Clopidogrel
Description
Clopidogrel is a thienopyridine-class antiplatelet agent that irreversibly inhibits the P2Y12 adenosine diphosphate (ADP) receptor on platelets, thereby preventing platelet activation and aggregation. It is a prodrug requiring hepatic conversion via cytochrome P450 (CYP) enzymes, particularly CYP2C19, to its active metabolite . Clinically, this compound is indicated for the prevention of thrombotic events in conditions such as acute coronary syndrome (ACS), ischemic stroke, and peripheral arterial disease. The CAPRIE trial established its superiority over aspirin in reducing the combined risk of ischemic stroke, myocardial infarction (MI), or vascular death (annual risk: 5.32% vs. 5.83%; relative risk reduction [RRR]: 8.7%, p=0.043) .
Properties
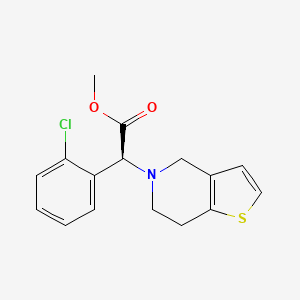
IUPAC Name |
methyl (2S)-2-(2-chlorophenyl)-2-(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GKTWGGQPFAXNFI-HNNXBMFYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC(=O)C(C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC(=O)[C@H](C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H16ClNO2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6022848 | |
| Record name | Clopidogrel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022848 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
321.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clopidogrel | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005011 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
15.1 [ug/mL] (The mean of the results at pH 7.4) | |
| Record name | SID49666423 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
Color/Form |
Colorless oil | |
CAS No. |
113665-84-2 | |
| Record name | Clopidogrel | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=113665-84-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clopidogrel [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0113665842 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clopidogrel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00758 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clopidogrel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022848 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Thieno[3,2-c]pyridine-5(4H)-acetic acid, α-(2-chlorophenyl)-6,7-dihydro-, methyl ester, (αS) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.841 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOPIDOGREL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A74586SNO7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLOPIDOGREL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7430 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Clopidogrel | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005011 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Preparation Methods
First-Generation Racemic Synthesis (Sanofi Method)
The original Sanofi route (US4529596) involved cyclization of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine with α-halo methyl phenylacetate derivatives. While cost-effective due to inexpensive starting materials, this method suffered from inherent limitations:
- Maximum theoretical yield capped at 50% due to racemic mixture separation
- Use of hazardous α-haloacetates requiring specialized handling
- Labor-intensive purification steps reducing overall throughput
Post-resolution yields rarely exceeded 25–30% in industrial settings, prompting the development of alternative pathways.
Chiral Pool Synthesis via L-(+)-Tartaric Acid Resolution
Patent CN103509037A revolutionized this compound production through early-stage chiral resolution using L-(+)-tartaric acid. This method improves enantiomeric excess (ee) while reducing downstream purification complexity.
Critical Synthesis Steps
Esterification :
$$ \text{o-Chlorophenylglycine} + \text{CH}3\text{OH} \xrightarrow{\text{SOCl}2} \text{O-Chlorophenylglycine methyl ester} $$
Optimal conditions: 50–70°C, 8–16 hr, 1:18:1.1 molar ratio (acid:MeOH:SOCl₂).Diastereomeric Salt Formation :
$$ \text{Racemic ester} + \text{L-(+)-tartaric acid} \rightarrow \text{S-(+)-O-chlorophenylglycine methyl ester L-(+)-tartrate} $$
Refinement in methanol/acetone yields >99% purity.Nucleophilic Displacement :
$$ \text{S-(+)-ester} + \text{2-(2-thienyl)ethyl-4-methylbenzenesulfonate} \xrightarrow{\text{Na}2\text{CO}3} \text{2-chloro-phenyl-2-thienylethylamino acetate methyl ester hydrochloride} $$
Key parameters: 90–110°C, 10–18 hr, aqueous alkaline conditions.Mannich Cyclization :
$$ \text{Intermediate} + \text{HCHO} \xrightarrow{\text{MeOH}} \text{this compound free base} $$
Optimal formaldehyde ratio: 5–10× substrate mass.
