Nystatin
Description
Discovery and Early Characterization of Nystatin
The story of this compound is a testament to persistent and collaborative scientific inquiry. Discovered in the mid-20th century, it was a breakthrough at a time when fungal infections posed a significant, and often untreatable, threat to human health. sciencehistory.orgsmithtownmatters.com
In 1950, the microorganism responsible for producing this compound was identified from a soil sample. chemistryviews.org This sample was collected from the dairy farm of a friend of microbiologist Elizabeth Lee Hazen. sciencehistory.orgwikipedia.org The bacterium, a member of the actinomycetes, was named Streptomyces noursei in honor of Jessie Nourse, the wife of the farm's owner. wikipedia.orgasmblog.org Hazen was systematically screening soil organisms for any sign of antifungal activity, a process inspired by the recent success of antibiotics like penicillin. sciencehistory.org The culture from this particular soil sample demonstrated promising activity against two key fungi, Candida albicans and Cryptococcus neoformans, while showing manageable toxicity in preliminary tests. sciencehistory.org
The discovery of this compound was the result of a remarkable long-distance collaboration between two scientists at the New York State Department of Health's Division of Laboratories and Research: Elizabeth Lee Hazen and Rachel Fuller Brown. chemistryviews.orgmit.edu Hazen, a microbiologist based in New York City, was responsible for culturing microorganisms from soil samples and performing the initial tests for antifungal activity. sciencehistory.orgmtholyoke.edu
When she found a promising culture, she would send it in a mason jar via mail to Brown, a chemist located at the division's headquarters in Albany. sciencehistory.orgchemistryviews.org Brown's role was to perform the difficult task of isolating and purifying the active chemical compound from the culture using painstaking solvent extraction methods. sciencehistory.org After isolating the active agent, she would mail it back to Hazen for further testing. sciencehistory.org This partnership led to the identification of the compound initially named "fungicidin," a name later changed to this compound to honor the New York State laboratory where it was discovered. sciencehistory.org They announced their discovery in 1950 at a meeting of the National Academy of Sciences. chemistryviews.org
The discovery of this compound occurred in an era when treatments for systemic fungal infections were severely lacking. mdpi.com While the antibacterial revolution, spearheaded by penicillin, was well underway, fungal diseases remained a major challenge. sciencehistory.orgmit.edu The widespread use of broad-spectrum antibacterial agents often had the unintended side effect of eliminating the body's natural bacteria that compete with fungi, leading to an increase in fungal infections like candidiasis. smithtownmatters.commtholyoke.edu Before this compound, there were no broadly effective and safe antifungal antibiotics available for human use. chemistryviews.orgasmblog.org The development of this compound, therefore, marked a pivotal moment, providing the first reliable weapon against a range of debilitating and often fatal fungal pathogens. smithtownmatters.commdpi.com
Pioneering Contributions of Hazen and Brown
This compound's Classification within Polyene Macrolide Antibiotics
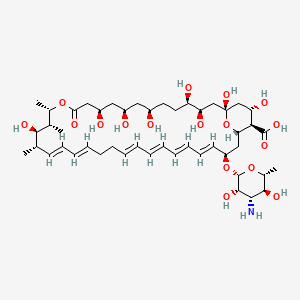
This compound is chemically classified as a polyene macrolide antibiotic, a group of compounds known for their antifungal properties. cfmot.dewikipedia.org Its structure is key to its function.
The molecule consists of a large macrolactone ring, specifically a 38-membered ring, which is a defining feature of macrolides. researchgate.netmdpi.com One side of this large ring contains multiple hydroxyl groups, making it hydrophilic, while the other side features a system of conjugated double bonds, making it lipophilic. wikipedia.org This amphipathic nature is crucial for its biological activity. This compound is specifically categorized as a tetraene because it contains a chromophore of four conjugated double bonds. researchgate.netvt.edu The structure also includes a distinct deoxysugar, D-mycosamine, attached to the macrolactone ring by a β-glycosidic bond. wikipedia.orgresearchgate.net
Table 1: Key Chemical Features of this compound
| Feature | Description | Reference |
| Class | Polyene macrolide antibiotic | cfmot.de |
| Macrolactone Ring | 38-membered ring | researchgate.net |
| Polyene System | Classified as a tetraene (four conjugated double bonds) | researchgate.netvt.edu |
| Key Functional Groups | Multiple hydroxyl groups, exocyclic carboxyl group | researchgate.netmdpi.com |
| Attached Sugar | D-mycosamine (an aminoglycoside) | wikipedia.orgwikipedia.org |
| Molecular Formula | C47H75NO17 | wikipedia.org |
Significance in Mycological and Pharmaceutical Research
The discovery of this compound had profound and lasting significance in both mycology (the study of fungi) and pharmaceutical science. cfmot.de It was the very first polyene macrolide antifungal discovered and provided a much-needed tool to treat infections caused by yeasts and other fungi. wikipedia.orgmdpi.com
In pharmaceutical research, this compound's success spurred further investigation into microbial sources for new drugs and helped establish the polyenes as a major class of antifungal agents. mdpi.comasm.org This led to the later discovery of other important polyenes like Amphotericin B. mdpi.com Although this compound's use is primarily topical due to toxicity when administered systemically, its discovery laid the groundwork for developing subsequent generations of antifungal therapies. drugbank.comoup.com
In mycological and cell biology research, this compound became an invaluable tool. Its mechanism of action involves binding to ergosterol, a sterol unique to fungal cell membranes. wikipedia.orgcfmot.de This specific interaction has been exploited by researchers to study the structure and function of cell membranes and the role of sterols within them. cfmot.de The compound's ability to selectively disrupt fungal membranes without similarly affecting most bacterial or animal cells (which contain cholesterol instead of ergosterol) makes it a standard reference agent in antifungal efficacy studies. wikipedia.orgcfmot.de Research continues into developing new formulations of this compound to improve its delivery and potentially expand its therapeutic applications. oup.com
Properties
IUPAC Name |
(1S,3R,4R,7R,9R,11R,15S,16R,17R,18S,19E,21E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28-,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VQOXZBDYSJBXMA-NQTDYLQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1C=CC=CCCC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(C(CCC(CC(CC(CC(=O)OC(C(C1O)C)C)O)O)O)O)O)O)O)C(=O)O)OC3C(C(C(C(O3)C)O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@@H](C[C@H]2[C@@H]([C@H](C[C@](O2)(C[C@H]([C@@H](CC[C@H](C[C@H](C[C@H](CC(=O)O[C@H]([C@@H]([C@@H]1O)C)C)O)O)O)O)O)O)O)C(=O)O)O[C@H]3[C@H]([C@H]([C@@H]([C@H](O3)C)O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C47H75NO17 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID80872323 | |
| Record name | (7R,10R)-8,9-Dideoxy-28,29-dihydro-7,10-dihydroxyamphotericin B | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80872323 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
926.1 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
MW: 926.12 /Form not specified/, Mg/ml at about 28 °C: methanol 11.2, ethanol 1.2, chloroform 0.48, carbon tetrachloride 1.23, benzene 0.28, toluene 0.285, acetone 0.390, ethyl acetate 0.75, ethylene glycol 8.75, Insol in ether, In water, 3.60X10+2 mg/L at 24 °C | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Light yellow powder, Yellow to tan powder | |
CAS No. |
34786-70-4, 1400-61-9 | |
| Record name | Nystatin A1 | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=34786-70-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nystatin A1 | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034786704 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (7R,10R)-8,9-Dideoxy-28,29-dihydro-7,10-dihydroxyamphotericin B | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80872323 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Nystatin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.014.317 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NYSTATIN A1 | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/W1LX4T91WI | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
Gradually decomp above 160 °C without melting by 250 °C | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Molecular and Cellular Mechanisms of Nystatin's Antifungal Action
Interaction with Fungal Cell Membranes
The primary target of nystatin is the fungal cell membrane, a critical structure for maintaining cellular integrity and function. nih.gov this compound's interaction with this membrane is the initial and most crucial step in its antifungal activity.
