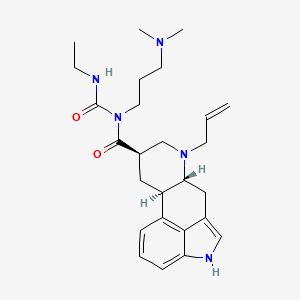
Cabergoline
Description
Historical Context and Evolution of Cabergoline in Research
This compound was synthesized by researchers at the Italian pharmaceutical company Farmitalia-Carlo Erba in Milan as part of investigations into semi-synthetic ergot derivatives. pharmacoj.com It was patented in 1980 and subsequently approved for medical use in 1993. wikipedia.org Early research focused on its potent and long-lasting dopamine D2 receptor agonist activity and its inhibitory effect on prolactin secretion, initially demonstrated in in vitro studies using rat lactotroph cells and in vivo in reserpinized rats. fda.govwikipedia.org This foundational research established its potential for treating conditions associated with elevated prolactin levels.
The evolution of this compound research has seen a shift from initial characterization of its basic pharmacological properties to broader investigations into its therapeutic potential and underlying mechanisms in various conditions beyond hyperprolactinemia. pharmacoj.comclinicaltrials.eu Comparative studies with older dopamine agonists like bromocriptine have been a significant part of this evolution, highlighting this compound's higher efficacy and more favorable pharmacological profile in many cases. medwave.cloup.comnih.gov Research has also explored its effects on different receptor subtypes and potential off-target actions. wikipedia.orgfishersci.senih.gov
Current Research Landscape and Significance of this compound Studies
The current research landscape for this compound is diverse, encompassing studies on its established applications as well as explorations into novel therapeutic areas. Its significance in research is underscored by its role as a key tool for investigating dopamine receptor function, particularly D2-like receptors, and their involvement in various physiological and pathological processes. drugbank.compharmacoj.comdroracle.ai
Current research areas include:
Prolactinomas and Hyperprolactinemia: Ongoing studies continue to evaluate this compound's efficacy in normalizing prolactin levels, reducing tumor size, and achieving biochemical and radiological remission in patients with prolactinomas. clinicaltrials.eunih.govendocrinolrespract.org Research also investigates factors influencing treatment response and strategies for managing resistant cases. nih.govendocrine-abstracts.org
Parkinson's Disease: While its use in Parkinson's disease has evolved, research continues to explore the role of dopamine agonists, including this compound, in managing motor symptoms and understanding dopaminergic pathways involved in the disorder. pharmacoj.compharmacoj.com
Other Pituitary Adenomas: Investigations are exploring this compound's potential in treating other types of pituitary tumors, such as Cushing's disease (ACTH-producing adenomas) and acromegaly (GH-producing adenomas), particularly in cases where these tumors co-secrete prolactin or express dopamine receptors. clinicaltrials.euelsevier.esresearchgate.netjst.go.jp
Neuroprotection: Studies are examining potential neuroprotective effects of this compound, particularly in the context of oxidative stress and excitotoxicity, which are implicated in neurodegenerative diseases. plos.org Research suggests a D2 receptor-mediated mechanism for this effect. plos.org
Reproductive Biology: Beyond hyperprolactinemia, this compound is being investigated for its effects on reproductive processes in various species, including its potential as a reproductive control agent in canids due to prolactin's role in maintaining pregnancy in these animals. unl.edu
The significance of these studies lies in refining treatment strategies, understanding the complex pharmacology of dopamine receptors, identifying potential new applications for this compound, and elucidating the underlying mechanisms of diseases where dopamine signaling is involved. pharmacoj.compharmacoj.comclinicaltrials.eupatsnap.com
Methodological Approaches in this compound Research
Research on this compound employs a variety of methodological approaches, ranging from in vitro studies characterizing receptor binding and cellular effects to in vivo studies in animal models and clinical investigations in human subjects.
Key methodological approaches include:
Receptor Binding Studies: These in vitro studies are crucial for determining this compound's affinity and activity at different dopamine and other receptors (e.g., D1, D2, D3, D4, D5, 5-HT1A, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, alpha-1, alpha-2 adrenergic). drugbank.comfda.govwikipedia.orgfishersci.se Techniques like radioligand binding assays are commonly used.
Cell Culture Studies: In vitro studies using various cell lines, such as pituitary lactotroph cells or neuronal cultures, are used to investigate the direct effects of this compound on hormone secretion, cell viability, and intracellular signaling pathways (e.g., ERK1/2 pathway). fda.govnih.govplos.orgoup.com
Animal Models: Research utilizes animal models (e.g., rats, mice, monkeys, coyotes) to study this compound's effects on prolactin levels, tumor growth (in xenograft models), motor symptoms, and reproductive processes in a physiological context. fda.govwikipedia.orgplos.orgunl.eduresearcher.life
Analytical Methods: Various analytical techniques are employed for the determination of this compound concentration in biological samples (e.g., plasma, serum) and pharmaceutical preparations. These include High-Performance Liquid Chromatography (HPLC), tandem Mass Spectrometry (MS-MS), UV-Visible Spectroscopy, and High-Performance Thin Layer Chromatography (HPTLC). endocrine-abstracts.orgunl.eduijprs.comresearchgate.net Mass spectrometry, particularly HPLC/tandem mass spectrometry, offers high sensitivity for detecting low picogram levels of this compound. endocrine-abstracts.orgunl.edu
Clinical Studies: Academic research heavily relies on clinical studies, including randomized controlled trials, retrospective studies, and systematic reviews, to evaluate this compound's efficacy and effects in human populations with various conditions. pharmacoj.compharmacoj.comclinicaltrials.eumedwave.cloup.comnih.govnih.govelsevier.esresearchgate.netjst.go.jpresearcher.lifenih.gov These studies often involve measuring hormone levels (e.g., prolactin, growth hormone, ACTH), assessing tumor volume via imaging (e.g., MRI), and evaluating clinical outcomes. clinicaltrials.eunih.govendocrinolrespract.orgendocrine-abstracts.orgresearchgate.netjst.go.jp
Molecular Techniques: Advanced techniques like single-cell RNA sequencing are being applied to analyze the cellular composition and transcriptional changes in tissues, such as prolactinomas, from this compound-treated patients to understand the drug's effects at a molecular level. nih.govoup.com This includes investigating its impact on tumor cells, immune cells (e.g., CD8+ T cells), and stromal cells. nih.govoup.com
These diverse methodologies allow researchers to investigate this compound from molecular interactions to clinical outcomes, contributing to a comprehensive understanding of its properties and potential applications in academic research.
Research Findings Highlights:
Research has demonstrated that this compound is highly effective in normalizing prolactin levels in patients with hyperprolactinemia and prolactinomas. pharmacoj.compharmacoj.comoup.comnih.gov In studies comparing this compound to bromocriptine, this compound has shown superior efficacy in achieving prolactin normalization and resolving associated symptoms. medwave.clnih.gov For instance, one comparative trial showed prolactin normalization in 77% of patients treated with this compound compared to 59% with bromocriptine. fda.gov
This compound treatment has also been shown to reduce the size of prolactin-secreting pituitary adenomas. clinicaltrials.euoup.comnih.govendocrinolrespract.org A scoping review indicated that this compound is effective in achieving both biochemical and radiological remission in prolactinoma cases. nih.gov Studies have evaluated tumor volume reduction over time, with some research suggesting greater reduction with longer treatment duration. nih.govendocrinolrespract.org
Beyond prolactinomas, research explores this compound's effects in other conditions. Studies in Cushing's disease have investigated its ability to lower ACTH levels and induce regression of ACTH-producing pituitary adenomas, with some cases showing normalization of urinary free cortisol and tumor shrinkage. elsevier.esresearchgate.net In acromegaly, research on combination therapy with somatostatin analogues and this compound has shown potential in normalizing IGF-I levels and decreasing tumor volume in some patients, particularly those resistant to somatostatin analogues alone. jst.go.jp
Investigations into the neuroprotective effects of this compound in cultured cortical neurons exposed to oxidative stress have shown that this compound can prevent cell death, a process mediated by dopamine D2 receptors and involving the suppression of the ERK signaling pathway. plos.org
Data Table Example (Illustrative based on search findings - specific numerical data points are synthesized from multiple sources and are representative, not exhaustive):
| Research Area | Key Finding | Relevant Receptor(s) Studied | Methodologies Used |
| Hyperprolactinemia | High rate of prolactin normalization and symptom resolution. pharmacoj.compharmacoj.comoup.comnih.gov | D2 | Clinical trials, systematic reviews, immunoassays for prolactin measurement. fda.govpharmacoj.comoup.comnih.govendocrine-abstracts.org |
| Prolactinoma Tumor Size | Significant tumor volume reduction. clinicaltrials.euoup.comnih.govendocrinolrespract.org | D2 | Clinical studies, MRI for tumor volume assessment. clinicaltrials.eunih.govendocrinolrespract.org |
| Cushing's Disease | Potential to lower ACTH and shrink adenomas in some cases. elsevier.esresearchgate.net | D2 | Case reports, retrospective studies, measurement of cortisol/ACTH, MRI. elsevier.esresearchgate.net |
| Acromegaly (Adjunctive) | May normalize IGF-I and reduce tumor volume in resistant cases. jst.go.jp | D2 | Retrospective clinical studies, measurement of GH/IGF-I, tumor volume assessment. jst.go.jp |
| Neuroprotection (in vitro) | Prevents neuronal cell death under oxidative stress via D2 receptor. plos.org | D2 | Cell culture studies, viability assays, signaling pathway analysis. plos.org |
| Receptor Binding | High affinity for D2, also binds D3, D4, 5-HT2B, etc. wikipedia.orgfishersci.se | D2, D3, D4, 5-HT subtypes | In vitro receptor binding assays. wikipedia.orgfishersci.se |
Properties
IUPAC Name |
(6aR,9R,10aR)-N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)-7-prop-2-enyl-6,6a,8,9,10,10a-hexahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C26H37N5O2/c1-5-11-30-17-19(25(32)31(26(33)27-6-2)13-8-12-29(3)4)14-21-20-9-7-10-22-24(20)18(16-28-22)15-23(21)30/h5,7,9-10,16,19,21,23,28H,1,6,8,11-15,17H2,2-4H3,(H,27,33)/t19-,21-,23-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KORNTPPJEAJQIU-KJXAQDMKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCNC(=O)N(CCCN(C)C)C(=O)C1CC2C(CC3=CNC4=CC=CC2=C34)N(C1)CC=C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCNC(=O)N(CCCN(C)C)C(=O)[C@@H]1C[C@H]2[C@@H](CC3=CNC4=CC=CC2=C34)N(C1)CC=C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C26H37N5O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
85329-89-1 (diphosphate) | |
| Record name | Cabergoline [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0081409907 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID6022719 | |
| Record name | Cabergoline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
451.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Cabergoline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Insoluble, 6.40e-02 g/L | |
| Record name | Cabergoline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00248 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cabergoline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
81409-90-7 | |
| Record name | Cabergoline | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=81409-90-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cabergoline [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0081409907 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cabergoline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00248 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cabergoline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6022719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (6aR,9R,10aR)-N-[3-(dimethylamino)propyl]-N-(ethylcarbamoyl)-7-prop-2-enyl-6,6a,8,9,10,10a-hexahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CABERGOLINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/LL60K9J05T | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Cabergoline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
102-104 °C, 102 - 104 °C | |
| Record name | Cabergoline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00248 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cabergoline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Pharmacological Foundations of Cabergoline
Mechanism of Action and Receptor Interactions
Cabergoline exerts its therapeutic effects primarily through the activation of dopamine receptors, particularly the D₂ subtype. This interaction triggers a cascade of intracellular events that modulate various physiological processes.
Dopamine D₂ Receptor Agonism: High Affinity and Selectivity
This compound is distinguished by its high affinity and selectivity for dopamine D₂ receptors. wikipedia.orgdrugbank.comfda.govnih.govbioline.org.br This potent agonism at D₂ receptors is central to its mechanism of action, particularly in the inhibition of prolactin secretion from pituitary lactotrophs. wikipedia.orgpacehospital.compharmacoj.comfda.govnih.gov Studies have demonstrated that this compound is more potent than other dopamine agonists like bromocriptine in inhibiting prolactin secretion and exhibits a longer receptor occupancy. nih.gov The stimulation of D₂ receptors in the nigrostriatal pathway is also associated with improvements in coordinated muscle activity.
