nih.govmdpi.comfrontiersin.orgresearchgate.netnih.gov. Higher doses, including 7.5 mg/kg and 10 mg/kg, have also been employed in mice nih.gov. The duration of this compound administration typically ranges from 7 to 14 days mdpi.comresearchgate.netmdpi.comfrontiersin.orgnih.gov. Parameters commonly assessed in these models include fecal weight, fecal water content, defecation frequency, intestinal length, and intestinal transit rate or time mdpi.comfrontiersin.orgmdpi.comscielo.brfrontiersin.orgscielo.brresearchgate.netnih.govjmb.or.kr.
Table 1: Effects of this compound in Animal Models of Constipation
| Parameter | Effect of this compound Treatment (vs. Control) | References |
| Defecation Frequency | Decreased | mdpi.comfrontiersin.orgmdpi.comfrontiersin.orgscielo.br |
| Fecal Water Content | Decreased | mdpi.comfrontiersin.orgmdpi.comfrontiersin.orgscielo.br |
| Fecal Quantity | Decreased | mdpi.comfrontiersin.org |
| Intestinal Transit Time | Prolonged/Delayed | mdpi.comresearchgate.netmdpi.comscielo.brfrontiersin.orgscielo.brresearchgate.netnih.govcambridge.orgjmb.or.kr |
| Intestinal Length | Decreased (in some studies) | mdpi.com |
Assessment of gastrointestinal transit rate in animal models often involves methods such as the charcoal meal transit test. In this technique, a non-absorbable marker like charcoal is orally administered, and the distance it travels through the intestines within a specific timeframe is measured jmb.or.kr. This compound administration consistently leads to a significant reduction in the intestinal transit rate mdpi.comfrontiersin.orgmdpi.comscielo.brjmb.or.kr. For example, in one study, the intestinal transit rate was notably reduced to approximately 38% in the this compound-treated group compared to 81% in the normal control group of mice jmb.or.kr. Another metric, the Oroanal Transit Time (OATT), also demonstrates a substantial increase in this compound-treated animals, with one study reporting a 90.5% increase in the this compound group, resulting in an OATT of 16.0 ± 1.9 hours compared to 8.4 ± 1.4 hours in controls scielo.br.
Interstitial Cells of Cajal (ICC) are specialized pacemaker cells crucial for regulating gastrointestinal motility. Research indicates that this compound administration is associated with a reduction in the number or expression levels of ICC biomarkers, such as c-kit and anoctamin-1 (ANO1) scielo.brfrontiersin.orgscielo.brnih.govkosfaj.orgresearchgate.net. Furthermore, in vitro studies have shown that this compound can induce apoptosis of ICC in a time- and concentration-dependent manner, with an observed IC50 of approximately 12 µM at 48 hours researchgate.net. This observed decrease in ICC is directly linked to the impaired colonic motility characteristic of this compound-induced constipation models scielo.brscielo.br.
Gastrointestinal Transit Rate Assessment
In Vitro Ion Channel Studies for Cardiotoxicity
In vitro studies using isolated cells or expression systems are vital for understanding the direct effects of this compound on cardiac ion channels, which can be relevant to its pharmacological profile.
This compound has been identified as a potent inhibitor of the human ether-à-go-go-related gene (hERG) potassium channel, which carries the rapidly activating delayed rectifier potassium current (IKr) in cardiac myocytes. Inhibition of hERG can prolong cardiac repolarization. Studies have reported varying inhibitory concentrations (IC50) for this compound on hERG channels. In Chinese hamster ovary (CHO) cells, the IC50 for hERG channel blockade was approximately 40 nmol/L (40 nM) for inhibition of tail currents jacc.org. Other studies using HEK293 cells have reported IC50 values of 0.390 µM (390 nM) semanticscholar.orgnih.govmdpi.comnih.gov. Additional reported values for hERG inhibition range from 33 nM to 88 nM semanticscholar.orgnih.gov, and between 15.7–20.5 µg/L, which corresponds to approximately 33-43 nM based on this compound's molar mass tandfonline.com.
