Vincristine
Description
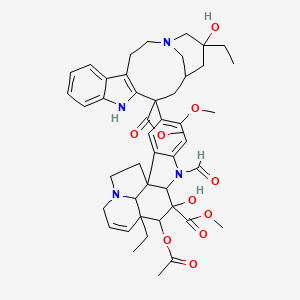
Molecular Architecture and Stereochemical Configuration
IUPAC Nomenclature and Systematic Classification
Vincristine, systematically named (1R,9R,10S,11R,12R,19R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.0⁴,¹².0⁵,¹⁰]nonadeca-4(12),5,7,9-tetraen-13-yl]-8-formyl-10-hydroxy-5-methoxy-8,16-diazapentacyclo[10.6.1.0¹,⁹.0²,⁷.0¹⁶,¹⁹]nonadeca-2,4,6,13-tetraene-10-carboxylate , belongs to the class of vinca alkaloids derived from the Madagascar periwinkle (Catharanthus roseus) . Its molecular formula, C₄₆H₅₆N₄O₁₀ , reflects a dimeric structure composed of catharanthine (a tetrahydrocarbazole moiety) and vindoline (an indole-containing segment) linked via a carbon-carbon bond . The systematic classification places this compound under the L01CA02 code in the Anatomical Therapeutic Chemical (ATC) system as an antineoplastic vinca alkaloid .
Structural Breakdown of IUPAC Name:
| Component | Description |
|---|---|
| 1R,9R,10S,11R,12R,19R | Stereochemical descriptors for six chiral centers in the vindoline moiety |
| 13S,15S,17S | Stereochemical configuration of the catharanthine-derived fragment |
| Diazatetracyclo[...] | Bridged bicyclic system with nitrogen atoms in the catharanthine unit |
| Diazapentacyclo[...] | Pentacyclic framework with fused indole and piperidine rings |
Three-Dimensional Conformational Analysis
X-ray crystallographic studies reveal that this compound adopts a rigid, bent conformation due to steric constraints imposed by its polycyclic framework . Key features include:
- Chair-like conformation of the eleven-membered carbomethoxyverbanamine ring in the catharanthine moiety, which directs the C18′ substituent into a sterically favorable axial position .
- Planar orientation of the vindoline indole ring, facilitating π-π stacking interactions with tubulin dimers .
- Hydrogen-bonding network between the C3 acetyloxy group and adjacent hydroxyl groups, stabilizing the tertiary structure .
Molecular dynamics simulations further demonstrate that this compound’s C18′ methoxycarbonyl group rotates freely in solution but becomes fixed upon binding to tubulin, inducing conformational strain in microtubules .
Critical Stereochemical Centers and Their Biological Implications
This compound contains nine stereogenic centers , with the C18′ configuration (S) being indispensable for antimitotic activity .
The C2′ hydroxyl group (R configuration) enhances solubility by participating in hydrogen-bonding interactions with aqueous solvents, while the C16 methoxy group (S) modulates membrane permeability . Alterations to these centers, as seen in synthetic analogs like vinorelbine , significantly alter pharmacokinetic profiles and toxicity .
