Colchicine
Description
Colchicine is a naturally occurring alkaloid derived from Colchicum autumnale, historically recognized for its medicinal properties, particularly in treating gout and familial Mediterranean fever . First isolated in 1820, its molecular structure—characterized by a tropolone ring linked to a trimethoxybenzene group—was elucidated in the early 20th century . This compound exerts its primary therapeutic effects by binding to tubulin, inhibiting microtubule polymerization, and disrupting cellular processes such as mitosis and inflammation . Its cytotoxicity and apoptosis-inducing activity are mediated through caspase activation and G2/M cell cycle arrest, as demonstrated in Vero cells (EC₅₀ values: ~0.02–0.05 μM) .
Properties
IUPAC Name |
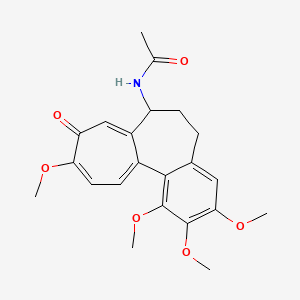
N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IAKHMKGGTNLKSZ-INIZCTEOSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)NC1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)OC)OC)OC)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(=O)N[C@H]1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)OC)OC)OC)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H25NO6 | |
| Record name | COLCHICINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/4925 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5024845 | |
| Record name | Colchicine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5024845 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
399.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Colchicine appears as odorless or nearly odorless pale yellow needles or powder that darkens on exposure to light. Used to treat gouty arthritis, pseudogout, sarcoidal arthritis and calcific tendinitis. (EPA, 1998) | |
| Record name | COLCHICINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/4925 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
Solubility |
greater than or equal to 100 mg/mL at 70 °F (NTP, 1992), 1 g dissolves in 22 mL water, 220 mL ether, 100 mL benzene; freely sol in alcohol or chloroform; practically insoluble in petroleum ether, SOL IN METHANOL; SLIGHTLY SOL IN CARBON TETRACHLORIDE, At 25 °C, 4.5 g/100 g water | |
| Record name | COLCHICINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/4925 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | COLCHICINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3044 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
Beta-lumicolchicine, Colchiceine, Colchicoside, N-deacetyl-N-formylcolchicine, For more Impurities (Complete) data for COLCHICINE (6 total), please visit the HSDB record page. | |
| Record name | COLCHICINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3044 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Pale yellow scales or powder; pale yellow needles when crystallized from ethyl acetate, Yellow plates from water + 1/2 mol of water of crystallization; yellow crystals from benzene | |
CAS No. |
64-86-8 | |
| Record name | COLCHICINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/4925 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Colchicine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=64-86-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Colchicine [USP:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000064868 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | colchicine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756702 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | colchicine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Acetamide, N-[(7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl]- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Colchicine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5024845 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Colchicine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.544 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Colchicine | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/SML2Y3J35T | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | COLCHICINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3044 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
288 to 302 °F (EPA, 1998), 142-150 °C | |
| Record name | COLCHICINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/4925 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | COLCHICINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3044 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
Total Synthesis via Catalytic Asymmetric Strategies
The 2021 gram-scale total synthesis of (−)-colchicine represents a landmark achievement in stereochemical control and step economy. This seven-step route achieves an overall yield of 27–36% through strategic bond disconnections and catalytic transformations. The synthesis begins with a modified Ir-catalyzed asymmetric amidation to install the C-7 acetamido group, achieving >98% enantiomeric excess. Subsequent Suzuki-Miyaura cross-coupling between a brominated tropolone derivative and a boronic ester constructs the biaryl framework, while a biomimetic phenol oxidative coupling forms the strained cycloheptatriene core. A Banwell-inspired cyclopropane ring cleavage finalizes the tricyclic structure, with crystallization in ethyl acetate yielding pharmaceutical-grade material.
This method’s efficiency derives from minimizing protecting group manipulations and leveraging transition-metal catalysis for stereoselectivity. Comparative analysis with earlier syntheses reveals a 40% reduction in step count while maintaining gram-scale practicality, addressing historical challenges in colchicine’s accessibility for clinical research.