Table 2: this compound's hERG (IKr) Current Inhibition (In Vitro)
| Cell Line / System | IC50 (nM) | Reference |
| CHO cells | ~40 | jacc.org |
| HEK293 cells | 390 | semanticscholar.orgnih.govmdpi.comnih.gov |
| Various | 33-88 | semanticscholar.orgnih.gov |
| Various | ~33-43 | tandfonline.com |
This compound has also been shown to inhibit various voltage-gated sodium channels (INa) in in vitro models. Inhibition of sodium channels can affect cardiac conduction. Studies indicate that this compound inhibits Nav1.5-mediated sodium current with an IC50 of 0.526 µM (526 nM) semanticscholar.orgnih.govmdpi.comnih.gov. Furthermore, this compound has demonstrated inhibitory effects on other sodium channel subtypes. For Nav1.7 channels, an IC50 of 1.86 ± 0.11 µM was reported for the resting state in HEK293 cells researchgate.netnih.gov. On recombinant Nav1.8 channels in ND7/23 cells, the IC50 was 0.60 ± 0.10 µM, with a higher potency observed on native Nav1.8 channels in dorsal root ganglion (DRG) neurons (0.11 ± 0.08 µM) researchgate.netnih.gov. Weaker potency was noted for Nav1.9 channels, with an IC50 of 3.48 ± 0.33 µM researchgate.netnih.gov. Other reported values for INa inhibition include 2900 nM using a high-throughput screening system in CHO cells and 239 nM via manual patch clamp in HEK293 cells semanticscholar.orgnih.gov.
Table 3: this compound's Sodium Channel (INa) Current Inhibition (In Vitro)
| Channel Subtype | Cell Line / System | IC50 (µM) / (nM) | Reference |
| Nav1.5 | N/A | 0.526 µM (526 nM) | semanticscholar.orgnih.govmdpi.comnih.gov |
| Nav1.7 (resting) | HEK293 cells | 1.86 ± 0.11 µM | researchgate.netnih.gov |
| Nav1.8 | Recombinant (ND7/23 cells) | 0.60 ± 0.10 µM | researchgate.netnih.gov |
| Nav1.8 | Native (DRG neurons) | 0.11 ± 0.08 µM | researchgate.netnih.gov |
| Nav1.9 | N/A | 3.48 ± 0.33 µM | researchgate.netnih.gov |
| INa (general) | HTS (CHO cells) | 2900 nM | semanticscholar.orgnih.gov |
| INa (general) | Manual patch clamp (HEK293 cells) | 239 nM | semanticscholar.orgnih.gov |
hERG (IKr) Current Inhibition Studies
In Silico Cardiac Electrophysiological Modeling
In silico cardiac electrophysiological modeling has been instrumental in understanding the potential cardiac effects of this compound, particularly at concentrations exceeding therapeutic levels. Studies employing human ventricular action potential models have confirmed that this compound exhibits a substantial safety margin at therapeutic exposures, typically up to 600 times its human Free Therapeutic Plasma Concentration (FTPC). mims.commims.comthermofisher.com
However, at extreme overdose concentrations, these in silico trials predict significant alterations in cardiac electrophysiology, including repolarization abnormalities and conduction slowing, which can lead to cardiac proarrhythmias. mims.commims.comthermofisher.com The primary mechanisms underlying this cardiac electrophysiological toxicity at overdose exposures are identified as the inhibition of the human Ether-à-go-go-Related Gene (hERG)-mediated IKr current and the sodium current (INa). mims.commims.comthermofisher.com this compound has been shown to inhibit hERG (IKr), INa, and ICa currents with specific half-maximal inhibitory concentration (IC50) values. mims.commims.comthermofisher.com These IC50 values are considerably higher than the FTPC, reinforcing the safety margin at standard therapeutic doses. mims.commims.comthermofisher.com
The observed effects on ion channels are summarized in the table below:
| Ion Channel | IC50 (µM) | Fold-Difference from FTPC (approx.) | Effect at Overdose Exposures | Source |
| hERG (IKr) | 0.390 | >1560 | Inhibition, leading to repolarization abnormalities | mims.commims.comthermofisher.com |
| INa | 0.526 | >1560 | Inhibition, leading to conduction slowing | mims.commims.comthermofisher.com |
| ICa | 4.091 | >1560 | Inhibition | mims.commims.comthermofisher.com |
Further in silico investigations have explored the potential for co-medications to influence this compound's cardiac effects. For instance, the combination of this compound with hydroxyzine in simulated action potential models appeared to mitigate abnormal action potential intervals compared to this compound administered alone. fishersci.ca
