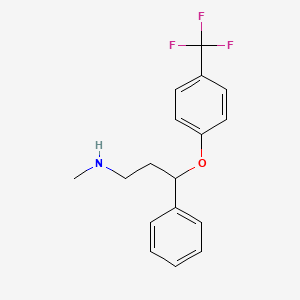
Fluoxetine
Description
Historical Context of Fluoxetine Discovery and Development
The development of this compound originated at Eli Lilly and Company in the early 1970s through a collaboration between Bryan Molloy and Ray Fuller. This research was partly influenced by the observation that the antihistamine diphenhydramine exhibited some antidepressant-like characteristics wikipedia.orgwikipedia.org. Starting with a compound structurally similar to diphenhydramine, 3-Phenoxy-3-phenylpropylamine, chemists synthesized numerous derivatives wikipedia.org.
A crucial step involved screening these compounds for their ability to selectively inhibit the reuptake of monoamine neurotransmitters, specifically serotonin, norepinephrine, and dopamine wikipedia.org. Utilizing a technique developed by neuroscientist Solomon Snyder, which involved using an extract of nerve endings from rat brains, researchers identified a compound that potently and selectively inhibited serotonin reuptake with minimal effects on norepinephrine and dopamine reuptake wikipedia.orgsciencehistory.org. This compound was later named this compound wikipedia.org.
The first academic article detailing this compound was published in 1974 wikipedia.org. Following years of research and development, an Investigational New Drug application for this compound was filed with the U.S. Food and Drug Administration (FDA) in February 1977 wikipedia.org. This compound first became available in the Belgian market in 1986, and the FDA granted its final approval in the U.S. in December 1987 wikipedia.org. Eli Lilly began marketing it as Prozac shortly thereafter wikipedia.org. The development of this compound is considered a significant breakthrough, being the first selective serotonin reuptake inhibitor (SSRI) approved by the FDA researchgate.netnih.gov.
Evolution of this compound as a Prototypical Selective Serotonin Reuptake Inhibitor (SSRI)
This compound's mechanism of action, primarily the inhibition of serotonin reuptake in presynaptic neurons by blocking the reuptake transporter protein, was central to its classification as a selective serotonin reuptake inhibitor (SSRI) nih.govdrugbank.commdpi.com. This selective action contrasted with earlier antidepressants like tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), which had broader effects on multiple neurotransmitter systems and were associated with more significant side effect profiles wikipedia.orgsciencehistory.org.
The concept of selectively targeting serotonin reuptake was a result of the evolving understanding of the role of serotonin (5-hydroxytryptamine or 5-HT) in mood regulation and depression, which gained traction in the early 1970s researchgate.netnih.gov. The hypothesis was that increasing the availability of serotonin in the synaptic cleft would help alleviate depressive symptoms researchgate.netnih.govdrugbank.commdpi.com. This compound's development was thus based on a planned strategy of rational drug design aimed at a specific biological target, the serotonin transporter (SERT) wikipedia.orgcambridge.org.
While the primary mechanism is SERT inhibition, research has also explored other interactions. This compound has shown mild activity at the 5-HT2A and 5-HT2C receptors nih.gov. At higher concentrations, it may also inhibit 5HT2C receptors, potentially contributing to increased synaptic norepinephrine and dopamine levels wikipedia.orgwikipedia.org. This compound also exhibits weak norepinephrine reuptake inhibition, which might have clinical relevance at higher doses wikipedia.org. Its active metabolite, northis compound, also contributes to elevating 5-HT levels researchgate.netfrontiersin.org. The relatively long half-life of this compound and its active metabolite distinguishes it from some other antidepressants wikipedia.org.
This compound's success and its more favorable side effect profile compared to older classes of antidepressants led to its rapid and widespread adoption, establishing it as a prototypical SSRI and paving the way for the development of other drugs in this class wikipedia.orgmdpi.com.
Current Research Landscape and Emerging Areas of Inquiry for this compound
Current academic research on this compound extends beyond its established uses in major depressive disorder, obsessive-compulsive disorder, bulimia nervosa, and panic disorder wikipedia.orgresearchgate.netnih.govdrugbank.com. Researchers are actively investigating its potential in a variety of other conditions and exploring its multifaceted biological effects.
Emerging areas of inquiry include the potential therapeutic role of this compound in neurological disorders such as Alzheimer's disease, where preclinical evidence suggests neuroprotective, antioxidant, and anti-inflammatory effects, as well as the promotion of neurogenesis and synaptic plasticity researchgate.net. Studies are exploring its impact on cognitive functions, although findings in this area remain inconsistent and require further investigation frontiersin.orgnih.gov.
Research is also delving into the effects of this compound on neurogenesis and neuroplasticity, noting its ability to stimulate neurogenesis and counteract declines caused by chronic stress, potentially linked to increases in brain-derived neurotrophic factor (BDNF) signaling frontiersin.orgnih.govresearchgate.net. The anti-inflammatory properties of this compound are another area of active research, with studies suggesting it can decrease neuroinflammation associated with various neurological disorders and inflammatory conditions frontiersin.org.
Furthermore, the potential application of this compound in treating conditions like selective mutism in children and its possible effects on complications related to COVID-19 are being explored in clinical trials clinicaltrials.eu. Investigations into the effects of early-life this compound exposure on neurodevelopment and behavior in animal models are also ongoing jneurosci.org.
At a more fundamental level, multi-omics studies are investigating the comprehensive impact of this compound across different brain regions, revealing region-specific changes in gene expression and chromatin state, and suggesting a global increase in energy metabolism as a potential shared mechanism of action nih.gov. Research is also examining the environmental impact of antidepressants like this compound on aquatic life, investigating effects on behavior and potential for bioaccumulation acs.org.
The ongoing research landscape for this compound highlights a continued effort to fully understand its complex pharmacological profile and explore its potential therapeutic applications beyond its initial indications.
Properties
IUPAC Name |
N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RTHCYVBBDHJXIQ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CNCCC(C1=CC=CC=C1)OC2=CC=C(C=C2)C(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H18F3NO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
59333-67-4 (hydrochloride) | |
| Record name | Fluoxetine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0054910893 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID7023067 | |
| Record name | Fluoxetine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023067 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
309.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
395.1°C at 760 mmHg | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
insoluble, 1.70e-03 g/L | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
54910-89-3, 57226-07-0 | |
| Record name | Fluoxetine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=54910-89-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Fluoxetine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0054910893 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | NSC-283480 | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=283480 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Fluoxetine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023067 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Benzenepropanamine, N-methyl-γ-[4-(trifluoromethyl)phenoxy] | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.125.370 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | FLUOXETINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/01K63SUP8D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
179 - 182 °C | |
| Record name | Fluoxetine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00472 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fluoxetine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014615 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Neurobiological Mechanisms of Fluoxetine Action
Serotonergic System Modulation
Fluoxetine's classification as an SSRI highlights its principal mode of action within the serotonergic system, which involves increasing the availability of serotonin in the synaptic cleft.
Inhibition of Serotonin Reuptake at the Presynaptic Terminal
This compound exerts its primary effect by blocking the reuptake of serotonin (5-hydroxytryptamine or 5-HT) at the presynaptic neuron terminal. This is achieved by inhibiting the serotonin transporter (SERT) protein. nih.govnih.govpharmgkb.org By binding to the reuptake pump on the neuronal membrane, this compound prevents the transport of serotonin back into the presynaptic neuron. wikipedia.org This inhibition leads to an increased concentration of serotonin in the synaptic cleft, thereby enhancing serotonergic neurotransmission. nih.govwikipedia.orgdrugbank.com This increased availability of serotonin allows for prolonged stimulation of postsynaptic serotonin receptors. nih.govspandidos-publications.com
Effects on Serotonin Receptor Subtypes (e.g., 5-HT1A, 5-HT2A, 5-HT2C)
While this compound's main action is on the serotonin transporter, it also exhibits activity, albeit typically weaker, at certain serotonin receptor subtypes. This compound has been shown to have mild activity at the 5-HT2A and 5-HT2C receptors. nih.gov Chronic administration of this compound has been shown to selectively interact with the 5-HT1A receptor, potentially enhancing its G protein coupling capability. oncotarget.com Activation of the 5-HT1A receptor, which is located presynaptically in areas like the dorsal raphe nucleus, can influence downstream signaling pathways. nih.govoncotarget.com Furthermore, it has been suggested that this compound's interaction with the 5-HT2C receptor may contribute to increases in norepinephrine and dopamine levels in the prefrontal cortex, particularly at higher concentrations. wikipedia.orgdrugbank.com Activation of postsynaptic 5-HT1A receptors has also been linked to the regulation of GSK-3β/β-catenin signaling, a pathway implicated in neurogenesis. oup.com
Impact on Serotonin Levels in Cerebrospinal Fluid
Studies have investigated the impact of this compound on serotonin levels in the cerebrospinal fluid (CSF). Low concentrations of serotonin have been observed in the CSF of patients with depression. nih.govdrugbank.com While direct measurements of serotonin increases in CSF due to this compound are complex, the inhibition of reuptake is understood to increase extracellular serotonin levels in various brain regions, including those that influence CSF composition. drugbank.comspandidos-publications.com Research has also explored the effect of this compound on neurosteroid levels in CSF, noting that this compound treatment can normalize decreased levels of allopregnanolone in the CSF of patients with major depression, and this normalization has shown a correlation with symptom improvement. nih.govquotidianosanita.it
Beyond Serotonin Reuptake Inhibition
Beyond its well-established role as a serotonin reuptake inhibitor, this compound engages in other neurobiological processes that contribute to its therapeutic profile, notably involving neurotrophic factors.