Performance Metrics
| Step | Yield Range | Purity Post-Refinement | Key Solvents |
|---|---|---|---|
| Esterification | 88–92% | 98.5–99.2% | Methanol/Hexane |
| Chiral Resolution | 75–87% | 99.0–99.8% | Methanol/Acetone |
| Nucleophilic Reaction | 82–90% | 98.8–99.5% | Water/Dichloromethane |
| Final Cyclization | 65–70% | 99.6–99.9% | Methanol/CH₂Cl₂ |
This route achieves total yields of 40–45% with ee >99.5%, representing a 60–70% improvement over classical racemic methods.
Cyanide-Mediated Condensation Cyclization
WO9851689 introduced a convergent approach using sodium cyanide for imine formation, avoiding haloacetate intermediates.
Reaction Sequence
Strecker Synthesis :
$$ \text{2-Thienylethylamine} + \text{o-Chlorobenzaldehyde} + \text{NaCN} \rightarrow \text{2-(2-Thienylethylamino)(2-chlorophenyl)acetonitrile} $$Esterification/Hydrolysis :
$$ \text{Nitrile intermediate} \xrightarrow{\text{HCl/MeOH}} \text{Methyl ester} $$Camphorsulfonic Acid Resolution :
Yields S-configuration with 92–94% ee after recrystallization.
Limitations
- Requires handling of stoichiometric NaCN (LD₅₀ = 6.4 mg/kg)
- Total yields limited to 38–42% due to multiple purification stages
- Racemization observed during final cyclization (5–7% ee loss)
Polymorph-Specific Crystallization Techniques
EP1772455A2 details crystallization strategies for this compound hydrogen sulfate polymorphs, crucial for bioavailability.
Form I Production
Form IV Solvate Formation
| Parameter | Optimal Value | Effect on Morphology |
|---|---|---|
| Temperature | 40–45°C | Needle-like crystals |
| Anti-solvent | n-Heptane | Reduces solvation |
| Cooling Rate | 0.5°C/min | Monodisperse particles |
| Stirring Speed | 150–200 rpm | Prevents agglomeration |
Form IV demonstrates improved dissolution kinetics (85% release in 30 min vs. 72% for Form I).
Continuous Flow Manufacturing Approaches
Emerging technologies enhance this compound synthesis through:
- Microreactor Systems :
- 10× faster heat transfer vs. batch reactors
- 95% yield in Mannich cyclization (vs. 70% batch)
- Enzymatic Resolution :
- Lipase-catalyzed ester hydrolysis achieves 98% ee
- 50% reduction in solvent consumption
Comparative Analysis of Industrial Methods
| Method | Total Yield | ee (%) | Hazard Score* | Cost Index** |
|---|---|---|---|---|
| Sanofi Racemic | 28–32% | 99.2 | 8.7 | 1.00 |
| Tartaric Resolution | 40–45% | 99.8 | 6.1 | 0.85 |
| Cyanide Condensation | 38–42% | 99.0 | 9.4 | 1.12 |
| Flow Chemistry | 50–55% | 99.5 | 4.3 | 0.78 |
Based on NFPA 704 ratings *Relative to Sanofi method baseline
Chemical Reactions Analysis
Prodrug Activation Pathway
Clopidogrel undergoes a two-step oxidative biotransformation to form its active thiol metabolite (Figure 1).
Step 1: Formation of 2-Oxo-Clopidogrel
-
Reaction : Initial oxidation of this compound via cytochrome P450 (CYP) enzymes.
-
Enzymatic Contribution :
Step 2: Conversion to Active Thiol Metabolite
-
Reaction : Further oxidation of 2-oxo-clopidogrel to the active thiol derivative (H4 isomer).