Extraction and Isolation Methods
Ultrasonic-Assisted Dual Aqueous Phase Extraction (UDAPE)
The CN102627576B patent details a UDAPE protocol combining ethanol-water phase separation with ultrasonic irradiation to enhance this compound recovery from plant biomass. Key operational parameters include:
| Parameter | Optimal Range |
|---|---|
| Ethanol concentration | 80–95% (v/v) |
| Solid-liquid ratio | 1:3–1:5 (plant:solvent) |
| Ultrasonic frequency | 40–140 kHz |
| Extraction temperature | 40–70°C |
| Salt additive | (NH₄)₂SO₄, Na₂SO₄ |
In a representative procedure, 3 kg of Gloriosa superba tubers treated with 95% ethanol and ammonium sulfate under 100 kHz ultrasound for 40 minutes yielded 314.15 g of crude extract (1.12 g/cm³ density). Phase separation exploits this compound’s preferential partitioning into the ethanol-rich upper phase, while ultrasonic cavitation disrupts plant cell walls, increasing diffusion rates by 60–80% compared to static extraction.
Supercritical Fluid Extraction (SFE) with CO₂ Modifiers
Supercritical CO₂ modified with 3% water demonstrates exceptional selectivity for this compound from Gloriosa seeds, achieving 93.6% extraction efficiency at 400 bar and 60°C. The biphasic solubility of this compound in scCO₂-water systems enables selective recovery, with the modifier reducing the dielectric constant to enhance tropolone solubility. Post-extraction, a tandem charcoal-alumina chromatography system purifies this compound to 99.82% purity, as validated by HPLC-UV at 254 nm.
Purification and Crystallization Techniques
Macroporous Resin Chromatography
D3520 and D201 macroporous resins effectively separate this compound from co-extracted phenolics and glycosides. Elution with 0.6% sulfuric acid achieves >95% recovery, leveraging this compound’s pKa (1.7) for ionic interactions. Post-adsorption, ethyl acetate recrystallization at 2–5°C produces needle-like crystals with 97.26–97.67% purity (HPLC), minimizing residual solvent levels below ICH Q3C limits.
Analytical Characterization
HPLC-UV Quantification
Reverse-phase C18 chromatography (60% acetonitrile in 0.1% formic acid) resolves this compound at 7.11 min (λ = 350 nm), enabling quantification down to 0.01 μg/mL. The patent method reports 97.26% purity using a 250 mm × 4.6 mm, 5 μm column at 1 mL/min.
LC-QToF/MS Profiling
High-resolution mass spectrometry identifies 42 Gloriosa metabolites alongside this compound, including colchicoside (m/z 548.2137) and 2-demethylthis compound (m/z 386.1600). Negative-mode ESI proves critical for detecting acidic tropolone derivatives, with mass accuracy <5 ppm across 50–1200 m/z.
Comparative Evaluation of Preparation Methods
| Method | Yield | Purity | Time | Scalability |
|---|---|---|---|---|
| Total Synthesis | 27–36% | >99% | 7 steps | Gram-scale |
| UDAPE | 1.56% (w/w) | 97.26–97.67% | 48 h | Industrial |
| SFE | 93.6% | 99.82% | 2 h | Pilot-scale |
Synthetic routes excel in purity but face cost barriers for large-scale production. UDAPE balances throughput and operational simplicity, while SFE offers superior selectivity but requires high capital investment.
Chemical Reactions Analysis
Photochemical Reactions
Colchicine undergoes significant structural changes under UV light due to its light-sensitive tropolone moiety. Key findings include:
Photoisomerization to Lumithis compound
Exposure to UV light induces skeletal rearrangement, converting this compound into lumithis compound isomers. This process involves intramolecular proton transfer and ring contraction/expansion .
| Starting Material | Exposure Time (h) | Isolation Yield (%) |
|---|---|---|
| This compound | 4 | 44 |
| N-butyl carbamate (4f ) | 4 | 31 |
| N-acetyl derivative (3b ) | 12 | 41 |
Table 1: UV-induced conversion rates of this compound derivatives
Lumithis compound lacks tubulin-binding activity due to disrupted conjugation in the tropolone ring . Femtosecond spectroscopy reveals excited-state intramolecular proton transfer (ESIPT) as the primary mechanism, with recovery times <100 ps .
Solvent-Dependent Reactivity and Aggregation
This compound’s conformational flexibility leads to solvent-specific behavior:
Self-Association in Chloroform
-
At 46 mM, forms dimers via C-ring stacking, altering NMR shifts (e.g., H-8: Δδ = 0.23 ppm) .
-
Dilution to 0.46 mM reverts to monomeric form, confirmed by DFT calculations (MAE <2 ppm) .