Neurotrophic Factor Modulation
This compound's effects extend to the modulation of neurotrophic factors, which are crucial for neuronal survival, growth, and plasticity.
Brain-Derived Neurotrophic Factor (BDNF) Signaling
Brain-Derived Neurotrophic Factor (BDNF) and its receptor, tropomyosin receptor kinase B (TrkB), are considered key players in the neuroplastic changes associated with the therapeutic effects of antidepressants, including this compound. frontiersin.orgresearchgate.netjneurosci.org Chronic administration of this compound has been widely reported to increase both BDNF mRNA and protein expression in vivo. researchgate.net Studies in primary cortical neurons have shown that this compound can induce both mRNA and protein expression of BDNF, as well as mRNA expression of immediate early genes related to the TrkB signaling pathway. researchgate.net This effect appears to involve TrkB receptor activation and, in some contexts, may be independent of the serotonin transporter. researchgate.net
BDNF binding to TrkB is typically associated with the activation of intracellular signaling pathways such as the Ras-MAPK, PI3-K, and PLCγ pathways, which are involved in processes like proliferation, differentiation, survival, and neurite extension. researchgate.net Research suggests that this compound's ability to enhance BDNF/TrkB signaling is fundamental to the activity-dependent synaptic plasticity that contributes to mood improvements. frontiersin.org Furthermore, studies indicate that this compound may directly bind to the TrkB receptor, facilitating its synaptic localization and activation by BDNF. frontiersin.org The increase in extracellular serotonin resulting from this compound's reuptake inhibition can act on receptors like 5-HT1A, which in turn can activate pathways leading to the expression of BDNF. oncotarget.com The reciprocal interaction between serotonin and BDNF may synergize the pharmacological effects of this compound. oncotarget.com this compound has also been shown to upregulate BDNF expression in astrocytes, suggesting a potential role of glial cells in its neurotrophic effects, possibly contributing to normalizing trophic and metabolic support to neurons. nih.gov Studies using BDNF heterozygous animals suggest that BDNF is crucial for this compound-induced effects. biorxiv.org
The effects of this compound on BDNF expression have been observed in various brain regions, including the hippocampus and prefrontal cortex. spandidos-publications.comjneurosci.orgbiorxiv.org Chronic this compound treatment has been shown to increase BDNF levels in the dorsal hippocampus, while acute treatment may increase levels in the ventral hippocampus, suggesting regional specificity in its effects. biorxiv.org
Below is a summary of research findings on this compound's impact on BDNF:
| Study Type | Finding | Citation |
| In vivo (chronic) | Increased BDNF mRNA and protein expression. | researchgate.net |
| In vitro (cortical neurons) | Induced BDNF mRNA and protein expression; increased immediate early genes related to TrkB signaling. | researchgate.net |
| Genetic models | BDNF crucial for this compound effects (BDNF +/- animals). | biorxiv.org |
| Receptor involvement | 5-HT1A receptor activation linked to BDNF expression. | oncotarget.com |
| Direct binding | May directly bind to TrkB receptor, facilitating activation by BDNF. | frontiersin.org |
| Glial cells | Upregulation of BDNF in astrocytes. | nih.gov |
| Regional specificity | Differential effects on BDNF levels in dorsal vs. ventral hippocampus (chronic vs. acute). | biorxiv.org |
Tropomyosin Receptor Kinase B (TrkB) Activation
This compound has been shown to activate the tropomyosin receptor kinase B (TrkB), the primary receptor for brain-derived neurotrophic factor (BDNF). This activation is considered fundamental to the activity-dependent synaptic plasticity observed with this compound treatment. Research suggests that this compound can directly bind to the TrkB receptor, allosterically promoting its signaling frontiersin.orgmdpi.com. This direct binding facilitates the synaptic localization of TrkB and enhances its activation by BDNF frontiersin.org. Different experimental approaches support the idea that enhanced BDNF/TrkB signaling is crucial for the mood improvements associated with this compound treatment frontiersin.org. Antidepressants, including this compound, are thought to facilitate the ability of BDNF to activate TrkB receptors in parvalbumin-positive (PV) interneurons through several mechanisms, including direct binding, inhibiting dephosphorylation of TrkB, and reducing TrkB endocytosis frontiersin.org. Studies using genetically modified mice have demonstrated that the plasticity-related and antidepressant-like responses to this compound are lost in animals with a mutation in the TrkB transmembrane domain that abolishes antidepressant binding, further supporting TrkB as a direct target frontiersin.org.
Vascular Endothelial Growth Factor (VEGF) Enhancement
Studies indicate that this compound can enhance the levels of Vascular Endothelial Growth Factor (VEGF). VEGF is a trophic factor known to regulate angiogenesis and also possesses neurotrophic and neuroprotective properties in the central and peripheral nervous systems researchgate.net. Chronic this compound administration has been shown to increase endothelial cell proliferation in the subgranular zone (SGZ) of the hippocampus, and VEGF receptor signaling has been identified as a mediator of this effect researchgate.netnih.gov. Research in patients with vascular cognitive impairment has also shown that this compound treatment can increase serum concentrations of VEGF nih.govnih.govresearchgate.net. This enhancement of VEGF may contribute to the remodeling of hippocampal circuitry and increased neurogenesis and angiogenesis researchgate.net.
Neurogenesis and Synaptogenesis
This compound treatment has been extensively studied for its effects on neurogenesis (the birth of new neurons) and synaptogenesis (the formation of new synapses), particularly in the adult brain.
Stimulation of Adult Neurogenesis (Hippocampus, Dentate Gyrus)
Chronic this compound treatment is known to stimulate neurogenesis in the adult hippocampus, specifically in the dentate gyrus (DG) frontiersin.orgkyoto-u.ac.jpnih.govmdpi.compnas.orgpnas.org. This increase in the production of new neurons is considered a potential mechanism underlying the behavioral effects of this compound kyoto-u.ac.jppnas.org. This compound has been shown to facilitate various stages of neurogenesis, including progenitor proliferation, survival, and the early phase of maturation frontiersin.org. It can also counteract the decline in neurogenesis caused by chronic stress frontiersin.orgfrontiersin.org. Studies using reporter mouse lines have indicated that this compound primarily targets an early progenitor cell class in the adult DG, increasing symmetric divisions and leading to an expansion of this cell population pnas.org. While increased hippocampal neurogenesis is a notable effect, some research suggests that functional modifications of existing neurons may also be necessary for the full action of antidepressants researchgate.net.
This compound also appears to promote neurogenesis of interneurons in the cerebral cortex frontiersin.orgkyoto-u.ac.jpnih.gov. Studies in adult marmosets showed increased expression of markers for immature neurons in the hippocampal dentate gyrus and increased generation of cortical interneurons following this compound treatment kyoto-u.ac.jpnih.gov.
Effects on Neuronal Plasticity and Network Remodeling
This compound promotes various forms of plasticity in the adult central nervous system, including structural remodeling of neurons and alterations in synaptic connectivity oup.commdpi.comnih.gov. These changes in neuroplasticity are thought to strengthen synaptic connectivity and contribute to the remodeling of neuronal circuits in emotional pathways mdpi.com. Chronic this compound treatment induces adaptive changes in forebrain structures, including modifications at glutamatergic synapses frontiersin.org. The neuroplastic effects have been observed in diverse neural systems, including the visual cortex and hippocampus biorxiv.org. This compound can restore in adult animals the plastic potential characteristic of earlier stages of postnatal life, which may facilitate the functional and structural recovery of neuronal networks nih.gov.
Alterations in Dendritic Spine Density
This compound treatment has been shown to increase dendritic spine density in various brain regions, including the hippocampus and medial prefrontal cortex frontiersin.orgoup.commdpi.com. Dendritic spines are the postsynaptic sites of most excitatory synapses, and changes in their density and morphology are indicative of synaptic plasticity plos.orgnih.gov. Chronic this compound treatment can lead to the development of robust and larger spines enriched with receptor subunits associated with enhanced synaptic responses frontiersin.org. Studies have observed an increase in dendritic spine density in the hippocampus of rodents and in cortical interneurons oup.commdpi.comfrontiersin.orgplos.orgnih.gov. For instance, chronic this compound treatment in mice induced the appearance of large-sized spines in the dentate gyrus plos.org. The effect on spine density can vary depending on the brain region, age, and duration of treatment frontiersin.orgmdpi.comfrontiersin.orgnih.gov.
Table 1: Observed Effects of this compound on Dendritic Spine Density
| Brain Region | Observed Effect on Spine Density | Reference |
| Hippocampus | Increase | frontiersin.orgmdpi.complos.org |
| Medial Prefrontal Cortex | Increase | frontiersin.orgmdpi.com |
| Somatosensory Cortex | Increase | mdpi.com |
| Hippocampal CA1 s.r. | Increase | frontiersin.org |
| Hippocampal CA1 s.l.m. | No change | frontiersin.org |
| Dentate Gyrus | Increase (in some studies) | plos.orgnih.gov |
| Cortical Interneurons | Increase | oup.com |
Myelination and Gene Expression
Research suggests that this compound can influence myelination and the expression of genes related to this process. Myelination, the formation of the myelin sheath around nerve fibers, is crucial for efficient neuronal communication and contributes to brain connectivity dntb.gov.ua. Studies in rats have shown that perinatal exposure to this compound can affect myelin-related gene expression in regions like the prefrontal cortex and basolateral amygdala, with effects potentially being sex-specific nih.govbiorxiv.org. Chronic this compound exposure has also been found to cause long-term changes in the expression of genes involved in myelination in the hippocampus of adult rats dntb.gov.uaresearchgate.net. Gene ontology analysis in one study revealed that upregulated genes induced by chronic this compound exposure were significantly enriched for genes involved in myelination dntb.gov.uaresearchgate.net. Specific myelin-related genes like Transferrin (Tf) and Ciliary neurotrophic factor (Cntf) have shown altered expression following this compound exposure dntb.gov.uaresearchgate.net. These findings suggest that this compound's effects extend to influencing the structural components that support neuronal network function.