The selective toxicity of this compound towards fungi is attributed to its high affinity for ergosterol, the predominant sterol in fungal cell membranes. patsnap.comdrugbank.com In contrast, mammalian cells contain cholesterol. While this compound can bind to cholesterol, its affinity for ergosterol is significantly higher. nih.govbiomolther.org This preferential binding is the cornerstone of this compound's therapeutic action, as it allows the drug to target fungal pathogens with minimal damage to host cells. patsnap.com The structural differences between ergosterol and cholesterol, though subtle, are sufficient to create a distinct molecular architecture for the this compound-sterol complexes, which is key to understanding the antibiotic's selective interaction with membranes. nih.govdrugbank.com
Studies have shown that the presence of cholesterol can compete with ergosterol for this compound binding, indirectly confirming that this compound can coassemble with cholesterol. nih.govdrugbank.com However, the biophysical consequences of these interactions differ significantly. The formation of this compound-ergosterol complexes leads to more profound membrane disruption compared to this compound-cholesterol complexes. nih.gov
Table 1: Comparative Binding Affinity of this compound
| Sterol | Primary Location | This compound Binding Affinity | Consequence of Binding |
|---|---|---|---|
| Ergosterol | Fungal Cell Membranes | High | Forms stable pores, leading to significant membrane permeabilization and cell death. drugbank.combiomolther.org |
| Cholesterol | Mammalian Cell Membranes | Low | Forms less stable complexes, resulting in minimal membrane disruption at therapeutic concentrations. nih.govwikipedia.org |
Upon binding to ergosterol, this compound molecules self-assemble to form pores or channels that span the fungal cell membrane. patsnap.comstudysmarter.co.ukontosight.ai This process is fundamental to its mechanism of action. The most widely accepted model suggests that this compound molecules, in conjunction with ergosterol, arrange into a barrel-like structure, creating a hydrophilic channel through the hydrophobic lipid bilayer. nih.govbiomolther.org Research indicates that these pores are composed of 4 to 12 this compound molecules. wikipedia.org
The formation of these transmembrane channels dramatically increases the permeability of the fungal cell membrane. nih.gov A two-stage mechanism has been proposed for this compound's activity in ergosterol-containing membranes. nih.gov At low concentrations, monomeric this compound molecules adsorb to the membrane surface and disrupt the lipid packing, leading to a general increase in permeability. nih.gov Once a critical surface concentration is reached, the mechanism shifts to the formation of stable, transmembrane aqueous channels. nih.gov The initial rate of this pore formation is dependent on the ergosterol-to-cholesterol ratio in the membrane. nih.gov
The formation of this compound-induced pores leads to a rapid and substantial leakage of essential ions and small molecules from the fungal cytoplasm. patsnap.comwikipedia.orgnih.gov The most significant of these is the efflux of potassium (K+) ions, which is a hallmark of polyene antibiotic action. wikipedia.orgmicrobiologyresearch.org Studies have demonstrated that lethal concentrations of this compound can cause the leakage of up to 90% of the non-bound intracellular potassium from Candida albicans. microbiologyresearch.org This massive loss of potassium ions disrupts the critical electrochemical gradients across the cell membrane, which are necessary for numerous cellular processes, including nutrient transport and the maintenance of membrane potential. drugbank.comnih.gov
The disruption of these gradients leads to cellular acidification and osmotic instability, contributing directly to fungal cell death. patsnap.comwikipedia.org Research has shown that even at half the minimal inhibitory concentration (MIC), this compound can cause a nearly 50% decrease in intracellular potassium within five minutes of exposure. oup.com
Table 2: this compound-Induced Potassium (K+) Leakage from Candida albicans
| This compound Concentration (vs. MIC) | Incubation Time (minutes) | Intracellular K+ Decrease (%) | Reference |
|---|---|---|---|
| 0.5 x MIC | 5 | ~50% | oup.com |
Beyond pore formation, this compound induces significant reorganization and biophysical changes within the fungal membrane. nih.govrsc.org The interaction of this compound with membrane lipids can alter the physical state of the bilayer. This compound's presence has been shown to be driven by highly ordered membrane domains, which help stabilize the this compound oligomers that form the pores. rsc.org
Furthermore, this compound affects the fluidity of the cell membrane. nih.govresearchgate.net Studies using fluorescence spectroscopy have indicated that this compound's fluorescence intensity responds to changes in lipid fluidity. nih.gov In some cellular contexts, this compound treatment can lead to a decrease in membrane fluidity, potentially by increasing the expression of proteins like caveolin-1. eco-vector.com This membrane reorganization is a crucial aspect of this compound's cytotoxic effect and is dependent on the specific lipid composition of the membrane. nih.govrsc.org The conjugated double bonds in this compound's structure can also contribute to lipid peroxidation, which alters the membrane's structural integrity and further contributes to ion leakage. wikipedia.org
Ionic Leakage and Disruption of Cellular Electrochemical Gradients
Fungicidal Versus Fungistatic Modes of Action at the Molecular Level
This compound exhibits both fungistatic (inhibiting fungal growth) and fungicidal (killing fungal cells) activity, depending on its concentration. drugbank.commdpi.com
Fungistatic Action: At lower concentrations, typically between 0.5 and 2 times the MIC, this compound's activity is primarily fungistatic. nih.gov At these levels, the pores formed may be transient or insufficient in number to cause catastrophic leakage, instead leading to a sublethal disruption of cellular processes that halts fungal replication. wikipedia.orgnih.gov
Fungicidal Action: At higher concentrations (generally ≥2 times the MIC), this compound's action becomes rapidly fungicidal. nih.govnih.gov The increased concentration leads to the formation of more numerous and stable pores, causing rapid and extensive leakage of intracellular contents, irreversible damage to the cell, and ultimately, cell death. nih.govwithpower.com This concentration-dependent activity is a key pharmacodynamic characteristic of the drug. nih.govmdpi.com
Table 3: Concentration-Dependent Activity of this compound against Candida Species
| This compound Concentration (vs. MIC) | Mode of Action | Molecular Rationale | Reference |
|---|---|---|---|
| 0.5x - 2x MIC | Fungistatic | Limited, transient pore formation; sublethal disruption of cellular functions. | nih.gov |
Cellular Consequences Leading to Fungal Cell Death
The primary mechanisms of membrane permeabilization and ion leakage trigger a cascade of secondary cellular events that culminate in fungal cell death. ontosight.ai The profound loss of intracellular potassium and the collapse of the membrane potential disrupt cellular homeostasis and inhibit essential enzymatic activities. wikipedia.orgplos.org
A growing body of evidence suggests that this compound, like other antifungals, can induce the production of reactive oxygen species (ROS) within the fungal cell. patsnap.comresearchgate.net This induction of oxidative stress further damages cellular components, including lipids, proteins, and nucleic acids, amplifying the drug's lethal effects. patsnap.commdpi.com
The cellular damage and stress induced by this compound can trigger programmed cell death (PCD), or apoptosis, in fungi. frontiersin.org This is a regulated process characterized by specific morphological and biochemical markers. The link between antifungal drug action, ROS production, and the initiation of fungal PCD pathways is an area of active research. researchgate.netfrontiersin.org It is thought that the antifungal drug may trigger PCD, with ROS formation being a key part of the apoptotic cascade. frontiersin.org Ultimately, the combination of direct membrane damage, loss of ionic homeostasis, oxidative stress, and the potential activation of PCD pathways leads to the comprehensive and effective killing of the fungal cell. patsnap.comontosight.ai
Table 4: Mentioned Compound Names
| Compound Name |
|---|
| This compound |
| Ergosterol |
| Cholesterol |
| Potassium |
| Caveolin-1 |
| Amphotericin B |
| Natamycin |
Antifungal Spectrum and Mechanistic Studies of Resistance Development
In Vitro Antifungal Activity Spectrum Against Fungal Pathogens
Nystatin demonstrates a broad spectrum of activity against various fungal pathogens in vitro. Its efficacy is particularly noted against yeasts and yeast-like fungi. nih.govuwb.edu.pl
Susceptibility Profiles of Candida Species
This compound shows favorable activity against most Candida species. asm.org Studies have indicated high susceptibility rates among Candida albicans and non-albicans Candida species. asm.org For instance, one study found that 87.2% of C. albicans and 85.9% of non-albicans Candida isolates were susceptible to this compound in vitro. asm.org Another study evaluating isolates from drug abusers with oral candidiasis reported that all Candida isolates tested were susceptible to this compound. nih.gov Minimal inhibitory concentration (MIC) values for this compound against C. albicans have been reported in the range of 1 to 4 µg/mL. unirioja.es In a study using the agar dilution method, MIC values for this compound against various Candida species ranged from 0.625 µg/mL to 1.25 µg/mL. oatext.com
Here is a summary of in vitro susceptibility data for this compound against Candida species based on the search results:
| Candida Species | Susceptibility Rate (%) | MIC Range (µg/mL) | Method | Source |
| C. albicans | 87.2 | 1-4, 0.625-1.25 | Kirby-Bauer, Agar Dilution | asm.orgunirioja.esoatext.com |
| Non-albicans Candida | 85.9 | Not specified | Kirby-Bauer | asm.org |
| All Candida isolates (from oral candidiasis) | 100 | Not specified | Broth Microdilution | nih.gov |
| C. tropicalis | 38.8 (Sensitive) | Not specified | Kirby-Bauer | dovepress.com |
| C. krusei | 27.7 (Sensitive) | Not specified | Kirby-Bauer | dovepress.com |
| Various Candida species | Not specified | 0.625-1.25 | Agar Dilution | oatext.com |
Note: Susceptibility criteria and methods may vary between studies.