Interaction with Other Dopamine Receptor Subtypes (D₁, D₅)
While its primary action is at D₂ receptors, receptor-binding studies indicate that this compound has low affinity for dopamine D₁ and D₅ receptors. wikipedia.orgdrugbank.comfda.govfda.gov The D₁ and D₅ receptors belong to the D₁-like receptor subfamily, which are associated with different signaling pathways compared to the D₂-like subfamily (D₂, D₃, D₄). medicaldialogues.innih.govtocris.com Although this compound exhibits some agonist activity at D₁ receptors, this is considered weak compared to its potent D₂ agonism. wikipedia.org
Adrenergic Receptor Affinity (α₁, α₂)
This compound also demonstrates affinity for adrenergic receptors, specifically α₁ and α₂ subtypes. wikipedia.orgdrugbank.comfda.gov Receptor-binding studies suggest that this compound has low affinity for both α₁ and α₂ adrenergic receptors. drugbank.comfda.govfda.gov While it may act as an antagonist at α₂-adrenergic receptors, particularly the α₂D subtype, its affinity for these receptors is considerably lower than for D₂ receptors. pharmacoj.com
Direct Inhibitory Effect on Prolactin Secretion in Pituitary Lactotrophs
A key pharmacological effect of this compound is its direct inhibitory action on prolactin secretion from lactotroph cells in the anterior pituitary gland. wikipedia.orgpacehospital.compharmacoj.comfda.govfda.govdroracle.airatguide.comdroracle.ai Prolactin secretion is tonically inhibited by dopamine released from tuberoinfundibular neurons, which acts on D₂ receptors on lactotrophs. fda.govfda.govdroracle.airatguide.comdroracle.ai As a potent D₂ receptor agonist, this compound mimics the action of dopamine, leading to a decrease in intracellular cAMP concentrations and reduced calcium influx, ultimately inhibiting prolactin synthesis and release. In vitro studies using rat pituitary lactotrophs have demonstrated this direct inhibitory effect. wikipedia.orgfda.govfda.govdroracle.airatguide.comdroracle.ai
Pharmacodynamics of this compound
The pharmacodynamics of this compound are characterized by its potent and long-lasting effects, primarily mediated through its high affinity for D₂ receptors. The duration of its prolactin-lowering effect is notably long, which is attributed to its slow elimination and long half-life. fda.govratguide.comnih.gov
Studies in healthy volunteers have shown that single doses of this compound can lead to a significant and prolonged suppression of prolactin levels. fda.govnih.gov For instance, a single dose of 1 mg in healthy female volunteers resulted in a mean maximum prolactin decrease of approximately 70%, with the effect persisting for up to 9 days and completely resolving after 23-28 days. nih.gov In hyperprolactinemic patients, a single dose of this compound demonstrated a markedly longer duration of prolactin decrease compared to bromocriptine. fda.gov
The selective inhibition of prolactin secretion is a hallmark of this compound's pharmacodynamics at therapeutic doses. fda.gov At higher doses, this compound can also exert central dopaminergic effects via D₂ receptor stimulation. pacehospital.com The pharmacodynamic effects of this compound, including the percentage decrease in plasma prolactin levels, have been shown to be comparable between tablet and solution formulations. nih.gov
This compound's long-lasting dopaminergic stimulatory effects have been observed in animal models, such as 6-hydroxydopamine-lesioned rats and MPTP-lesioned primates. nih.gov These effects contribute to its potential in managing conditions related to dopamine dysregulation. pharmacoj.com
| Receptor Subtype | Affinity/Interaction | Effect | Source Index |
| Dopamine D₂ | High Affinity | Agonist | wikipedia.orgdrugbank.comfda.govnih.govbioline.org.br |
| Dopamine D₃ | Significant Affinity | Agonist | wikipedia.orgpharmacoj.commedicaldialogues.in |
| Dopamine D₄ | Affinity | Agonist | wikipedia.orgpharmacoj.commedicaldialogues.in |
| Dopamine D₁ | Low Affinity | Agonist (weak) | wikipedia.orgdrugbank.comfda.govfda.govwikipedia.org |
| Dopamine D₅ | Low Affinity | Agonist | drugbank.com |
| Adrenergic α₁ | Low Affinity | Antagonist | wikipedia.orgdrugbank.comfda.govfda.gov |
| Adrenergic α₂ | Low Affinity | Antagonist (especially α₂D) | wikipedia.orgdrugbank.compharmacoj.comfda.govfda.gov |
| Serotonin 5-HT₁A | High Affinity | Agonist | wikipedia.orgpharmacoj.comnih.gov |
| Serotonin 5-HT₁B | Affinity | Agonist | wikipedia.orgnih.gov |
| Serotonin 5-HT₁D | High Affinity | Agonist | wikipedia.orgnih.gov |
| Serotonin 5-HT₂A | High Affinity | Agonist | wikipedia.orgpharmacoj.comnih.gov |
| Serotonin 5-HT₂B | High Affinity | Agonist | wikipedia.orgpharmacoj.comnih.gov |
| Serotonin 5-HT₂C | Affinity | Agonist | wikipedia.orgnih.gov |
| Serotonin 5-HT₇ | Moderate to Low Affinity | Antagonist | wikipedia.orgpharmacoj.com |
Note: Affinity and interaction profiles can vary depending on the specific study and methodology.
Dose-Dependent Suppression of Prolactin Secretion
Studies have demonstrated a dose-dependent relationship between this compound administration and the suppression of serum prolactin levels. In hyperprolactinemic women, increasing doses of this compound resulted in a greater percentage of patients achieving normalized prolactin levels. fda.govfda.govnih.govsolameds.us For instance, in a placebo-controlled study, after 4 weeks of treatment, prolactin was normalized in 29% of patients receiving 0.125 mg twice weekly, compared to 76% with 0.5 mg, 74% with 0.75 mg, and 95% with 1.0 mg twice weekly. fda.govfda.govnih.govsolameds.us Prolactin inhibition has been observed at doses as low as 0.2 mg in healthy volunteers, with doses of 0.5 mg or higher causing maximal suppression in most subjects. fda.govnih.govnih.govresearchgate.net Higher doses generally lead to prolactin suppression in a larger proportion of individuals. fda.govnih.govnih.gov
Here is a table illustrating the dose-dependent normalization of prolactin levels based on a study in hyperprolactinemic women:
| This compound Dose (twice weekly) | Percentage of Patients with Normalized Prolactin (after 4 weeks) |
| 0.125 mg | 29% |
| 0.5 mg | 76% |
| 0.75 mg | 74% |
| 1.0 mg | 95% |
Duration of Prolactin-Lowering Effect
This compound is characterized by a prolonged duration of its prolactin-lowering effect. Following a single oral dose, the suppression of serum prolactin levels can persist for an extended period. In hyperprolactinemic patients, a single dose of 0.6 mg of this compound resulted in a markedly longer duration of effect (14 days) compared to 2.5 mg of bromocriptine (24 hours). fda.govfda.gov In healthy volunteers and hyperprolactinemic patients, the effect has been observed to last up to 7-28 days after a single administration of 0.3-1.5 mg. medicinesauthority.gov.mtpfizer.com The duration of action is dose-related. fda.govmedicinesauthority.gov.mtnih.govnih.govpfizer.com The long-lasting effect is thought to be related to its slow elimination and long half-life. fda.govsolameds.us
Effects on Other Anterior Pituitary Hormones (GH, FSH, LH, ACTH, TSH) and Cortisol
While this compound primarily targets prolactin secretion, its effects on other anterior pituitary hormones and cortisol have also been investigated. Studies have indicated that this compound demonstrates a high specificity in inhibiting prolactin secretion with generally no significant changes observed in the plasma levels of growth hormone (GH), thyroid-stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and cortisol in non-acromegalic, hyperprolactinemic patients. karger.comnih.gov
However, in some studies, particularly with long-term therapy or in specific patient populations like those with macroprolactinomas, some effects on other axes have been noted. For instance, in a study of patients with idiopathic hyperprolactinemia on long-term this compound, a progressive decrease in ACTH and GH levels was observed in a subset of patients. psu.edu In patients with macroprolactinomas treated with this compound, recovery of gonadotrophin deficiency has been reported in a subset of patients, while severe GH deficiency and ACTH deficiency have shown less frequent recovery. researchgate.net The role of this compound in conditions like Cushing's disease (related to ACTH) appears limited, with studies showing inconsistent or no dose-dependent reductions in cortisol levels. nih.govelsevier.eselsevier.es
Pharmacokinetics of this compound
The pharmacokinetic profile of this compound describes its absorption, distribution, metabolism, and excretion.
Absorption Characteristics
Following oral administration, this compound is rapidly absorbed from the gastrointestinal tract. pharmacoj.commedicinesauthority.gov.mt
Peak Plasma Levels and Time to Peak
Peak plasma concentrations of this compound are typically reached within 0.5 to 4 hours after a single oral dose. pharmacoj.commedicinesauthority.gov.mt In studies with healthy adult volunteers receiving single oral doses ranging from 0.5 mg to 1.5 mg, mean peak plasma levels of 30 to 70 picograms (pg)/mL were observed within 2 to 3 hours. fda.govfda.govnih.govsolameds.us
Here is a summary of peak plasma levels and time to peak:
| Dose Range (single oral) | Mean Peak Plasma Levels (healthy volunteers) | Time to Peak Plasma Levels |
| 0.5 mg to 1.5 mg | 30 to 70 pg/mL | 2 to 3 hours |
Dose Proportionality of Plasma Levels
This compound plasma levels appear to be dose-proportional over a range of 0.5 mg to 7 mg in healthy adult volunteers and adult parkinsonian patients. fda.govfda.govnih.govsolameds.usresearchgate.netnih.govfda.gov This suggests that as the dose increases within this range, the concentration of the drug in the plasma increases proportionally.
The absolute bioavailability of this compound is unknown. fda.govfda.govnih.govsolameds.usresearchgate.netnih.govfda.gov A significant fraction of the administered dose undergoes a first-pass effect. fda.govsolameds.usresearchgate.netnih.govfda.gov
Steady-State Levels with Once-Weekly Dosing
Studies in healthy volunteers suggest that following a once-weekly dosing schedule, steady-state plasma levels of this compound are expected to be two to three times higher compared to levels achieved after a single dose. hres.cafda.govfda.gov This indicates an accumulation of the drug with repeated administration at weekly intervals. Mathematical modeling and clinical trial data further support that a weekly regimen, particularly when fractionated into more frequent smaller doses, can lead to higher minimum and maximum steady-state plasma concentrations compared to less frequent, larger doses, an effect termed "hyperfractionation." acta-endo.ro
First-Pass Effect
A significant fraction of an orally administered dose of this compound undergoes a first-pass effect. hres.cafda.govfda.govnih.govnih.govdrugbank.comresearchgate.netratguide.com The absolute bioavailability of this compound in humans is currently unknown. hres.cafda.govfda.govnih.govnih.govdrugbank.comresearchgate.net
Distribution Profile
This compound demonstrates extensive distribution throughout the body. fda.govfda.gov
Plasma Protein Binding
In in vitro experiments, this compound at concentrations ranging from 0.1 to 10 ng/mL is approximately 41% to 42% bound to plasma proteins. hres.cafda.govfda.govnih.govnih.govdrugbank.comresearchgate.net This binding is concentration-independent. fda.govfda.govnih.govnih.govdrugbank.comresearchgate.net Concomitant administration of highly protein-bound drugs is considered unlikely to significantly affect this compound's disposition. fda.govfda.govnih.govresearchgate.net
Tissue Distribution, including Pituitary Gland
Based on studies involving total radioactivity in animals, this compound and/or its metabolites show extensive tissue distribution. hres.cafda.gov Notably, radioactivity levels in the pituitary gland exceeded those in plasma by more than 100-fold. hres.cafda.gov Radioactivity was eliminated from the pituitary with a half-life of approximately 60 hours in animals, a finding consistent with the drug's prolonged prolactin-lowering effect. hres.cafda.gov Studies in rats also indicated significant concentrations of this compound in the pituitary compared to serum. ratguide.com Whole-body autoradiography studies in pregnant rats revealed no fetal uptake but high levels in the uterine wall. fda.gov Significant radioactivity was detected in the milk of lactating rats, suggesting potential exposure to nursing offspring. fda.gov
Blood-Brain Barrier Permeation
While not explicitly stated as readily crossing the intact blood-brain barrier (BBB) in all contexts, research indicates that this compound can influence BBB integrity. tandfonline.comresearchgate.net Studies have explored this compound's protective effect on BBB integrity, particularly in the context of inflammation induced by lipopolysaccharide (LPS). tandfonline.comresearchgate.netnih.gov In animal models, this compound administration reversed LPS-induced increases in neurological deficits and disrupted BBB integrity. tandfonline.comnih.gov In vitro studies using human brain microvascular endothelial cells (HBMECs) showed that this compound alleviated elevated cell permeability and declined trans-endothelial electrical resistance caused by LPS, accompanied by the upregulation of zonula occludens-1 (ZO-1), a key tight junction protein. tandfonline.comresearchgate.netnih.gov These findings suggest that this compound can impact the function and integrity of the BBB.