Anti-inflammatory and Neuroprotective Properties
Reduction of Neuroinflammation
Studies indicate that this compound can reduce neuroinflammation by modulating the levels of pro-inflammatory cytokines. Research in animal models of depression has shown that this compound treatment can suppress neuroinflammation in the hippocampus. frontiersin.orgnih.gov This involves reducing the activation of microglia and astrocytes and decreasing the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). frontiersin.orgnih.govexplorationpub.comnih.gov The anti-inflammatory effects may be mediated through multiple signaling pathways, including the inhibition of the p38 mitogen-activated protein kinase (MAPK) pathway and the disruption of nuclear factor-kappa B (NF-κB) signaling. frontiersin.orgnih.govfrontiersin.org
Table 1: Effects of this compound on Pro-inflammatory Cytokine Levels in Animal Models
| Study Context | Observed Effect on IL-1β | Observed Effect on TNF-α | Key Mechanism Involved | Source |
| Chronic unpredictable mild stress | Decreased | Decreased | Inhibition of p38 MAPK | frontiersin.orgnih.gov |
| LPS-induced microglial activation | Decreased | Decreased | Inhibition of NF-κB signaling | nih.gov |
| Experimental inflammation models | Decreased | Decreased | Reduction in mRNA levels | nih.gov |
Inhibition of Apoptosis and Neuronal Injury
This compound exhibits anti-apoptotic properties, contributing to the preservation of neuronal integrity and function. nih.govnih.gov It has been shown to mitigate neuronal injury induced by various insults, including chronic stress and ischemia/reperfusion. frontiersin.orgnih.govmdpi.com Mechanisms involved in the anti-apoptotic effects include the reduction of apoptosis mediators and oxidative substances. nih.gov Studies have demonstrated that this compound can decrease neuronal apoptosis by inhibiting pathways such as the p38 MAPK pathway and modulating proteins like caspase-3. frontiersin.orgnih.govaging-us.comnih.gov In models of early brain injury, this compound treatment significantly attenuated apoptosis and the expression of apoptosis-related proteins. nih.gov Furthermore, this compound has been shown to protect neurons against microglia-mediated neurotoxicity, partly by inhibiting microglial activation and the subsequent release of pro-inflammatory factors. nih.gov
Table 2: Effects of this compound on Neuronal Apoptosis and Injury
| Study Context | Observed Effect on Apoptosis | Involved Molecular Targets/Pathways | Source |
| Chronic unpredictable mild stress | Decreased | p38 MAPK, Caspase-3 | frontiersin.orgnih.gov |
| Rotenone-induced neurodegeneration | Inhibited | Caspase-3, ER stress | aging-us.com |
| Early brain injury after SAH | Attenuated | Notch1/ASK1/p38 MAPK signaling | nih.gov |
| Microglia-mediated neurotoxicity | Protected against | Microglial activation, NF-κB | nih.gov |
Modulation of Neurosteroid Levels
This compound has been found to modulate the levels of neurosteroids in the brain, which are known to act as allosteric modulators of neurotransmitter receptors, particularly the GABAA receptor. pnas.orgfrontiersin.orggoogle.com This modulation is hypothesized to contribute to the anxiolytic effects of this compound, potentially explaining rapid effects observed before significant serotonin reuptake inhibition occurs. novapublishers.comfrontiersin.orgresearchgate.net Research indicates that this compound can increase brain levels of neurosteroids such as allopregnanolone (3α, 5α-THP). pnas.orgfrontiersin.orgresearchgate.netresearchgate.net This increase may occur through direct interference with enzymes involved in neurosteroid synthesis, such as 3α-hydroxysteroid oxidoreductase, or by inhibiting competing pathways. pnas.orgfrontiersin.orgresearchgate.net Studies in both rats and depressed patients have shown elevated levels of allopregnanolone in the brain and cerebrospinal fluid following this compound treatment. pnas.orgfrontiersin.orgresearchgate.net
Table 3: Effects of this compound on Neurosteroid Levels
| Neurosteroid | Observed Effect (Animal Studies) | Observed Effect (Human Studies) | Proposed Mechanism | Source |
| Allopregnanolone (3α, 5α-THP) | Increased in brain | Increased in CSF and plasma | Modulation of neurosteroidogenic enzymes (e.g., 3α-HSD), inhibition of oxidation | pnas.orgfrontiersin.orgresearchgate.netresearchgate.net |
| 5α-androstane-3α,17β-diol | Elevated in brain/serum ratio | Not specified | Activation of 3α-hydroxysteroid dehydrogenase | jst.go.jp |
Glutamatergic Neurotransmission Modulation
While primarily known for its effects on the serotonergic system, this compound also appears to modulate glutamatergic neurotransmission. frontiersin.org Glutamate is the primary excitatory neurotransmitter in the mammalian nervous system and plays a crucial role in various brain functions, including mood and cognition. nih.gov The precise mechanisms by which this compound influences glutamatergic signaling are still under investigation, but this modulation may contribute to its therapeutic effects and potentially its impact on neuroplasticity. novapublishers.com
Sigma-1 Receptor Action
This compound has been shown to interact with the sigma-1 receptor. frontiersin.org The sigma-1 receptor is a chaperone protein located at the endoplasmic reticulum that modulates various cellular processes, including calcium signaling, protein folding, and neurotransmission. Activation of this receptor has been linked to anti-inflammatory and neuroprotective effects in certain cell culture models. explorationpub.com this compound's action at the sigma-1 receptor may contribute to its effects on neuroinflammation and neuroprotection, independent of its primary action on serotonin reuptake. frontiersin.orgexplorationpub.com
Functional Inhibition of Acid Sphingomyelinase (FIASMA) and the Ceramide System
This compound is recognized as a functional inhibitor of acid sphingomyelinase (FIASMA). pnas.org Acid sphingomyelinase is an enzyme involved in the hydrolysis of sphingomyelin into ceramide. nih.govdisprot.org Modulation of this enzyme and the resulting levels of ceramide can impact various cellular processes, including apoptosis, inflammation, and cell signaling. metabolomicsworkbench.orgciteab.comnih.gov By inhibiting acid sphingomyelinase, this compound can alter the balance of sphingolipids, potentially contributing to its observed anti-apoptotic and anti-inflammatory effects. pnas.org
Table 4: Overview of this compound's Actions on the Ceramide System
| Target | Type of Action | Downstream Effect | Source |
| Acid Sphingomyelinase (ASM) | Functional Inhibition | Altered sphingomyelin/ceramide balance | pnas.org |
| Ceramide Levels | Decreased (indirectly) | Potential impact on apoptosis, inflammation, signaling | metabolomicsworkbench.orgciteab.comnih.gov |
Impact on mTOR Pathway
Research into the neurobiological mechanisms of this compound action has increasingly focused on intracellular signaling pathways, including the mammalian target of rapamycin (mTOR) pathway. The mTOR pathway is recognized for its critical role in various neuronal functions, such as protein synthesis, synaptic plasticity, neurogenesis, and cell survival, processes implicated in the pathophysiology and treatment of mood disorders. oup.comcsic.es
Studies have demonstrated that chronic treatment with this compound can influence mTOR signaling in a region-dependent manner in the brain. Specifically, chronic administration of this compound has been shown to attenuate the reduction in mTOR phosphorylation induced by chronic unpredictable mild stress (CUMS) in the hippocampus and amygdala of mice. researchgate.netnih.govnih.govscite.ai This suggests that this compound can counteract stress-induced impairments in mTOR pathway activity in these key brain regions associated with mood regulation. However, this effect was not consistently observed in other regions like the frontal cortex or hypothalamus in some studies. researchgate.netnih.govnih.gov
Activation of the mTOR signaling pathway by chronic this compound treatment appears to be crucial for its antidepressant-like effects and its impact on synaptic plasticity. Research indicates that this compound-induced increases in synaptic protein levels, such as PSD-95 and synapsin I, in the hippocampus are mediated through the activation of the mTOR pathway. researchgate.netnih.govnih.gov These effects on synaptic proteins, which are vital for synaptic structure and function, were blocked by co-administration of rapamycin, a known inhibitor of mTOR. researchgate.netnih.govnih.govfrontiersin.org This provides strong evidence that the mTOR pathway is a necessary component for at least some of the neurobiological effects of this compound, particularly those related to synaptic plasticity in the hippocampus. nih.govfrontiersin.org
Upstream regulators of the mTOR pathway, including the PI3K/Akt and MAPK/ERK pathways, have also been implicated in the effects of antidepressants. Some studies suggest that this compound may influence these upstream molecules, which can subsequently lead to mTOR activation. frontiersin.orgoup.com The BDNF-TrkB signaling pathway is also considered an upstream activator that can converge on mTORC1 signaling, and this compound has been shown to affect BDNF levels and TrkB signaling, potentially contributing to mTOR activation. frontiersin.orgmdpi.com
While chronic this compound treatment consistently shows an impact on mTOR signaling in specific brain regions in vivo, some in vitro studies in cultured hippocampal neurons have presented findings suggesting that while this compound increases upstream regulators like phospho-Akt and phospho-ERK, its effects on synaptic proteins might occur through mechanisms independent of direct mTOR activation in that specific context. oup.com This highlights the complexity of this compound's mechanisms and potential differences between in vitro and in vivo conditions or regional variations in signaling reliance.