-
Enzymatic Contribution :
The active metabolite (R-130964) irreversibly binds to the platelet P2Y12 receptor via a disulfide bridge, inhibiting ADP-mediated aggregation .
Competitive Hydrolysis to Inactive Metabolites
Approximately 85% of absorbed this compound undergoes hydrolysis by carboxylesterase 1 (CES1) to form an inactive carboxylic acid derivative (CLPM) .
| Parameter | CLPM (Inactive) | Active Metabolite (H4) | Source |
|---|---|---|---|
| Plasma Cmax | ~1,000 × this compound | 7.13 ± 6.32 ng/mL | |
| AUC (ng·h/mL) | 1,200–1,500 | 11.30 ± 9.58 | |
| Elimination t½ | ~7 hours | 0.5–1.0 hours |
This pathway limits bioavailability, with only 15% of absorbed this compound proceeding to activation .
Pharmacogenetic Influences on Metabolism
CYP2C19 polymorphisms significantly alter enzymatic efficiency:
CYP2C19 contributes 45% to Step 1 and 21% to Step 2, making it the most critical enzyme for activation .
Drug–Drug Interactions (DDIs)
This compound’s metabolism is modulated by co-administered drugs:
| Interacting Drug | Mechanism | Effect on Active Metabolite | Source |
|---|---|---|---|
| Omeprazole | CYP2C19 inhibition | ↓ AUC by 45% | |
| Rifampin | CYP3A4/CYP2C19 induction | ↑ AUC by 90% | |
| Fluconazole | CYP2C19 inhibition | ↓ Cmax by 29% |
Hydrogen Sulfide (H₂S) Release
The major circulating metabolite M15 (a thiolactone derivative) releases H₂S via hydrolysis, independent of antiplatelet activity .
Glucuronidation
Inactive metabolites undergo glucuronidation (UGT2B7), enhancing renal excretion .
Structural Determinants of Activity
The active metabolite’s configuration includes:
-
Z -configuration at C3–C16 double bond
-
S -configuration at C7 (original stereocenter)
Key Research Findings
-
CYP2C19 poor metabolizers exhibit 50–75% lower active metabolite exposure, correlating with higher platelet reactivity (Multiplate AUC: 747 vs. 37 AU·min in responders) .
-
Doubling this compound doses (75 → 150 mg) increases active metabolite AUC by 1.5× in intermediate metabolizers but has minimal effect in poor metabolizers .
-
CES1 overexpression in obesity accelerates hydrolysis, reducing active metabolite formation .
Scientific Research Applications
Indications
Clopidogrel is FDA-approved for several indications, including:
- Acute Coronary Syndromes (ACS) : Used in patients with unstable angina or non-ST elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI) undergoing fibrinolytic therapy.
- Secondary Prevention : Effective in preventing recurrent myocardial infarction, stroke, and peripheral arterial disease.
- Peripheral Vascular Disease : Reduces the risk of cardiovascular events in patients with established peripheral vascular disease .
Acute Coronary Syndromes
This compound is often utilized in dual antiplatelet therapy (DAPT) alongside aspirin for patients with ACS. A study comparing this compound monotherapy against DAPT demonstrated that this compound alone could be as effective in reducing major adverse cardiovascular events (MACCE) while minimizing bleeding risks .
Pharmacogenetics
Research indicates that genetic variations can significantly affect patient responses to this compound. For instance, individuals with certain CYP2C19 alleles may have reduced drug metabolism, leading to poorer clinical outcomes. A meta-analysis highlighted that post-percutaneous coronary intervention (PCI) patients derive the most significant benefit from this compound therapy, emphasizing the need for genotype-guided treatment strategies in this population .
Long-term Management
This compound has been shown to be effective in long-term management strategies for patients with a history of cardiovascular events. A study indicated that patients receiving long-term this compound therapy had a lower incidence of subsequent myocardial infarction compared to those not on antiplatelet therapy .