Pharmacokinetics in Special Populations
Hepatic Impairment
This compound is extensively metabolized by the liver, and caution is advised when administering it to patients with hepatic impairment. fda.govhres.capfizer.commedicinesauthority.gov.mt Studies have investigated the pharmacokinetics of this compound in patients with varying degrees of hepatic dysfunction. In patients with mild hepatic impairment (Child-Pugh A), no significant effect on the mean area under the this compound plasma concentration-time curve (AUC) has been observed. fda.govpfizer.com Similarly, patients with mild-to-moderate hepatic dysfunction (Child-Pugh score ≤ 10) have shown no effect on mean this compound Cmax or AUC in some studies. fda.govfda.gov However, patients with severe hepatic insufficiency (Child-Pugh Class C or score > 10) exhibit a substantial increase in the mean this compound Cmax and AUC, necessitating careful monitoring. fda.govfda.govmedicinesauthority.gov.mt
| Hepatic Impairment Severity (Child-Pugh Score) | Effect on Mean this compound AUC |
| Mild (A) | No significant effect |
| Mild-to-Moderate (≤ 10) | No effect observed in some studies fda.govfda.gov |
| Moderate (B) | 1.5-fold increase pfizer.com |
| Severe (> 10 or C) | Substantial increase |
Pharmacokinetics of this compound were not altered in patients with moderate-to-severe renal insufficiency as assessed by creatinine clearance. fda.govfda.govhres.capfizer.commedicinesauthority.gov.mt
Renal Insufficiency
Studies have investigated the pharmacokinetics of this compound in patients with renal insufficiency. In patients with moderate-to-severe renal insufficiency, assessed by creatinine clearance, the pharmacokinetics of this compound were not found to be altered. fda.govpfizer.comhpra.iefda.gov However, the pharmacokinetics of this compound have not been studied in patients with end-stage renal failure or those undergoing hemodialysis. hpra.iemedicinesauthority.gov.mt Therefore, caution is advised when treating patients with end-stage renal failure or those on hemodialysis. hpra.iemedicinesauthority.gov.mtpacehospital.com
Hyperprolactinemia is a common finding in patients with kidney failure. journals.ac.za In cases where patients with kidney failure have symptomatic or markedly elevated prolactin levels, investigation for a prolactinoma is important. journals.ac.za Both this compound and bromocriptine may be used in patients with kidney failure. journals.ac.za this compound is recommended due to its greater efficacy in normalizing prolactin levels and reducing tumor size. journals.ac.za Studies on the use of dopaminergic agonists, including this compound, in the chronic kidney disease population are scarce, but their use appears to be safe in those with a clinical indication. nih.gov
Therapeutic Applications and Clinical Research
Hyperprolactinemic Disorders
Prolactin-Secreting Pituitary Adenomas (Prolactinomas)
Resolution of Galactorrhea
Galactorrhea, the inappropriate production of breast milk, is often associated with elevated levels of prolactin (hyperprolactinemia). Cabergoline is highly effective in addressing this symptom by lowering prolactin levels. In patients with microprolactinomas, this compound has been shown to lead to the resolution of galactorrhea in a significant percentage of cases. One study reported resolution of galactorrhea in 86% of patients with microprolactinomas treated with this compound. medscape.com Dopamine agonists, including this compound, are considered first-line therapy for hyperprolactinemia and the associated symptom of galactorrhea. aafp.orgnih.gov this compound's superior efficacy in normalizing prolactin levels contributes to its effectiveness in resolving galactorrhea compared to other dopamine agonists like bromocriptine. aafp.orgnih.govnih.gov
Comparison with Other Dopamine Agonists (e.g., Bromocriptine, Quinagolide)
This compound is often compared to other dopamine agonists, particularly bromocriptine and quinagolide, in the treatment of hyperprolactinemia and other conditions. Studies indicate that this compound is generally more effective at lowering prolactin levels and shrinking tumor size in patients with prolactinomas compared to bromocriptine. aafp.orgnih.govmedscape.combioscientifica.com For instance, this compound normalized prolactin levels in 87.7% of women with hyperprolactinemic amenorrhea in one study, compared to 67.7% in the bromocriptine group. bioline.org.br Tumor shrinkage was also observed more frequently with this compound. aafp.orguniupo.it
This compound is also noted for having a better tolerability profile than bromocriptine, with fewer reported adverse effects, particularly gastrointestinal symptoms like nausea and vomiting. nih.govbioline.org.br This improved tolerability, coupled with its longer half-life allowing for less frequent dosing, contributes to better patient compliance. medscape.comnih.gov
Quinagolide, a non-ergot dopamine agonist, has shown similar efficacy to bromocriptine in normalizing prolactin levels and reducing tumor size in patients with prolactinoma. nih.govuniupo.it However, this compound is likely more effective than quinagolide in hyperprolactinemic patients. nih.gov While quinagolide may result in fewer side effects compared to bromocriptine, it is probably inferior to this compound in this regard. nih.govbioline.org.br
| Feature | This compound | Bromocriptine | Quinagolide |
| Efficacy (Prolactin Lowering) | Higher aafp.orgnih.govnih.govmedscape.combioscientifica.combioline.org.br | Lower compared to this compound aafp.orgnih.govnih.govmedscape.combioscientifica.combioline.org.br | Similar to Bromocriptine, likely less than this compound nih.govuniupo.it |
| Tumor Shrinkage | More effective aafp.orgmedscape.combioscientifica.comuniupo.it | Less effective compared to this compound aafp.orgmedscape.combioscientifica.comuniupo.it | Observed, but possibly less than this compound uniupo.it |
| Tolerability | Generally better medscape.comnih.govbioline.org.br | Higher incidence of adverse effects nih.govbioline.org.br | Better than Bromocriptine, possibly inferior to this compound nih.govbioline.org.br |
| Dosing Frequency | Less frequent (once or twice weekly) medscape.comnih.gov | More frequent (daily, often divided) nih.govbioline.org.br | Once daily nih.gov |
Parkinson's Disease
This compound is also utilized in the management of Parkinson's disease (PD), a neurodegenerative disorder characterized by dopamine deficiency. It is effective as both monotherapy in early stages and as adjunctive therapy with levodopa in more advanced stages. karger.comresearchgate.netnih.gov this compound, a tetracyclic ergoline derivative, acts as a dopamine agonist with a strong predominance for D2 receptors. karger.com
Monotherapy in Early Stages
In the early stages of Parkinson's disease, this compound monotherapy has been investigated as an initial treatment option. Studies have shown that starting treatment with this compound can delay the need for levodopa and potentially reduce the frequency of motor complications such as dyskinesia and fluctuations compared to initiating treatment with levodopa. karger.comnih.govajmc.com
A 3- to 5-year trial involving 412 patients with early PD compared this compound monotherapy with levodopa. The study found that the development of motor complications was significantly less frequent in patients treated with this compound (22%) compared to levodopa recipients (34%). nih.gov The relative risk of developing motor complications was more than 50% lower with this compound. nih.gov While both treatments improved motor disability, the mean improvement in UPDRS Factor III scores was higher in the levodopa group in one study, although the proportion of patients showing clinical improvement was similar between the two groups at one year. neurology.org
Adjunctive Therapy with Levodopa in Advanced Stages
For patients with advanced Parkinson's disease experiencing motor fluctuations, this compound is used as an adjunct to levodopa therapy. karger.comresearchgate.netnih.gov In this setting, this compound can help to prolong the "on" phases (periods of good motor function) and reduce the duration of "off" phases (periods of poor motor function). karger.com
Clinical studies involving patients with advanced PD and motor complications have demonstrated the efficacy of this compound as an add-on treatment. A phase III placebo-controlled study with 188 patients showed that this compound treatment led to a statistically significant decrease in levodopa dosage compared to placebo and improved scores on the Unified Parkinson's Disease Rating Scale (UPDRS) for activities of daily living. nih.gov A combined analysis of studies involving over 1500 patients found that this compound significantly decreased "off" time and levodopa dose requirements. nsj.org.sa
Impact on Motor Fluctuations and Levodopa Requirements
This compound's role as an adjunctive therapy in advanced PD is particularly focused on managing motor fluctuations and reducing the reliance on levodopa. By providing continuous dopaminergic stimulation, this compound helps to smooth out the motor response and minimize the fluctuations between "on" and "off" states. karger.comcochrane.org
Studies have consistently shown that the addition of this compound to a levodopa regimen results in a significant reduction in the amount of daily "off" time. nih.govnsj.org.sacochrane.org This reduction in "off" time is often accompanied by a decrease in the required daily dosage of levodopa. nih.govnsj.org.sacochrane.org For example, one study reported a significant decrease in levodopa dosage (18% vs 3% for placebo) in patients treated with this compound. nih.gov This levodopa-sparing effect is considered a potential advantage of this compound, as it may help to delay or reduce the intensity of levodopa-induced dyskinesias. nih.govoup.com
| Study Type | Patient Population | Key Findings | Citations |
| Monotherapy (Early PD) | De novo PD patients | Delayed need for levodopa, lower incidence of motor complications vs levodopa | karger.comnih.govajmc.com |
| Adjunctive Therapy (Advanced PD) | PD with motor complications | Reduced "off" time, decreased levodopa requirements, improved motor function | karger.comnih.govnsj.org.sacochrane.org |
| Comparison with Bromocriptine | Hyperprolactinemia, Advanced PD | Generally more effective and better tolerated | aafp.orgnih.govmedscape.combioline.org.br |
Other Investigational and Emerging Therapeutic Applications
Beyond its established uses in hyperprolactinemia and Parkinson's disease, this compound is being investigated for its potential therapeutic benefits in other conditions.
This compound is being studied in various pituitary disorders beyond prolactinomas, including non-functioning pituitary adenomas, Cushing's disease, and acromegaly. clinicaltrials.eu Research is exploring its potential to normalize hormone levels, reduce tumor size, and prevent tumor recurrence in these conditions. clinicaltrials.eu For instance, this compound has shown promise in treating Cushing's disease by potentially modulating ACTH release and reducing tumor size in some cases. elsevier.es
There is also research exploring the use of this compound in the treatment of certain types of breast cancer and as a preventive measure for ovarian hyperstimulation syndrome in fertility treatments. clinicaltrials.eu
Furthermore, this compound is being investigated as a potential treatment for chronic pain associated with endometriosis. childrenshospital.orgnih.gov The rationale behind this application is based on the hypothesis that this compound, as a dopamine receptor agonist, may inhibit angiogenesis, a process implicated in the development and maintenance of endometriosis lesions and associated pain. childrenshospital.orgnih.gov Pilot studies suggest that this compound may be an effective adjunct treatment for pelvic pain in adolescents and young women with surgically-proven endometriosis. childrenshospital.orgnih.gov
This compound has also been investigated for its role in managing Restless Legs Syndrome (RLS) symptoms, showing potential in reducing sensory and motor symptoms and improving sleep quality. pharmacoj.com
Cushing's Disease and ACTH-Secreting Pituitary Adenomas
Cushing's disease (CD) is a form of Cushing's syndrome caused by an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma. jcrpe.orgfrontiersin.org Transsphenoidal surgery is the primary treatment, but medical therapy is often required for patients who are not cured by surgery, are not surgical candidates, or experience recurrence. jcrpe.orgfrontiersin.org
This compound, a D2 dopamine receptor agonist, has shown effectiveness in treating prolactinomas, and the presence of D2 receptors in corticotroph tumors has led to its investigation in CD. elsevier.esresearchgate.net Functional D2 receptors have been demonstrated in approximately 60% of corticotroph pituitary tumors. jcrpe.org Short-term treatment with this compound has shown effectiveness in normalizing ACTH and cortisol secretion in a subset of these tumors. jcrpe.org
In a retrospective analysis of 30 patients with CD treated with this compound monotherapy, complete response (sustained normalization of urinary free cortisol) was achieved in 36.6% of patients within 3-6 months. researchgate.net After long-term therapy (mean 37 months), 30% of patients maintained a complete response. researchgate.net Another study reported that short-term treatment with this compound improved cortisol secretion in 50% of CD subjects, with complete normalization of urinary free cortisol in 36.6% of cases. jcrpe.org Long-term follow-up (mean 37 months) demonstrated sustained effectiveness in 30% of subjects, primarily those with persistent or recurrent CD. jcrpe.org One study found a 25% complete response to this compound monotherapy in 12 CD patients who had unsuccessful transsphenoidal surgery. jcrpe.org
A case report described the efficacy of this compound in treating Cushing's disease caused by an ACTH-secreting macroadenoma, where the adenoma showed a relatively high expression level of dopamine D2 receptor mRNA. nih.gov this compound treatment gradually reduced ACTH levels and led to shrinkage of the remnant pituitary mass. nih.gov
| Study Type | Number of Patients | Response Type | Percentage of Patients | Follow-up Duration |
| Retrospective Analysis researchgate.net | 30 | Complete Response | 36.6% | 3-6 months |
| Retrospective Analysis researchgate.net | 30 | Complete Response | 30% | Mean 37 months |
| Study by Godbout et al. jcrpe.org | Not specified | Improved Cortisol | 50% | Short-term |
| Study by Godbout et al. jcrpe.org | Not specified | UFC Normalization | 36.6% | Short-term |
| Study by Godbout et al. jcrpe.org | Not specified | Sustained Effectiveness | 30% | Mean 37 months |
| Study by Vilar et al. jcrpe.org | 12 | Complete Response | 25% | 6 months |
Acromegaly and GH-Secreting Pituitary Adenomas
Acromegaly is typically caused by a growth hormone (GH)-secreting pituitary adenoma, leading to excessive production of GH and insulin-like growth factor I (IGF-I). aem-sbem.comoup.com While somatostatin analogs (SSAs) are a common medical treatment, a significant proportion of patients remain resistant to SSA therapy. aem-sbem.comscielo.br Dopamine agonists have a role in the medical management of acromegaly, and this compound, a long-acting dopamine agonist, has been investigated for this application. nih.govresearchgate.net Dopamine agonists can inhibit GH overproduction in acromegaly patients, supported by in-vitro studies. aem-sbem.comscielo.br Both GH-producing adenomas and mixed PRL-GH-producing adenomas have binding sites for dopamine. aem-sbem.comscielo.br
A multicenter, prospective study evaluated the effect of long-term this compound administration in 64 patients with acromegaly. oup.comnih.gov Treatment with this compound suppressed plasma IGF-I below 300 micrograms/L in 39% of cases and between 300-450 micrograms/L in another 28%. oup.comnih.gov In patients with pretreatment plasma IGF-I concentrations less than 750 micrograms/L, IGF-I suppression below 300 micrograms/L was achieved in 53% of cases, and between 300-450 micrograms/L in another 32%. oup.comnih.gov For patients with pretreatment plasma IGF-I concentrations above 750 micrograms/L, the suppression rates were lower, with 17% achieving levels below 300 micrograms/L and 21% between 300-450 micrograms/L. oup.comnih.gov
In patients with GH-/PRL-cosecreting adenomas, 50% suppressed plasma IGF-I levels below 300 micrograms/L, and 31% achieved levels between 300-450 micrograms/L. oup.comnih.govresearchgate.net This contrasts with 35% and 27%, respectively, in patients with pure GH-secreting adenomas. oup.comnih.govresearchgate.net Similar results were observed concerning GH secretion. oup.comnih.govresearchgate.net Tumor shrinkage was demonstrated in 13 of 21 patients, with a mass reduction by more than half in 5 cases of GH-/PRL-cosecreting adenomas. oup.comnih.govresearchgate.net
The addition of this compound to SSA therapy in acromegaly patients resistant to SSA alone normalized IGF-I levels in a considerable number of patients with moderately elevated IGF-I, regardless of serum prolactin levels. aem-sbem.com In one study, IGF-I levels reduced by 32% compared with baseline, and IGF-I normalization was achieved in 42% of patients (21/50). aem-sbem.com A substantial reduction in tumor size was also observed with combined therapy. aem-sbem.com A meta-analysis revealed that this compound monotherapy normalized IGF-I levels in over one-third of acromegaly patients. scielo.br
| Patient Group (Multicenter Study oup.comnih.gov) | IGF-I < 300 µg/L | IGF-I 300-450 µg/L |
| All Acromegaly Patients (n=64) | 39% | 28% |
| Pretreatment IGF-I < 750 µg/L | 53% | 32% |
| Pretreatment IGF-I > 750 µg/L | 17% | 21% |
| GH-/PRL-Cosecreting Adenomas (n=16) | 50% | 31% |
| Pure GH-Secreting Adenomas | 35% | 27% |
| Study Type | Intervention | IGF-I Normalization | Tumor Size Reduction |
| Study on SSA-Resistant Patients aem-sbem.com | This compound add-on to SSA | 42% (21/50) | Substantial |