Pharmacological Profile of Fluoxetine
Pharmacokinetics
The pharmacokinetics of fluoxetine are notable for its relatively slow elimination and the formation of an active metabolite, contributing to its prolonged effects.
Absorption and Bioavailability
This compound is well absorbed following oral administration. hres.ca Despite extensive absorption, hepatic first-pass metabolism reduces its oral bioavailability to below 90%. mdpi.comdrugbank.com Some sources indicate bioavailability between 60% and 80%. wikipedia.org Peak plasma concentrations are typically reached within 6 to 8 hours after a single oral dose. hres.camims.com Food does not appear to significantly affect the systemic bioavailability of this compound, although it may slightly delay absorption. hres.ca
Distribution and Tissue Accumulation
This compound is highly lipophilic and exhibits a large volume of distribution (Vd), ranging from 12 to 100 L/kg, which indicates substantial accumulation in tissues. mdpi.comdrugbank.compharmgkb.orgscielo.br Compared to other SSRIs, this compound has the highest volume of distribution. mdpi.com It is highly bound to plasma proteins, primarily albumin and α₁-acid glycoprotein, with approximately 94-95% protein binding. drugbank.comwikipedia.orgmims.com This high protein binding allows the drug and its active metabolite, northis compound, to distribute to the brain. drugbank.com this compound is widely distributed throughout the body and readily crosses the blood-brain barrier. mims.com While it accumulates in several tissues, its accumulation in the brain is reported to be significantly lower compared to some other SSRIs, with a brain to plasma ratio of 2.6:1 in patients. mdpi.compharmgkb.org Tissue distribution studies in animals have shown this compound to be most highly concentrated in the lungs, with distribution coefficients relative to whole blood being significantly higher in the lungs (60x), liver (38x), spleen (20x), brain (15x), heart (10x), and kidneys (9x). nih.gov
Metabolism and Metabolites
This compound undergoes extensive metabolism in the liver, primarily through the cytochrome P450 enzyme system. pharmgkb.orgresearchgate.netfda.gov
The primary metabolic pathway for this compound is N-demethylation, which produces the only identified active metabolite, northis compound. hres.capharmgkb.orgresearchgate.netfda.gov Northis compound is also an SSRI and contributes to the long duration of action of this compound. hres.caresearchgate.net this compound is administered as a racemic mixture of two enantiomers, R- and S-fluoxetine. pharmgkb.org S-fluoxetine is slightly more potent in inhibiting serotonin reuptake than R-fluoxetine. pharmgkb.orgmedcraveonline.com This difference in potency is more pronounced for the active metabolite, with S-northis compound having approximately 20 times greater reuptake blocking potency than R-northis compound. pharmgkb.orgmedcraveonline.com The formation of northis compound from this compound is stereoselective. researchgate.net
Multiple cytochrome P450 (CYP) enzymes are involved in the metabolism of this compound to its N-desmethyl metabolites, including CYP2D6, CYP2C19, CYP2C9, CYP3A4, and CYP3A5. mdpi.compharmgkb.orgmedcraveonline.comnih.gov While several enzymes contribute to the N-demethylation of this compound, CYP2D6, CYP2C9, and CYP3A4 appear to be the major contributing enzymes for phase I metabolism. drugbank.com In vivo studies in humans indicate that CYP2D6 plays a significant role. researchgate.net However, this compound and northis compound are inhibitors of CYP2D6-mediated reactions. mdpi.compharmgkb.orgmedcraveonline.com Consequently, the involvement of CYP2C19, CYP2C9, CYP3A4, and CYP3A5 in this compound metabolism becomes more prominent during chronic administration as CYP2D6 activity is reduced due to this inhibition. mdpi.compharmgkb.org In vitro studies have also shown this compound to have inhibitory efficacy against CYP2C19, CYP2C9, and CYP3A4. mdpi.compharmgkb.orgmedcraveonline.com In addition to N-demethylation, this compound and potentially northis compound undergo O-dealkylation mediated by CYP2C19 and CYP3A4, producing para-trifluoromethylphenol, which is subsequently metabolized to hippuric acid. drugbank.comnih.gov
The cytochrome P450 isoforms, particularly CYP2D6, exhibit genetic variations that impact their catalytic activity. mdpi.compharmgkb.org CYP2D6 is a highly polymorphic enzyme, and genetic variability in its function can be clinically important regarding this compound metabolism. wikipedia.orgnih.gov Studies have demonstrated that individuals with genetically predicted CYP2D6 poor metabolizer status (having two no-function alleles) tend to have higher steady-state plasma concentrations of this compound and lower concentrations of northis compound. researchgate.net This genetic polymorphism contributes to the interindividual variability observed in this compound plasma concentrations. nih.govresearchgate.net The frequency of CYP2D6 poor metabolizers varies among ethnic groups, being approximately 5-10% in Caucasians and 1% in East Asians. bpac.org.nz
Table 1: Key Pharmacokinetic Parameters of this compound
| Parameter | Value Range / Description | Source(s) |
| Oral Bioavailability | <90%, 60-80% | mdpi.comdrugbank.comwikipedia.org |
| Time to Peak Plasma Conc | 6-8 hours | hres.camims.com |
| Plasma Protein Binding | 94-95% | drugbank.comwikipedia.orgmims.com |
| Volume of Distribution | 12-100 L/kg | mdpi.comdrugbank.compharmgkb.orgscielo.br |
| Brain:Plasma Ratio | 2.6:1 | mdpi.compharmgkb.org |
| Primary Metabolism | Hepatic, N-demethylation to northis compound | hres.capharmgkb.orgresearchgate.netfda.gov |
| Major Metabolite | Northis compound (active) | hres.capharmgkb.orgresearchgate.netfda.gov |
| Elimination Half-life (this compound) | 1-3 days (acute), 4-6 days (chronic) | hres.cadrugbank.comfda.gov |
| Elimination Half-life (Northis compound) | 4-16 days | hres.cadrugbank.comfda.gov |
| Primary Elimination Route | Hepatic metabolism to inactive metabolites, urinary excretion | hres.cafda.gov |
Table 2: Cytochrome P450 Enzymes Involved in this compound Metabolism
| Enzyme | Role in this compound Metabolism | Notes | Source(s) |
| CYP2D6 | Major contributor to N-demethylation, highly polymorphic | Inhibited by this compound and northis compound; significant role in S-northis compound formation. pharmgkb.orgmedcraveonline.comresearchgate.net | mdpi.commims.compharmgkb.orgresearchgate.netmedcraveonline.comnih.govresearchgate.netheraldopenaccess.us |
| CYP2C19 | Involved in N-demethylation and O-dealkylation | Contribution increases with chronic dosing due to CYP2D6 inhibition; inhibited by this compound. mdpi.comdrugbank.compharmgkb.orgmedcraveonline.comnih.gov | mdpi.comdrugbank.compharmgkb.orgmedcraveonline.comnih.govnih.govresearchgate.netheraldopenaccess.us |
| CYP2C9 | Involved in N-demethylation | Contribution increases with chronic dosing due to CYP2D6 inhibition; preferentially catalyzes R-fluoxetine demethylation; inhibited by this compound. mdpi.comdrugbank.compharmgkb.orgmedcraveonline.comresearchgate.net | mdpi.comdrugbank.compharmgkb.orgmedcraveonline.comnih.govresearchgate.netheraldopenaccess.us |
| CYP3A4 | Involved in N-demethylation and O-dealkylation | Contribution increases with chronic dosing due to CYP2D6 inhibition; inhibited by this compound. mdpi.comdrugbank.compharmgkb.orgmedcraveonline.comnih.gov | mdpi.comdrugbank.compharmgkb.orgmedcraveonline.comnih.govnih.govheraldopenaccess.us |
| CYP3A5 | Involved in N-demethylation | Contribution increases with chronic dosing due to CYP2D6 inhibition. mdpi.compharmgkb.orgmedcraveonline.com | mdpi.compharmgkb.orgmedcraveonline.comnih.govheraldopenaccess.us |
Self-Inhibition of Metabolism
This compound and its active metabolite, northis compound, are known inhibitors of certain cytochrome P450 (CYP) enzymes, particularly CYP2D6. wikipedia.orgnih.govmedcraveonline.com This inhibitory activity can lead to the self-inhibition of their own metabolism. wikipedia.orguzh.ch As a result, the elimination half-life of this compound can increase with chronic use compared to a single dose. wikipedia.org This self-inhibition contributes to the non-linear pharmacokinetics observed at higher doses. mdpi.comnih.gov While CYP2D6 is a major enzyme involved in the metabolism of this compound to northis compound, other enzymes such as CYP2C9, CYP2C19, CYP3A4, and CYP3A5 also play a role, and their influence may become more significant during chronic administration when CYP2D6 activity is diminished due to inhibition by this compound and northis compound. medcraveonline.comdrugbank.compharmgkb.org
Elimination and Half-Lives
This compound is extensively metabolized in the liver, primarily through oxidative metabolism and conjugation. pharmgkb.orgpharmgkb.org The main metabolic pathway involves the N-demethylation of this compound to its active metabolite, northis compound. nih.govpharmgkb.org Approximately 2.5% of the administered dose is excreted unchanged in the urine. nih.gov Both this compound and northis compound have long elimination half-lives, which is a distinguishing feature compared to other antidepressants. wikipedia.org The elimination half-life of this compound typically ranges from 1 to 3 days after acute administration and increases to 4 to 6 days after chronic use. wikipedia.orgdrugbank.comnih.gov The half-life of northis compound is even longer, ranging from 4 to 16 days after both acute and chronic administration. uzh.chdrugbank.commedicines.org.uk These long half-lives contribute to the significant accumulation of this compound and northis compound in the body during chronic treatment and result in a prolonged presence of the drug for several weeks after discontinuation. drugbank.commedicines.org.uknih.gov
Non-linear Pharmacokinetics at Higher Doses
The pharmacokinetics of this compound exhibit non-linear characteristics, particularly at higher doses. mdpi.comnih.govnih.gov This is indicated by a disproportionate increase in plasma levels as the dosage is increased. uzh.chmdpi.comnih.gov This non-linearity is attributed, in part, to the self-inhibition of metabolism by this compound and northis compound, primarily affecting CYP2D6. wikipedia.orguzh.ch As enzyme activity becomes saturated or inhibited at higher drug concentrations, the rate of metabolism does not increase proportionally with the dose, leading to higher-than-predicted plasma concentrations. mdpi.comfda.gov This characteristic is important to consider when adjusting doses, as it can lead to significant increases in drug exposure. uzh.chmdpi.com
Pharmacodynamics
The pharmacodynamic profile of this compound is primarily defined by its interaction with neurotransmitter transporters and receptors.