Activity Against Non-Candida Fungi (e.g., Rhodotorula, Torulopsis, Trichosporon, Aspergillus)
This compound also exhibits activity against certain non-Candida fungi. Studies have shown its effectiveness against species like Rhodotorula, Torulopsis, and Trichosporon. acspublisher.comcabidigitallibrary.orgresearchgate.net For example, one study on fungal isolates from bovine mastitis reported 100% sensitivity to this compound among Rhodotorula rubra and Torulopsis sp. isolates. acspublisher.com Trichosporon spp. have also shown susceptibility to this compound in vitro, with reported mean MICs of 0.54 mg/L. researchgate.net
Against Aspergillus species, the activity of conventional this compound appears to be less potent compared to other polyenes or its liposomal formulation. One study comparing liposomal this compound with conventional this compound and other antifungals against Aspergillus isolates found that conventional this compound had higher geometric mean MICs (9.51 µg/ml) compared to liposomal this compound (2.30 µg/ml) and amphotericin B formulations. nih.gov Aspergillus terreus was found to be significantly less susceptible to polyene drugs, including this compound, than other Aspergillus species. nih.gov
Here is a summary of in vitro activity data for this compound against selected non-Candida fungi:
| Fungal Species | Susceptibility / MICs | Source |
| Rhodotorula rubra | 100% sensitive (in bovine mastitis isolates) | acspublisher.com |
| Torulopsis sp. | 100% sensitive (in bovine mastitis isolates) | acspublisher.com |
| Trichosporon spp. | Mean MIC 0.54 mg/L | researchgate.net |
| Geotrichum candidum | Inhibited well, constant MIC values at 1.25 µg/mL | oatext.com |
| Trichosporon mucoides | Inhibited well, constant MIC values at 1.25 µg/mL | oatext.com |
| Aspergillus species | Geometric mean MIC 9.51 µg/ml; A. terreus less susceptible | nih.gov |
Mechanisms of Fungal Resistance to Polyenes
Resistance to polyene antifungals like this compound is generally considered rare compared to other antifungal classes, although resistant isolates, particularly among less common Candida species such as C. lusitaniae, C. glabrata, and C. guilliermondii, have been reported. asm.org Mechanisms of resistance primarily involve alterations affecting the interaction between the drug and its target, ergosterol. asm.orgnih.gov
Alterations in Ergosterol Biosynthesis Pathways
Changes in the ergosterol biosynthesis pathway are a primary mechanism of resistance to polyenes. mdpi.comejgm.co.ukasm.orgnih.gov Modifications, either quantitative or qualitative, in the sterol content of the fungal cell membrane can influence the amount or availability of ergosterol for polyene binding. ejgm.co.ukasm.org Studies using polyene-resistant strains have shown a marked decrease in membrane ergosterol content, with ergosterol being replaced by biosynthetic precursors such as lanosterol, fecosterol, lichesterol, and episterol. ejgm.co.uk This decrease in ergosterol content can lead to decreased susceptibility to polyenes. asm.org
Mutations in specific genes involved in ergosterol biosynthesis, such as ERG genes, have been linked to polyene resistance. nih.govasm.org For instance, mutations in the ERG6 gene, which encodes a sterol C24-methyltransferase, have been shown to lead to alterations in susceptibility to various antifungal agents, including reduced sensitivity to this compound in Candida glabrata. mdpi.comnih.gov Deletion of ERG6 in C. lusitaniae resulted in increased resistance to amphotericin B and increased sensitivity to fluconazole, possibly due to decreased ergosterol content leading to fewer polyene targets but increased membrane permeability to azoles. asm.org In Saccharomyces cerevisiae, targeted deletion of ERG3 has been coupled with resistance to both azoles and this compound. nih.gov
Modification of Drug Target Structures
Resistance can also arise from modifications to the structure of the drug target itself, ergosterol, or the surrounding membrane environment, which can reduce the affinity of the polyene for ergosterol. ejgm.co.ukasm.org While the primary target is ergosterol, changes in the ratio of sterols to phospholipids or reorientation or masking of ergosterol within the cell membrane could potentially hinder polyene binding. ejgm.co.uk Although less common than alterations in biosynthesis, structural changes that prevent effective drug-target interaction are a recognized resistance mechanism for various antibiotics and could theoretically apply to polyenes interacting with ergosterol. skintherapyletter.comnih.govreactgroup.org
Insights from Mutagenesis and Laboratory-Induced Resistance Studies
Much of the understanding regarding the mechanisms of resistance to polyenes in fungal species has been derived from studies utilizing mutants generated in laboratory settings. nih.gov These studies often involve techniques such as growing cells in the presence of increasing concentrations of antifungal agents (multistep mutants), exposing cells to a gradient concentration, or creating mutants through one-step mutation using mutagenic agents. nih.gov
Laboratory studies have shown that polyene resistance can result from an alteration of sterols in the cell membrane of mutants. microbiologyresearch.org For instance, analysis of this compound-resistant Candida mutants isolated in vivo indicated a total absence of ergosterol and an increased level of a possible precursor. researchgate.net Successive culturing of these resistant strains in a medium supplemented with ergosterol induced a sensitivity to this compound similar to that of the wild-type strain. researchgate.net Resistance was recovered after subculture in medium without ergosterol, highlighting the role of ergosterol content in this compound sensitivity. researchgate.net
Genetic analysis of this compound-resistant mutants of Saccharomyces cerevisiae has identified several genes involved in resistance. cambridge.orgcambridge.org Studies have described primary genes, including dominant (e.g., NYSA, NYSD, NYSE, NYSF) and recessive (e.g., nysB, nysC) genes, responsible for varying levels of this compound resistance. cambridge.org Modifier genes have also been identified that can increase the level of resistance conferred by primary genes. cambridge.org For example, a dominant modifier gene was shown to increase resistance conferred by NYSF, and a recessive modifier gene enhanced resistance due to NYSA. cambridge.org These studies indicate that this compound resistance can be a multigenic trait. cambridge.org
In Dictyostelium discoideum, spontaneous this compound resistance mutations have been categorized into complementation groups, such as nysA, nysB, and nysC. core.ac.uknih.govias.ac.in Mutations in nysB and nysC are associated with alterations in sterol composition, while nysA mutants exhibit wild-type sterols. ias.ac.in Studies using these mutants have also revealed cross-resistance phenotypes. For example, this compound-resistant mutants of Dictyostelium caveatum selected through mutagenesis were found to be cross-resistant to pisatin and other isoflavonoid phytoalexins. ias.ac.in
Pharmacological Research in Pre-clinical and in Vitro Models
In Vitro Pharmacodynamic Characteristics
In vitro pharmacodynamic studies investigate how Nystatin interacts with fungal pathogens over time and at different concentrations. These studies provide insights into the concentration-effect relationship, the rate and extent of fungal killing, and the persistence of antifungal activity after drug exposure.
Concentration-Effect Relationship Studies
Studies examining the concentration-effect relationship of this compound against Candida species have demonstrated concentration-dependent fungicidal activity. nih.govresearchgate.netasm.org This means that increasing concentrations of this compound lead to a greater rate and/or extent of fungal killing. For most tested isolates, fungistatic activity is typically observed at concentrations between 0.5 and 2 times the Minimum Inhibitory Concentration (MIC). nih.gov Rapid fungicidal activity is generally seen at concentrations equal to or greater than 2 times the MIC. nih.gov However, for some species like Candida krusei, fungicidal endpoints may require concentrations of 4 times the MIC or higher. nih.gov The rate of kill for polyenes like this compound is more dependent on concentration than the extent of kill due to their efficient killing mechanism. nih.gov
Time-Kill Kinetics and Postantifungal Effects (PAFE)
Time-kill kinetics studies assess the rate at which this compound kills fungal cells over a specific period. These studies have shown that this compound exhibits concentration-dependent killing against Candida species. nih.govresearchgate.netoup.comcapes.gov.brnih.govasm.org As this compound concentrations increase, the time required to achieve a significant reduction in fungal colony-forming units (CFU) decreases. nih.govoup.comms-editions.cl
The Postantifungal Effect (PAFE) refers to the suppression of fungal growth that persists after the drug concentration has fallen below the MIC. This compound has been shown to produce a pronounced PAFE against Candida species. nih.govresearchgate.netcapes.gov.brasm.orgoup.comoup.com Studies have indicated that even short exposure periods (e.g., 30 minutes) to this compound at concentrations ranging from 25% to 100% of the MIC can result in a PAFE lasting for several hours (e.g., 5-6 hours). nih.govoup.com The PAFE of this compound is concentration-dependent, with higher concentrations generally leading to longer PAFEs. oup.com
Table 1: Representative Time-Kill Kinetics Data for this compound Against Candida Species (Illustrative based on search results)
| This compound Concentration (x MIC) | Time (h) | Change in log₁₀ CFU/mL (vs. initial inoculum) | Observed Effect |
| Control | 24 | Growth | Growth |
| 0.5 | 24 | ≤ 0 | Fungistatic |
| 1 | 24 | ≤ 0 | Fungistatic |
| 2 | 4 | ≥ -3 | Fungicidal |
| 4 | 2 | ≥ -3 | Rapid Fungicidal |
Note: Specific values vary depending on the fungal isolate and experimental conditions.
Table 2: Representative Postantifungal Effect (PAFE) Data for this compound Against Candida albicans (Illustrative based on search results)
| This compound Concentration During Exposure (x MIC) | Exposure Time (min) | PAFE Duration (h) |
| 0.25 | 30 | Significant PAFE observed nih.gov |
| 0.5 | 30 | 5-6 nih.govoup.com |
| 1 | 30 | 5-6 nih.govoup.com |
Note: Specific values vary depending on the fungal isolate and experimental conditions.
Absorption and Distribution Studies in Non-Human Biological Systems
Studies in non-human biological systems, particularly animal models, have investigated the absorption and distribution characteristics of this compound. Following oral administration, systemic absorption of this compound is minimal, and detectable plasma concentrations are generally not attained. drugbank.comoup.commedicaldialogues.inmedicinenet.combjmu.edu.cn This limited absorption restricts its efficacy primarily to topical, oral, and gastrointestinal infections. drugbank.commedicaldialogues.inmedicinenet.com Due to the lack of systemic absorption, this compound does not undergo significant distribution in the body after oral administration. drugbank.commedicaldialogues.in
Research with liposomal formulations of this compound has explored the possibility of systemic administration and improved distribution. Studies in rabbit models of cryptococcal meningitis, for instance, have shown that while liposomal this compound can result in detectable plasma concentrations, achieving significant concentrations in the cerebrospinal fluid (CSF) can be challenging. nih.gov Brain tissue levels above the MIC have been observed in some animal studies with liposomal formulations. nih.gov
Assessment of Toxicity in In Vitro Cell Lines and Animal Models
Toxicity assessment in pre-clinical models is crucial for understanding the potential adverse effects of this compound on host cells and tissues.