Solvent Effects on Reactivity
| Solvent | Aggregation Observed? | Key Structural Impact |
|---|---|---|
| Chloroform | Yes (dimerization) | Alters H-bonding at C9 carbonyl |
| DMSO | No | Stabilizes monomeric form |
| Water | Yes | Enhances π-π stacking |
Table 2: Solvent influence on this compound reactivity
Metabolic and Toxicological Reactions
This compound’s toxicity arises from metabolic interactions and reactive intermediates:
CYP3A4/P-glycoprotein Interactions
-
Inhibitors (e.g., erythromycin) increase plasma levels by 300%, risking mitotic arrest and organ failure .
-
Grapefruit juice potentiates toxicity via CYP3A4 inhibition .
Reactive Metabolites
-
Demethylation at C1/C2 produces oxidative byproducts implicated in hepatotoxicity .
-
Tubulin-colchicine complexes form irreversible adducts, disrupting microtubule dynamics .
Degradation Products
-
Hydrolysis under alkaline conditions yields inactive colchiceine (IC >10 μM vs. 3 nM for native this compound) .
This compound’s chemical reactivity is central to its dual role as a therapeutic agent and toxin. Its photolability, synthetic complexity, and metabolic vulnerabilities necessitate precise handling in both laboratory and clinical settings. Ongoing research focuses on stabilizing its active conformation and mitigating off-target reactions through targeted derivatization .
Scientific Research Applications
Historical Context and Mechanism of Action
Colchicine's primary mechanism involves the disruption of microtubule formation, which affects cellular processes such as inflammation and immune response. It inhibits the activation of the NLRP3 inflammasome and modulates leukocyte function, leading to reduced inflammatory responses . This mechanism underlies its efficacy in various conditions beyond its traditional uses.
Cardiovascular Applications
This compound has gained attention for its role in managing cardiovascular diseases. Clinical trials have demonstrated its effectiveness in reducing cardiovascular events, particularly after myocardial infarction (MI). Notable studies include:
- COLCOT Trial : Involving 4,745 patients post-MI, this trial showed that this compound (0.5 mg/day) significantly decreased the risk of major cardiovascular events over 22.7 months (5.5% vs. 7.1%, P = 0.02) .
- Recurrent Pericarditis : this compound has been shown to effectively prevent recurrences in patients with pericarditis, with one study reporting a recurrence rate drop from 50.6% to 24% (P = 0.003) after treatment .
Gout and Familial Mediterranean Fever
This compound remains a first-line treatment for gout flares and Familial Mediterranean Fever due to its anti-inflammatory properties. Its use in these conditions is well-established, providing rapid relief from symptoms.
Oncology
Recent studies have explored this compound's potential in cancer therapy:
- Cancer Treatment : this compound's ability to inhibit cell division makes it a candidate for cancer treatments. It has been investigated for its effects on various cancers, including breast cancer and non-small cell lung cancer .
- Combination Therapies : this compound is being studied in combination with other chemotherapeutic agents to enhance efficacy while minimizing side effects .
Infectious Diseases
This compound has emerged as a potential treatment for COVID-19:
- COVID-19 Studies : A case-control study indicated that this compound improved outcomes in COVID-19 patients receiving standard care, showing lower mortality rates (47.1% vs. 80.8%, P = 0.0003) and reduced intubation rates . The COLCORONA trial further supports its potential benefits in managing COVID-19-related inflammation .
Summary of Clinical Trials
| Trial Name | Condition | Participants | Dosage | Key Findings |
|---|---|---|---|---|
| COLCOT | Myocardial Infarction | 4,745 | 0.5 mg/day | Reduced major cardiovascular events (5.5% vs 7.1%) |
| CORE | Recurrent Pericarditis | 84 | Variable | Decreased recurrence rate (24% vs 50.6%) |
| COLCORONA | COVID-19 | 4,488 | Standard care + this compound | Lower mortality (47.1% vs 80.8%) |
Mechanism of Action
Colchicine exerts its effects primarily through the inhibition of microtubule polymerization . By binding to tubulin, this compound disrupts the formation of microtubules, which are essential for various cellular processes, including cell division, intracellular transport, and maintenance of cell shape . This disruption leads to the downregulation of multiple inflammatory pathways and modulation of innate immunity . This compound also inhibits the activation of the NLRP3 inflammasome, reducing the release of pro-inflammatory cytokines .
Comparison with Similar Compounds
Key Findings:
- Pyrazolo[4,3-d]pyrimidines : Compounds 9 , 11 , and 13 inhibited tubulin polymerization with IC₅₀ values of 0.42–0.49 μM, rivaling combretastatin A-4 (CA-4) and demonstrating >94% inhibition of colchicine binding at 5 μM .