Selectivity for Serotonin Transporter (SERT/SLC6A4)
This compound is classified as a selective serotonin reuptake inhibitor (SSRI). wikipedia.orgnih.gov Its primary mechanism of action involves the potent inhibition of the serotonin transporter (SERT), also known as SLC6A4. pharmgkb.orgnih.govnih.govpsychopharmacologyinstitute.combiologists.com SERT is responsible for the reuptake of serotonin from the synaptic cleft back into the presynaptic neuron, a process that terminates the action of serotonin. biologists.comwikipedia.org By blocking SERT, this compound increases the concentration and prolongs the presence of serotonin in the synaptic space, thereby enhancing serotonergic neurotransmission. wikipedia.orgnih.govnih.gov This selective inhibition of serotonin reuptake is considered the main contributor to its antidepressant effects. nih.govnih.gov
Minimal Activity on Noradrenergic and Dopaminergic Reuptake (at therapeutic doses)
At therapeutic doses, this compound exhibits minimal or no appreciable inhibitory activity on the reuptake of norepinephrine and dopamine. wikipedia.orgnih.govnih.govmedcentral.com This selectivity for the serotonin transporter distinguishes it from tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors (SNRIs), which inhibit the reuptake of both serotonin and norepinephrine. medscape.org While this compound is primarily a SERT inhibitor, some research suggests that at higher concentrations, it may interact with other systems, such as acting as an antagonist at 5HT2C receptors, which could indirectly influence norepinephrine and dopamine levels in certain brain regions like the prefrontal cortex. wikipedia.orgpsychopharmacologyinstitute.com However, its direct effect on noradrenergic and dopaminergic reuptake at typical therapeutic doses is considered negligible. wikipedia.orgnih.govnih.govmedcentral.com
Clinical Efficacy and Therapeutic Applications
Major Depressive Disorder (MDD)
Fluoxetine is an approved treatment for major depression in both adults and children. wikipedia.org
Efficacy in Adult and Pediatric Populations
In adult populations, meta-analyses of clinical trials have indicated that this compound is more effective than placebo in the treatment of MDD. wikipedia.orgpharmaceutical-journal.com Studies have shown significant improvement in depressive symptoms in adults treated with this compound. dovepress.com
The efficacy of this compound in children and adolescents with MDD has been established in placebo-controlled clinical trials. fda.govnih.gov Studies involving pediatric outpatients diagnosed with MDD have shown that this compound produced a statistically significantly greater mean change from baseline compared to placebo on measures of depression severity, such as the Children's Depression Rating Scale-Revised (CDRS-R). fda.govnih.govopenrepository.com For instance, a meta-analysis of randomized controlled trials in children and adolescents with MDD found a mean change from baseline in CDRS-R favoring this compound treatment. nih.govopenrepository.com The efficacy of this compound for MDD has been demonstrated in pediatric outpatients aged 8 to 18 years. fda.gov
Comparison with Placebo and Other Antidepressants
Clinical trials have consistently shown this compound to be more effective than placebo in treating MDD in adults. wikipedia.orgpharmaceutical-journal.comnih.gov A large meta-analysis of 522 double-blind, randomized controlled trials found that all 21 antidepressants included, including this compound, were more efficacious than placebo for the acute treatment of adults with MDD. pharmaceutical-journal.comox.ac.uk
When compared to other antidepressants, particularly older tricyclic antidepressants (TCAs), this compound has shown comparable efficacy in treating depressive symptoms. nih.govnih.gov Some studies have found no statistically significant difference in efficacy between this compound and TCAs as a class. nih.gov However, some meta-analyses suggest that while all antidepressants are more effective than placebo, there are differences in efficacy among them, with some, like escitalopram and sertraline, showing higher response rates than others, including this compound, in adults. pharmaceutical-journal.comox.ac.uk Conversely, this compound has been found to be more tolerable than TCAs, leading to potentially better patient compliance. nih.govnih.gov
In pediatric populations, while this compound has demonstrated efficacy over placebo, a meta-analysis suggested that the effect size in children and adolescents is smaller than that observed in adults. mrctcenter.org This analysis indicated that the largest reduction in CDRS-R score compared to placebo was seen with sertraline, followed by this compound. mrctcenter.org
Rapid Onset of Antidepressant Effect
While the full therapeutic effects of this compound typically become evident over several weeks, some studies suggest that early improvements in depression symptoms may occur sooner. consensus.app Analyses of clinical trial data have indicated statistically significant improvement in depression symptoms with this compound compared to placebo as early as the first week of treatment. nih.govconsensus.appnih.gov However, the probability of achieving a full clinical response may not differ significantly from placebo in the very initial phase. consensus.app Continued improvement is generally observed over a longer period. consensus.app
Role in Treatment-Resistant Depression (in combination with Olanzapine)
For patients with treatment-resistant depression (TRD), defined generally as a failure to respond to at least two antidepressant trials, the combination of this compound and the atypical antipsychotic olanzapine has shown superior efficacy compared to monotherapy with either agent. dovepress.compsychiatryonline.orgresearchgate.netresearchgate.netmedigraphic.com This combination is an FDA-approved medication for bipolar depression, including cases of TRD. nih.gov
Studies have shown that the olanzapine/fluoxetine combination can lead to greater improvement in depressive symptoms and higher response and remission rates compared to this compound alone in patients with TRD. dovepress.comresearchgate.netmedigraphic.com The improvement with the combination therapy can also occur earlier than with this compound monotherapy. researchgate.netresearchgate.net Clinical trials have demonstrated rapid reduction in depression rating scale scores with the olanzapine/fluoxetine combination in patients who had responded inadequately to antidepressant monotherapy. dovepress.com
Obsessive-Compulsive Disorder (OCD)
This compound is effective in the treatment of obsessive-compulsive disorder (OCD) in adults. wikipedia.org
Efficacy in Adult and Pediatric Populations
The efficacy of this compound for the treatment of obsessions and compulsions in adult patients with OCD has been established in clinical trials. fda.gov Studies have shown significant reduction in OCD symptom severity with this compound treatment. fda.gov
This compound is also effective for treating OCD in children and adolescents. wikipedia.org Its efficacy in this population has been demonstrated in placebo-controlled clinical trials. fda.govnih.govkglmeridian.com Studies in pediatric patients with OCD have shown that this compound is associated with significantly greater reduction of OCD symptom severity compared with placebo, as measured by scales such as the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS). fda.govnih.govkglmeridian.com Meta-analyses of SSRIs in the treatment of childhood and adolescent OCD have indicated that this compound is more effective than placebo. psychiatryonline.org
Here is a summary of some key efficacy findings:
| Condition | Population | Key Finding | Source(s) |
| Major Depressive Disorder (MDD) | Adults | More effective than placebo. wikipedia.orgpharmaceutical-journal.comnih.gov Comparable efficacy to TCAs, but generally better tolerated. nih.govnih.gov | wikipedia.orgpharmaceutical-journal.comnih.govnih.gov |
| Major Depressive Disorder (MDD) | Children/Adolescents | Statistically significantly greater improvement than placebo on depression severity scales. fda.govnih.govopenrepository.com Effect size may be smaller than in adults. mrctcenter.org | fda.govnih.govopenrepository.commrctcenter.org |
| Treatment-Resistant Depression (with Olanzapine) | Adults | Superior efficacy to monotherapy; greater and earlier improvement in depressive symptoms. dovepress.compsychiatryonline.orgresearchgate.netresearchgate.netmedigraphic.com | dovepress.compsychiatryonline.orgresearchgate.netresearchgate.netmedigraphic.com |
| Obsessive-Compulsive Disorder (OCD) | Adults | Effective in reducing obsessions and compulsions. wikipedia.orgfda.gov | wikipedia.orgfda.gov |
| Obsessive-Compulsive Disorder (OCD) | Children/Adolescents | Significantly greater reduction in OCD symptom severity compared to placebo. fda.govnih.govkglmeridian.com More effective than placebo. psychiatryonline.org | fda.govnih.govkglmeridian.compsychiatryonline.org |
Bulimia Nervosa and Binge Eating Disorder
This compound has demonstrated efficacy in the management of bulimia nervosa, particularly in reducing the frequency of binge eating and vomiting episodes. nih.govnih.gov Research indicates that this compound is superior to placebo in decreasing these bulimic symptoms. nih.govnih.gov For instance, an 8-week double-blind trial involving 387 bulimic women showed that this compound significantly reduced the frequency of weekly binge-eating and vomiting episodes at the study's end. nih.gov A large multicenter study confirmed the superiority of this compound compared to placebo, with a notable reduction in both binge eating crises (67% vs. 33%) and purging phenomena (56% vs. 5%). Lower doses were not as effective in this study.