Stereochemical Impact on Tubulin Binding:
Properties
Key on ui mechanism of action |
The antitumor activity of Vincristine is thought to be due primarily to inhibition of mitosis at metaphase through its interaction with tubulin. Like other vinca alkaloids, Vincristine may also interfere with: 1) amino acid, cyclic AMP, and glutathione metabolism, 2) calmodulin-dependent Ca2+-transport ATPase activity, 3) cellular respiration, and 4) nucleic acid and lipid biosynthesis. Vinca alkaloids are cell cycle specific agents ... /which/ block mitosis and produce metaphase arrest. Biological activities of these drugs can be explained by their ability to bind specifically tubulin, and to block ability of the protein to polymerize into microtubules ... through disruption of microtubules of mitotic apparatus, cell division is arrested in metaphase. In absence of intact mitotic spindle, chromosomes may disperse throughout cytoplasm ... or may occur in unusual groupings ... inability to segregate chromosomes correctly during mitosis presumably leads to cellular death. /Vinca alkaloids/ Although the mechanism of action has not been definitely established, vincristine appears to bind to or crystallize critical microtubular proteins of the mitotic spindle, thus preventing their proper polymerization and causing metaphase arrest. In high concentrations, the drug also exerts complex effects on nucleic acid and protein synthesis. Vincristine exerts some immunosuppressive activity. |
|---|---|
CAS No. |
57-22-7 |
Molecular Formula |
C46H56N4O10 |
Molecular Weight |
825.0 g/mol |
IUPAC Name |
methyl (10S,11R,12R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-8-formyl-10-hydroxy-5-methoxy-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate |
InChI |
InChI=1S/C46H56N4O10/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3/t28-,37?,38?,39-,42+,43-,44?,45+,46+/m1/s1 |
InChI Key |
OGWKCGZFUXNPDA-DLBZMDDPSA-N |
impurities |
3'-hydroxyvincristine; 4'-deoxyvincristine; N-desmethylvinblastine; deacetylvincristine; deacetylvinblastine; vinblastine; leurosine; formylleurosine |
SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Isomeric SMILES |
CC[C@@]1(C[C@@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7[C@@](C=CC9)([C@H]([C@@](C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Canonical SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Appearance |
White to off-white, odorless amorphous or crystalline powder |
Color/Form |
Blades from methanol |
melting_point |
424 to 428 °F (NTP, 1992) 218-220 °C |
Other CAS No. |
57-22-7 |
physical_description |
Vincristine appears as a white crystalline solid. Melting point 218 °C. Used as an antineoplastic. |
shelf_life |
STERILE SOLN IN EITHER H2O OR PHYSIOLOGICAL SALINE STORED IN REFRIGERATOR FOR UP TO 2 WK WITHOUT SIGNIFICANT LOSS OF POTENCY |
solubility |
WHITE TO SLIGHTLY YELLOW, AMORPHOUS OR CRYSTALLINE POWDER; ODORLESS, HYGROSCOPIC; FREELY SOL IN WATER /VINCRISTINE SULFATE USP/ |
Synonyms |
cellcristin Citomid Farmistin Leurocristine Oncovin Oncovine Onkocristin PFS, Vincasar Sulfate, Vincristine Vincasar Vincasar PFS Vincristin Bristol Vincristin medac Vincristine Vincristine Sulfate Vincrisul Vintec |
Origin of Product |
United States |
Preparation Methods
Oxidation of Vinblastine to Vincristine
Reaction Conditions and Reagents
The oxidation of vinblastine (VLB) to this compound represents a semi-synthetic pathway leveraging the structural similarity between the two alkaloids. This method employs an acidic ferrous perchlorate-hydrogen peroxide system under rigorously controlled conditions. Key parameters include:
Procedural Details from US4303584A
A representative procedure involves dissolving vinblastine sulfate (142 mg) in acetonitrile, mixing with 70% perchloric acid, and adding the solution to a chilled (-20°C) ferrous perchlorate suspension. Hydrogen peroxide in acetonitrile is then introduced over 25 minutes, maintaining the temperature at -20°C. Post-reaction quenching with sodium bisulfite and extraction with methylene dichloride yields crude this compound, which is purified via preparative thin-layer chromatography (TLC) using silica plates. This method achieves yields up to 35%, a significant improvement over traditional chromic acid oxidations.
Table 1: Key Parameters in Vinblastine Oxidation
| Parameter | Details | Source |
|---|---|---|
| Temperature Range | -20°C to -40°C | |
| Solvent | Acetonitrile | |
| Oxidizing System | Fe(ClO₄)₂·6H₂O + H₂O₂ + HClO₄/CH₃COOH | |
| Yield | Up to 35% |
Total Synthesis of this compound
Stereoselective Coupling Strategy
The 2004 total synthesis by Fukuyama et al. hinges on coupling demethylvindoline with an eleven-membered carbomethoxyverbanamine precursor. Demethylvindoline is synthesized via oxidation of 17-hydroxy-11-methoxytabersonine, followed by regioselective acetylation using a mixed anhydride method.
Critical Synthetic Steps
- Oxidation and Acetylation : 17-Hydroxy-11-methoxytabersonine undergoes oxidation to form demethylvindoline, which is acetylated at the C17 position.