| Meta-analysis scielo.br | This compound monotherapy | >33% | Not specified |
Restless Legs Syndrome
Restless Legs Syndrome (RLS) is a neurological disorder characterized by an irresistible urge to move the legs, often accompanied by uncomfortable sensations. Dopaminergic agents are a common treatment approach for RLS. karger.com this compound, a long-acting dopamine agonist, has been investigated for its efficacy in treating RLS. karger.comfrontiersin.orgjwatch.org
A double-blind, placebo-controlled study involving 85 patients with moderate to severe idiopathic RLS evaluated this compound over 5 weeks, followed by a 47-week open-label phase. jwatch.org During the randomized period, this compound treatment resulted in significant improvement of RLS symptoms at bedtime and during the night compared to placebo, and patients reported improved satisfaction with sleep. jwatch.org Benefit continued in the open-label phase, with 81% of subjects reporting no or only mild symptoms. jwatch.org
A network meta-analysis of RLS treatments found that this compound showed the greatest efficacy in relieving RLS symptoms compared to other evaluated drugs, with a mean difference in International RLS Study Group Severity Scale (IRLS) scores of -11.98 (95% CI -16.19 to -7.78) compared to placebo. frontiersin.org
A placebo-controlled study with polysomnography (CATOR study) in patients with moderate to severe RLS found that single-evening this compound was superior to placebo in improving the periodic leg movements during sleep arousal index (PLMS-AI), sleep efficiency, PLMS index, PLM index, and total sleep time. nih.gov Improvements in IRLS total score and other severity and quality of life measures were also greater with this compound compared to placebo. nih.gov
| Study Type | Number of Patients | Comparison | Key Findings |
| Placebo-Controlled Study jwatch.org | 85 | Placebo | Significant improvement in RLS symptoms and sleep satisfaction. |
| Network Meta-Analysis frontiersin.org | Not specified | Various | Showed greatest efficacy in reducing IRLS scores (MD -11.98 vs placebo). |
| Placebo-Controlled PSG Study (CATOR) nih.gov | 43 (40 evaluated) | Placebo | Superior to placebo in improving PLMS-AI, sleep efficiency, and other PSG measures; greater improvement in IRLS and QoL scores. |
Breast Cancer
Research has explored the potential role of this compound in breast cancer, particularly in the context of chemoprevention. Studies in animal models have investigated the effect of this compound on breast cancer incidence and latency. csic.esresearchgate.netnih.gov
A research group found that this compound increased latency and decreased breast cancer incidence in a mouse model deficient in Brca1/P53. csic.esresearchgate.net A single dose was sufficient to potentiate post-lactational gland involution and decrease epithelial proliferation, leading to less tumor susceptibility in mice. csic.es These results suggest that this compound could be a potential protective agent for breast cancer prevention. csic.es The mechanism may involve enhancing the protective effect of pregnancy against breast cancer by potentiating post-lactational involution. researchgate.netnih.gov Histological analysis in mice showed accelerated post-lactational involution with increased apoptosis and altered gene expression. researchgate.netnih.gov Long-term follow-up revealed decreased epithelial proliferation and ductal epithelial area. researchgate.net
In a study involving ten postmenopausal patients with metastatic breast cancer, this compound significantly suppressed plasma prolactin levels. karger.com In a small Phase II trial involving 20 breast cancer patients, this compound was used to inhibit pituitary prolactin secretion, and two patients experienced extended disease control. frontiersin.org
| Study Type | Model/Patients | Key Findings |
| Mouse Model (Brca1/P53-deficient) csic.esresearchgate.netnih.gov | Mice | Increased latency and decreased breast cancer incidence; potentiated post-lactational involution; decreased epithelial proliferation. |
| Study in Metastatic Breast Cancer Patients karger.com | 10 postmenopausal patients | Significant suppression of plasma prolactin levels. |
| Small Phase II Trial frontiersin.org | 20 breast cancer patients | Two patients experienced extended disease control with this compound. |
Ovarian Hyperstimulation Syndrome Prevention
Ovarian Hyperstimulation Syndrome (OHSS) is a complication that can arise during assisted reproductive technologies (ART) due to controlled ovarian stimulation. ijifm.com The use of dopamine receptor agonists, such as this compound, has been suggested for the prevention of OHSS. ijifm.com
A systematic review and meta-analysis of randomized trials comparing this compound to no treatment in IVF/ICSI cycles found a statistically significant reduction in the incidence of OHSS in the this compound group. nih.gov Data from seven studies involving 858 women showed that this compound reduced the risk of moderate-severe OHSS (RR 0.38, 95% CI 0.29-0.51). nih.gov this compound is effective in reducing moderate to severe OHSS, particularly when compared to placebo, making it a suitable option for high-risk women. researchgate.net
However, some studies suggest that this compound may be less effective than other treatments, such as calcium gluconate, in preventing mild OHSS. researchgate.net A randomized, double-blind, parallel group study evaluating this compound versus placebo in high-risk patients undergoing IVF-ICSI found no significant difference in the incidence rate of moderate OHSS between the groups. ijifm.com
| Study Type | Comparison | Number of Studies | Number of Women | Key Findings |
| Systematic Review and Meta-analysis nih.gov | This compound vs. No Treatment | 7 | 858 | Statistically significant reduction in the incidence of OHSS, specifically moderate-severe OHSS (RR 0.38, 95% CI 0.29-0.51). nih.gov |
| Systematic Review and Meta-analysis researchgate.net | This compound vs. Placebo | Not specified | 919 | Effective in reducing moderate to severe OHSS, especially compared to placebo. researchgate.net |
| Randomized, Double-Blind, Parallel Group Study ijifm.com | This compound vs. Placebo | 1 | 110 | No significant difference in the incidence rate of moderate OHSS. ijifm.com |
Endometriosis and Related Pain/Infertility
Endometriosis is a condition where tissue similar to the uterine lining grows outside the uterus, often causing pain and infertility. worldscientific.com Research is exploring the potential of this compound in managing endometriosis-associated pain and improving fertility in affected women. worldscientific.comchildrenscolorado.org
Recent studies suggest that this compound may reduce pelvic pain and endometrioma size in women with endometriosis. worldscientific.com A study aimed to evaluate the effectiveness of this compound on pain symptoms associated with endometriosis in women experiencing infertility. this compound, by reducing prolactin levels, may help decrease the growth of endometrial tissue and alleviate pain.
A prospective comparative study investigated this compound versus dienogest in women with symptomatic endometrioma. worldscientific.com While the study found a significant decrease in pain score with dienogest and a percentage reduction in endometrioma size, it also referenced previous studies. worldscientific.com A prospective randomized study comparing this compound to an LHRH agonist in women with endometriosis reported a greater than 25% reduction in endometrioma size in 64.1% of women receiving this compound compared to 21% of those receiving LHRH agonists. longdom.org This study also indicated that this compound and the LHRH agonist were similar in reducing severe pain. longdom.org Another randomized controlled trial on women with pelvic pain due to endometriosis showed that this compound and medroxyprogesterone acetate were equally effective in decreasing chronic pelvic pain. worldscientific.com
A parallel-design randomized clinical trial comparing this compound to dydrogesterone in infertile women with endometriosis found that this compound resulted in significant pain reduction over a short period and also promoted pregnancies. longdom.org The reduction in visual analog scale score of pain in those given this compound was three times that in those given dydrogesterone. longdom.org
| Study Type | Comparison | Patient Population | Key Findings |
| Study on Pain and Fertility | This compound vs. Placebo | Women with endometriosis and infertility | Aims to evaluate effectiveness on pain and fertility. |
| Prospective Comparative Study worldscientific.com | This compound vs. Dienogest | Women with symptomatic endometrioma | Dienogest showed significant pain reduction and endometrioma size decrease; references studies showing this compound reduces pain and endometrioma size. |
| Prospective Randomized Study longdom.org | This compound vs. LHRH agonist | Women with endometriosis | Greater reduction in endometrioma size with this compound (64.1% vs 21%); similar efficacy in reducing severe pain. |
| Randomized Controlled Trial worldscientific.com | This compound vs. Medroxyprogesterone acetate | Women with pelvic pain due to endometriosis | Equally effective in decreasing chronic pelvic pain. |
| Parallel-Design Randomized Clinical Trial longdom.org | This compound vs. Dydrogesterone | Infertile women with endometriosis | Significant pain reduction and promoted pregnancies with this compound; greater pain reduction compared to dydrogesterone. |
| Patient Preference Clinical Trial ijrcog.org | Medical (incl. This compound) vs. Surgical | 20 infertile women with endometriosis | Medical management preferred; all participants on medical management had pain reduction and most had cyst size reduction; pregnancy occurred in 14.3% of those on medication. |
Migraine Prevention
Research suggests that dopamine agonists, including this compound, may exert beneficial effects in the headache phase of migraine, and studies in rodents propose that D2 receptor activation could influence central nociceptive sensitization in chronic migraine. plos.orgplos.orgnih.gov The findings from this pilot study support further investigation into the use of this compound for migraine prevention, particularly in episodic migraine. nih.govplos.orgmedrxiv.org
Below is a summary of the findings from the pilot study on this compound for migraine prevention:
| Study Group | Intervention | Baseline Monthly Migraine Days (Mean ± SD) | Reduction in Monthly Migraine Days (Mean ± SE) | p-value |
| Entire Group | This compound | 13.6 ± 4.1 | Not specified as overall difference not significant | - |
| Entire Group | Placebo | 14.0 ± 5.3 | Not specified as overall difference not significant | - |
| Episodic Migraine | This compound | - | -5.4 ± 1.3 | 0.04 |
| Episodic Migraine | Placebo | - | -1.8 ± 0.9 | |
| Chronic Migraine | This compound | - | Not significantly different | 0.6 |
| Chronic Migraine | Placebo | - | Not significantly different |
Nelson's Syndrome
Nelson's syndrome is a condition that can develop following bilateral adrenalectomy for Cushing's disease, characterized by an enlarging pituitary tumor and elevated levels of adrenocorticotropic hormone (ACTH). patient.infoscielo.brendocrine-abstracts.org While surgical intervention and radiotherapy are primary management strategies, medical treatments have shown inconsistent results. patient.infoscielo.br
Case reports have indicated that this compound, a dopamine receptor antagonist, may induce remission in Nelson's syndrome. patient.info Its potential efficacy is attributed to its higher affinity and specificity for D2 receptors, which are expressed in corticotroph pituitary tumors, and its longer half-life compared to other dopamine agonists like bromocriptine. psu.edumdpi.com
Studies have reported cases where this compound treatment led to a decline in ACTH levels and resolution or stabilization of pituitary adenomas in patients with Nelson's syndrome. patient.infoscielo.brendocrine-abstracts.orgnih.govkarger.comnih.gov For instance, one case report described a young man with Nelson's syndrome who showed normalized plasma ACTH levels and disappearance of a pituitary microadenoma after one year of this compound treatment. nih.gov Another case reported clinical and biochemical stabilization of Nelson's syndrome with long-term low-dose this compound treatment in a woman over a 6-year period, with a dramatic decrease in ACTH levels and a stable residual pituitary tumor. nih.gov Complete remission of a pituitary macroadenoma in a patient with Nelson's syndrome after long-term this compound treatment has also been reported. karger.com The favorable clinical response to this compound in this setting may be explained by the specific expression pattern of D2 receptors in some corticotroph tumors. endocrine-abstracts.org
While medical therapies for Nelson's syndrome have generally not shown consistent effectiveness, case reports highlight this compound as a potential therapeutic alternative capable of inducing remission or achieving clinical and biochemical stabilization. patient.infoendocrine-abstracts.orgnih.govnih.gov
Methamphetamine Dependence
This compound, possessing dopaminergic properties, has been explored as a potential treatment for stimulant dependence, including methamphetamine use disorder. researchgate.netresearchgate.netnih.gov It has been hypothesized that dopamine agonists like this compound may help reduce cravings and the risk of relapse by influencing dopaminergic transmission in the mesolimbic pathway. lenus.ie
A randomized, double-blind, placebo-controlled clinical trial investigated the effects of this compound on abstaining from methamphetamine in individuals with methamphetamine use disorder. Sixty male subjects were randomized to receive either this compound or placebo over a 12-week follow-up period. researchgate.netresearchgate.netnih.gov The study compared urine test results for methamphetamine use between the two groups. researchgate.netnih.gov
The findings of this study indicated an association between treatment with this compound and remaining sober from methamphetamine. researchgate.netresearchgate.net While the placebo group showed a trend of increasing positive urine tests that did not reach statistical significance by the end of the study, the this compound group maintained a significantly higher number of negative urine tests from early stages (weeks 7-8) through the end of the study (p = 0.043). researchgate.netresearchgate.netnih.gov At the end of the 12-week follow-up, 70% of subjects in the this compound group had a negative urine test for methamphetamine compared to 43% in the placebo group. researchgate.net
This study concluded that this compound demonstrated an effect in reducing methamphetamine use. researchgate.netresearchgate.net The long-acting property of this compound makes it a candidate for the treatment of methamphetamine use, and further studies are suggested to expand the understanding of this effect. researchgate.net However, it is worth noting that a systematic review of reviews on stimulant use disorder treatments found that the literature does not consistently support the use of dopamine agonists for this purpose, although few studies specifically focused on methamphetamine use. lenus.ie
Below is a summary of the urine test results for methamphetamine use from the clinical trial:
| Treatment Group | Percentage of Negative Urine Tests at 12 Weeks |
| This compound | 70% |
| Placebo | 43% |
Adverse Effects and Safety Profile in Academic Research
Common Adverse Events
Common adverse events reported in academic studies frequently involve the gastrointestinal, neurological, and psychiatric systems pharmacoj.comdrugs.comnih.govplos.orgnih.gov. These effects are often mild to moderate in severity and may diminish with continued treatment or dose adjustment drugs.comnih.govnih.gov.
Gastrointestinal Disturbances (Nausea, Constipation, Dry Mouth, Dyspepsia, Vomiting)
Gastrointestinal side effects are among the most frequently reported with cabergoline pharmacoj.comdrugs.comnih.govplos.orgnih.govdroracle.aipatsnap.com. Studies have indicated high frequencies of these effects, particularly in patients treated for Parkinson's disease pharmacoj.com. Nausea, constipation, and dry mouth are commonly observed pharmacoj.comdrugs.com. Other reported gastrointestinal disturbances include dyspepsia, vomiting, abdominal pain, gastritis, diarrhea, and flatulence drugs.complos.orgdroracle.aipatsnap.com. In one study of patients with Parkinson's disease, 53% reported gastrointestinal side effects, with nausea (30%), constipation (22%), and dry mouth (10%) being very frequent pharmacoj.com.
Neurological and Psychiatric Effects (Headache, Dizziness, Fatigue, Drowsiness, Syncope, Hallucinations, Dyskinesia, Confusion, Impulse Control Disorders)
This compound can induce a variety of neurological and psychiatric effects nih.govpharmacoj.comnih.govdroracle.airesearchgate.netnih.govmedicinesauthority.gov.mtmpa.sepfizer.com. Common neurological adverse events include headache, dizziness, fatigue, and drowsiness drugs.complos.orgdroracle.aipatsnap.commedicinesauthority.gov.mt. Syncope has also been reported nih.govkarger.com. Psychiatric effects can encompass hallucinations, confusion, and impulse control disorders (ICDs) nih.govmpa.sehres.ca. ICDs, such as pathological gambling, hypersexuality, compulsive shopping, and compulsive eating, have been linked to dopamine agonists like this compound, potentially mediated by effects on dopamine reward pathways hres.cahra.nhs.ukbioscientifica.com. While some research suggests this compound may predispose patients to depression and anxiety, other studies in patients with prolactinoma have found no significant difference in the prevalence of these disorders compared to control groups hra.nhs.ukhamidiyemedj.comhamidiyemedj.com. This compound has been associated with somnolence, and sudden sleep onset episodes have been reported, particularly in patients with Parkinson's disease medicinesauthority.gov.mtmpa.sepfizer.comhres.ca.
