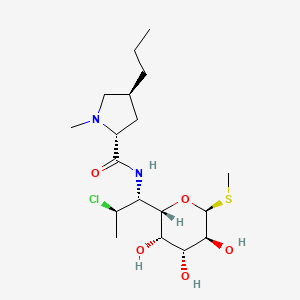
Clindamycin
Description
Clindamycin is a lincosamide antibiotic that inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit, effective against Gram-positive aerobes and anaerobes . It exhibits both antimicrobial and anti-inflammatory properties, making it a cornerstone in dermatology (e.g., acne vulgaris) and systemic infections (e.g., intra-abdominal, bone/joint, and malaria) . Its pharmacokinetics include high tissue penetration and metabolism into active derivatives like N-demethylthis compound .
Properties
IUPAC Name |
(2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KDLRVYVGXIQJDK-AWPVFWJPSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCC1CC(N(C1)C)C(=O)NC(C2C(C(C(C(O2)SC)O)O)O)C(C)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCC[C@@H]1C[C@H](N(C1)C)C(=O)N[C@@H]([C@@H]2[C@@H]([C@@H]([C@H]([C@H](O2)SC)O)O)O)[C@H](C)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H33ClN2O5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
21462-39-5 (mono-hydrochloride), 58207-19-5 (mono-HCl, mono-hydrate) | |
| Record name | Clindamycin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0018323449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID2022836 | |
| Record name | Clindamycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
425.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Color/Form |
Yellow, amorphous solid | |
CAS No. |
18323-44-9 | |
| Record name | Clindamycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=18323-44-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clindamycin [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0018323449 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clindamycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clindamycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.038.357 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLINDAMYCIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3U02EL437C | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLINDAMYCIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3037 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
Reaction Mechanism
- Vilsmeier Reagent Formation : POCl₃ reacts with DMF to generate chloroiminium ions, which selectively chlorinate lincomycin’s 7(R)-hydroxyl group.
- Hydrolysis and Crystallization : The chlorinated intermediate undergoes alkaline hydrolysis, followed by hydrochloric acid-mediated crystallization to produce this compound hydrochloride alcoholate.
Process Optimization
- Solvent System : Acetone enhances reaction homogeneity and product solubility.
- Catalysis : Pyridine and triethylamine accelerate esterification, achieving 85–90% conversion.
- Deprotection : Sodium carbonate dissociates protected intermediates, improving purity to >98%.
Advantages :
- Eliminates TPPO byproducts
- Higher regioselectivity for 7(R)-chlorination
- Scalable for industrial production.
Enzymatic and Hybrid Synthesis Approaches
Recent innovations explore biocatalysis to streamline synthesis:
Lipase-Catalyzed Acylation
Candida antarctica lipase B (CAL-B) enables one-step synthesis of this compound esters (e.g., palmitate) with 90% regioselectivity for the 2-hydroxyl group.
Hybrid Protection-Deprotection Strategies
Patent CN107652332B introduces a sodium carbonate-mediated deprotection step that reduces hydrolysis-induced degradation:
- Isopropylidene Protection : Triethyl orthoformate and p-toluenesulfonic acid in acetone yield protected intermediates.
- Controlled Hydrolysis : Glacial acetic acid/hydrochloric acid mixture minimizes side reactions (conversion: 95%).
Comparative Analysis of Industrial Methods
Key Findings :
- The POCl₃ method balances efficiency and scalability, dominating current production.
- Enzymatic routes, though nascent, offer sustainability advantages for derivative synthesis.
Chemical Reactions Analysis
Clindamycin undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized to form sulfoxide and N-demethylated metabolites.
Reduction: Reduction reactions are less common but can occur under specific conditions.
Substitution: The Mitsunobu substitution reaction is a key step in its synthesis.
Common reagents used in these reactions include chlorinating agents, oxidizing agents, and reducing agents. The major products formed from these reactions are this compound hydrochloride and its metabolites .
Scientific Research Applications
Clindamycin has a wide range of scientific research applications:
Chemistry: It is used as a model compound in studying antibiotic synthesis and modification.
Biology: this compound is used to study bacterial protein synthesis and resistance mechanisms.
Medicine: It is widely used to treat bacterial infections, including those caused by methicillin-resistant Staphylococcus aureus (MRSA) and anaerobic bacteria
Mechanism of Action
Clindamycin works by binding to the 50S ribosomal subunit of bacteria, inhibiting protein synthesis. This action prevents the elongation of the peptide chain during translation, effectively stopping bacterial growth . This compound targets the bacterial ribosome, disrupting the transpeptidation reaction and inhibiting early chain elongation .
Comparison with Similar Compounds
Tetracyclines vs. Clindamycin
- Mechanism : Tetracyclines (e.g., doxycycline) inhibit the 30S ribosomal subunit, whereas this compound targets the 50S subunit. Both reduce Cutibacterium acnes in acne but via distinct pathways .