- Coupling Reaction : Demethylvindoline reacts with the carbomethoxyverbanamine precursor, achieving stereochemical control at C18′.
- Piperidine Ring Formation : Sequential deprotection and intramolecular cyclization yield the this compound skeleton.
Table 2: Total Synthesis Yield and Key Steps
| Step | Description | Yield | Source |
|---|---|---|---|
| Demethylvindoline Prep | Oxidation of tabersonine derivative | 62% | |
| Coupling Reaction | Stereoselective C18′ bond formation | 48% | |
| Final Cyclization | Piperidine ring closure | 75% |
Alternative Synthetic Routes
1,4-Dihydropyridine-Mediated Synthesis
US5047528A discloses a method using 1,4-dihydropyridine compounds to reduce vindoline-carbomethoxyvelbanamine intermediates. For example, sodium borohydride reduction of a dihydropyridine derivative yields this compound alongside by-products like 3',4'-dehydrovinblastine (17%) and leurosine (9%).
By-Product Analysis
The reduction process generates multiple alkaloids, necessitating rigorous chromatographic separation. Reverse-phase HPLC quantifies yields, with this compound constituting 23% of the crude product.
Table 3: By-Products in Alternative Synthesis
| By-Product | Yield | Structure | Source |
|---|---|---|---|
| 3',4'-Dehydrovinblastine | 17% | Δ³',⁴' unsaturation | |
| Leurosine | 9% | C18′ epimer | |
| Catharine | 7.5% | N-Oxide derivative |
Comparative Analysis of Preparation Methods
Chemical Reactions Analysis
Vincristine undergoes various chemical reactions, including oxidation, reduction, and substitution . Common reagents used in these reactions include oxidizing agents, reducing agents, and nucleophiles . The major products formed from these reactions depend on the specific conditions and reagents used . For example, oxidation of this compound can lead to the formation of different oxidized derivatives, while reduction can yield reduced forms of the compound .
Scientific Research Applications
Clinical Applications
Vincristine is approved for treating various malignancies, including:
- Acute Lymphoblastic Leukemia (ALL)
- Hodgkin's and Non-Hodgkin Lymphomas
- Neuroblastoma
- Wilms Tumor
- Rhabdomyosarcoma
- Kaposi Sarcoma
FDA-Approved Indications
| Cancer Type | FDA Approval Year |
|---|---|
| Acute Lymphoblastic Leukemia | 1963 |
| Wilms Tumor | 1963 |
| Hodgkin's Lymphoma | 1963 |
| Non-Hodgkin Lymphoma | 1963 |
| Rhabdomyosarcoma | 1963 |
Off-Label Uses
In addition to its approved indications, this compound is used off-label for:
- Central Nervous System (CNS) tumors
- Ewing Sarcoma
- Medulloblastoma
- Bladder Cancer
- Ovarian Cancer
Combination Therapies
This compound is often used in combination with other chemotherapeutic agents to enhance efficacy while minimizing adverse effects. Notable combination regimens include:
- CHOP : Cyclophosphamide, Doxorubicin, this compound, Prednisolone
- CVP : Cyclophosphamide, this compound, Prednisolone
- CISCA : Cisplatin, Doxorubicin, Vinblastine, Bleomycin
Case Studies and Clinical Trials
Numerous studies underscore this compound's effectiveness across different cancer types. A notable trial reported that this compound administered weekly to children with advanced cancer resulted in a 68% objective tumor response rate across various malignancies, including ALL and Hodgkin's disease .
Clinical Trial Insights
| Study Focus | Findings |
|---|---|
| This compound in Advanced Cancer | 68% response rate in pediatric patients |
| Combination Therapy with this compound | Enhanced efficacy in lymphoma treatments |
Pharmacokinetics and Challenges
This compound's pharmacokinetic profile reveals rapid distribution and significant protein binding (approximately 75%). However, its clinical use is limited by neurotoxicity and other side effects such as alopecia and constipation. Recent advancements focus on improving its delivery through nanotechnology-based formulations to enhance targeting and reduce toxicity .