Moreover, studies have explored the role of this compound in preventing relapse in patients with bulimia nervosa who have responded to acute treatment. psychiatryonline.orgresearchgate.net A 52-week study found that patients who continued treatment with this compound exhibited a longer time to relapse compared to those receiving placebo. psychiatryonline.orgresearchgate.net Quantitative analysis in this study indicated that the efficacy of this compound treatment was statistically superior to placebo across various measures, including the frequency of vomiting and binge eating episodes. psychiatryonline.orgresearchgate.net
For binge eating disorder (BED), this compound has also shown effectiveness in reducing binge-eating frequency. nih.gov A 6-week, placebo-controlled trial in outpatients with BED demonstrated that this compound led to a significantly greater reduction in the frequency of binge eating compared to placebo. nih.gov This study also reported significant reductions in body mass index and weight with this compound treatment. nih.gov However, some more recent trials with longer durations (16-20 weeks) have not found this compound to be effective in reducing binge eating or weight in BED. psychiatryonline.org
While this compound has shown promise in reducing binge eating episodes in BED, some research suggests that its impact on eating disorder psychopathology may be less pronounced compared to psychotherapy like Cognitive Behavioral Therapy (CBT). psychiatryonline.orgqucosa.de Some studies did not find increased treatment efficacy when this compound was added to CBT, although binge remission rates were better in groups treated with CBT and this compound than with medication alone. psychiatryonline.org
Panic Disorder (with or without agoraphobia)
This compound has been associated with statistically significant improvements in panic attack symptoms and broader symptom measures in patients with panic disorder. cambridge.orgaafp.org Studies have shown that this compound is effective in reducing the frequency of panic attacks compared to placebo. cambridge.orgaafp.orgpsychiatryonline.org For example, one study reported that the estimated odds of achieving at least a 50% reduction from baseline in the number of full panic attacks were significantly higher for this compound-treated patients compared to the placebo group at both 6 weeks and end-point. cambridge.org Similarly, the odds of achieving panic-free status were also higher with this compound treatment. cambridge.org
Beyond panic attack frequency, this compound treatment has been linked to significant reductions in anxiety, phobic avoidance, and depressive symptoms in individuals with panic disorder. aafp.orgpsychiatryonline.org A study comparing different doses of this compound to placebo found that a higher dose was associated with significantly greater improvement across a range of symptom domains, including anxiety, phobia, and depression. aafp.orgpsychiatryonline.org
Comparative studies have also assessed the efficacy of this compound against other pharmacotherapies for panic disorder. A pilot double-blind clinical trial comparing this compound and clomipramine found that both drugs produced similar antipanic effectiveness, with favorable responses observed in a high percentage of patients in both treatment groups. bjbms.org
Premenstrual Dysphoric Disorder (PMDD)
This compound has been found to be effective in reducing the symptoms associated with Premenstrual Dysphoric Disorder (PMDD). nih.govresearchgate.netaafp.org Research, including controlled and open-label clinical trials, has demonstrated a decrease in PMDD symptoms during treatment with this compound. nih.govresearchgate.net
Studies have investigated different dosing strategies for this compound in PMDD, including continuous daily dosing and intermittent luteal-phase dosing. nih.govresearchgate.netaafp.orgcapes.gov.br Premenstrual daily dosing with this compound has been shown to effectively treat mood, physical, and social functioning symptoms related to PMDD. capes.gov.br One placebo-controlled trial using computerized diaries found that a specific dose of this compound demonstrated significant improvement in mean symptom scores compared with placebo. capes.gov.br Both this compound groups in this study showed a significant advantage over placebo for mood-related symptoms, and the higher dose also showed a significant advantage for physical symptoms such as breast tenderness, bloating, and joint/muscle pain. capes.gov.br
While continuous dosing is effective, preliminary findings suggest that intermittent luteal-phase dosing may also be a suitable treatment strategy for selected patients with PMDD. nih.govresearchgate.net Several studies have indicated that serotoninergic antidepressants, including this compound, are effective when used intermittently during the luteal phase of the menstrual cycle. aafp.org
Other Investigational and Off-Label Uses
This compound has been explored for its potential therapeutic effects in conditions beyond its primary indications.
Post-Traumatic Stress Disorder (PTSD)
A meta-analysis of randomized controlled trials concluded that this compound could be an effective treatment for PTSD, showing higher responder status and less severe PTSD symptoms at trial endpoint compared to placebo groups. nih.govnsj.org.sa Another double-blind, randomized, placebo-controlled study conducted in various regions found that this compound was associated with greater improvement from baseline in PTSD symptom scores than placebo, with statistically significant differences observed relatively early in treatment. psychiatrist.compsychiatrist.com Compared with placebo, this compound was also associated with significantly greater improvement in total symptom scores and intrusive and hyperarousal subscores, as well as improvements in measures of global impression, anxiety, and depression. psychiatrist.compsychiatrist.com
Generalized Anxiety Disorder (GAD)
While widely used in clinical practice for Generalized Anxiety Disorder (GAD), the evidence supporting this compound's efficacy specifically for GAD in some populations has been considered limited. nih.govnih.govresearchgate.net Reviews of clinical trials, particularly in Chinese patients, have noted that while short-term efficacy had been established in open-label, head-to-head controlled trials comparing this compound to other anxiolytics, there was a lack of strong evidence from placebo-controlled studies. nih.govnih.govresearchgate.net These reviews often highlight the high risk of bias in the included studies, small sample sizes, and the absence of placebo control groups as limitations. nih.govnih.govresearchgate.net
Some research suggests that this compound may have a rapid onset of action in GAD, potentially within 1-2 weeks, and may be effective in maintenance treatment. nih.govnih.govresearchgate.net However, definitive recommendations for this compound as a reliable first-line treatment for GAD, particularly in certain populations, have been cautious due to the limitations in the available research. nih.govnih.govresearchgate.net
Impulsive Aggression
This compound has been investigated for its potential to reduce impulsive aggressive behavior. nih.govjwatch.orgresearchgate.netfrontiersin.orgfrontiersin.org Central serotonergic system dysfunction is thought to be related to impulsive aggressive behavior, and pharmacologic enhancement of serotonin activity could potentially reduce this behavior. nih.govresearchgate.netfrontiersin.org
A double-blind, randomized, placebo-controlled trial in individuals with Intermittent Explosive Disorder (IED) and histories of impulsive aggressive behavior found that this compound treatment resulted in a sustained reduction in aggression and irritability scores. nih.govresearchgate.net This reduction was apparent relatively early in the treatment course. nih.govresearchgate.net this compound was also found to be superior to placebo in the proportion of responders based on global improvement scales. nih.govresearchgate.net Closer examination of the data revealed that a percentage of this compound-treated subjects achieved full or partial remission of impulsive aggressive behaviors. nih.govresearchgate.net
Another study evaluating the anti-aggressive efficacy of this compound in patients with personality disorders and a history of impulsive aggression demonstrated that this compound led to a significant reduction in scores for verbal aggression, aggression against objects, and irritability compared with placebo. jwatch.org These findings suggest that SSRIs like this compound can diminish impulsive aggression independently of their antidepressant action. jwatch.org While effective, research indicates that this compound may lead to full or partial remission of impulsive aggressive behaviors in a notable percentage of individuals with IED, but not all. nih.govresearchgate.net
Social Anxiety Disorder (limited efficacy)
Research into the efficacy of this compound for Social Anxiety Disorder (SAD), also known as social phobia, has yielded varied results. Some studies suggest that this compound may be effective in treating SAD, with observed improvements in measures of social anxiety and phobic avoidance. nih.gov For instance, a 12-week open clinical trial involving 16 patients with a primary diagnosis of social phobia reported significant improvement in symptoms from baseline to endpoint in the majority of patients who completed the trial. nih.gov Another study in children and adolescents aged 7 to 17 with SAD indicated that this compound was significantly effective and well tolerated, with the beneficial effect increasing over time. psychiatry-psychopharmacology.compsikofarmakoloji.org Factors such as younger age, lower baseline social anxiety scores, absence of a family history of depression and/or anxiety disorders, and lower maternal social anxiety scores were suggested to predict a better outcome in this population. psychiatry-psychopharmacology.compsikofarmakoloji.org
However, other studies have presented less conclusive findings. Two placebo-controlled trials of this compound for SAD have reported negative results. psychiatrist.com While a large two-site trial found efficacy for both this compound and cognitive-behavioral therapy (CBT) over placebo in patients with generalized social phobia, the response rates for this compound alone were comparable to those for CBT alone or CBT plus placebo. psychiatrist.com
| Study Type | Population | Duration | Key Finding | Citation |
| Open Clinical Trial | Adults with Social Phobia | 12 weeks | Significant improvement in social anxiety and phobic avoidance in responders. | nih.gov |
| Naturalistic Retrospective Study | Children/Adolescents with SAD | 8 and 12 weeks | Significantly effective, efficacy increased over time. Predictors of better outcome identified. | psychiatry-psychopharmacology.compsikofarmakoloji.org |
| Randomized Controlled Trial (compared to CBT) | Adults with Generalized Social Phobia | 14 weeks | Efficacy over placebo, but comparable to CBT alone or CBT plus placebo. Response rate 50.9%. | psychiatrist.com |
| Placebo-Controlled Trials | Adults with SAD | Not specified | Two studies reported negative findings. | psychiatrist.com |
Cataplexy
This compound has a role in the treatment of cataplexy, a symptom often associated with narcolepsy. jtsm.org It is noted for its effectiveness in reducing cataplexy attacks. jtsm.org While systematic research evidence specifically supporting antidepressants for cataplexy may be limited, this compound, as a selective serotonin reuptake inhibitor (SSRI), is frequently used in the treatment of cataplexy in narcolepsy. bioprojet.nldovepress.com Limited data from Class III and Class IV evidence studies have reported minor improvements in cataplexy with this compound. bioprojet.nl A pilot study evaluating this compound in six patients with poorly controlled cataplexy showed a mean reduction of 92% in the number of cataplectic episodes per week after 20 weeks of treatment. nih.govneurology.org