- Efficacy : In acne, this compound’s anti-inflammatory effects complement its antibacterial activity, achieving comparable efficacy to tetracyclines. However, tetracyclines are preferred for moderate-to-severe cases due to broader anti-inflammatory action .
- Resistance : Tetracycline resistance in C. acnes is rising, but this compound remains effective in regions without widespread resistance .
Table 1: Topical this compound vs. Azithromycin in Acne
| Parameter | This compound 1% Gel | Azithromycin 2% Gel |
|---|---|---|
| Efficacy (Lesion Reduction) | 68% | 72% |
| Resistance Development | Low (Region-dependent) | Not observed |
| Adverse Effects | Mild irritation | Similar irritation |
Intra-Abdominal Infections
This compound is often combined with aminoglycosides (e.g., gentamicin) or β-lactams:
- Cefoxitin: this compound + gentamicin showed similar efficacy to cefoxitin monotherapy in perforated appendicitis (cure rates: 89% vs. 87%) .
- Imipenem: In severe intra-abdominal infections, imipenem monotherapy outperformed this compound + tobramycin (cure rates: 92% vs. 85%) due to broader Gram-negative coverage .
- Meropenem : Comparable efficacy to this compound + tobramycin in advanced appendicitis, but meropenem requires fewer doses .
Malaria Treatment
This compound + quinine is a second-line therapy for uncomplicated falciparum malaria:
- Artesunate + this compound : Similar 28-day parasitological failure rates (RR 0.57, 95% CI 0.26–1.24) but longer parasite clearance time (16.7 hours longer) with this compound + quinine .
- Quinine Monotherapy: this compound + quinine reduced parasitological failure risk by 86% (RR 0.14, 95% CI 0.07–0.29) in 3-day regimens .
Biofilm Disruption in Bacterial Vaginosis
- Dequalinium Chloride (DQC) : At 8.11 µg/mL, DQC and this compound both reduced Gardnerella biofilm biomass by 50%. However, DQC was superior in biomass reduction (p < 0.05), while this compound better inhibited metabolic activity .
Resistance Patterns
Macrolide-Lincosamide Resistance
- Methylase-Mediated Resistance : Constitutive methylase production in Streptococcus spp. and Staphylococcus spp. confers cross-resistance to this compound and macrolides (e.g., erythromycin) .
- Inducible Resistance : Staphylococcus aureus may develop inducible this compound resistance during therapy, necessitating D-zone testing before use .
Biological Activity
Clindamycin is an antibiotic belonging to the lincosamide class, primarily effective against anaerobic bacteria and certain protozoa. It is widely used in clinical settings for treating various infections, including skin and soft tissue infections, respiratory tract infections, and certain types of bone infections. This article delves into the biological activity of this compound, supported by data tables, case studies, and relevant research findings.
This compound exerts its antibacterial effects by inhibiting bacterial protein synthesis. It binds to the 50S ribosomal subunit of susceptible bacteria, thereby blocking peptide bond formation during translation. This mechanism is similar to that of macrolides but differs in its binding site preference, making this compound effective against a range of Gram-positive cocci and anaerobic bacteria.
Key Points:
- Target : 50S ribosomal subunit.
- Effect : Inhibition of protein synthesis.
- Spectrum : Effective against anaerobes and some protozoa.
Clinical Applications
This compound is indicated for various infections, particularly those caused by anaerobic bacteria. Its effectiveness is highlighted in several case studies:
Case Study Highlights:
- Skin and Soft Tissue Infections : this compound has shown significant efficacy in treating cellulitis and abscesses caused by Staphylococcus aureus, including methicillin-resistant strains (MRSA).
- Bone Infections : In osteomyelitis cases, this compound demonstrated favorable outcomes when combined with surgical intervention.
- Periodontal Disease : A study indicated that this compound could improve glycemic control in diabetic patients with periodontal disease, showing a mean reduction in HbA1c levels .
Efficacy Against Specific Pathogens
This compound's activity against various pathogens can be summarized in the following table:
Resistance Patterns
Resistance to this compound can occur through various mechanisms, including:
- Methylation of adenine residues in the 23S rRNA, which alters the binding site.
- Efflux pumps that expel the antibiotic from bacterial cells.
Monitoring resistance patterns is crucial, especially in hospital settings where resistant strains may emerge.
Adverse Effects and Considerations
While this compound is generally well-tolerated, it can lead to side effects such as gastrointestinal disturbances and a risk of C. difficile-associated diarrhea. The incidence of C. difficile infection has been noted to increase with this compound use, necessitating careful patient monitoring .
Important Considerations:
- Caution in prescribing for patients with a history of gastrointestinal disorders.
- Monitoring for signs of C. difficile infection during treatment.
Q & A
Q. What statistical methods resolve contradictions in this compound’s efficacy for bacterial vaginosis trials?
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