- Tetrazole Derivatives : Compound 4l showed 2-fold greater tubulin polymerization inhibition than its isomer 5b (IC₅₀: 1.2 vs. 2.4 μM), though both had similar antiproliferative effects .
- Dibenzo[b,f]oxepine-Fluoroazobenzenes : All synthesized photoswitches exhibited lower binding energies than this compound (-36.0 kJ/mol), suggesting higher affinity .
Table 1: Comparative Tubulin Inhibition and Binding Data
*Estimated from docking studies.
Structural Mimicry and Modifications
- Trimethoxyphenyl Group : Critical for binding. Compounds like 9ІV-c and imidazole-substituted P5 replicate this compound’s trimethoxybenzene orientation, achieving similar inhibitory profiles (IC₅₀: 0.42–5.3 μM) .
- Biphenyl Analogues : TKB (2,3,4-trimethoxy-4'-methyl-1,1'-biphenyl) fully occupies the this compound binding pocket, whereas TCB’s protruding methyl group reduces efficacy .
- Isoxazoline and Pyrazoline Derivatives : Modifications to ring B in compounds 3a–d and 4a–b enhanced hydrophobic interactions with Cysβ241, improving binding stability .
Toxicity and Selectivity
- Gloriosine : Exhibited lower toxicity (predicted LD₅₀: 6 mg/kg) than this compound (LD₅₀: ~2 mg/kg) while maintaining similar β-tubulin binding affinity (-27.8 vs. -26.13 kcal/mol) .
- Azetidin-2-one Derivatives : Compound 6 showed cancer cell line specificity but required further validation of tubulin effects .
Biological Activity
Colchicine is a potent alkaloid derived from the plant Colchicum autumnale, primarily known for its anti-inflammatory and anti-mitotic properties. This article explores the biological activity of this compound, focusing on its mechanisms of action, therapeutic applications, and recent research findings.
This compound exerts its effects primarily through the disruption of microtubule formation, which is crucial for various cellular processes. The key mechanisms include:
- Microtubule Disruption : this compound binds to soluble tubulin, preventing polymerization into microtubules. This action halts mitosis at metaphase, leading to cell cycle arrest .
- Inflammation Modulation : this compound downregulates multiple inflammatory pathways, including:
- Effects on Immune Cells : this compound inhibits macrophage chemotaxis and neutrophil superoxide production, thereby reducing oxidative stress and inflammation .
Therapeutic Applications
This compound has been utilized in various clinical settings beyond its traditional use in gout treatment. Notable applications include:
- Cardiovascular Diseases : Recent trials have demonstrated that this compound significantly reduces inflammation in patients with ischemic heart disease and heart failure. For instance, the COLCOT trial showed that this compound reduced cardiovascular events post-myocardial infarction .
- COVID-19 : A case-control study indicated that this compound improved outcomes in COVID-19 patients by lowering mortality rates and inflammatory markers such as CRP and D-dimer .
- Familial Mediterranean Fever (FMF) : this compound remains a first-line treatment for FMF, effectively preventing attacks and complications associated with this genetic disorder .
Research Findings
Recent studies provide robust evidence supporting this compound's efficacy across various conditions:
Case Studies
- COVID-19 Outcomes :
- This compound Poisoning :
Q & A
Basic: What analytical components are essential for assessing colchicine purity and stability in pharmaceutical research?
Answer: Standard analytical workflows include chromatographic peak detection (HPLC/UPLC), mass spectral peak picking (LC-MS), and impurity profiling to quantify degradation products. Data preprocessing steps (baseline correction, noise reduction) ensure reproducibility. Quantitative analysis requires calibration curves validated against certified reference materials. For stability studies, accelerated degradation experiments under varied pH/temperature conditions are paired with metabolomic data integration to identify degradation pathways .
Basic: How can single-factor experiments optimize this compound extraction from plant sources?
Answer: Single-factor testing evaluates isolated variables (e.g., ethanol concentration, extraction time) on yield. For example, ethanol concentrations between 50–70% and extraction times of 20–40 minutes are tested, with this compound quantified via UV-spectroscopy. This identifies preliminary ranges for advanced optimization methods like response surface methodology (RSM) .
Basic: What statistical methods are used to analyze this compound’s dose-dependent effects in plant polyploidy studies?