Cardiovascular Effects (Hypotension, Peripheral Edema, Palpitations, Arrhythmias, Angina Pectoris, Vasoconstriction)
Cardiovascular effects of this compound include hypotension, particularly orthostatic hypotension nih.govdrugs.comnih.govhres.ca. Peripheral edema has been commonly reported in studies involving patients with Parkinson's disease treated with higher doses hres.ca. Other potential cardiovascular effects mentioned in research include palpitations, arrhythmias, angina pectoris, and vasoconstriction nih.govnih.govnih.govmedicinesauthority.gov.mthamidiyemedj.comresearchgate.net. A mild hypotensive effect, with slight decreases in mean systolic and diastolic blood pressures, has been observed in some long-term studies nih.gov.
Serious and Long-Term Adverse Events
While generally well-tolerated, especially at lower doses, this compound has been associated with serious and long-term adverse events, particularly with chronic use and higher cumulative doses nih.govnih.govoup.com.
Fibrotic Reactions (Pulmonary, Pericardial, Cardiac Valvular, Retroperitoneal Fibrosis)
Ergot-derived dopamine agonists, including this compound, have been linked to fibrotic reactions nih.govnih.govoup.comfishersci.sefrontiersin.org. These can affect various tissues, leading to pulmonary fibrosis, pericardial fibrosis, cardiac valvular fibrosis, and retroperitoneal fibrosis nih.govnih.govoup.comfishersci.sefrontiersin.org. Cardiac valvular fibrosis, particularly affecting the tricuspid, mitral, and aortic valves, has been a significant concern, especially with the higher doses used in Parkinson's disease drugs.comfrontiersin.orgnih.govmedcraveonline.comoup.com. Studies in patients with Parkinson's disease have shown an increased risk of valvular regurgitation and fibrotic changes in heart valves drugs.comnih.govmedcraveonline.com. While the risk appears lower at the doses typically used for hyperprolactinemia, some studies suggest a possible association, particularly with tricuspid regurgitation, although the clinical significance of this finding is debated and warrants further investigation drugs.comnih.govnih.govoup.comresearchgate.netuliege.be. Research evaluating pulmonary side effects in patients treated for prolactinoma with this compound has generally not shown an increased risk of pleuropulmonary fibrosis at typical doses researchgate.netcapes.gov.brviamedica.pl. Mediastinal fibrosis has also been reported in rare cases frontiersin.org.
Sudden Onset of Sleep
Sudden onset of sleep is a less common but serious adverse event associated with dopamine agonists, including this compound, particularly in patients with Parkinson's disease nih.govmedicinesauthority.gov.mtmpa.sepfizer.comhres.ca. This can occur during daily activities, sometimes without warning signs, posing a risk to the patient and others mpa.sepfizer.com. Patients treated with this compound who experience somnolence or sudden sleep episodes are advised to refrain from activities requiring alertness, such as driving or operating machinery, until these symptoms resolve medicinesauthority.gov.mtmpa.sepfizer.com.
Safety in Specific Populations
The safety and tolerability of this compound can vary depending on patient demographics and underlying health conditions. Academic research has provided insights into its use in pediatric, geriatric, pregnant, lactating, and hepatically impaired individuals.
Pediatric Patients
The safety and effectiveness of this compound in pediatric patients have not been definitively established. hres.camayoclinic.orgfda.govdrugs.commedicinesauthority.gov.mtmpa.se Limited data exist regarding the experience of treating hyperprolactinemia in children and adolescents. jcrpe.org While some studies in pediatric and adolescent patients with prolactinomas have shown that this compound effectively lowers prolactin levels and may reduce tumor mass, potential adverse effects, including mental disorders and behavioral problems, have been reported even at low doses. nih.gov In a review of 21 cases of hyperprolactinemia in children and adolescents, both bromocriptine and this compound appeared equally efficacious in lowering prolactin levels, and no significant side effects were observed, except for mild nausea and drowsiness in some patients. jcrpe.org However, the experience with dopamine agonist therapy in children is limited compared to adults. jcrpe.org Careful individual dose adjustments are required in this population to enable therapy adherence. nih.gov
Pregnancy and Lactation
The use of this compound during pregnancy requires careful consideration. While animal reproduction studies have not consistently shown evidence of fetal damage, adequate and well-controlled studies in pregnant women are lacking. fda.govdrugs.com this compound is classified as FDA pregnancy category B, meaning animal studies have not demonstrated a risk to the fetus, but human data are insufficient. drugs.com Some studies suggest that fetal exposure to this compound in early pregnancy does not appear to increase the risk of miscarriage or fetal malformation. endocrine-abstracts.orgresearchgate.netscielo.br An observational study following pregnancy outcomes over 12 years, including 256 pregnancies with this compound exposure, reported major congenital malformations or abortion in a percentage comparable to or slightly lower than the general population. drugs.com Neonatal abnormalities were recorded in a small percentage of infants, with no apparent pattern in type or severity. drugs.comresearchgate.net Despite these findings, due to its long half-life, some recommend discontinuing this compound one month prior to intended conception. drugs.com this compound is generally not recommended for use during breastfeeding as it suppresses lactation. nih.gov The prolactin-lowering action of this compound suggests it will interfere with lactation. fda.gov Serious adverse events, including hypertension, myocardial infarction, seizures, stroke, or psychiatric disorders, have been reported in postpartum women treated with this compound for lactation inhibition, although these are uncommon. medicinesauthority.gov.mtnih.govpfizer.com Two systematic reviews found this compound generally well-tolerated for lactation suppression, with dizziness, headache, nausea, and vomiting occurring occasionally. nih.gov However, the U.S. Food and Drug Administration (FDA) does not indicate this compound for the inhibition or suppression of physiologic lactation due to risks associated with the similar drug bromocriptine when used for this purpose. nih.govlacted.orgfda.gov
Patients with Hepatic Impairment
This compound is extensively metabolized by the liver, necessitating caution and careful monitoring when administering it to patients with hepatic impairment. hres.camedicinesauthority.gov.mtfda.govpacehospital.comguidetopharmacology.org The use of this compound is not recommended in patients with severe hepatic impairment (Child-Pugh Class C). medicinesauthority.gov.mtmpa.sepfizer.com In patients with moderate hepatic impairment (Child-Pugh Class B), increased monitoring is recommended. pfizer.com Studies have shown an increase in mean this compound AUC (area under the plasma concentration-time curve) in patients with severe hepatic insufficiency compared to those with mild impairment or normal hepatic function after a single 1 mg dose. medicinesauthority.gov.mtmpa.sepfizer.comcbg-meb.nl While no specific dosage adjustments are required for patients with mild to moderate hepatic impairment, the drug should be used cautiously in this population. pacehospital.com Regular liver function tests are mandatory during treatment as this compound can potentially elevate liver enzymes.
Monitoring Strategies for Adverse Events
Comprehensive monitoring strategies are essential to identify and manage potential adverse events associated with this compound therapy. Monitoring should be tailored to the individual patient, considering their medical history, concomitant medications, and the duration of treatment.
Regular monitoring of serum prolactin levels is advised to determine the lowest effective dosage and assess treatment response. hres.ca Once a normal serum prolactin level is achieved and maintained, periodic monitoring is still recommended to determine if or when treatment reinstitution is necessary. hres.ca
Given the potential for cardiovascular effects, particularly cardiac valvulopathy, especially with long-term or high-dose use, a cardiovascular evaluation, including an echocardiogram, is recommended before initiating long-term treatment to assess for pre-existing valvular disease. fda.govpacehospital.compfizer.comhealthline.com If fibrotic valvular disease is detected, this compound treatment should not be initiated. fda.govpfizer.com Repeat echocardiography may be considered periodically during long-term therapy, particularly at higher doses. derbyshiremedicinesmanagement.nhs.uk Attention should be paid to signs and symptoms of cardiac failure or valvulopathy, such as shortness of breath, difficulty breathing when lying down, cough, chest pain, or swelling of the extremities. pacehospital.comhealthline.comderbyshiremedicinesmanagement.nhs.ukmedlineplus.gov
Monitoring for fibrotic and serosal inflammatory disorders, such as pleural effusion, pulmonary fibrosis, pericarditis, and retroperitoneal fibrosis, is also important, especially with long-term administration. fda.govfda.govpacehospital.comcbg-meb.nl Symptoms to monitor for include dyspnea, persistent cough, chest pain, pain in the back, side, or groin, or abdominal masses or tenderness. pacehospital.comhealthline.commedlineplus.gov Erythrocyte sedimentation rate (ESR) has been found to be abnormally increased in association with pleural effusion/fibrosis, and chest x-ray examination may be recommended in cases of unexplained ESR increases. hres.capacehospital.com
Patients should be monitored for neurological and psychiatric adverse events, including somnolence, dizziness, headache, nervousness, insomnia, anxiety, aggression, psychotic disorder, delusions, and impulse control disorders such as pathological gambling, increased libido, hypersexuality, compulsive spending or buying, and compulsive eating. hres.capacehospital.commedlineplus.govdroracle.aiclinicaltrials.eu Patients should be warned about the potential for somnolence and sudden sleep onset episodes. hres.capacehospital.com
Monitoring for gastrointestinal adverse effects such as nausea, vomiting, heartburn, constipation, flatulence, and anorexia is also part of clinical practice. hres.capacehospital.commedlineplus.govdroracle.ai
In patients with hepatic impairment, careful monitoring of liver function tests is necessary due to the drug's metabolism. pacehospital.compfizer.com
Regular monitoring of blood pressure is advised, particularly during the initial days of administration, due to the potential for orthostatic hypotension. pfizer.comfda.govpfizer.comcbg-meb.nl
| Population | Key Safety Considerations | Monitoring Recommendations |
| Pediatric Patients | Safety and effectiveness not established. Potential for mental/behavioral issues. Limited data. | Careful individual dose adjustments. Monitor for mental disorders and behavioral problems. |
| Geriatric Patients | Limited data, but no special risk indicated. Increased likelihood of age-related organ dysfunction. | Caution in dose selection, potentially starting low. Monitor for adverse events, considering comorbidities. |
| Pregnancy | FDA Category B. Studies suggest no increased risk of miscarriage/malformation with early exposure, but data are limited. | Discontinuation one month prior to intended conception often recommended. Monitor pregnancy outcomes if exposure occurs. |
| Lactation | Suppresses lactation. Not indicated for this use by FDA due to potential risks (though some studies suggest tolerability). | Generally not recommended. Monitor for potential adverse effects if used off-label for lactation suppression. |
| Patients with Hepatic Impairment | Extensively metabolized by liver. Not recommended in severe impairment. Increased AUC in severe impairment. | Caution and careful monitoring. Increased monitoring in moderate impairment. Regular liver function tests. Not recommended in severe impairment (Child-Pugh C). |
Note: This table summarizes key points from the text and is not exhaustive.
Drug Interactions and Concomitant Therapies
Pharmacodynamic Interactions
Pharmacodynamic interactions occur when the effects of cabergoline are altered by other drugs acting on the same or different physiological systems. Given this compound's primary mechanism of action as a dopamine agonist, co-administration with dopamine antagonists can reduce its therapeutic efficacy pfizer.commedscape.commims.com. Examples of dopamine antagonists include antipsychotics such as phenothiazines, butyrophenones, thioxanthenes, and the antiemetic metoclopramide pfizer.commedscape.commims.com. These agents counteract the effects of this compound by blocking dopamine receptors medscape.commims.com.
This compound, being an ergot derivative, may also possess vasoconstrictive properties mims.comhres.ca. Concomitant use with other vasoconstrictors or antihypertensive agents requires caution due to the potential for additive effects, which could lead to excessive hypotension or increased vasoconstricting activities mims.comhres.cadrugbank.compdr.net. Specific examples of drugs that may increase the vasoconstricting activities when combined with this compound include certain beta-blockers like atenolol and alprenolol, as well as other agents like atomoxetine, antipyrine, antrafenine, arformoterol, benzphetamine, benorilate, benoxaprofen, acetylsalicylic acid, aceclofenac, and acemetacin drugbank.com. Combining this compound with agents like dexfenfluramine, midodrine, propylhexedrine, pseudoephedrine, phendimetrazine, phentermine, phenylephrine, rizatriptan, and yohimbine may lead to additive vasospasm and an increased risk of hypertension medscape.com. Conversely, this compound may decrease the vasodilatory effects of nitroglycerin medscape.commedtigo.com.
Pharmacokinetic Interactions
Pharmacokinetic interactions involve alterations in the absorption, distribution, metabolism, or excretion of this compound by other drugs. While this compound is extensively metabolized in the liver, primarily through hydrolysis, cytochrome P450 (CYP450)-mediated metabolism is considered minimal drugsporphyria.netnih.govbccancer.bc.ca. Despite minimal CYP450 metabolism, some interactions involving these enzymes have been suggested. For instance, clarithromycin, a macrolide antibiotic, has been shown to increase the plasma concentration of this compound, potentially through the inhibition of both P-glycoprotein and CYP3A4 drugsporphyria.net. Similarly, itraconazole, a known CYP3A4 inhibitor, has been suggested to inhibit this compound metabolism via CYP3A4 drugsporphyria.net. Grapefruit juice, a moderate inhibitor of CYP3A4, has also been shown to increase this compound plasma concentration, further indicating a contribution of CYP3A4 to its metabolism drugsporphyria.net.
Conversely, some drugs may increase the metabolism of this compound, such as alpelisib and apremilast drugbank.com. Drugs that can increase the serum concentration of this compound include astemizole, asunaprevir, ambrisentan, arsenic trioxide, apixaban, and acetazolamide drugbank.com. Although CYP450 metabolism is minimal, caution is advised when co-administering this compound with drugs metabolized mainly by CYP450 enzymes, such as erythromycin and ketoconazole, as increased plasma levels of this compound may occur wikipedia.orgmims.com. However, this compound is not listed as an inducer or inhibitor of any major CYP enzymes drugsporphyria.net.