Hemolytic Activity Research
Hemolytic activity, the ability of a compound to lyse red blood cells, is a significant concern with polyene antifungals like this compound due to their interaction with cholesterol in mammalian cell membranes. google.comsemanticscholar.org In vitro studies have demonstrated that this compound exhibits hemolytic activity. google.comsemanticscholar.orgnih.govasm.orgmdpi.com The degree of hemolysis is concentration-dependent. google.com Studies comparing this compound with its derivatives have shown that modifications to the this compound molecule can influence its hemolytic potential. semanticscholar.orgasm.orgmdpi.com For example, some semisynthetic derivatives have shown reduced hemolytic activity compared to native this compound. semanticscholar.orgmdpi.com Liposomal formulations have also been investigated as a strategy to reduce the hemolytic toxicity of this compound. google.com Studies have shown that certain formulations can increase the concentration of this compound required to cause 50% hemolysis compared to free this compound. google.com
Table 3: Comparative Hemolytic Activity (Illustrative based on search results)
| Compound | Concentration Causing 50% Hemolysis (µg/mL) |
| Pure this compound | ~55 - 250 google.comnih.gov |
| This compound Formulation (with Pluronic F127) | ~125 - 180 google.com |
| Certain this compound Derivatives | > 50 (some derivatives) semanticscholar.orgasm.orgmdpi.com |
Note: Specific values vary depending on the study and experimental conditions.
Cytotoxicity in Epithelial Cell Models
The cytotoxicity of this compound has been evaluated in various in vitro cell lines, including epithelial cells. Studies using human keratinocyte cell lines (e.g., HaCat) and reconstituted human oral epithelium (RHOE) models have shown that this compound can exhibit toxicity to these cells. researchgate.netmostwiedzy.plresearchgate.netnih.gov This cytotoxicity is related to this compound's interaction with cholesterol in mammalian cell membranes. drugbank.comgoogle.combiorxiv.orgsemanticscholar.org
Research on this compound derivatives has aimed to reduce host cell toxicity while maintaining antifungal efficacy. Studies have indicated that certain derivatives of this compound A1, particularly those with modifications at the amino group, show reduced toxicity against human keratinocytes and oral epithelial cells compared to the parent compound. researchgate.netmostwiedzy.plresearchgate.netnih.gov For instance, lactate dehydrogenase (LDH) activity, a marker of cell damage, was found to be significantly lower in RHOE models treated with certain this compound derivatives compared to native this compound. researchgate.netmostwiedzy.plnih.gov Similarly, studies on Vero E6 kidney cells have indicated that this compound is only slightly toxic up to certain concentrations (e.g., 250 µg/ml), while higher concentrations can lead to reduced cell viability. biorxiv.orgoup.com
Table 4: Comparative Cytotoxicity in Epithelial Cell Models (Illustrative based on search results)
| Compound | Cell Line/Model | Indicator of Toxicity | Observation |
| This compound A1 | Human Keratinocytes (HaCat) | Cell Viability (MTT assay) | Markedly more toxic than novel derivatives researchgate.netmostwiedzy.plresearchgate.net |
| This compound A1 | Reconstituted Human Oral Epithelium (RHOE) | LDH Activity | Higher LDH release compared to derivatives researchgate.netmostwiedzy.plnih.gov |
| This compound | Vero E6 cells | Cell Viability (Trypan blue exclusion, XTT test) | Cytotoxic at high concentrations biorxiv.orgoup.com |
| Certain this compound Derivatives | Human Keratinocytes (HaCat), RHOE | Cell Viability, LDH Activity | Reduced toxicity compared to this compound A1 researchgate.netmostwiedzy.plresearchgate.netnih.gov |
Nystatin Chemical Modifications and Structure-activity Relationship Sar Research
Biosynthetic Engineering for Nystatin Analogues
Biosynthetic engineering of the Streptomyces noursei strain that produces this compound A1 has been employed to generate novel polyene macrolides with structural differences in various regions. asm.orgresearchgate.netntnu.no This approach allows for targeted modifications within the biosynthetic pathway, leading to the production of this compound analogues with altered properties. ntnu.noresearchgate.net
Modifications within the Polyol Region
The polyol region of this compound, specifically the C-7 to C-10 positions, has been identified as having a significant influence on antifungal activity. asm.orgresearchgate.net Biosynthetic engineering targeting this region has involved the removal or introduction of hydroxyl groups. researchgate.netnih.gov For instance, modifications in the C-9-C-10 region of the heptaene this compound analogue S44HP, such as the introduction of a C-9 hydroxyl group through inactivation of the dehydratase domain in module 15 (DH15), have been shown to drastically decrease both antifungal and hemolytic activities of the resulting analogues. researchgate.netnih.gov Conversely, the single removal of the C-10 hydroxyl group by inactivating the P450 monooxygenase NysL had only a marginal effect on these activities. researchgate.netnih.gov Studies comparing the positions of hydroxyl groups in different polyene antibiotics suggest that the most active compounds have hydroxyl groups at positions C-8 and C-9 or C-7 and C-10, while those with hydroxyl groups at both C-7 and C-9 exhibit lower activity. asm.orgresearchgate.net Modifications in the polyol region (C-5, C-7, and C9+C10) have generally resulted in compounds with reduced antifungal activity and toxicity, although the specific effect varies depending on the modification. core.ac.uk
Alterations at the C-16 Exocyclic Group
The exocyclic carboxyl group at the C-16 position is another target for modification. nih.gov Replacing the C-16 carboxyl group with a methyl group, for example, through the inactivation of the P450 monooxygenase NysN, has been explored. researchgate.net Research indicates that the replacement of the C-16 carboxyl with a methyl group does not significantly impact the in vitro antifungal activity of antibiotics that lack modifications at the amino group of the mycosamine. asm.orgresearchgate.net However, in N-modified derivatives, the activity is modulated by the presence of either a CH3 or COOH group at C-16, as well as the structure of the modifying substituent. asm.orgresearchgate.net
Investigation of Glycosylation and Mycosamine Moiety Impact
The mycosamine sugar moiety, attached at the C-19 position, is crucial for the biological activity of this compound. mdpi.comnih.govnih.gov The absence of mycosamine can lead to a dramatic reduction in antifungal activity. nih.gov Biosynthetic studies have focused on the genes involved in mycosamine biosynthesis and attachment, such as nysDI, nysDII, and nysDIII. nih.gov While mycosamine is important for antifungal activity, it also contributes significantly to the hemolytic activity (toxicity) of this compound. nih.gov This makes the mycosamine moiety a potential target for modifications aimed at reducing toxicity while retaining antifungal efficacy. nih.gov The C2' hydroxyl of mycosamine is particularly important, as modifications at this position in Amphotericin B analogues have shown improved selectivity for ergosterol over cholesterol. mdpi.com Increasing the extent of glycosylation by adding a second sugar moiety, such as to the C4' hydroxyl of mycosamine, has been shown in some polyene analogues to lead to higher solubility and reduced hemolytic toxicity. mdpi.comresearchgate.net
Semisynthetic Derivatives and Their Antifungal Activity
Semisynthetic approaches involve chemical modifications of the this compound molecule. These methods offer an alternative or complementary strategy to biosynthetic engineering for generating this compound derivatives with potentially improved properties. asm.orggoogle.com Modifications have been explored at various positions, including the C-16 carboxyl group and the amino group of the mycosamine moiety. asm.orgnih.gov
Semisynthetic amides of this compound A1 have been synthesized and evaluated for their antifungal activity and toxicity. mdpi.comnih.gov Some novel this compound derivatives have demonstrated potent antifungal activity comparable to that of this compound itself. mdpi.com For example, certain amide derivatives, such as those containing ethylenediamine and N-(2-hydroxyethyl)-ethylenediamine moieties, have shown notable findings in comparative studies with Amphotericin B derivatives. mdpi.com Studies have also shown that introducing a side chain with a tertiary amino group on the amide moiety can improve water solubility and, in some cases, increase antifungal activity. mdpi.com The introduction of a positively charged group at the C16 position can disrupt the zwitterionic interaction between the C16 carboxyl group and the mycosamine amino group, thereby increasing solubility. mdpi.com
Research on semisynthetic derivatives has also highlighted the potential for reduced host cell toxicity while maintaining promising antifungal activity. google.commdpi.com Some this compound derivatives have shown good antifungal effects against Candida albicans with low toxicity in in vitro models. mdpi.comresearchgate.net For instance, one derivative (compound 10 in a specific study) exhibited a minimum inhibitory concentration (MIC) of 2 µg/mL against C. albicans and was identified as a highly effective antifungal derivative. researchgate.net Another study found that certain this compound derivatives with an amide linker showed low hemotoxicity and formed water-soluble salts, exhibiting antifungal activity against a broad spectrum of Candida species, filamentous fungi, dermatophytes, and multidrug-resistant strains. google.com
Data on the antifungal activity of semisynthetic this compound derivatives against Candida albicans can illustrate the impact of chemical modifications:
| Derivative Type | Example Compound | MIC against C. albicans (µg/mL) | Reference |
| Parent this compound A1 | This compound A1 | Lower than derivatives | researchgate.net |
| N-substituted derivative | Compound 10 | 2 | mdpi.comresearchgate.net |
| N-substituted derivatives | Compounds 9, 11 | 4 | mdpi.com |
| Amide derivative | Compound 12 | Similar to this compound | mdpi.com |
| Mono-N-alkylated derivative | Compound 3 | Varied | mdpi.com |
| N-(hydroxyethyl)amino)ethyl fragment | Compounds 3, 4 | Relatively high | researchgate.net |
| N-(2-hydroxyethyl)amides | Compounds 5, 9 | Relatively high | researchgate.net |
This table illustrates that while some semisynthetic modifications may result in slightly higher MIC values compared to the parent this compound A1, they can still exhibit good antifungal activity, often coupled with reduced toxicity. mdpi.comresearchgate.net
Elucidation of Structure-Function Relationships
Understanding the relationship between the chemical structure of this compound and its biological function, particularly its interaction with fungal cell membranes, is critical for the rational design of improved analogues. SAR studies aim to identify which structural features are essential for antifungal activity and which contribute to toxicity. asm.orgcore.ac.uk
Impact of Structural Changes on Membrane Interaction