Emerging Trends
Research continues to explore this compound's role in combination therapies and novel formulations. For instance, liposomal formulations like Marqibo have been developed to prolong circulation time and improve dosing efficacy for patients with relapsed ALL . Additionally, ongoing studies are investigating its use in treating solid tumors and enhancing its therapeutic index through innovative drug delivery systems .
Mechanism of Action
Vincristine exerts its effects by binding to tubulin, a protein that is essential for the formation of microtubules . By inhibiting microtubule formation, this compound disrupts the mitotic spindle, leading to cell cycle arrest at the metaphase stage . This inhibition of cell division ultimately results in the death of rapidly dividing cancer cells . Additionally, this compound may interfere with nucleic acid and protein synthesis by blocking the utilization of glutamic acid .
Comparison with Similar Compounds
Comparison with Similar Compounds
Vincristine belongs to the Vinca alkaloid family, which includes vinblastine, vindesine, vinorelbine, and vinflunine. These compounds share a core structure but differ in substituents, leading to variations in potency, toxicity, and clinical applications.
Mechanistic Differences in Tubulin Binding and Microtubule Dynamics
- This compound vs. Vinblastine : While both inhibit tubulin polymerization, vinblastine exhibits stronger antiproliferative effects in cell cultures. For example, in L-cells, 40 nM vinblastine completely inhibited growth, whereas this compound caused only 25% inhibition . However, this compound has a lower Ki (0.085 µM) for tubulin binding compared to vinblastine (0.178 µM), indicating higher intrinsic activity against microtubule assembly .
- This compound vs. Vinorelbine: Vinorelbine induces tubulin self-association into spiral aggregates but forms fewer large polymers compared to this compound . This may explain its distinct clinical profile in non-small-cell lung cancer (NSCLC) .
Clinical Efficacy and Toxicity Profiles
- Neurotoxicity: this compound’s neurotoxicity is more pronounced than vinblastine’s, which predominantly causes myelosuppression .
- Combination Therapy : this compound’s synergy with methotrexate is schedule-dependent. Administering methotrexate before this compound enhances efficacy, whereas the reverse sequence increases toxicity .
Resistance and Novel Formulations
- P-gp Overexpression : this compound is a substrate for P-glycoprotein (P-gp), a drug efflux pump linked to multidrug resistance. Liposomal this compound circumvents this by maintaining high intracellular drug concentrations .
- Novel Derivatives: Semisynthetic compounds like vinepidine show comparable tubulin inhibition (Ki: 0.079 µM) to this compound but weaker in vivo activity, highlighting the role of non-tubulin targets (e.g., calmodulin) in efficacy .
Comparative Cytotoxicity in Preclinical Models
Table 1. IC50 Values (µM) of Vinca Alkaloids in Human Cancer Cell Lines
| Cell Line | This compound | Vinblastine | Vindesine | Vinorelbine | |
|---|---|---|---|---|---|
| B16 Melanoma | 0.02 | 0.01 | 0.03 | 0.05 | |
| L-cells | 0.10 | 0.04 | 0.08 | 0.12 | |
| UKF-NB-3 (Neuroblastoma) | 0.015 | N/A | N/A | N/A |
- Neuroblastoma : this compound’s potency (IC50: 0.015 µM) is enhanced by pirinixic acid derivatives, which sensitize resistant cells by inhibiting survival pathways .
Q & A
Q. What experimental models are most effective for studying Vincristine-induced neurotoxicity, and how should variables be controlled?
this compound’s neurotoxic effects are best studied using compartmentalized in vitro models (e.g., microfluidic chambers) to isolate axonal and somatic responses. For example, a concentration of 1 μM this compound applied to axonal compartments induces progressive degeneration, while the same concentration in somatic compartments shows no effect . Key variables to control include exposure duration, drug concentration gradients, and neuronal subtype specificity (e.g., dorsal root ganglion vs. cortical neurons). Include negative controls (e.g., untreated axons) and validate results via morphological (microscopy) and functional (electrophysiology) assays .