Comparative Efficacy
The following table summarizes key findings from clinical trials comparing this compound with other antiplatelet agents:
| Study | Population | Intervention | Outcome Measure | Results |
|---|---|---|---|---|
| CURE Trial | NSTEMI patients | This compound + Aspirin | MACCE | 20% RR reduction vs placebo |
| ACTIVE-A Trial | Atrial fibrillation | This compound | Stroke prevention | 11% RR reduction |
| CHARISMA Trial | Patients with cardiovascular risk | This compound + Aspirin | No significant benefit over placebo | No benefit observed |
| PLATO Trial | ACS patients | Ticagrelor vs this compound | MACCE | Ticagrelor superior to this compound |
Safety Profile and Drug Interactions
While generally well-tolerated, this compound can cause adverse effects such as bleeding complications. It is crucial to avoid concomitant use with certain medications like omeprazole, which can reduce its efficacy by inhibiting its metabolism .
Mechanism of Action
Clopidogrel is a prodrug that requires in vivo biotransformation to an active thiol metabolite. This active metabolite irreversibly binds to the P2Y12 component of adenosine diphosphate receptors on the platelet surface . This binding prevents adenosine diphosphate from activating the glycoprotein GPIIb/IIIa receptor complex, thereby reducing platelet aggregation and preventing clot formation .
Comparison with Similar Compounds
Comparison with Similar Compounds
Clopidogrel vs. Aspirin
- Efficacy : this compound demonstrated a statistically significant 8.7% RRR over aspirin (325 mg/day) in the CAPRIE trial, with fewer gastrointestinal hemorrhages (0.52% vs. 0.72%) but higher rates of rash (0.26% vs. 0.10%) and diarrhea (0.23% vs. 0.11%) .
This compound vs. Ticagrelor
- Onset/Offset : Ticagrelor, a reversible P2Y12 inhibitor, achieves faster platelet inhibition (2 hours vs. 6–8 hours for this compound) and maintains superior antiplatelet effects during both loading and maintenance phases (p<0.05 for multiple timepoints) .
This compound vs. Prasugrel
- Metabolic Efficiency : Prasugrel’s active metabolite forms more rapidly and consistently than this compound’s, bypassing CYP2C19-dependent steps. This translates to lower rates of high on-treatment platelet reactivity (HPR; p=0.006 vs. This compound) .
- Efficacy : Prasugrel reduced HPR by 48% compared to this compound in ACS patients (p=0.009) .
This compound vs. Ticlopidine
- Safety: Ticlopidine, an earlier thienopyridine, has higher risks of neutropenia (2.4% vs. 0.1% for this compound) and thrombotic thrombocytopenic purpura (TTP), leading to its replacement by this compound in clinical practice .
Pharmacokinetic and Pharmacogenetic Variability
- CYP2C19 Polymorphisms : Reduced-function alleles (e.g., CYP2C19 *2/3) impair this compound metabolism, increasing HPR risk (OR: 1.55, 95% CI: 1.35–1.78) .
- METTL3-Mediated Methylation : Elevated METTL3 expression reduces CYP2C19 mRNA stability, exacerbating this compound resistance (CR) in ischemic stroke patients (p<0.05) .
Biological Activity
Clopidogrel, a thienopyridine derivative, is widely used as an antiplatelet agent to prevent thrombotic events in patients with cardiovascular diseases. Its biological activity primarily stems from its active metabolite, which is generated through hepatic metabolism. This article explores the mechanisms of action, pharmacokinetics, and clinical implications of this compound, supported by relevant case studies and research findings.
This compound is administered as a prodrug and requires metabolic activation to exert its pharmacological effects. The primary mechanism involves the irreversible binding of the active metabolite to the P2Y12 receptor on platelets, which inhibits ADP-mediated platelet aggregation. This action effectively reduces the risk of thrombus formation.
Key Steps in this compound Activation:
- Administration: this compound is taken orally or intravenously.