Resistance to Cabergoline Therapy
Definition of Resistance
Defining resistance to dopamine agonists, including cabergoline, has been a subject of some variation in clinical literature. Generally, this compound resistance is defined as the failure to achieve normalization of serum prolactin (PRL) levels and/or a significant reduction in tumor size after treatment with a sufficient dose of this compound for a specified duration. elsevier.esthieme-connect.comoup.comfrontiersin.org
Specific criteria for defining resistance can vary across studies. Some definitions consider biochemical resistance as the failure to normalize PRL levels with a this compound dose up to 2 mg/week (or 3 mg/week in cases where lower doses were not tested) for at least 3 months. elsevier.es Morphological resistance is often defined as the failure to achieve at least a 50% reduction in the major tumor diameter under the same dose and duration criteria. elsevier.esthieme-connect.com Other definitions suggest a failure to normalize PRL despite doses exceeding 3 mg per week, with some authors using 4 mg as a cutoff point. researchgate.net A widely accepted definition in the context of prolactinomas is the failure to achieve normal PRL levels and a ≥50% reduction in tumor size at maximally tolerated doses. nih.gov
Prevalence of Resistance
Estimates of this compound resistance in patients with prolactinomas range from approximately 10% to 15% in terms of failure to normalize PRL levels and/or reduce tumor size by at least 50%. thieme-connect.come-enm.org Some studies report a prevalence of around 10% for this compound resistance. nih.gov In a multicenter study, the prevalence of resistance to this compound, defined as failure to normalize prolactin levels at or above a cutoff dose for at least six months, was reported as 3.4% among centers. karger.comuliege.be Another study identified a prevalence of this compound resistance around 2.5% in their series. endocrine-abstracts.org
Interactive Table 1: Reported Prevalence of this compound Resistance
| Study/Source | Definition of Resistance | Prevalence Estimate |
| General Literature | Failure to normalize PRL and/or ≥50% tumor size reduction at maximally tolerated doses | 10-15% thieme-connect.come-enm.org |
| General Literature | Failure to normalize PRL and/or ≥50% tumor size reduction | ~10% nih.gov |
| Multicenter Study | Failure to normalize PRL at or above cutoff dose for ≥ 6 months | 3.4% karger.comuliege.be |
| Single Center Study | Failure to normalize PRL and/or ≥50% tumor size reduction under maximally tolerated doses (≥3mg/week this compound) | ~2.5% endocrine-abstracts.org |
| Study based on CAB dose up to 2mg/week | Failure to normalize PRL (biochemical) or reduce tumor size by ≥50% (morphological) with CAB dose up to 2mg/week for ≥3 months | Varied patterns observed (isolated MR, combined MR+BR) elsevier.es |
Factors that may be associated with a higher risk of this compound resistance include male gender, larger tumor size, and tumor invasiveness. researchgate.netendocrine-abstracts.org
Molecular Mechanisms of Resistance
The molecular mechanisms underlying this compound resistance are not fully understood but are thought to involve alterations in the dopamine D2 receptor (D2R) and downstream signaling pathways. thieme-connect.comnih.gov
Decreased Dopamine D2 Receptor Expression and Activity
A primary mechanism implicated in this compound resistance is decreased expression and/or activity of the dopamine D2 receptor. thieme-connect.comresearchgate.netresearchgate.net this compound exerts its therapeutic effects primarily by binding to and activating D2 receptors on prolactin-secreting cells, inhibiting prolactin synthesis and release, and suppressing tumor cell proliferation. thieme-connect.comfrontiersin.org Reduced levels of functional D2 receptors on the cell surface can diminish the effectiveness of this compound. oup.comresearchgate.net Studies have shown that lower D2 receptor gene transcription and reduced receptor activity are associated with resistance. thieme-connect.com Decreased D2 receptor expression levels can lead to the failure of dopamine agonists to inhibit prolactin and contribute to resistance in prolactinomas. researchgate.net
Modifications in D2 Receptor Subtypes (D2S, D2L)
The dopamine D2 receptor exists in two main isoforms generated by alternative splicing: the short isoform (D2S) and the long isoform (D2L). nih.govfrontiersin.org These isoforms differ in the presence or absence of 29 amino acids in the third intracellular loop and may have distinct signaling properties. nih.govnih.gov While both isoforms can transduce dopamine signals, their relative expression levels and functional coupling to downstream pathways may play a role in this compound resistance. plos.orgoup.com
Some research suggests that a lower messenger RNA expression of D2S and a lower D2S/D2L ratio may be confirmed in dopamine agonist-resistant pituitary tumors. nih.govfrontiersin.org Conversely, one study surprisingly correlated resistance to dopamine agonists in prolactinomas with reduced messenger expression of the D2L receptor subtype. frontiersin.org The D2S isoform may play a more pivotal role in the control of hormone secretion and cell growth inhibition, and a lack of D2S expression or an increased D2L/D2S ratio might contribute to dopamine agonist resistance. oup.com
Interactive Table 2: D2 Receptor Isoform Expression in Dopamine Agonist Resistance
| D2 Receptor Isoform | Observed Expression in Resistant Tumors | Potential Role in Resistance |
| D2S | Lower mRNA expression nih.govfrontiersin.org | May be crucial for hormone inhibition and growth suppression; decreased expression contributes to resistance. oup.com |
| D2L | Increased D2L/D2S ratio nih.govfrontiersin.org or reduced mRNA expression frontiersin.org | May have different signaling properties affecting responsiveness. plos.orgoup.com |
Lowered Inhibitory G Protein Expression
Dopamine D2 receptors are G protein-coupled receptors that primarily couple to inhibitory G proteins (Gi/Go) to mediate their effects, such as inhibiting adenylyl cyclase activity and reducing intracellular cAMP levels. frontiersin.orgfrontiersin.org Alterations in the expression or function of these inhibitory G proteins can impair D2 receptor signaling and contribute to this compound resistance. thieme-connect.com
A decrease in the levels of the G protein that couples the D2 receptor to adenylyl cyclase has been suggested to further reduce the ability of dopamine to inhibit prolactin secretion. frontiersin.org Specifically, a reduction in Gi2 protein expression, but not Go and Gs, has been described in this compound-resistant prolactinomas compared to sensitive ones. frontiersin.org
Role of Filamin A
Filamin A (FLNA) is a cytoskeleton protein that plays a role in regulating the expression and signaling of various G protein-coupled receptors, including the dopamine D2 receptor. nih.govfrontiersin.orgnih.govthieme-connect.com FLNA acts as a scaffold protein, anchoring receptors to the actin cytoskeleton and influencing their plasma membrane localization and signal transduction. nih.govfrontiersin.org
Reduced expression of FLNA has been observed in dopamine agonist-resistant prolactinomas. nih.govoup.com Studies have demonstrated a causal relationship between FLNA and D2R expression levels. nih.gov Silencing of FLNA in dopamine agonist-sensitive prolactinomas has been shown to result in a significant reduction of D2R expression and an abrogation of dopamine-induced signals, including the inhibition of prolactin release and antiproliferative effects. oup.com Conversely, overexpression of FLNA in dopamine agonist-resistant prolactinomas has been shown to restore D2R expression and responsiveness to dopamine agonists. oup.com These findings strongly suggest a crucial role for FLNA in regulating D2R expression and signaling in tumorous lactotrophs, and that its reduced expression contributes to this compound resistance. oup.com
Interactive Table 3: Molecular Factors Implicated in this compound Resistance
| Molecular Factor | Observation in Resistant Tumors | Proposed Mechanism |
| Decreased Dopamine D2 Receptor (D2R) Expression | Reduced levels on cell surface, lower gene transcription thieme-connect.comresearchgate.netresearchgate.net | Diminished binding and activation by this compound, leading to reduced therapeutic effect. oup.comresearchgate.net |
| Altered D2S/D2L Ratio | Lower D2S mRNA and/or lower D2S/D2L ratio, or reduced D2L mRNA nih.govfrontiersin.org | Imbalance in receptor isoforms may affect signaling efficiency and responsiveness. plos.orgoup.com |
| Lowered Inhibitory G Protein (Gi/Go) Expression | Reduced Gi2 protein expression frontiersin.org | Impaired coupling of D2R to downstream inhibitory pathways, reducing signal transduction. frontiersin.orgfrontiersin.org |
| Reduced Filamin A (FLNA) Expression | Lower protein expression nih.govoup.com | Impaired D2R membrane localization and signaling due to loss of scaffolding function. nih.govfrontiersin.orgoup.com |
Other Intracellular Signaling Pathways and Escape Mechanisms (e.g., AKT phosphorylation)
Beyond the primary interaction with the dopamine D₂ receptor, other intracellular signaling pathways and escape mechanisms can contribute to this compound resistance. This compound has been shown to suppress the activation of the AKT/mTOR signaling pathway, which is involved in cell growth and proliferation, potentially inducing autophagy in prolactinoma cells nih.govnih.gov. Studies have indicated that this compound treatment can lead to significantly lower phosphorylation levels of AKT and mTOR in prolactinoma cell lines compared to control groups nih.govnih.gov. This suggests that the inhibition of the AKT/mTOR pathway might play a role in this compound-induced autophagy nih.gov.
However, in some contexts, the activation status of pathways like PI3K/AKT/mTOR and RAF/MEK/ERK has been implicated in aggressive pituitary adenomas and potential resistance mechanisms nih.gov. For instance, in everolimus-resistant pituitary tumor cells, this compound treatment was found to reduce AKT phosphorylation frontiersin.orgfrontiersin.org. The combined treatment of this compound and everolimus further reduced cell proliferation and AKT phosphorylation in these cells, suggesting a potential synergistic effect frontiersin.orgfrontiersin.org. Conversely, a recent study indicated that this compound treatment can induce the upregulation of NDFIP1 in pituitary neuroendocrine tumor cells, which correlates with tumor size and contributes to reduced this compound sensitivity, potentially through the activation of the mTOR pathway, as supported by increased phosphorylation of p-AKT and p-4EBP1 researchgate.net.
This compound's effects on intracellular signaling may also involve interactions with serotonin receptors. An in vitro study in a mouse muscle cell model, which expressed serotonin receptors but not dopamine D₂ receptors, demonstrated that this compound stimulated glucose uptake and activated insulin receptor signaling (IRS-1 and Akt phosphorylation) and AMPK pathways through serotoninergic receptors (5-HT2RA and 5-HT2RB) endocrine-abstracts.org. This suggests that this compound can influence intracellular pathways via receptors other than D₂ under certain conditions endocrine-abstracts.org.
Angiogenesis and Cell Proliferation
Histological studies of dopamine agonist-resistant prolactinomas have noted increased angiogenesis, cellular atypia, increased proliferation, and increased invasiveness escholarship.orgendocrine-abstracts.org. Resistance to this compound is associated with a failure of the drug to adequately inhibit cell proliferation and reduce tumor size mdpi.comthieme-connect.com. The increased proliferation observed in resistant tumors may be linked to alterations in intracellular signaling pathways that regulate the cell cycle and growth nih.gov.
Clinical Predictors of Resistance
Several clinical factors have been identified as predictors of resistance to dopamine agonists, including this compound. The two main predictive factors are male gender and tumor invasiveness nih.govendocrine-abstracts.org. Studies have shown that male patients with resistant prolactinomas tend to have larger tumors and higher baseline prolactin levels compared to females endocrine-abstracts.org. Large tumor volume, particularly macroadenomas and giant tumors, is consistently associated with a higher risk of resistance mdpi.comendocrine-abstracts.org. Tumor invasiveness, such as cavernous sinus invasion, is also a significant predictor mdpi.comescholarship.orgendocrine-abstracts.org.
Other factors that may be taken into consideration include prolonged time to prolactin normalization and the presence of cystic, hemorrhagic, and/or necrotic components within the tumor on MRI scans before treatment initiation mdpi.comnih.govresearchgate.net. High baseline prolactin levels and high contrast capture on MRI are also suggested as potential factors nih.govresearchgate.net. Some studies also suggest that polymorphisms of the DRD2 gene, such as the DRD2 Ncol-T+ alleles, may be associated with this compound resistance, potentially leading to reduced or dysfunctional DRD2 mRNA or protein escholarship.org.
Management Strategies for Resistant Cases
The management of this compound-resistant prolactinomas requires a multimodal approach endocrine-abstracts.orgendocrine-abstracts.org. Strategies include increasing the this compound dose, switching to other dopamine agonists, or pursuing surgical intervention nih.govmdpi.comscielo.brfrontiersin.orgnih.gov.
Increased this compound Dosing
For patients who show a partial response or resistance to conventional doses of this compound, a stepwise increase in the dose is often the first management strategy, provided it is tolerated and there are no adverse effects nih.govscielo.brnih.gov. This approach can be successful in achieving prolactin normalization in a significant proportion of patients scielo.brendocrine-abstracts.org. Studies have shown that increasing the this compound dose above typical levels (e.g., >2 mg/week or >3.5 mg/week) can lead to hormonal control in some patients who were initially resistant nih.govscielo.brendocrine-abstracts.orgendocrine-abstracts.orgaustinpublishinggroup.comomet-endojournals.ru. However, the benefit of increasing doses beyond a certain threshold (e.g., >7 mg/week) may be limited scielo.brendocrine-abstracts.org.
Switching to Other Dopamine Agonists
Switching to another dopamine agonist is another option for managing this compound resistance escholarship.orgfrontiersin.org. While this compound is generally more effective and better tolerated than bromocriptine nih.govscielo.brnih.gov, some patients who are resistant to this compound may achieve a satisfactory response with bromocriptine scielo.broatext.combioscientifica.com. Quinagolide, a non-ergot derived D₂ receptor selective agonist, is also available in some regions and may be considered thieme-connect.comscielo.br.
Radiotherapy
Radiotherapy is considered a treatment option for patients with this compound-resistant prolactinomas, particularly those with aggressive tumors or those who have not responded adequately to surgery nih.govoup.com. It is typically reserved for cases where medical therapy and surgical intervention have been unsuccessful in controlling tumor growth or prolactin secretion nih.govoup.com.
Studies have evaluated the effectiveness of both conventional fractionated radiotherapy and stereotactic radiosurgery in managing resistant prolactinomas oup.com. While radiotherapy can help control tumor growth, its effect on normalizing prolactin levels is often delayed and may not be achieved in all patients nih.govfrontiersin.org. Some reports indicate that normalization of prolactin levels may occur in approximately one-third of patients after several years following radiotherapy nih.gov. Radiotherapy also carries the risk of long-term side effects, including hypopituitarism, which can develop years after treatment frontiersin.org.
In a multicenter study of 92 patients with this compound-resistant prolactinomas, 14.1% received postoperative radiotherapy researchgate.net. Another study noted that while surgery demonstrated usefulness for disease control and reducing this compound dose in resistant macroprolactinomas, radiotherapy did not show a significant benefit in this context e-enm.org. However, other sources suggest that aggressive prolactinomas and metastatic pituitary neuroendocrine tumors should receive multimodality therapy including high dose this compound, surgery, and radiation therapy (preferably stereotactic radiosurgery where suitable) mdpi.com.
Novel Therapeutic Approaches (e.g., Temozolomide, Metformin, Everolimus combination)
For this compound-resistant prolactinomas, particularly aggressive or malignant cases, novel therapeutic approaches are being explored, including the use of chemotherapy agents and targeted therapies thieme-connect.combham.ac.uk.