Answer: Randomized block designs with one-way ANOVA and post-hoc Duncan tests (α = 0.05) compare treatment groups. For example, this compound concentrations (0.025–0.8%) are tested on seed germination rates, with SPSS or R used for variance analysis.
Advanced: How does response surface methodology (RSM) improve this compound extraction efficiency?
Answer: RSM with Box-Behnken designs models interactions between variables (e.g., ethanol concentration, temperature). A quadratic regression equation predicts optimal conditions (e.g., 60% ethanol, 33 min, 50°C), validated via confirmatory experiments. Design-Expert® software calculates response maxima and interaction plots .
Advanced: How can researchers reconcile contradictory data on this compound’s concentration-dependent effects across plant species?
Answer: Discrepancies (e.g., reduced germination at 0.8% in Calendula vs. callus death at 0.1% in Echinochloa) require species-specific toxicity thresholds. Dose-response curves and LC50 calculations, paired with histochemical viability assays (e.g., TTC staining), clarify mechanisms.
Basic: What experimental designs validate this compound’s anti-inflammatory mechanisms in vitro?
Answer: Microtubule polymerization assays (spectrophotometry at 350 nm) quantify inhibition rates. Cell-based models (e.g., neutrophil chemotaxis) use IC50 calculations, while ELISA/Western blotting measures cytokine suppression (e.g., IL-1β, TNF-α) .
Advanced: How can multi-omics data integration address this compound stability challenges?
Answer: Combining LC-MS metabolomics (to identify degradation metabolites) with NMR structural analysis and transcriptomic profiling (e.g., oxidative stress pathways) validates degradation mechanisms. Machine learning tools (e.g., MetaboAnalyst) correlate stability with environmental factors .
Basic: What are key considerations for designing this compound clinical trials in cardiovascular diseases?
Answer: Double-blind, placebo-controlled RCTs with primary endpoints (e.g., cardiovascular mortality) require stratification by comorbidities. The LoDoCo trial design (0.5 mg/day, median follow-up 29 months) exemplifies dose standardization and endpoint selection .
Advanced: How do researchers analyze this compound’s efficacy in heterogeneous patient cohorts (e.g., PFAPA syndrome)?
Answer: Multivariable logistic regression adjusts for covariates (age, genetic mutations). ROC curves identify predictive biomarkers (e.g., CRP levels), while Kaplan-Meier analysis evaluates time-to-relapse. Exclusion criteria must address confounding treatments (e.g., prior tonsillectomy) .
Basic: What protocols ensure reproducibility in this compound-induced polyploidy studies?
Answer: Sterile in vitro cultures with MS media, this compound exposure (24–72 hours), and flow cytometry (ploidy verification) are standard. Survival rates and regeneration frequencies are tracked, with 0.05% this compound for 48 hours yielding optimal polyploidy (42.9%) in Echinochloa.
Advanced: How can pharmacokinetic-pharmacodynamic (PK-PD) modeling optimize this compound dosing?
Answer: Non-linear mixed-effects modeling (NONMEM®) integrates plasma concentration-time profiles (Cmax, AUC) with neutrophil inhibition data. Bayesian forecasting tailors doses for renal-impaired patients, minimizing toxicity risks .
Basic: What metrics assess this compound’s impact on plant morphology in mutation studies?
Answer: Morphometric parameters (plant height, leaf area) are measured digitally (ImageJ®). For Matricaria chamomilla, 0.8% this compound reduces height by 28.7% versus controls, with Duncan tests confirming significance (p < 0.05).
Advanced: How do researchers validate this compound’s anti-fibrotic effects in hepatic cirrhosis models?
Answer: Histopathological scoring (Ishak scale) of liver biopsies quantifies collagen deposition. Hydroxyproline assays and RT-PCR for fibrogenic markers (e.g., TGF-β1) are paired with survival analysis (log-rank tests), as in 14-year RCTs showing 56% 10-year survival with this compound .
Basic: What methodologies detect this compound impurities in formulation stability studies?
Answer: Forced degradation (heat, light, oxidation) followed by LC-UV/LC-MS identifies impurities. Thresholds follow ICH Q3A guidelines, with mass spectral libraries (e.g., NIST) confirming structural identities .
Advanced: How can Bayesian adaptive trials improve this compound dose-finding in rare diseases?
Answer: Adaptive designs use real-time efficacy-toxicity trade-offs (e.g., escalation with overdose control). For familial Mediterranean fever, posterior probabilities guide dose adjustments, reducing sample size versus traditional RCTs.
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