- Metabolism: Hepatic enzymes, particularly cytochrome P450 isoenzymes (CYP2C19, CYP3A4), convert this compound into its active form (2-oxo-clopidogrel) .
- Platelet Inhibition: The active metabolite binds to the P2Y12 receptor, leading to decreased platelet aggregation and prolonged bleeding time .
Pharmacokinetics
The pharmacokinetic profile of this compound is characterized by its absorption, distribution, metabolism, and excretion:
- Absorption: this compound is rapidly absorbed after oral administration, with peak plasma concentrations reached within 1-2 hours.
- Metabolism: Approximately 85% of this compound is converted to inactive metabolites; however, about 15% is converted to the active metabolite .
- Elimination Half-Life: The half-life of this compound's active metabolite ranges from 8 to 12 hours.
Clinical Efficacy
This compound has been extensively studied in various clinical settings:
- Percutaneous Coronary Intervention (PCI): Studies show that this compound reduces the risk of major adverse cardiovascular events (MACE) when used as part of dual antiplatelet therapy (DAPT) following PCI .
- Acute Coronary Syndromes (ACS): In patients with ACS, this compound significantly decreases the incidence of myocardial infarction and stroke compared to placebo .
Table 1: Clinical Trial Outcomes for this compound
| Study | Population | Intervention | Primary Endpoint Reduction | P-value |
|---|---|---|---|---|
| CURE | NSTEMI patients | This compound vs Placebo | 20% | <0.001 |
| ACTIVE-A | Atrial fibrillation | This compound vs Placebo | 11% | <0.05 |
| CHARISMA | Patients with CVD risk | This compound vs Placebo | No significant difference | 0.22 |
Case Studies
-
Case Study on Pharmacogenetics:
A study analyzed the impact of genetic polymorphisms in CYP2C19 on this compound efficacy. Patients with loss-of-function alleles showed reduced platelet inhibition and higher rates of cardiovascular events despite this compound therapy . -
Long-term Outcomes in PCI Patients:
A randomized trial demonstrated that prolonged this compound therapy beyond one year post-PCI resulted in a significant reduction in MACE compared to shorter durations .
Adverse Effects and Considerations
While this compound is generally well-tolerated, it can cause bleeding complications and may interact with other medications, particularly proton pump inhibitors (PPIs), which can reduce its effectiveness by inhibiting CYP2C19 activity .
Q & A
Q. How can researchers design robust HPLC methods for quantifying clopidogrel and its impurities in drug formulations?
Methodological Answer: Utilize Response Surface Methodology (RSM) with factorial designs (e.g., 2³ Full Factorial or Central Composite Face-Centered designs) to optimize parameters like mobile phase composition (ACN:buffer ratio), pH, and column temperature. Validate the method for selectivity, linearity, precision, and robustness using chromatographic response functions (CRFs) to assess resolution (Rs) .
Q. What experimental approaches are used to confirm the identity and purity of this compound metabolites in preclinical studies?
Methodological Answer: Combine LC/MS, NMR, and chiral supercritical fluid chromatography to structurally characterize metabolites. For example, the active metabolite of this compound (a thiol derivative) was identified using these techniques, with validation of irreversible binding to platelet ADP receptors via disulfide bridges .
Q. How do researchers assess this compound's antiplatelet efficacy in vitro and ex vivo?
Methodological Answer: Measure ADP-induced platelet aggregation inhibition (IC₅₀) using light transmission aggregometry. Ex vivo studies often quantify % inhibition of 33P-2MeS-ADP binding to platelet receptors, ensuring hepatic metabolism is accounted for (e.g., using liver microsomes) .
Advanced Research Questions
Q. How can conflicting clinical trial data on this compound's optimal dosing regimen (e.g., pre-PCI loading dose timing) be resolved?