Temozolomide: Temozolomide, an oral alkylating agent, has emerged as a significant treatment option for aggressive pituitary tumors and carcinomas, including those that are resistant to conventional therapies like this compound, surgery, and radiotherapy mdpi.comfrontiersin.orgoup.com. It is often considered when other treatment modalities have failed nih.govoup.com. Studies have shown that temozolomide can lead to tumor reduction and a decrease in prolactin levels in a subset of patients with resistant prolactinomas mdpi.comfrontiersin.org. Response rates to temozolomide in aggressive/malignant prolactinomas can vary, but some reports indicate tumor reduction in a significant percentage of patients and a reduction in serum prolactin levels spandidos-publications.com. However, complete remission is less common, and resistance to temozolomide can also develop frontiersin.orgoup.com.
Metformin: The role of metformin, a biguanide commonly used for type 2 diabetes, in the treatment of this compound-resistant prolactinomas has been investigated, primarily based on in vitro studies suggesting it can reduce prolactin secretion karger.comnih.gov. However, clinical studies evaluating the effect of adding metformin to ongoing high-dose this compound treatment in patients with resistant prolactinomas have shown inconsistent results karger.comnih.gov. A pilot study did not observe a consistent inhibitory effect of metformin on prolactin levels in this patient group, although a few patients showed a significant decrease at certain time points karger.comnih.govoup.com. Further studies with larger cohorts and potentially higher doses may be needed to fully define the efficacy of metformin in this setting karger.com.
Everolimus combination: Everolimus, an mTOR inhibitor, has been explored as a potential therapeutic agent for aggressive prolactin-secreting pituitary adenomas, particularly those resistant to conventional treatments spandidos-publications.comoup.com. The mTOR pathway is implicated in the development of pituitary tumors spandidos-publications.comoup.com. Studies, including in vitro experiments and case reports, suggest that everolimus may exhibit antiproliferative actions and could be a novel option for some aggressive, resistant tumors oup.com. Combining everolimus with this compound has also been investigated. In vitro studies in pituitary tumor cells have shown that this compound might overcome an escape mechanism induced by everolimus, suggesting a potential benefit of the combination therapy in inhibiting cell proliferation and AKT phosphorylation frontiersin.orgendocrine-abstracts.org. Clinical reports also exist where the addition of everolimus to this compound led to decreased prolactin levels and tumor regression in a patient with a resistant prolactinoma oup.com.
Other novel approaches are also being explored, such as the use of somatostatin analogs and estrogen modulators, although data on their efficacy in this compound-resistant cases are still limited bham.ac.ukresearchgate.net. Hydroxychloroquine in combination with this compound has also shown promise in a case report of a patient with a dopamine agonist-resistant lactotroph pituitary neuroendocrine tumor frontiersin.org.
Summary of Novel Therapeutic Approaches in this compound Resistance
| Therapeutic Agent/Combination | Mechanism of Action | Findings in this compound Resistance | Level of Evidence (Based on provided snippets) |
| Temozolomide | Alkylating agent | Can induce tumor reduction and decrease prolactin; often used after failure of other therapies; resistance can occur. mdpi.comfrontiersin.orgoup.comspandidos-publications.com | Moderate (Clinical studies, case reports) |
| Metformin | Biguanide (potential prolactin reduction) | Clinical studies show inconsistent results; some patients may show partial response; in vitro data more promising. karger.comnih.govoup.com | Low (Pilot clinical studies, in vitro data) |
| Everolimus (alone or with this compound) | mTOR inhibitor | May have antiproliferative effects in aggressive tumors; combination with this compound may overcome resistance mechanisms in vitro and show clinical benefit. oup.comfrontiersin.orgendocrine-abstracts.org | Low (Case reports, in vitro studies) |
| Hydroxychloroquine (with this compound) | Traditionally anti-malarial/anti-rheumatic (mechanism in PitNETs being studied) | Case report showed normalization of prolactin and no recurrence in a resistant case. frontiersin.org | Very Low (Single case report) |
Preclinical and Translational Research
In Vitro Studies on Cellular Mechanisms
In vitro studies have demonstrated that cabergoline exerts a direct inhibitory effect on the secretion of prolactin by rat pituitary lactotroph cells. This is primarily mediated through the stimulation of dopamine D2 receptors on these cells.
This compound also displays affinity for other receptors, including dopamine D3 and D4 receptors, as well as several serotonin receptor subtypes (5-HT1A, 5-HT1D, 5-HT2A, and 5-HT2B). However, its affinity for dopamine D1, alpha1- and alpha2-adrenergic, and 5-HT1- and 5-HT2-serotonin receptors is low.
Research using pituitary neuroendocrine tumor (PitNET) cell lines, such as MMQ cells, has investigated this compound's effects on cell proliferation and signaling pathways. This compound significantly reduced cell proliferation and the ratio of phosphorylated AKT (p-AKT) to total AKT in MMQ cells. This suggests an impact on pro-survival pathways. In these cells, this compound was also shown to counteract the increase in AKT phosphorylation induced by the mTOR inhibitor everolimus, thereby potentially overcoming an escape mechanism from everolimus's antiproliferative effects.
Studies on normal cell lines, such as Rat Embryonic Fibroblast (REF) cells, have indicated that this compound can cause increased cell proliferation in a dose-dependent manner.
In cultured cortical neurons, this compound has demonstrated neuroprotective effects against oxidative stress. This neuroprotection appears to be mediated through a D2 receptor-dependent mechanism, rather than solely through radical scavenging. Adequate pretreatment time with this compound was necessary for this protective effect.
This compound has also been shown to inhibit lipid accumulation in white 3T3L1 cells, a murine adipose cell line. This effect was associated with the stimulation of phosphorylated AMPK (pAMPK) and a decrease in proteins like PPARγ, leptin, and FAS, suggesting a promotion of the oxidative pathway.
Interactive Data Table: In Vitro Effects of this compound
| Cell Type | Observed Effect | Proposed Mechanism / Associated Pathway | Source |
| Rat Pituitary Lactotrophs | Direct inhibition of prolactin secretion | Stimulation of dopamine D2 receptors | |
| MMQ cells (PitNET cell line) | Reduced cell proliferation | Reduction of p-AKT/total-AKT ratio | |
| MMQ cells (PitNET cell line) | Counteraction of everolimus-induced increased AKT phosphorylation | Overcoming escape mechanism from everolimus antiproliferative effects | |
| REF cells (Normal cell line) | Increased cell proliferation (dose-dependent) | Underlying mechanisms still under investigation; potential direct interaction with chromosomes or high cell sensitivity | |
| Cultured Cortical Neurons | Neuroprotection against oxidative stress | D2 receptor-mediated mechanism, reducing excitotoxicity | |
| 3T3L1 cells (Murine Adipose) | Inhibition of lipid accumulation | Stimulation of pAMPK; decrease in PPARγ, leptin, FAS; promotion of oxidative pathway |
Animal Models of Disease and this compound Efficacy
This compound's efficacy has been evaluated in various animal models. In reserpinized rats, this compound decreased serum prolactin levels, demonstrating its prolactin-inhibiting effect in vivo.
Studies in rats have also investigated the effects of this compound on lipid accumulation in adipose and skeletal muscle tissues. In male Wistar rats on a high-fat and fructose-rich diet, this compound administration counteracted lipid accumulation. This was associated with increased pACC Ser72 and stimulated PKA-Cα in soleus tissues.
In models of Parkinson's disease, this compound has been shown to reverse levodopa-induced dyskinesias in Parkinsonian monkeys.
This compound has been studied for its effects on the reproductive cycle in female dogs. It was found to be more efficient than bromocriptine in inducing oestrus, resulting in higher induction, pregnancy, and whelping rates. This compound also reduced the inter-oestrus interval in normal cycling bitches.
In dairy cows, a single intramuscular injection of this compound at drying-off significantly reduced milk leakage the day after dry-off compared to placebo and antibiotic treatment. This compound-treated cows also showed a reduced risk of new intramammary infections by major pathogens across the dry period and postcalving.
Studies in cats with hypersomatotropism (HST) and concurrent diabetes mellitus (DM) have evaluated this compound as a treatment option. This compound monotherapy was effective in reducing IGF-1 excess in a subset of these cats and normalized IGF-1 concentrations in some cases. It also improved diabetes control and was associated with remission of DM in a percentage of treated cats. The median pituitary height at the start of treatment was lower in cats that experienced a reduction in IGF-1.
Genetic effects of this compound have been investigated in mice. In vivo studies showed that this compound administration led to a significant reduction in mitotic index percentage, while increasing micronucleus formation and chromosomal aberrations.
Interactive Data Table: this compound Efficacy in Animal Models
| Animal Model | Condition Studied | Observed Efficacy | Source |
| Reserpinized Rats | Elevated prolactin levels | Decreased serum prolactin levels | |
| Wistar Rats (High-fat/fructose diet) | Adipose and skeletal muscle lipid accumulation | Counteracted lipid accumulation; increased pACC Ser72 and stimulated PKA-Cα in soleus tissue | |
| Parkinsonian Monkeys | Levodopa-induced dyskinesias | Reversal of dyskinesias | |
| Female Dogs | Oestrus induction | More efficient than bromocriptine in inducing oestrus; higher pregnancy and whelping rates; reduced inter-oestrus interval | |
| Dairy Cows | Milk leakage and intramammary infections at drying-off | Reduced milk leakage; reduced risk of new intramammary infections | |
| Cats with HST and DM | Hypersomatotropism and diabetes mellitus | Reduced IGF-1 excess; normalized IGF-1; improved diabetes control; associated with DM remission | |
| Mice | Genetic effects | Reduced mitotic index; increased micronucleus formation and chromosomal aberrations |
Molecular and Genetic Research on this compound Targets
This compound's primary molecular target is the dopamine D2 receptor, for which it has high affinity. It also binds with lower affinity to dopamine D3 and D4 receptors, and certain serotonin receptors
Clinical Trials and Methodological Considerations
Design of Clinical Trials (Randomized, Double-Blind, Placebo-Controlled, Comparative)
Clinical trials involving cabergoline frequently utilize rigorous designs to minimize bias and establish the drug's effects. Randomized, double-blind, placebo-controlled trials are considered the gold standard for evaluating new interventions. For instance, a pilot study assessing this compound for endometriosis-associated pain was designed as a randomized, double-blind, placebo-controlled trial. nih.gov In this study, participants were randomly assigned to receive either this compound or a placebo, with both patients and investigators unaware of the assigned treatment. nih.gov Similarly, a pilot trial investigating this compound as a preventive migraine treatment also employed a randomized, double-blind, placebo-controlled, parallel design. plos.orgresearchgate.net In this migraine study, this compound and placebo were encapsulated to ensure they were visually identical, and block randomization was used to allocate participants. plos.org Another randomized clinical trial evaluated this compound's effectiveness in women with uterine fibroids using a double-blind design where participants were randomly allocated to either the this compound or placebo group. jimc.ir
Comparative studies are also conducted to assess this compound against existing standard therapies. The endometriosis pilot study, for example, compared this compound to norethindrone acetate (NETA), a standard clinical therapy for the condition. nih.gov
While randomized controlled trials are prominent, other study designs are also utilized. An open-label intervention study assessed the safety and efficacy of this compound for idiopathic restless legs syndrome over 26 weeks, notably without a control group. nih.gov Retrospective cohort studies have also been conducted, such as one evaluating this compound in non-irradiated patients with acromegaly. oup.com Another retrospective cohort study examined this compound's impact on laboratory and clinical outcomes in patients with hyperprolactinemic polycystic ovary syndrome and microprolactinoma. springermedizin.de A naturalistic prospective comparison study assessed this compound in psychiatric patients with antipsychotic-induced hyperprolactinemia, comparing a treatment group to a control group who declined this compound. cambridge.org
Patient Selection and Cohort Characteristics
Patient selection criteria and the characteristics of the study cohorts are crucial for defining the generalizability of trial results. Studies on this compound for specific conditions enroll patients based on diagnostic criteria relevant to the disease being investigated.
In the endometriosis pilot study, participants were women with surgically confirmed endometriosis. nih.gov The migraine prevention trial enrolled adults with at least 6 monthly migraine days. plos.orgresearchgate.net For studies in hyperprolactinemia, patients with pathological hyperprolactinemia are included, with cohorts often comprising individuals with microadenomas, macroadenomas, or idiopathic hyperprolactinemia. oup.com Some studies specifically include patients who have shown intolerance or resistance to other dopamine agonists like bromocriptine. oup.com In a study on this compound for Cushing's disease, inclusion criteria included the persistence of the disease after surgery and elevated urinary cortisol excretion. oup.com
Cohort characteristics, such as age, gender distribution, and baseline disease severity, are typically reported. For instance, in a study on idiopathic restless legs syndrome, the cohort consisted of 302 patients, with 73% being women and a mean age of 61 years. nih.gov A multi-center cohort study on this compound for acromegaly included 69 patients on monotherapy, with a median age at diagnosis of 50.5 years, and a distribution of 45 males and 24 females. oup.com In the study of this compound for antipsychotic-induced hyperprolactinemia, the cohort comprised 84 chronic psychiatric patients, with a significant majority being female (77/7). cambridge.org A retrospective study on hyperprolactinemia treated with this compound included 455 patients (102 males and 353 females) across nine Belgian centers, with varying proportions of microadenoma, macroadenoma, and idiopathic hyperprolactinemia. oup.com
Outcome Measures (Biochemical, Clinical, Radiological)
Clinical trials evaluating this compound utilize a range of outcome measures to assess its effects, encompassing biochemical, clinical, and radiological parameters.