Q. What methodologies are recommended for pharmacokinetic analysis of this compound in biological samples?
High-performance liquid chromatography (HPLC) coupled with mass spectrometry (LC-MS/MS) is the gold standard for quantifying this compound in plasma. A vortex-assisted dispersive liquid-liquid microextraction (DLLME) protocol improves recovery rates (≥90%) by optimizing solvent ratios (e.g., chloroform:acetonitrile) and pH conditions (pH 9.0) . Calibration curves should span 0.1–100 ng/mL, with intra-day and inter-day precision maintained at <15% .
Q. How can researchers standardize dosing protocols for this compound in preclinical cancer studies?
Preclinical dosing should align with human equivalent doses (HEDs) calculated via body surface area normalization. For murine models, a typical HED range is 0.5–1 mg/kg administered intravenously weekly. Monitor hematological toxicity (e.g., neutropenia) and neurotoxicity (e.g., gait abnormalities) as endpoints. Use syngeneic or patient-derived xenograft (PDX) models to replicate human pharmacokinetic variability .
Q. What criteria distinguish reliable in vitro efficacy data for this compound across cancer cell lines?
Use cell lines with documented this compound sensitivity (e.g., leukemia Jurkat cells, IC₅₀: 2–5 nM) and resistance (e.g., MCF-7 breast cancer, IC₅₀: >50 nM). Ensure assays include:
Q. How should researchers address batch-to-batch variability in this compound for in vitro studies?
Source this compound from accredited suppliers (e.g., Sigma-Aldrich, Selleckchem) and verify purity (>98%) via certificate of analysis (CoA). Pre-test each batch in a reference cell line (e.g., Jurkat) to confirm IC₅₀ consistency. Normalize data to a positive control (e.g., paclitaxel) to mitigate inter-experimental variability .
Advanced Research Questions
Q. How can high-throughput CRISPR screens identify synergistic drug combinations with this compound?
A CRISPR knockout library (e.g., GeCKO v2) can screen 18 cancer cell lines against 8 drugs, including this compound, to identify gene targets enhancing efficacy. For example, EP300 knockout synergizes with this compound in neuroblastoma models. Use a dual-readout system (cell viability and apoptosis) and validate hits via RNAi or pharmacological inhibition (e.g., JQAD1, an EP300 degrader) . Analyze data with SynergyFinder to quantify combination indices (CI < 0.3 indicates strong synergy) .
Q. What experimental strategies resolve conflicting data on this compound’s efficacy in solid tumors vs. hematological cancers?
Conflicting data often arise from tumor microenvironment (TME) differences. To address this:
- Compare drug penetration using 3D spheroid vs. 2D monolayer cultures.
- Profile ATP-binding cassette (ABC) transporter expression (e.g., P-gp) via qPCR.
- Use intravital imaging to assess this compound distribution in orthotopic models. Apply meta-analysis frameworks (e.g., PRISMA) to harmonize datasets and identify confounding variables .
Q. How can researchers model this compound resistance mechanisms in in vivo systems?
Develop resistance by exposing PDX models to escalating this compound doses over 6–8 weeks. Profile resistance markers via RNA-seq (e.g., upregulated βIII-tubulin, MAPK pathway activation) and validate with CRISPR-Cas9 knockout. Use single-cell sequencing to identify resistant subclones and test combination therapies (e.g., this compound + MAPK inhibitors) .
Q. What advanced imaging techniques optimize this compound delivery studies in the blood-brain barrier (BBB)?
Two-photon microscopy tracks this compound extravasation in real-time using fluorescent analogs (e.g., BODIPY-Vincristine). Combine with dynamic contrast-enhanced MRI to quantify BBB permeability changes in glioblastoma models. Validate delivery efficiency via LC-MS/MS of brain homogenates .
Q. How should multi-omics datasets be analyzed to uncover this compound’s off-target effects?
Integrate transcriptomic (RNA-seq), proteomic (TMT labeling), and metabolomic (LC-MS) data using platforms like MetaboAnalyst or IPA. Focus on pathways enriched in neurotoxicity (e.g., axon guidance, microtubule dynamics) and validate candidates (e.g., MAP1B, CRMP2) via siRNA knockdown. Use machine learning (e.g., random forest) to prioritize biomarkers .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