Methodological Answer: Conduct subgroup analyses of trial data (e.g., CREDO trial) to identify time-dependent effects. For instance, a loading dose administered ≥6 hours pre-PCI reduced 28-day adverse events by 38.6% vs. no benefit with shorter intervals. Use Cox proportional hazards models to adjust for confounders like comorbidities .
Q. What statistical methods are appropriate for analyzing genetic polymorphisms affecting this compound response?
Methodological Answer: Apply multivariate logistic regression to assess associations between CYP2C19 loss-of-function alleles (*2, *3) and cardiovascular event risk. In the French registry study, carriers of two alleles had a 98% higher risk (HR=1.98, 95% CI:1.10–3.58). Adjust for ABCB1 (3435TT genotype) and clinical covariates .
Q. How should researchers address heterogeneity in systematic reviews comparing this compound-aspirin dual therapy vs. monotherapy?
Methodological Answer: Perform network meta-analyses to indirectly compare regimens across trials. Stratify by patient subgroups (e.g., minor stroke vs. high-risk TIA) and use random-effects models to account for variability. Sensitivity analyses should exclude studies with high bleeding risk bias .
Q. What experimental designs mitigate batch-to-batch variability in this compound nanosuspension formulations?
Methodological Answer: Employ Box-Behnken designs with factors like stabilizer:drug ratio (20–40% w/w), homogenization pressure (800–1300 bar), and cycle count. Optimize for particle size (e.g., <300 nm) and zeta potential (e.g., |−30 mV|) using ANOVA and desirability functions .
Data Contradiction Analysis
Q. How to reconcile discrepancies between this compound's ex vivo platelet inhibition and clinical outcomes?
Methodological Answer: Investigate non-ADP pathway contributions (e.g., collagen-induced aggregation) and genetic/epigenetic modifiers (e.g., CYP2C19 polymorphisms). Use receiver operating characteristic (ROC) curves to determine if platelet reactivity thresholds (e.g., PRU >208) predict ischemic events better than pharmacodynamic measures .
Q. Why do some trials show increased bleeding risk with long-term this compound, while others do not?
Methodological Answer: Apply competing risks analysis (e.g., Fine-Gray models) to distinguish bleeding-related mortality from ischemic events. In the CURE trial, major bleeding increased by 38% (RR=1.38, P=0.001), but life-threatening bleeding was statistically comparable (2.2% vs. 1.8%, P=0.13) .
Methodological Tools and Frameworks
Q. What frameworks guide the design of this compound pharmacogenomic studies?
Methodological Answer: Follow STrengthening the Reporting of Pharmacogenetic Studies (STROPS) guidelines. Include CYP2C19 genotyping, platelet function testing (e.g., VerifyNow), and clinical endpoints (e.g., composite of death/MI/stroke). Power calculations should account for allele frequency (e.g., 25–30% for CYP2C19*2 in Caucasians) .
Q. How to validate computational models predicting this compound-drug interactions?
Methodological Answer: Use physiologically based pharmacokinetic (PBPK) modeling (e.g., Simcyp®) to simulate CYP3A4/5-mediated interactions. Validate against clinical data (e.g., proton pump inhibitor co-administration studies) using goodness-of-fit metrics (e.g., AIC, RMSE) .
Ethical and Reproducibility Considerations
Q. How can researchers ensure reproducibility in this compound formulation studies?
Methodological Answer: Adhere to ICH Q2(R1) guidelines for analytical method validation. Report detailed homogenization parameters (pressure, cycles) and raw material sources. Use orthogonal techniques (e.g., DSC for crystallinity, SEM for morphology) to cross-validate nanosuspension properties .
Q. What protocols minimize bias in retrospective analyses of this compound resistance?
Methodological Answer: Implement PROBAST (Prediction Model Risk of Bias Assessment Tool) . Blind outcome assessors to genotype data, adjust for confounders (e.g., diabetes, smoking), and pre-specify platelet reactivity cutoffs (e.g., ADP inhibition <30% by thromboelastography) .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