This compound's mechanism of action involves binding to ergosterol in the fungal cell membrane and forming pores, leading to membrane permeabilization and cell death. wikipedia.orgdrugbank.com Structural changes in the this compound molecule can significantly impact its interaction with membrane lipids and, consequently, its antifungal activity and toxicity. mdpi.comnih.govresearchgate.net
The polyene region, with its conjugated double bonds, is involved in hydrophobic interactions with membrane sterols like ergosterol. researchgate.net The number of conjugated double bonds influences antifungal activity; for example, Amphotericin B, with seven conjugated double bonds, generally has higher antifungal activity than this compound, which has four conjugated double bonds and a diene, presumably due to more efficient hydrophobic interaction. mdpi.comnih.gov
The polyol region also plays a role in membrane interaction. Modifications in this region can affect both antifungal and hemolytic activities. core.ac.uk
The mycosamine moiety and the exocyclic carboxyl group contribute to the amphoteric character of this compound and are important for biological activity and toxicity. mdpi.com The zwitterionic interaction between the C16 carboxyl group and the mycosamine amino group can influence solubility and potentially membrane interaction. mdpi.com
Studies using model membranes, such as Langmuir monolayers and liposomes, have provided insights into how this compound and its derivatives interact with lipid bilayers containing ergosterol (mimicking fungal membranes) and cholesterol (mimicking mammalian membranes). mdpi.comnih.gov These studies have shown that this compound exhibits higher affinity for ergosterol compared to cholesterol, which underlies its selective toxicity. drugbank.comnih.govfishersci.ca The presence of phospholipids in the membrane can also play a key role in the interaction and the resulting antifungal activity and toxicity. nih.gov
Structural modifications can alter the ability of this compound derivatives to permeabilize membranes. For instance, some mono-N-alkylated derivatives have shown membrane activity comparable to the original antibiotics, and their selectivity for ergosterol-containing membranes has correlated well with their in vitro activity and cytotoxicity. mdpi.com However, for other derivatives, such as amides of this compound, a clear correlation between membrane activity in model systems and in vitro biological activity has not always been observed. mdpi.com
The formation of membrane-spanning pores by this compound is a key aspect of its function. drugbank.comfishersci.ca The size and characteristics of these pores can be influenced by the structure of the polyene molecule and the composition of the membrane. mdpi.com While the barrel-stave model has been proposed for pore formation, the exact mechanism remains a topic of debate, and other factors, such as the influence of polyenes on membrane biophysical properties and organization, are also considered important. mdpi.comresearchgate.net Structural modifications can affect the oligomerization state of this compound in the membrane and the stability and ion selectivity of the formed pores. mdpi.com
Correlation between Chemical Structure and Antifungal Potency
The antifungal activity of polyene antibiotics like this compound is closely linked to specific regions of their chemical structure. The polyene macrolide ring, particularly the conjugated double bond system, and the polyol region play significant roles in the interaction with ergosterol and subsequent membrane disruption. researchgate.netresearchgate.net
Research has highlighted the importance of the C-7 to C-10 polyol region for antifungal activity. asm.orgresearchgate.net Studies comparing this compound analogues with modifications in this region have shown that the position of hydroxyl groups significantly influences potency. Compounds with hydroxyl groups at positions C-8 and C-9 or C-7 and C-10 tend to exhibit higher antifungal activity. researchgate.netnih.gov Conversely, antibiotics with hydroxyl groups at both C-7 and C-9 have demonstrated lower activity. researchgate.netnih.gov
Modifications to the mycosamine sugar moiety, specifically the amino group, can also affect antifungal activity. nih.govnih.gov While the replacement of the C-16 carboxyl group with a methyl group did not significantly impact the in vitro antifungal activity of derivatives without modifications at the mycosamine amino group, the activity of N-modified derivatives was influenced by both the C-16 modification and the structure of the substituent on the amino group. researchgate.netnih.gov For instance, some N-substituted this compound derivatives have shown weaker antifungal activity compared to the parent compound against Candida strains. pg.edu.pl
The conjugated polyene chain is essential for the interaction with sterols in the fungal membrane. mdpi.com The length and configuration of this lipophilic region contribute to the molecule's ability to insert into the lipid bilayer and form pores.
Modifications Influencing Solubility and Selective Toxicity in Research Models
A major challenge with this compound is its poor aqueous solubility, which limits its formulation options and systemic use. mdpi.commdpi.comnih.govpg.edu.pl Furthermore, its toxicity to mammalian cells, primarily due to some interaction with cholesterol, necessitates the development of modifications that enhance selective toxicity towards fungal cells. mdpi.comnih.govpg.edu.pl
Chemical modifications have been explored to improve both solubility and the activity/toxicity ratio. Amidation of the C16-carboxylic group is one such strategy. nih.govresearchgate.net New this compound amides have been synthesized with considerably higher solubility in water compared to the parent antibiotic. nih.gov For some of these amide derivatives, solubility exceeding 100 g/L has been reported, a significant improvement over this compound's solubility of 0.36 g/L. nih.gov
Modifications at the amino group of the mycosamine moiety have also been investigated for their effect on selective toxicity. pg.edu.pl While some N-substituted this compound derivatives showed weaker antifungal activity, they were found to be less toxic to mammalian cells in research models, with some exhibiting non-hemolytic properties. pg.edu.pl However, studies suggest that introducing large steric hindrance at the nitrogen atom of this compound may not significantly improve selective toxicity in vitro. pg.edu.pl
Research using model membranes containing either ergosterol (fungal) or cholesterol (mammalian) helps to assess the selective toxicity of this compound derivatives. mdpi.comnih.gov An increased ratio of selective permeabilization of ergosterol-containing membranes versus cholesterol-containing membranes generally correlates with an improved activity/toxicity ratio observed in cell-based assays. nih.gov Some new water-soluble this compound derivatives have demonstrated a prominent improvement in safety in these studies and are considered promising candidates for further drug development. nih.gov
The development of solid dispersions with carrier agents like maltodextrin has also been explored to improve this compound's solubility and enhance its antifungal efficacy, particularly against Candida spp. biofilms in research models. mdpi.com
Interactive Table 1: Summary of Selected this compound Modifications and Their Effects in Research Models
| Modification Site | Type of Modification | Effect on Antifungal Activity | Effect on Solubility | Effect on Mammalian Toxicity (in research models) | Source |
| C-7 to C-10 Polyol Region | Hydroxyl group position alteration | Significant influence; activity varies based on position (e.g., C8/C9 or C7/C10 generally higher potency than C7/C9) | Not explicitly detailed in source | Not explicitly detailed in source | researchgate.netnih.gov |
| Mycosamine Amino Group | N-substitution | Can lead to weaker antifungal activity | Not explicitly detailed in source | Can lead to reduced toxicity (e.g., non-hemolytic) | pg.edu.pl |
| C16-Carboxylic Group | Amidation | Potent antifungal activity comparable to this compound | Considerably higher water solubility (e.g., >100 g/L) | Improved safety profile | nih.govmdpi.com |
| C16-Carboxylic Group | Replacement with methyl group | Did not significantly affect activity without amino group modification | Not explicitly detailed in source | Not explicitly detailed in source | researchgate.netnih.gov |
Advanced Analytical Methodologies for Nystatin Research
Spectrophotometric Analytical Techniques
Spectrophotometric methods are widely used for the analysis of Nystatin, leveraging its light-absorbing and fluorescent properties.
UV/VIS Absorption and Fluorescence Spectroscopy
UV/VIS absorption spectroscopy is a fundamental technique for this compound analysis. The absorption spectrum in the UV/VIS region exhibits a vibronic structure with characteristic maximums (λmax). redalyc.org Studies have reported λmax values for this compound in methanol at approximately 292 nm, 304 nm, and 320 nm, which are related to the conjugated double bonds in its macrolactone ring. redalyc.org These absorption bands are affected by factors such as the solvent used. For instance, in a dioxane-water solution (7:3), this compound shows three well-defined optical absorption maximums. scispace.com
Fluorescence spectroscopy can also be applied to this compound. While absorption spectra show a clear vibronic structure, fluorimetric analysis may not always comply with the mirror image rule. redalyc.org The fluorescence decay of this compound can be two-fold exponential, suggesting the presence of multiple fluorophores, with their contribution influenced by the solvent's nature and its effect on the flexibility sites of the tetraenic chromophore. epa.gov UV irradiation can lead to a decrease in fluorescence intensity and changes in the absorption spectrum, attributed to the trans-cis isomerization of the tetraenic chain. epa.gov
UV/VIS spectrophotometry is also utilized for the quantitative determination of this compound in pharmaceutical formulations, often employing solvents like a dioxane-water mixture (7:3) or phosphate-citrate buffer pH 5.5, which are suitable for dissolving this compound prior to analysis. researchgate.net However, the low solubility of this compound can pose challenges for bioanalytical analysis. scispace.comresearchgate.net
Photochemical Transformation in Analytical Contexts
This compound is known to be sensitive to light, and this photosensitivity can be exploited or must be considered in analytical methods. scispace.comresearchgate.netnih.gov Controlled photochemical transformation of this compound solutions can be used as a basis for quantitative analysis. scispace.comresearchgate.net By irradiating a this compound solution, its optical absorbance changes, and this change can serve as an analytical signal proportional to the this compound concentration. scispace.comresearchgate.net The effect of irradiation is observed across all absorption maximums, but it is often most pronounced at specific wavelengths, such as 322 nm. researchgate.net Studies have shown that this compound undergoes photodegradation in a two-step consecutive manner upon UV irradiation. nih.govresearchgate.net
Chromatographic Separation and Quantification Methods
Chromatographic techniques, particularly High-Performance Liquid Chromatography (HPLC) and Liquid Chromatography-Mass Spectrometry (LC-MS), are essential for separating, identifying, and quantifying this compound and its related substances, including degradation products.