| Study Type | Population | Duration | Key Finding | Citation |
| Pilot Study | Patients with Cataplexy | 20 weeks | Mean reduction of 92% in cataplectic episodes per week. | nih.govneurology.org |
| Evidence Studies | Patients with Cataplexy | Not specified | Limited data suggests minor improvements in cataplexy. | bioprojet.nl |
Alcohol Dependence
The efficacy of this compound in treating alcohol dependence, particularly in patients with comorbid depression, has been investigated. A 12-week double-blind, placebo-controlled trial involving patients with comorbid major depressive disorder and alcohol dependence demonstrated that the this compound group showed significantly greater improvement in depressive symptoms and lower total alcohol consumption compared to the placebo group. nih.gov A 1-year follow-up study of patients from a previous 3-month trial also indicated that the this compound group continued to show less depression and less drinking than the placebo group. researchgate.net
However, the results are not consistently positive across all studies. A placebo-controlled, double-blind study of this compound in male patients with severe alcohol dependence without other Axis I disorders did not find a reduction in clinically significant relapse rates. nih.gov In this study, supportive living arrangements after hospital discharge were associated with reduced relapse rates, rather than this compound treatment. nih.gov Another placebo-controlled trial in alcohol-dependent subjects without comorbid major depression found no significant effects of this compound on alcohol consumption, although it did reduce depression scores in subjects with current major depression. psychiatryonline.org
| Study Type | Population | Duration | Key Finding | Citation |
| Double-Blind, Placebo-Controlled Trial | Patients with Comorbid Depression and Alcohol Dependence | 12 weeks | Significant reduction in depressive symptoms and alcohol consumption compared to placebo. | nih.gov |
| 1-Year Follow-up Study | Patients with Comorbid Depression and Alcohol Dependence | 1 year | Continued less depression and less drinking compared to placebo group. | researchgate.net |
| Placebo-Controlled, Double-Blind Study | Male Patients with Severe Alcohol Dependence (no other Axis I disorders) | 12 weeks | No reduction in clinically significant relapse rates. Supportive living arrangements were beneficial. | nih.gov |
| Placebo-Controlled Trial | Alcohol-Dependent Subjects (without comorbid depression) | 12 weeks | No significant effect on alcohol consumption; reduced depression scores in those with current major depression. | psychiatryonline.org |
Trichotillomania
Studies investigating the efficacy of this compound for trichotillomania (hair pulling disorder) have largely shown limited benefit. Several studies have reported no significant changes in hair-pulling severity compared to waitlist or placebo groups. mdpi.com For example, a long-term, double-blind, placebo-controlled crossover trial involving adult chronic hair pullers found no significant differences between this compound and placebo treatments across various measures of hair pulling and urge to pull. nih.gov Similarly, an 18-week placebo-controlled, double-blind crossover study did not demonstrate the short-term efficacy of this compound in treating trichotillomania. psychiatryonline.org
In comparative studies, behavioral therapy, such as habit reversal, has demonstrated superiority over this compound in reducing trichotillomania symptoms. jwatch.orgnih.gov A 12-week randomized, waiting-list controlled study comparing behavioral therapy and this compound found that the behavioral therapy group showed a significantly greater reduction in symptoms and higher effect sizes than the this compound and waiting-list groups. nih.gov
| Study Type | Population | Duration | Key Finding | Citation |
| Long-Term, Double-Blind, Placebo-Controlled Crossover Trial | Adult Chronic Hair Pullers | 31 weeks | No significant differences between this compound and placebo for measures of hair pulling/urge. | nih.gov |
| Placebo-Controlled, Double-Blind Crossover Study | Adult Chronic Hair Pullers | 18 weeks | Short-term efficacy not demonstrated. | psychiatryonline.org |
| Randomized, Waiting-List Controlled Study (vs. Behavioral Therapy) | Patients with Trichotillomania | 12 weeks | Behavioral therapy was superior to this compound in reducing symptoms. | jwatch.orgnih.gov |
Selective Mutism
This compound has shown promise in the treatment of selective mutism, particularly in children and adolescents. An 8-week open-label clinical study involving 17 children with selective mutism reported that a significant majority (76%) showed improvement in measures of mutism, depression, and anxiety. jkacap.org A 9-week open trial with 21 children also found that 76% improved, exhibiting diminished anxiety and increased speech in public settings. nih.gov Improvement in this study was inversely correlated with age. nih.gov
A double-blind, placebo-controlled study involving 15 children with elective mutism (selective mutism) who did not respond to a placebo run-in period indicated that subjects treated with this compound were significantly more improved than placebo-treated subjects on parents' ratings of mutism and global change. nih.gov However, clinician and teacher ratings did not show significant differences between the treatment groups, and most subjects in both groups remained symptomatic at the end of the study. nih.gov While evidence for SSRIs in selective mutism is considered limited, this compound has the most empirical evidence among SSRIs for this condition. jpn.ca
| Study Type | Population | Duration | Key Finding | Citation |
| Open-Label Clinical Study | Children with Selective Mutism | 8 weeks | 76% of subjects showed statistical improvement in mutism, depression, and anxiety measures. | jkacap.org |
| Open Trial | Children with Selective Mutism | 9 weeks | 76% of children improved with diminished anxiety and increased speech; improvement inversely correlated with age. | nih.gov |
| Double-Blind, Placebo-Controlled Study | Children with Elective Mutism | 12 weeks | Significant improvement on parents' ratings of mutism and global change compared to placebo. | nih.gov |
Borderline Personality Disorder
The efficacy of this compound in the treatment of Borderline Personality Disorder (BPD) has been explored, particularly in addressing symptoms such as dysphoria and impulsive aggression. Some investigators hypothesized that this compound's antidepressant properties and serotonin reuptake inhibition could help treat BPD symptoms and potentially reduce impulsivity. the-hospitalist.org Case series and a double-blind, placebo-controlled trial have demonstrated this compound's efficacy in BPD, with one study finding its greatest impact on "anger". the-hospitalist.org
However, other research suggests that while this compound treatment can lead to a substantial reduction in impulsive aggression and severity of depression in women with BPD, other treatments, such as olanzapine monotherapy or the olanzapine-fluoxetine combination, may be associated with a significantly greater rate of improvement over time on measures of depression and impulsive aggression. nih.govpsychiatrist.com A study examining the effect of adding this compound to dialectical behavior therapy (DBT) for BPD found no significant additional benefits from this compound compared to placebo when added to this psychosocial treatment. psychiatrist.comresearchgate.net
| Study Type | Population | Duration | Key Finding | Citation |
| Double-Blind, Placebo-Controlled Trial | Women with Borderline Personality Disorder | 8 weeks | Led to substantial reduction in impulsive aggression and depression; olanzapine/OFC showed greater improvement rate. | nih.govpsychiatrist.com |
| Double-Blind, Placebo-Controlled Study (adjunctive to DBT) | Patients with Borderline Personality Disorder | 12 weeks | Adding this compound to DBT did not provide significant additional benefits compared to placebo. | psychiatrist.comresearchgate.net |
| Case Series and Double-Blind, Placebo-Controlled Trial | Patients with Borderline Personality Disorder | Not specified | Demonstrated efficacy, with greatest impact potentially on anger. | the-hospitalist.org |
Raynaud Phenomenon
The use of this compound in treating Raynaud Phenomenon, a condition characterized by reduced blood flow to the fingers and toes, has been debated, with limited evidence supporting its routine use. droracle.ainih.gov While some small studies suggest that SSRIs like this compound may help reduce the frequency and severity of Raynaud's attacks, the evidence is considered insufficient for routine use. droracle.ai Studies typically used higher doses than a low dose of 10 mg daily, which is not a recommended treatment due to limited evidence and potential for suboptimal dosing. droracle.ai
A 6-week crossover study comparing this compound with nifedipine in patients with primary or secondary Raynaud's phenomenon showed a reduction in attack frequency and severity with both treatments, but the effect was statistically significant only in the this compound-treated group. researchgate.netccjm.org Subgroup analysis in this study indicated that the greatest response to this compound was seen in females and in patients with primary Raynaud's phenomenon. researchgate.net However, a 2016 review found limited evidence for the efficacy of this compound in treating the condition. droracle.ai Despite some positive findings, there is insufficient scientific evidence to recommend this compound as a treatment in Raynaud's phenomenon secondary to systemic sclerosis. nih.gov
| Study Type | Population | Duration | Key Finding | Citation |
| Crossover Study (vs. Nifedipine) | Patients with Primary or Secondary Raynaud's Phenomenon | 6 weeks | Reduction in attack frequency and severity, statistically significant only with this compound; greatest response in females and primary RP. | researchgate.netccjm.org |
| Review of Pharmacologic Approaches | Patients with Raynaud's Phenomenon | Not specified | Limited evidence for the efficacy of this compound. | droracle.ai |
| Small Studies | Patients with Raynaud's Phenomenon | Not specified | Suggest this compound may help reduce frequency and severity of attacks, but evidence is insufficient for routine use. | droracle.ai |
Autism Spectrum Disorder (tentative evidence in adults)
Research into the efficacy of this compound for treating core symptoms of Autism Spectrum Disorder (ASD), particularly repetitive behaviors, has yielded inconsistent results, with some studies showing promise primarily in adults. While studies in children with ASD have generally not shown a significant effect of this compound on repetitive behaviors, a double-blind placebo-controlled trial in adults with ASD reported improvements. thetransmitter.orgpsychiatryonline.orgmdpi.com
However, it is important to note that the evidence in adults is considered limited but promising, and further research is needed. psychiatryonline.orgmdpi.com
Premature Ejaculation
This compound has been investigated for its efficacy in treating premature ejaculation (PE). Studies have indicated that this compound can delay the onset of ejaculation in men with PE.