Preclinical Studies on Central Opioid Action
This compound is characterized as a piperidine derivative opioid ligand that primarily activates peripheral opioid receptors, with minimal penetration into the central nervous system (CNS) at therapeutic doses. wikipedia.orguni.lu This peripheral selectivity is largely attributed to its low oral absorption and its efficient efflux from the CNS by P-glycoprotein. wikipedia.orguni.lu
Despite its peripheral action at normal doses, preclinical studies have investigated the potential for central opioid effects under specific conditions. High doses of this compound administered to mice, rats, and rhesus monkeys have been shown to induce a mild physical dependence, with observable mild opiate withdrawal symptoms upon abrupt cessation of long-term treatment. wikipedia.orgresearchgate.net
The co-administration of this compound with P-glycoprotein inhibitors, such as quinidine, has been demonstrated to facilitate its passage across the blood-brain barrier, resulting in central morphine-like effects, including respiratory depression. researchgate.net Moreover, at extremely high doses (exceeding 70 mg), this compound can saturate P-glycoprotein, thereby overcoming the efflux mechanism and potentially leading to psychoactive effects. researchgate.net
While animal studies generally indicate that this compound lacks significant analgesic properties at typical therapeutic doses (2 to 16 mg/kg), some research in animal models of pain has reported antinociceptive effects following systemic or intraspinal administration. wikipedia.orgnih.govnih.gov For example, topical application of this compound at the site of inflammation in a thermal injury-induced hyperalgesia rat model produced a dose-dependent antihyperalgesic effect, which was reversible by naloxone, confirming a peripheral opioid-mediated action. nih.gov
Studies on Novel this compound Analogs and Opioid Receptor Agonists
This compound functions as a µ-opioid receptor (MOR) agonist, exhibiting notable affinity and selectivity for the cloned human µ-opioid receptor over the δ-opioid receptor. fishersci.sewikipedia.orgnih.gov Automated docking studies have suggested that this µ/δ selectivity may stem from the differential accommodation of this compound's two phenyl groups within distinct lipophilic pockets of the µ- and δ-opioid receptors. wikipedia.org
The structural characteristics of this compound, particularly its 4-phenylpiperidine scaffold, have served as a basis for the design and synthesis of novel analogs aimed at developing new µ-opioid receptor agonists. wikipedia.orgnih.govnih.gov Researchers have synthesized compounds based on the chemical structures of this compound and diphenoxylate, with the intent of creating potent MOR agonists. nih.gov
Key examples of these novel this compound analogs include:
Compound 5: This analog incorporates two 4-phenylpiperidine scaffolds, and its synthesis has been optimized to achieve excellent yields. wikipedia.orgnih.govnih.gov
Compounds 6 and 7: Each of these analogs features a single 4-phenylpiperidine scaffold. wikipedia.orgnih.govnih.gov
These synthesized analogs are considered promising candidates for future development as MOR agonists. nih.gov Beyond direct structural analogs, research has also explored compounds with mixed opioid receptor activity. For instance, FBNTI, a novel compound, has been identified as a potent delta opioid receptor (DOR) antagonist that also acts as a MOR agonist through an allosteric mechanism, demonstrating a significantly higher affinity for DOR compared to MOR. wikipedia.org Another compound, MuDelta, designed as a mixed µ opioid receptor agonist and δ opioid receptor antagonist, has been investigated for its ability to normalize gastrointestinal motility without inducing constipation. Preclinical studies with MuDelta showed very low plasma levels after oral administration and demonstrated its capacity to normalize gastrointestinal transit in stressed mice over a broader dose range than this compound. nih.gov
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