High-Performance Liquid Chromatography (HPLC) Development and Validation
HPLC is a widely used method for the analysis of this compound in various matrices, including pharmaceutical formulations and biological samples. Developing and validating robust HPLC methods is crucial for quality control and stability studies. A typical HPLC method for this compound involves reversed-phase chromatography. sci-hub.senih.govuobasrah.edu.iq
Method development often focuses on achieving adequate separation of this compound from its degradation products and excipients. nih.govuobasrah.edu.iqscielo.br For instance, a method for evaluating this compound stability in an ointment achieved resolutions higher than 2 for this compound and its degradation products using an Inerstil ODS-3 column with isocratic elution (methanol:water) and UV detection at 305 nm. scielo.brscielo.br Another validated RP-HPLC method used an Ion Pac column (Arcus EP-C18) with a mobile phase of ammonium acetate buffer/methanol mixture (30:70) and UV detection at 305 nm, demonstrating linearity over a range of 5–500 µg/mL. nih.govuobasrah.edu.iq
Validation of HPLC methods for this compound typically includes assessing parameters such as specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, and robustness, following guidelines from regulatory bodies like ICH. nih.govuobasrah.edu.iqscielo.br For the RP-HPLC method mentioned above, the LOD and LOQ were determined to be 0.01 and 0.025 µg/mL, respectively. nih.govuobasrah.edu.iq
HPLC methods have also been developed for the determination of this compound in biological fluids like saliva. sci-hub.se A validated isocratic RP-HPLC method for this compound in human saliva used a Luna™ C18 column with a mobile phase of MeOH, H2O, and DMF (70:20:10, v/v/v) and detection by both UV and fluorescence, showing linearity from 0.78 to 50 μg/ml. sci-hub.se The use of dimethylformamide in the mobile phase can help reduce peak tailing. sci-hub.se
Here is a summary of parameters from a validated HPLC method for this compound:
| Parameter | Value | Citation |
| Column | Ion Pac; Arcus EP-C18; 5µm, 4.6×250 mm | uobasrah.edu.iq |
| Mobile Phase | Ammonium acetate 0.05 M buffer/Methanol (30:70) | uobasrah.edu.iq |
| Flow Rate | 1.0 mL/min | uobasrah.edu.iq |
| Detection Wavelength | 305 nm | uobasrah.edu.iq |
| Linearity Range | 5–500 µg/mL | uobasrah.edu.iq |
| LOD | 0.01 µg/mL | uobasrah.edu.iq |
| LOQ | 0.025 µg/mL | uobasrah.edu.iq |
| Retention Time | ~8 min | uobasrah.edu.iq |
Liquid Chromatography-Mass Spectrometry (LC-MS) Applications
LC-MS, particularly LC-MS/MS, offers high sensitivity and selectivity for the analysis of this compound, especially in complex matrices or when analyzing low concentrations. This technique is valuable for pharmacokinetic studies and the detection of this compound residues.
LC-MS methods have been developed for the quantitative determination of this compound in human plasma. nih.govresearchgate.net One such method utilized solid-phase extraction (SPE) on C18 cartridges for sample preparation, followed by isocratic chromatography on a reversed-phase column and detection by tandem mass spectrometry with electrospray ionization (ESI) in positive selected ion monitoring (SIM) mode. nih.govresearchgate.net The method monitored the molecular ion [M+H]+ at m/z 926.6 for this compound. nih.govresearchgate.net This method was validated and successfully applied to quantify this compound in human plasma in pharmacokinetic trials. nih.govresearchgate.net
LC-MS/MS is also employed for the detection of this compound residues in food products like muscle, milk, and eggs. fda.gov.twnih.gov A screening method for veterinary drug residues, including this compound, in animal-derived foods used LC-MS/MS after extraction and purification. fda.gov.twnih.gov Chromatographic separation was achieved on a C18 column, and the method demonstrated linearity and acceptable recovery and precision for this compound at relevant concentration levels. nih.gov
LC-MS/MS can also be used for the identification of this compound or this compound-like compounds by analyzing their fragmentation patterns. researchgate.net The main fragmentation mode involves the loss of water molecules. researchgate.net
Physico-Chemical Characterization Techniques (e.g., Thermal Analysis, Particle Size Analysis)
A comprehensive understanding of this compound requires the application of physico-chemical characterization techniques to assess properties such as thermal behavior, crystallinity, and particle size.
Thermal analysis techniques like Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) provide information about the thermal stability and phase transitions of this compound. DSC thermograms of crystalline this compound typically show an endothermic peak corresponding to its melting point, which has been reported around 163 °C in some studies. researchgate.net Commercial this compound samples, being mixtures of related compounds, may show a wide, single endothermal peak in DSC, indicating similarities among samples but also suggesting impurity rather than polymorphism. redalyc.org TGA can reveal the mass loss profile of this compound upon heating, indicating decomposition stages. redalyc.orgpreprints.org Studies have shown that this compound exhibits relatively high thermal stability up to approximately 150 °C. researchgate.net The thermogravimetric curves of this compound show gradual mass loss in several stages at higher temperatures. preprints.org
Particle size analysis is particularly important for characterizing this compound formulations, especially those involving nanoparticles or microparticles, as particle size can significantly impact properties like dissolution, absorption, and efficacy. Techniques such as dynamic light scattering (DLS) and laser diffraction are used to determine the average particle size and particle size distribution. dovepress.comoup.com For example, studies on this compound-loaded nanoparticles have measured particle sizes using a Malvern Zetasizer, reporting values in the nanometer range. dovepress.comnih.gov Laser diffraction has been used to analyze the particle size distribution of this compound nanosuspensions, showing a significant reduction in median particle size compared to conventional suspensions. oup.com Scanning Electron Microscopy (SEM) is often used in conjunction with particle size analysis to visualize the morphology and size of this compound particles in formulations. preprints.orgnih.gov
Other physico-chemical techniques applied to this compound research include X-ray Diffraction (XRD) to assess crystallinity nih.govpreprints.org and Fourier Transform Infrared Spectroscopy (FTIR) for structural characterization and evaluating compatibility with other materials in formulations. nih.govpreprints.org
Here is a table summarizing typical particle size ranges observed in this compound formulations:
| Formulation Type | Typical Particle Size Range (nm) | Measurement Method | Citation |
| This compound Spanlastics | 202.2 ± 10.31 to 376.7 ± 41.9 | DLS | dovepress.com |
| This compound Nanosuspension | Median 137 nm (after milling) | Laser Diffraction | oup.com |
| This compound-Loaded PLGA NPs | 108.63 ± 4.5 to 168.8 ± 5.65 | SEM | nih.gov |
| This compound-Loaded PLGA-GlcN NPs | 208.76 ± 16.85 | SEM | nih.gov |
| This compound Loaded Nanogel | 278 to 401 | Zetasizer | sifisheriessciences.com |
| Nanoparticulate this compound | < 2000 | Static Light Scattering | google.com |
Microbiological Assays for In Vitro Potency Determination
Microbiological assays are fundamental techniques for determining the in vitro potency of antibiotics, including the antifungal agent this compound. These methods rely on the inhibitory effect of the antibiotic on the growth of a susceptible microorganism under controlled conditions. Unlike chemical methods, microbiological assays assess the biological activity of the compound, which is crucial for polyene macrolides like this compound, where structural integrity directly impacts function micromasterlab.comekb.eg. The activity (potency) of an antibiotic is demonstrated by its inhibitory effect on microorganisms, and a reduction in this activity can indicate changes not detectable by chemical means micromasterlab.com.
Two primary methods are commonly employed for the microbiological assay of this compound: the agar diffusion method (cylinder-plate or plate assay) and the turbidimetric method (tube assay) micromasterlab.comuspnf.com. These methods are recognized and described in major pharmacopoeias, such as the United States Pharmacopeia (USP) and the European Pharmacopoeia micromasterlab.comuspnf.com.
The agar diffusion method is based on the principle of diffusion of the antibiotic from a reservoir (typically a cylinder or a well) through a solidified agar medium inoculated with a susceptible test organism micromasterlab.comuspnf.com. A zone of inhibition, where microbial growth is prevented, forms around the reservoir. The diameter of this zone is proportional to the concentration or activity of the antibiotic micromasterlab.com. This method requires careful control of factors such as the critical rates of antibiotic diffusion, the growth rates of the standard organism, and the minimal inhibitory coefficient levels for the organism micromasterlab.com. A standard strain, such as Saccharomyces cerevisiae ATCC 2601 or ATCC 9763, is commonly used as the test organism for this compound assays micromasterlab.comexodocientifica.com.brhimedialabs.comexodocientifica.com.brtmmedia.incabidigitallibrary.org. Antibiotic Assay Medium No. 12, also known as this compound Assay Agar, is a suitable medium for this method, providing essential nutrients for the test organism micromasterlab.comexodocientifica.com.brhimedialabs.com. The assay often utilizes a balanced (symmetrical) two-dose parallel line assay model for quantification, involving statistical analysis methods like regression analysis and analysis of variance (ANOVA) to determine the potency ekb.egscimedjournal.orgresearcher.lifedoaj.org. Studies have shown that this assay design is satisfactory for routine quality control testing and quantitation of this compound in raw materials and pharmaceutical dosage forms scimedjournal.orgresearcher.lifedoaj.org. For instance, in one study evaluating the suitability of the agar diffusion method for this compound potency determination, the routine inspection methodology yielded good results, with mean values of the variance ratio for regression and parallelism squares reported as 534.349 ± 212.546 and 0.596 ± 0.345, respectively scimedjournal.orgresearcher.lifedoaj.org.