A double-blind placebo-controlled study involving 17 patients with premature ejaculation demonstrated that the intravaginal ejaculation latent period was significantly longer in the group receiving this compound compared to the placebo group. nih.gov Other studies comparing this compound to other SSRIs like citalopram and sertraline have also shown that this compound is effective in increasing intravaginal ejaculation latency time, although some studies suggest other SSRIs may have a greater effect. tandfonline.comauajournals.orgbrieflands.com A study comparing this compound and citalopram found a significant increase in ejaculation time with both drugs compared to placebo, with no significant difference in effectiveness between this compound and citalopram. Another study comparing this compound, sertraline, and clomipramine found that while all three medications and placebo increased intravaginal ejaculation latency, clomipramine was the most efficacious, followed by sertraline and then this compound. auajournals.org
COVID-19 (potential therapeutic role)
Research has explored the potential therapeutic role of this compound in the context of COVID-19, particularly regarding its potential to mitigate severe outcomes. clinicaltrials.eufrontiersin.org
Studies suggest that this compound may reduce the risk of intubation or death in patients with COVID-19, potentially through mechanisms beyond its primary serotonergic activity. frontiersin.org this compound has been found to block the production of Interleukin-6 (IL-6), a pro-inflammatory cytokine involved in the cytokine storm associated with severe COVID-19. clinicaltrials.eufrontiersin.org It may also interfere with platelet activation and aggregation by limiting serotonin uptake, potentially reducing inflammation. mdpi.com Observational studies have correlated antidepressant therapy, including this compound, with a decreased risk of intubation or death in hospitalized COVID-19 patients. frontiersin.orgucv.ve One observational cohort study showed that COVID-19 patients treated with an average dose of 20 mg of this compound had a lower risk of intubation and death. frontiersin.org
Cancer Therapy Repurposing
This compound is being investigated for its potential to be repurposed for cancer therapy, exhibiting potential oncolytic effects through various mechanisms. nih.gov
Studies suggest that this compound may have activity against several types of tumors, including brain tumors (glioblastoma), breast, liver, colon, and cervical cancers. nih.govwilliamscancerinstitute.com Its ability to cross the blood-brain barrier makes it potentially useful for treating brain tumors like glioblastoma. nih.govwilliamscancerinstitute.com In laboratory models, combining this compound with temozolomide, a standard glioblastoma chemotherapy, has shown synergistic effects, leading to increased cancer cell death and tumor regression in mice. williamscancerinstitute.combraintumor.org this compound may target tumor metabolism, inhibit signaling pathways like epidermal growth factor receptor (EGFR), induce endoplasmic reticulum stress, and activate apoptotic proteins. williamscancerinstitute.combraintumor.org It has also shown potential in overcoming drug resistance in breast cancer models and increasing sensitivity to chemotherapy agents like cisplatin in cervical cancer. nih.govwilliamscancerinstitute.com Preclinical studies also suggest this compound's potential as a novel treatment for neuroendocrine prostate cancer by inhibiting cell viability and neuroendocrine differentiation through targeting the AKT pathway. frontiersin.org
Cognitive Function Enhancement in Specific Pathologies
The impact of this compound on cognitive function is complex and appears to vary depending on the presence of underlying pathologies. While some studies suggest potential for cognitive enhancement in specific conditions, the effects are not universally observed and may be influenced by factors such as the specific pathology, dosage, and duration of treatment. frontiersin.org
Q & A
Q. What established methodologies are recommended for assessing Fluoxetine's pharmacokinetics in preclinical models?
To evaluate this compound's absorption, distribution, metabolism, and excretion (ADME), researchers should employ high-performance liquid chromatography (HPLC) or mass spectrometry for precise quantification in biological samples. Tissue distribution studies require organ-specific sampling at multiple time points, validated against standardized protocols to ensure reproducibility . Experimental designs should include control groups for endogenous compound interference and use species-specific metabolic profiles to account for interspecies variability.
Q. How can the PICOT framework structure clinical trials investigating this compound's efficacy in treatment-resistant depression?
Using the PICOT framework:
- Population : Adults diagnosed with major depressive disorder (MDD) unresponsive to two prior antidepressants.
- Intervention : this compound (20–80 mg/day) over 8 weeks.
- Comparison : Placebo or active comparator (e.g., sertraline).
- Outcome : Change in Hamilton Depression Rating Scale (HAM-D) scores .
- Time : 12-week follow-up. This design ensures clarity in hypothesis testing and minimizes confounding variables .
Q. What validated behavioral assays are used to assess this compound's anxiolytic effects in rodent models?
The elevated plus maze (EPM) and forced swim test (FST) are gold standards. For reproducibility:
- Standardize testing conditions (e.g., lighting, time of day).
- Include blinded scoring of immobility time (FST) or open-arm exploration (EPM).
- Control for baseline anxiety levels using genetic or environmental manipulations (e.g., chronic mild stress) .
Advanced Research Questions
Q. How can conflicting data on this compound's impact on synaptic plasticity be reconciled across studies?
Contradictions often arise from methodological differences:
- Dosage : Low-dose this compound (5 mg/kg) may enhance hippocampal neurogenesis, while high doses (20 mg/kg) impair it.
- Exposure duration : Acute vs. chronic administration differentially affects BDNF signaling.
- Model systems : Human iPSC-derived neurons vs. rodent models show variability in serotonin transporter (SERT) expression. Meta-analyses should stratify results by these variables and assess publication bias using funnel plots .
Q. What experimental designs are optimal for studying this compound's neurodevelopmental effects in autism spectrum disorder (ASD) models?
Fractional factorial designs allow multiplexed testing of environmental factors (e.g., this compound, lead exposure) across genetic backgrounds. For example:
- Expose human iPSC-derived neural progenitors to this compound (1 µM) during critical neurodevelopmental windows.
- Combine transcriptomic (RNA-seq) and metabolomic (LC-MS) profiling to identify pathway-specific effects (e.g., synaptic function, lipid metabolism) .
- Validate findings in in vivo models with conditional SERT knockout to isolate serotoninergic mechanisms.
Q. How do multi-omics approaches clarify this compound's role in lipid metabolism dysregulation?
Integrate transcriptomics, lipidomics, and proteomics:
- Transcriptomics : Identify this compound-induced upregulation of FASN (fatty acid synthase) in hepatic cells.
- Lipidomics : Quantify triglycerides and phospholipids via tandem mass spectrometry.
- Proteomics : Assess PPAR-α/γ activity to link gene expression changes to metabolic outcomes. Data integration tools (e.g., weighted gene co-expression networks) can pinpoint causal pathways .
Q. What statistical methods address heterogeneity in this compound's therapeutic response across demographic subgroups?
Apply mixed-effects models to account for covariates like age, sex, and genetic polymorphisms (e.g., SLC6A4 variants). Cluster analysis can identify responder/non-responder subgroups based on metabolomic profiles or HAM-D score trajectories. Sensitivity analyses should test robustness against missing data .
Methodological Considerations
- Data Contradiction Analysis : Use PRISMA guidelines for systematic reviews to evaluate this compound studies. Assess risk of bias via Cochrane tools and perform subgroup analyses by dose, duration, and population .
- Reproducibility : Share raw data and code in repositories like Zenodo or Figshare. Pre-register protocols on Open Science Framework (OSF) to reduce selective reporting .
- Ethical Frameworks : Adhere to FINER criteria (Feasible, Interesting, Novel, Ethical, Relevant) when designing studies involving vulnerable populations (e.g., adolescents, pregnant individuals) .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