Biochemical outcomes often involve measuring hormone levels relevant to the condition being treated. In studies for hyperprolactinemia and prolactinomas, normalization of serum prolactin (PRL) levels is a primary biochemical endpoint. oup.comfrontiersin.org Studies in acromegaly assess the normalization of insulin-like growth factor-1 (IGF-1) and growth hormone (GH) levels. oup.comnih.gov For conditions like polycystic ovary syndrome (PCOS) with elevated prolactin, changes in total testosterone and dehydroepiandrosterone-sulphate levels are evaluated. springermedizin.de In Cushing's disease, the effectiveness of this compound is assessed by its ability to control cortisol secretion, often measured through urinary cortisol excretion. oup.com
Clinical outcome measures focus on the symptomatic response to this compound. For conditions like restless legs syndrome, efficacy is evaluated using scales such as the RLS-6 and the International RLS Rating Scales, assessing the severity of symptoms at different times. nih.gov In the endometriosis study, measures of pelvic pain and pain relief were collected. nih.gov For migraine prevention trials, the change in monthly migraine days is a key clinical outcome. plos.orgresearchgate.netmedrxiv.org Studies in hyperprolactinemia also assess clinical symptoms such as menstrual disorders, galactorrhea, and sexual dysfunction, sometimes using scales like the UKU side effects rating scale. cambridge.org Patient global impression of change scales are also used to capture the patient's perception of treatment effectiveness. nih.govplos.orgresearchgate.net
Radiological outcome measures are particularly relevant in studies involving pituitary adenomas. Tumor size reduction or shrinkage is a critical radiological endpoint in patients with prolactinomas or acromegaly. oup.comfrontiersin.orgnih.gov Radiological remission in prolactinoma is assessed alongside biochemical remission. nih.govresearchgate.net Imaging studies, such as MRI, are used to evaluate changes in tumor volume and size. frontiersin.org
Long-Term Efficacy and Safety Assessments
The long-term efficacy and safety of this compound are important aspects evaluated in clinical research, although specific safety profiles and adverse effects are excluded from this discussion. Assessments over extended periods are conducted to understand the sustained benefits and potential long-term considerations of this compound treatment.
Studies have investigated the long-term effectiveness of this compound in various conditions. In patients with Parkinson's disease, observational studies have examined the efficacy of long-term this compound use in treating motor symptoms such as akinesia and dyskinesia. karger.com For hyperprolactinemia, long-term this compound treatment has been assessed for its efficacy in normalizing prolactin levels and its impact on associated symptoms. cambridge.orgoup.com Some studies report on the duration of this compound treatment, with median durations of several months to years observed in cohorts. oup.comoup.comelsevier.es The maintenance of biochemical control over extended periods is a key aspect of long-term efficacy assessment. oup.comnih.gov
Long-term assessments also include monitoring for the durability of response after this compound withdrawal in conditions like prolactinoma. frontiersin.org Studies track the recurrence of hyperprolactinemia following discontinuation of the drug over several months. frontiersin.org
Methodological Challenges and Limitations in this compound Clinical Research
Clinical research on this compound, like other pharmacological studies, faces various methodological challenges and limitations that can influence the interpretation and generalizability of findings.
One significant challenge is patient recruitment and retention, particularly for studies requiring specific or rare patient populations. fastercapital.com Delays in recruitment can impact study timelines. fastercapital.com
Methodological drawbacks identified in some this compound studies include small sample sizes, which can limit the statistical power to detect significant effects. oup.com The application of variable criteria for defining disease control across different studies can also make comparisons challenging. oup.com The inclusion of patients who have previously received other treatments, such as pituitary radiotherapy, can confound results regarding the efficacy of this compound as a medical treatment. oup.com
Retrospective study designs, while providing valuable real-world data, have limitations. Management and monitoring decisions in such studies may be based on clinician preference rather than a standardized protocol, potentially introducing variability. oup.com Unclear or incomplete documentation in medical records can also reduce the amount of retrievable information. dovepress.com
Defining and assessing outcomes can also present challenges. For instance, there can be contradicting and inadequate evidence on the efficacy of this compound in achieving radiological and biochemical remission in prolactinoma, and it is not always clear which patients will have an adequate response. nih.govresearchgate.net The interpretation of elevated serum prolactin levels can also be complex due to various factors. researchgate.net
Furthermore, ensuring blinding in clinical trials, especially in open-label studies, is a limitation that can introduce bias. nih.gov While some studies successfully blind participants and investigators, others operate under open-label conditions. nih.gov
Challenges also exist in the assessment of long-term outcomes, including tracking patients over extended periods and accounting for potential changes in treatment regimens or patient status outside the study protocol.
Ethical and Societal Implications of Cabergoline Research
Informed Consent in Clinical Trials
In the context of cabergoline clinical trials, informed consent is a fundamental ethical and legal requirement. It is the process by which potential participants learn about and understand the purpose, benefits, and potential risks of the trial before agreeing to participate clinicalleader.compaho.org. This process must be voluntary, and participants must be competent to give consent paho.org. The information provided should be specific to the proposed procedure or treatment course, allowing individuals to make a sound decision clinicalleader.compaho.org. Ethical approval from relevant committees, such as a Medical Research Center, is typically obtained, and in some cases, a waiver of informed consent may be granted under specific conditions and with ethical approval dovepress.comdovepress.com. Participants should have the opportunity to ask questions and clarify any doubts without pressure, and they should retain the freedom to revoke their consent clinicalleader.com.
Reporting of Adverse Events and Patient Safety
Ensuring patient safety through the diligent reporting of adverse events is a critical ethical obligation in this compound research and clinical use. Researchers are required to monitor for side effects, assess efficacy, and ensure ongoing safety throughout studies lotilabs.com. In clinical trials, adverse events and serious adverse events are typically monitored and reported plos.orgnih.gov. While some studies have reported adverse events associated with this compound, the severity profiles can vary depending on the condition being studied and the comparator treatment neurology.org. For instance, in a study comparing this compound to levodopa in Parkinson's disease, adverse events occurred in a notable percentage of patients in both groups, with similar severity profiles neurology.org. Another study on this compound for migraine prevention also reported adverse effects in participants receiving the drug, though none were serious plos.org. The systematic reporting and evaluation of these events are essential for understanding the safety profile of this compound and protecting current and future patients. Regulatory bodies and ethical committees play a vital role in overseeing these reporting mechanisms.
Access to Treatment and Global Health Equity
The development and availability of this compound raise questions regarding access to treatment and global health equity. While medical advancements offer potential benefits, ensuring these benefits are accessible to people worldwide, regardless of their socioeconomic status or geographical location, presents a significant challenge ihf-fih.orgweforum.org. Global health equity emphasizes the intrinsic value of health for well-being and calls for equal respect for all human life nih.gov. Achieving this requires collaborative actions at local, national, regional, and international levels, while respecting ethical integrity ihf-fih.org. Disparities in access to healthcare, including necessary medications like this compound for relevant conditions, can perpetuate structural inequality and health inequities thehastingscenter.org. Efforts to promote global health equity involve advocating for strategies that bridge healthcare delivery gaps and address the needs of vulnerable populations ihf-fih.orgweforum.orgthehastingscenter.org. This includes considering how research findings translate into accessible and affordable treatments globally.
Future Directions in Cabergoline Research
Development of Novel Formulations or Delivery Systems
The development of novel drug delivery systems and formulations for cabergoline presents a significant opportunity for innovation and growth in the market. datahorizzonresearch.com Exploring sustained-release or controlled-release formulations could potentially enhance patient compliance by reducing dosing frequency, thereby improving therapeutic outcomes and minimizing potential side effects. datahorizzonresearch.com Advancements are anticipated in this compound's delivery systems, including the development of injectable forms for patients who may have difficulty with oral administration. globalgrowthinsights.com Intranasal delivery is another area of investigation, with studies exploring microemulsion formulations to potentially enhance brain delivery by bypassing the blood-brain barrier. researchgate.netfrontiersin.org Research has shown that intranasal microemulsion formulations can deliver this compound selectively and in higher amounts to the brain compared to other routes in animal models. researchgate.net The stability of this compound tablets is also being studied, with research indicating that the use of non-hygroscopic excipients, film coating, and aluminum packaging materials are important for preservation during stability studies. researchgate.net
Personalized Medicine Approaches and Predictive Biomarkers
The increasing focus on personalized medicine and the development of companion diagnostics create opportunities for optimizing and targeting this compound-based treatments. datahorizzonresearch.com Personalized medicine aims to tailor medical procedures based on individual biological traits, or biomarkers, to optimize medical efficiency. openaccessjournals.com Predictive biomarkers, in particular, are associated with drug response and are used to guide clinical decisions. openaccessjournals.com While the search for specific biomarkers predicting this compound response is ongoing, research in personalized medicine for pituitary adenomas, the primary indication for this compound, is advancing. mdpi.com Biomarkers with predictive value for managing pituitary tumors are being identified, particularly concerning their clinical and radiological characteristics. mdpi.com The integration of RNA sequencing and gene expression analysis into clinical trials is increasing to identify biomarkers predictive of treatment responses. mdpi.com
Understanding Long-Term Outcomes and Quality of Life
Understanding the long-term outcomes of this compound treatment and its impact on patients' quality of life remains a crucial area of research. Studies have investigated the long-term use of this compound in conditions like Parkinson's disease, showing effectiveness in treating motor symptoms and improving quality of life and disability in activities of daily living. nih.govkarger.com In patients with antipsychotic-induced hyperprolactinemia, long-term this compound treatment has been shown to be effective and safe in the majority of patients, improving sexual function and quality of life beyond the reversal of typical symptoms. cambridge.org For prolactinomas, while biochemical control with dopamine agonist therapy can improve quality of life, achieving a quality of life comparable to healthy controls may require prolonged biochemical control. nih.gov Research indicates that poor quality of life in prolactinoma is multifactorial, related to biochemical control, side effects of therapy, and sellar mass effect, highlighting the need to target persistent symptoms and reduce side effects to improve quality of life. nih.gov
Investigating Mechanisms of Action Beyond D2 Receptors
While this compound's primary mechanism of action involves potent agonism at dopamine D2 receptors, research is exploring its interactions with other receptors and their potential contributions to its therapeutic effects and side effects. pharmacoj.comdrugbank.comfda.gov this compound also exhibits affinity for dopamine D3 and D4 receptors, as well as serotonin receptors (5-HT1A, 5-HT2A, 5-HT2B, and 5-HT2C) and alpha-2 adrenergic receptors. pharmacoj.com Its binding to these receptors results in diverse effects. pharmacoj.com For instance, it acts as a partial or complete agonist on D2, D3, and D4 dopaminergic receptors, as well as serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors, contributing to its therapeutic benefits. pharmacoj.com Conversely, it acts as an antagonist on serotonin 5-HT7 receptors and alpha-2 adrenergic receptors. pharmacoj.com Further investigation into these interactions is crucial for a comprehensive understanding of this compound's pharmacodynamics and for identifying potential new therapeutic targets or strategies to mitigate side effects. pharmacoj.com Studies are also exploring the complex interplay between dopamine agonists like this compound, prolactin, and glucose metabolism, with some research suggesting effects on glucose metabolism that may be partly independent of prolactin levels. nih.gov
Addressing Unmet Needs in Resistant and Aggressive Diseases
Addressing unmet needs in diseases that are resistant or aggressive despite this compound treatment is a critical area of ongoing research. This compound resistance in prolactinomas, defined by uncontrolled hyperprolactinemia or insufficient tumor shrinkage despite adequate doses, remains a significant clinical challenge. researchgate.netnih.govbioscientifica.com Studies are investigating the clinical presentation, management, and outcomes of patients with resistant prolactinomas, noting that males often present with more aggressive disease. researchgate.netnih.gov Genetic factors, such as germline SDHB pathogenic variants, are being identified as potential contributors to aggressive and this compound-resistant macroprolactinomas. frontiersin.org For aggressive pituitary tumors, including resistant prolactinomas, newer therapeutic strategies beyond conventional medical treatments, surgery, and radiotherapy are being explored. researchgate.netfrontiersin.org These include investigating the potential of temozolomide, which has shown impressive responses in some aggressive and resistant cases, particularly those with low/intermediate MGMT expression. frontiersin.org Early diagnosis of genetic forms associated with aggressive disease could also help improve outcomes. researchgate.net
Q & A
Basic Research Questions
Q. What experimental models are appropriate for investigating Cabergoline’s dopamine receptor selectivity and mechanism of action?
- Methodological Answer : Utilize in vitro competitive binding assays with radiolabeled dopamine receptors (D2 and D5 subtypes) to measure this compound’s affinity and intrinsic activity. Dose-response curves can quantify receptor activation thresholds. In vivo models (e.g., rodent prolactinoma) should measure serum prolactin suppression as a functional endpoint. Ensure assays include controls for cross-reactivity with other receptors (e.g., serotonin) to confirm specificity .
Q. How can researchers determine the optimal this compound dose for prolactin suppression in hyperprolactinemia?
- Methodological Answer : Conduct dose-escalation studies in animal models or clinical cohorts, measuring serum prolactin levels weekly. Use nonlinear mixed-effects modeling (NONMEM) to correlate dose with hormonal response, adjusting for covariates like body weight and baseline prolactin. Validate findings with pharmacokinetic/pharmacodynamic (PK/PD) simulations to identify minimal effective and maximal tolerated doses .
Q. What biomarkers are validated for monitoring this compound’s therapeutic efficacy in endocrine disorders?
- Methodological Answer : IGF-I normalization (for acromegaly) and prolactin suppression (for hyperprolactinemia) are primary biomarkers. Secondary endpoints include tumor volume reduction (MRI) and patient-reported outcomes (e.g., headache resolution). Ensure assays meet CLIA/CAP certification standards to minimize variability .
Advanced Research Questions
Q. How can researchers reconcile contradictory findings on this compound’s association with valvular heart disease?
- Methodological Answer : Apply multivariate Cox regression in large-scale cohort studies to adjust for confounders (e.g., age, hypertension, cumulative dose). Stratify analyses by dose thresholds (e.g., <3 mg/week vs. ≥3 mg/week) and validate echocardiographic endpoints (e.g., valve thickening) with blinded adjudication. Meta-analyses should assess publication bias via funnel plots and Egger’s test .
Q. What statistical approaches are suitable for analyzing this compound’s synergistic effects with somatostatin analogs in acromegaly?
- Methodological Answer : Use interaction terms in mixed-effects regression models to evaluate additive vs. synergistic effects on IGF-I suppression. Individual patient data (IPD) meta-analyses can pool data from heterogeneous studies, adjusting for baseline IGF-I, tumor size, and prolactin levels. Sensitivity analyses should exclude outliers to ensure robustness .
Q. How to design longitudinal studies evaluating this compound’s long-term neuroendocrine and cardiovascular outcomes?
- Methodological Answer : Implement a prospective, matched cohort design with annual echocardiography and hormonal profiling. Use time-dependent covariates in survival analysis to account for cumulative exposure. Power calculations should assume a 5% annual incidence of subclinical valvulopathy, requiring ≥500 patient-years of follow-up .
Methodological Considerations
- Contradiction Analysis : When studies report conflicting results (e.g., valvulopathy risk), perform subgroup analyses by dose, duration, and population characteristics. Use the Bradford Hill criteria to assess causality .
- Research Design : For preclinical studies, adhere to ARRIVE guidelines for experimental rigor. In clinical research, prioritize randomized adaptive trials for dose-finding and PROBE designs for long-term safety .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