The turbidimetric method, on the other hand, depends on the inhibition of microbial growth in a liquid medium containing a uniform solution of the antibiotic uspnf.com. The extent of growth inhibition is determined by measuring the turbidity or light transmittance of the medium after incubation using a spectrophotometer exodocientifica.com.brhimedialabs.com. The concentration of the antibiotic is then determined by comparing the amount of growth obtained with that of reference standard solutions exodocientifica.com.brhimedialabs.com. This method is appropriate when the test samples are clear exodocientifica.com.brhimedialabs.com. Antibiotic Assay Medium No. 13, also referred to as this compound Assay Broth or Fluid Sabouraud Medium, is commonly used for turbidimetric assays of antifungals like this compound, facilitating the growth of test organisms such as Saccharomyces cerevisiae exodocientifica.com.brtmmedia.inhimedialabs.com.
Research findings highlight the importance of validating these microbiological assay methods to ensure statistically valid potency determinations ekb.eg. Validation parameters typically include selectivity, linearity, precision (intra-assay and inter-assay variation), accuracy, and robustness (evaluating variations in pH, incubation time, and temperature) ekb.eg. Studies have demonstrated acceptable validation parameters for these assays when applied to this compound in pharmaceutical preparations ekb.eg. For example, linearity with a correlation coefficient (r²) ≥ 0.98 and relative standard deviation (RSD) values within acceptable limits for precision and accuracy have been reported ekb.eg.
Microbiological assays are also utilized in research to investigate the antifungal activity of this compound in various contexts, such as determining minimum inhibitory concentrations (MIC) and minimum fungicidal concentrations (MFC) against Candida albicans redalyc.orgnih.gov. Broth microdilution techniques, as outlined by standards like those from the National Committee for Clinical Laboratory Standards, are employed for MIC determination nih.gov. The MIC is typically defined as the lowest concentration of the drug that results in complete inhibition of visible fungal growth nih.gov. Time-kill studies, which assess the rate and extent of antifungal activity over time, are also conducted using microbiological methods, involving serial dilutions and plating to determine colony counts nih.gov.
Here is a summary of typical parameters and findings from microbiological assays for this compound potency determination:
| Assay Method | Test Organism | Medium | Principle | Key Measurement | Typical Validation Parameters Evaluated | Representative Finding (Agar Diffusion) |
| Agar Diffusion | Saccharomyces cerevisiae | Antibiotic Assay Medium No. 12 | Inhibition zone diameter proportional to concentration | Zone of Inhibition Diameter | Selectivity, Linearity, Precision, Accuracy, Robustness | Mean variance ratio for regression: 534.349 ± 212.546 scimedjournal.orgresearcher.lifedoaj.org |
| Turbidimetric | Saccharomyces cerevisiae | Antibiotic Assay Medium No. 13 | Inhibition of microbial growth measured by turbidity/transmittance | Light Transmittance/Turbidity | Selectivity, Linearity, Precision, Accuracy, Robustness | Acceptable validation parameters for linearity, precision, and accuracy |
Microbiological assays remain essential tools for the quality control and research of this compound, providing a direct measure of its biological potency against susceptible fungi.
Emerging Research Directions and Novel Nystatin Applications
Nanotechnology in Nystatin Delivery Research
Nanotechnology offers a promising avenue to augment the effectiveness of this compound. By reformulating the drug into nano-sized particles, researchers are addressing its solubility challenges and improving its antifungal activity.
Nanosuspensions and nanoemulsions are at the forefront of this compound delivery research. These formulations consist of this compound particles reduced to the nanometer scale, which can significantly enhance the drug's dispersion and interaction with fungal cells. scienceopen.comresearchgate.net
One approach involves high-energy wet-media milling of a commercial this compound suspension to create a nanosuspension. oup.com This process has been shown to reduce the median particle size from 6577 nm to as small as 137 nm. oup.comnih.gov Another method utilizes high-pressure homogenization, which has demonstrated better milling yield compared to wet milling. cognit.ca
Nanoemulsions, which are thermodynamically stable systems of oil, water, emulsifiers, and co-emulsifiers, serve as another effective delivery vehicle. scienceopen.com Researchers have successfully developed this compound-loaded nanoemulsions using components like liquid paraffin oil as the oil phase and PEG400 as a co-emulsifier. scienceopen.com The use of exopolysaccharides (EPS) as a natural emulsifier is also being explored. scienceopen.com These nanoformulations are designed to improve drug solubility, promote absorption, and extend the duration of action. scienceopen.comresearchgate.net
Table 1: Characteristics of this compound Nanoformulations
| Formulation Type | Preparation Method | Key Components | Average Particle Size |
|---|---|---|---|
| Nanosuspension | Wet-media milling | Commercial this compound suspension | 137 nm nih.gov |
| Nanosuspension | High-pressure homogenization | This compound, various stabilizers | < 1.9 µm (for 90% of particles) cognit.ca |
| Nanoemulsion | Ultrasonic method | This compound, liquid paraffin oil, PEG400, Exopolysaccharides (EPS) | 131.1 ± 4.32 nm scienceopen.comresearchgate.net |
Magnetic nanocomposites represent a sophisticated strategy for the targeted and controlled release of this compound. These composites typically involve loading this compound onto magnetic nanoparticles, such as iron oxide nanoparticles, which can then be guided to the infection site using an external magnetic field. le.ac.ukresearchgate.net
One method involves the in situ co-precipitation of iron with ammonia in the presence of cellulose fibers, which act as capping and stabilizing agents, to form magnetic nanocellulose fiber composites. le.ac.uk Another approach utilizes chitosan to coat the magnetic nanoparticles before loading this compound. researchgate.netnih.govresearchgate.net This functionalization not only allows for magnetically directed therapy but also aims to decrease drug-related side effects. researchgate.net
These magnetic nanocomposites exhibit a porous structure, which allows for a high drug-loading capacity. le.ac.uk For instance, magnetic nanocellulose fibers have shown a loading capacity of around 17.8%. le.ac.uk Furthermore, these formulations provide sustained drug release over extended periods, with studies showing release for up to 8 to 30 hours. le.ac.ukresearchgate.net
The transition to nanoparticulate formulations has demonstrated a significant enhancement of this compound's antifungal activity in both laboratory and animal studies. nih.govoup.com
In vitro, this compound nanosuspensions have shown superior growth inhibition of Candida albicans compared to conventional suspensions at concentrations ranging from 100 to 5000 µg/mL. oup.comoup.com Similarly, nanoemulsions have drastically lowered the minimum inhibitory concentration (MIC) against C. albicans to as low as 0.125 µg/mL. scienceopen.com Magnetic nanocomposites loaded with this compound have also displayed prominent inhibitory activity against C. albicans. le.ac.ukresearchgate.net
In vivo studies using murine models of oral candidiasis have corroborated these findings. nih.govoup.com Mice treated with a this compound nanosuspension showed lower oral burdens of C. albicans and superior survival rates compared to those treated with a conventional suspension. oup.comnih.govoup.com Importantly, a pharmacokinetic study indicated no detectable systemic absorption of the nanosized this compound after oral administration, suggesting a reduced risk of systemic toxicity. oup.com In rat models of vulvovaginal candidiasis, nanoemulsion formulations have also demonstrated good therapeutic ability. scienceopen.com
Table 2: Enhanced Antifungal Activity of this compound Nanoformulations
| Formulation | Model | Key Finding | Reference |
|---|---|---|---|
| Nanosuspension | In vitro (C. albicans) | Superior growth inhibition at 100-5000 µg/mL vs. conventional suspension. | oup.com |
| Nanoemulsion | In vitro (C. albicans) | MIC of 0.125 µg/mL. | scienceopen.com |
| Nanosuspension | In vivo (Murine oral candidiasis) | Lower oral fungal burden and improved survival. | nih.govoup.com |
| Nanoemulsion | In vivo (Rat vulvovaginal candidiasis) | Effective therapeutic ability. | scienceopen.com |
Magnetic Nanocomposites for Controlled Release
Immunomodulatory Research in Pre-Clinical Models
Beyond its direct antifungal action, emerging research in pre-clinical models suggests that this compound possesses immunomodulatory properties. Studies indicate that this compound can influence the host's immune response to fungal infections. nih.govoup.comoup.com
In a rat model of vulvovaginal candidiasis caused by C. albicans, treatment with this compound led to a significant increase in the levels of interferon-gamma (IFN-γ), interleukin-17 (IL-17), and IgG in vaginal epithelial cells compared to untreated infected rats. nih.gov This suggests that this compound plays a protective role by up-regulating the IFN-γ-related cellular response and the IL-17 signaling pathway. nih.gov Furthermore, this compound treatment was found to protect the ultrastructure of the vaginal epithelium, partially by preserving mitochondrial integrity and reducing fungal adhesion and invasion. nih.gov
Other studies have shown that polyenes like this compound can induce the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and IFN-γ. oup.comoup.com This pro-inflammatory nature is thought to be mediated through a Toll-like receptor 2 (TLR2)-dependent mechanism. nih.gov Interestingly, a lipid formulation of this compound (NYT-IL) was found to induce lower levels of pro-inflammatory cytokines and higher levels of the anti-inflammatory cytokine IL-10 compared to standard this compound in mice, suggesting that formulation can modulate its immunomodulatory effects. oup.com
Combination Studies with Other Antimicrobial Agents or Therapies (e.g., Silver Nanoparticles, Photodynamic Therapy)
To enhance antifungal efficacy and combat resistance, researchers are investigating this compound in combination with other antimicrobial agents and therapies.
Studies have explored the use of this compound with silver nanoparticles (AgNPs) . AgNPs themselves have antifungal properties and have been shown to be effective against this compound-resistant C. albicans biofilms. semanticscholar.orgresearchgate.net The combination of this compound with nanoparticles is proposed to increase the bioavailability and efficacy of the drug. mdpi.com
Antimicrobial photodynamic therapy (aPDT) is another modality being combined with this compound. frontiersin.org aPDT involves using a photosensitizer and light to generate reactive oxygen species that kill microbes. A meta-analysis of studies on oral candidiasis concluded that the combination of PDT and this compound is more effective than this compound alone and results in a lower risk of recurrence. nih.gov In animal models of oral candidiasis induced by fluconazole-resistant C. albicans, the combination of a photosensitizer-mediated aPDT and this compound promoted a significant reduction in fungal viability and oral lesions. frontiersin.org
Genetic Engineering for Yield Improvement and Diversification of Polyketide Libraries
The production of this compound is dependent on the bacterium Streptomyces noursei. ontosight.ai Genetic and metabolic engineering of this organism is a key area of research aimed at increasing production yields and creating novel antifungal compounds. ontosight.ainih.gov
Scientists are focused on optimizing fermentation processes and employing strain improvement techniques to boost this compound output. ontosight.ai This includes strategies like UV mutagenesis to select for high-yielding mutant strains. mdpi.com One such study obtained a mutant strain with a this compound potency that was over 1.5 times higher than the original strain after optimizing fermentation conditions. mdpi.com
Furthermore, biosynthetic engineering of the this compound polyketide synthase (PKS) genes in S. noursei is being used to generate new polyene macrolides. nih.govucd.ieasm.org The this compound PKS is a large, modular enzyme responsible for building the complex polyketide structure. asm.orgnih.gov By inactivating specific genes (like nysL, nysN) and domains (like DH15) within the PKS pathway, researchers have successfully created new this compound-related analogues with altered chemical structures, such as modified hydroxyl groups. nih.govasm.org These new compounds are then tested for their antifungal and hemolytic activities to identify candidates with an improved therapeutic index. nih.gov This approach not only aims to enhance this compound's properties but also contributes to the diversification of polyketide libraries, which could lead to the discovery of entirely new drugs. asm.orgnih.govasm.org
Exploratory Research in Non-Medical Applications (e.g., Agricultural, Veterinary, Material Science)
Beyond its established medical uses, the antifungal properties of this compound have prompted exploratory research into a variety of non-medical fields. Scientists are investigating its potential in agriculture to protect crops, in veterinary medicine to treat animals, and in material science to create novel functional materials.
Agricultural Applications
Research in agriculture has focused on this compound's potential as a biopesticide, particularly for controlling post-harvest fungal diseases that lead to significant crop losses.
Recent studies have confirmed the inhibitory effects of this compound on the growth and development of Botrytis cinerea, the fungus responsible for gray mold, a primary cause of post-harvest loss in table grapes. researchgate.netmdpi.com The application of this compound was found to disrupt the membrane permeability of B. cinerea, leading to cellular leakage and death. researchgate.netmdpi.com Transcriptome analysis revealed that this compound treatment altered the expression of genes related to the fungus's cellular machinery and metabolic processes, ultimately reducing its pathogenicity. researchgate.netmdpi.com These findings suggest this compound could be a viable agent for managing gray mold on post-harvest table grapes. researchgate.net
This compound has also been proposed for broader use in preventing post-harvest losses in various crops. mdpi.com For instance, regulations in some regions have permitted the use of this compound on the skin of bananas to prevent mold. fao.orgwikipedia.org However, its application in plant tissue culture has shown limitations. In a study on blueberry cultivars, this compound added to the culture medium to control contamination was found to inhibit plantlet growth and, at low concentrations, even induced plantlet death, suggesting a level of phytotoxicity. kspbtjpb.org
Table 1: Summary of Research Findings for this compound in Agricultural Applications
| Application Area | Target Organism/System | Key Research Findings | Reference |
|---|---|---|---|
| Post-Harvest Fruit Protection | Botrytis cinerea (Gray Mold) on Table Grapes | Effectively inhibits mycelial growth and spore germination. Reduces the severity of gray mold by disrupting fungal cell membrane permeability and altering gene expression. | researchgate.netmdpi.com |
| Food Preservation | Fungi on Banana Skin | Permitted for use on the skin (but not the flesh) of bananas as a preservative to prevent mold growth. | fao.orgwikipedia.org |
| Plant Tissue Culture | Blueberry (Vaccinium sp.) | Exhibited phytotoxicity, causing growth retardation and death of plantlets, limiting its use for contamination control in this context. | kspbtjpb.org |
Veterinary Applications
In veterinary medicine, this compound is used to treat fungal infections, primarily those caused by Candida species. Its use in many animal species is considered "off-label" or "extra-label," meaning it is used in a manner not specifically described on the product label, a common practice in veterinary medicine. vcacanada.com
This compound is utilized to treat fungal infections of the mouth and gastrointestinal tract in cats, dogs, reptiles, and birds. vcacanada.com It is also a component in topical and otic (ear) preparations for dogs and cats to treat skin and ear infections caused by susceptible yeasts. monvet.competplace.comresearchgate.net For poultry, this compound can be added to feed or drinking water to treat and prevent candidiasis (also known as crop mycosis or thrush), an opportunistic infection that can occur after the disruption of normal microflora. apsnbiotech.commsdvetmanual.com
Beyond candidiasis, in vitro research has explored other potential applications. One study investigated the effects of this compound on Babesia gibsoni, a protozoan parasite that infects canine red blood cells. The study found that this compound could destroy the parasite through its ionophorous activity without harming the host's red blood cells or leukocytes in the lab setting. nih.gov
Table 2: Summary of Research Findings for this compound in Veterinary Applications
| Animal Group | Application | Target Pathogen | Key Research Findings | Reference |
|---|---|---|---|---|
| Small Animals (Dogs, Cats, Birds, Reptiles) | Oral and Gastrointestinal Infections | Candida spp. | Used off-label to treat fungal infections in the mouth or GI tract. | vcacanada.com |
| Dogs and Cats | Dermatological and Otic Infections | Yeast and Fungi (e.g., Candida) | Ingredient in topical ointments and ear drops for skin and ear infections. | monvet.competplace.comresearchgate.net |
| Poultry (Turkeys, Chickens) | Prophylaxis and Treatment of Candidiasis | Candida albicans | Administered via feed or water to control crop mycosis, especially after antibiotic use. | apsnbiotech.commsdvetmanual.com |
| Canine (In Vitro) | Antiprotozoal Activity | Babesia gibsoni | Demonstrated in vitro activity against the parasite through its ionophorous mechanism. | nih.gov |
Material Science Applications
The unique properties of this compound have attracted interest in material science for creating advanced materials with antifungal capabilities.
One significant area of research is the development of active food packaging. Scientists have successfully incorporated this compound into biodegradable polymer composites to create antifungal films. researchgate.netzenodo.org In one study, this compound was melt-mixed with Polycaprolactone (PCL) and then with Polylactic acid (PLA) to produce composite films. These films demonstrated good activity against a range of pathogenic and foodborne fungi, including Candida and Penicillium species, with a sustained release of this compound for up to four days. researchgate.netzenodo.org This research highlights the potential for using this compound to develop biodegradable packaging that can extend the shelf-life of food products. researchgate.netzenodo.org Similarly, this compound has been studied for retarding spoilage in cottage cheese. nih.gov
In a different application, this compound has been investigated as a corrosion inhibitor for mild steel in acidic environments. researchgate.net Electrochemical and computational studies suggested that the this compound molecule could adsorb onto the steel surface, acting as an effective mixed-type inhibitor to slow down the corrosion process. researchgate.net
This compound has also been used in the preservation of cultural artifacts. Notably, a derivative of this compound was applied to protect wood panel paintings that were damaged by mold following a major flood in Florence, Italy, in 1966. wikipedia.org Furthermore, the compound has been used to create various polymeric films and hydrogels, often as a model drug to test drug release properties for biomedical systems, which nonetheless contributes to the fundamental understanding of its behavior within polymer matrices. brieflands.comnih.govnih.gov
Table 3: Summary of Research Findings for this compound in Material Science
| Application Area | Material System | Key Research Findings | Reference |
|---|---|---|---|
| Antifungal Food Packaging | Biodegradable Polymer Composites (PLA/PCL) | Successfully incorporated into films via melt-processing. The resulting composites exhibited sustained antifungal activity against food spoilage fungi. | researchgate.netzenodo.org |
| Corrosion Inhibition | Mild Steel in Hydrochloric Acid | Acts as an effective mixed-type corrosion inhibitor by adsorbing onto the metal surface. | researchgate.net |
| Art Preservation | Wood Panel Paintings | Used to prevent the spread of mold on works of art. | wikipedia.org |
| Polymer Science | Polymeric Films and Hydrogels | Incorporated into various polymer matrices (e.g., Eudragit, Chitosan) to create functional films, demonstrating its compatibility with film-forming materials. | brieflands.comnih.gov |
Q & A
Q. How can researchers optimize in vitro models for this compound’s mechanism of action studies?
- Methodological Answer : Use polarized epithelial monolayers (e.g., Caco-2) to simulate intestinal barriers. Quantify membrane depolarization via transepithelial electrical resistance (TEER) measurements. Correlate with sterol-binding assays to confirm target engagement .
Contradictions and Knowledge Gaps
- Key Contradiction : this compound’s prophylactic efficacy in immunocompromised patients remains disputed, with meta-analyses showing no mortality benefit over placebo (RR 0.85) and inferiority to fluconazole (RR 0.37) .
- Knowledge Gap : Limited data exist on this compound’s antiviral mechanisms beyond sterol binding. Multidisciplinary studies integrating virology, pharmacokinetics, and nanotechnology are needed .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


