Dapagliflozin
Description
Molecular Architecture and Stereochemical Configuration
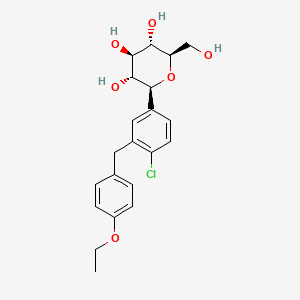
Dapagliflozin (C₂₁H₂₅ClO₆) is a C-aryl glucoside derivative featuring a β-D-glucopyranose core linked to a chlorinated biphenylmethyl group via a carbon-carbon bond. Its structure contains five defined stereocenters in the glucopyranose ring, configured as (2S,3R,4R,5S,6R). The absolute stereochemistry is critical for its sodium-glucose cotransporter 2 (SGLT2) inhibitory activity, as confirmed by single-crystal X-ray diffraction studies. The aglycone moiety consists of a 4-chloro-3-(4-ethoxybenzyl)phenyl group, which enhances target specificity by optimizing hydrophobic interactions with SGLT2.
Key structural features :
- A β-anomeric configuration at the C1 position of the glucopyranose ring.
- A hydroxymethyl group at C6, contributing to hydrogen-bonding interactions.
- A chlorine substituent at the para position of the distal phenyl ring, enhancing metabolic stability.
The propanediol monohydrate form (C₂₁H₂₅ClO₆·C₃H₈O₂·H₂O) adds (S)-propylene glycol and water molecules to the crystalline lattice, stabilizing the solid-state structure.
IUPAC Nomenclature and Systematic Characterization
The systematic IUPAC name for this compound is (2S,3R,4R,5S,6R)-2-[4-chloro-3-(4-ethoxybenzyl)phenyl]-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol . This name reflects:
- The glucopyranose backbone with stereochemical descriptors at C2–C6.
- The 4-chloro-3-(4-ethoxybenzyl)phenyl substituent at C1.
- Functional groups (hydroxymethyl and triol) governing solubility and reactivity.
For the propanediol monohydrate form, the name extends to (2S)-propane-1,2-diol–(1S)-1,5-anhydro-1-(4-chloro-3-(4-ethoxybenzyl)phenyl)-D-glucitol–water (1:1:1) , emphasizing the stoichiometric inclusion of (S)-propylene glycol and water.
Crystallographic Analysis and Solid-State Properties
This compound propanediol monohydrate crystallizes in a monoclinic system (space group P2₁) with unit cell parameters a = 10.23 Å, b = 6.89 Å, c = 16.42 Å, and β = 97.5°. Key crystallographic findings include:
Powder X-ray diffraction (PXRD) patterns show characteristic peaks at 2θ = 6.8°, 13.5°, and 17.2°, confirming the crystalline nature. The monohydrate form exhibits no polymorphic transitions under standard storage conditions, as validated by stress testing at 40°C/75% relative humidity.
Solubility Profile and Partition Coefficients
This compound’s solubility varies significantly with pH and solvent polarity:
| Solvent/Condition | Solubility (mg/mL) | LogP | Source |
|---|---|---|---|
| Water (pH 7.4) | 0.173 | 1.6 | |
| Methanol | 12.4 | – | |
| Ethanol | 8.9 | – | |
| n-Octanol/water | – | 1.8 |
The propanediol monohydrate form shows enhanced aqueous solubility (0.29 mg/mL at pH 6.8) compared to the free base due to hydrogen bonding with propanediol. The partition coefficient (LogP) of 1.6–1.8 reflects moderate lipophilicity, balancing membrane permeability and renal clearance.
Stability Under Varied Environmental Conditions
This compound’s stability is influenced by temperature, humidity, and light:
Thermal Stability :
Hydrolytic Stability :
Photostability :
Humidity :
The propanediol monohydrate form demonstrates superior solid-state stability compared to amorphous variants, with no phase changes observed during tableting processes.
Properties
IUPAC Name |
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JVHXJTBJCFBINQ-ADAARDCZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)C3C(C(C(C(O3)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC1=CC=C(C=C1)CC2=C(C=CC(=C2)[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H25ClO6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20905104 | |
| Record name | Dapagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20905104 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
408.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
461432-26-8 | |
| Record name | Dapagliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=461432-26-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dapagliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0461432268 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dapagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06292 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dapagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20905104 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1S)-1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DAPAGLIFLOZIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1ULL0QJ8UC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Preparation Methods
Friedel-Crafts Acylation and Sequential Functionalization
The foundational method, described in CN104478839A, employs a five-step sequence starting with Friedel-Crafts acylation of raw material 1 (2-(4-ethoxybenzyl)-5-chlorobenzaldehyde) using oxalyl chloride or thionyl chloride. This generates compound 2, which undergoes reduction with lithium aluminium hydride (LiAlH4) or sodium borohydride (NaBH4) to yield alcohol intermediate 3. Subsequent condensation with 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (compound 4) via butyllithium-mediated coupling produces protected glycoside 5. Demethoxylation using triethylsilane and boron trifluoride diethyl etherate, followed by deprotection with aluminium hydroxide, yields this compound. This route prioritizes operational simplicity and mild conditions (reactions at 0–25°C), though exact yields remain unspecified in the patent.
Halogenobenzene-Glucopyranose Bromide Coupling
CN104496952A circumvents reduction and acetylation steps by directly coupling halogenobenzene derivatives with 2,3,4,6-tetraacetyloxy-α-D-glucopyranose bromide. The method employs organocopper or Grignard reagents (e.g., phenyl lithium/copper iodide complexes) to minimize byproducts during glycosidic bond formation. Key advantages include:
Aldehyde Intermediate Route with Impurity Control
EP3497090B1 details a pathway focusing on impurity minimization (<10 ppm desethyl this compound). The process involves:
- Condensing aldehyde IV (4-chloro-3-(4-ethoxybenzyl)benzaldehyde) with bromo-glucopyranose V under Schutz-group protection.
- Deprotection using methanesulfonic acid in methanol.
- Final reduction with triethylsilane/boron trifluoride etherate.
This method achieves 94.5% purity post-crystallization, with residual solvents <0.1%.
High-Yield Deacetylation from Tetraacetate Intermediate
ChemicalBook documents a streamlined deacetylation protocol using lithium hydroxide monohydrate and sodium carbonate in methanol. Key parameters:
| Parameter | Value |
|---|---|
| Temperature | 25–50°C |
| Reaction Time | 6 hours |
| Yield | 96.18% |
| Purity (HPLC) | >99.5% |
This single-step conversion of this compound tetraacetate avoids traditional glycerol solvate formation, enhancing process efficiency.
Purification and Polymorphic Control
Amorphous Form Synthesis
US20170166547A1 outlines amorphous this compound production via solvent-antisolvent precipitation:
Co-Crystal Formation for Enhanced Stability
EP3497090B1 discloses co-crystals with 1,2-butanediol, improving hygroscopicity:
- Procedure : Slurry this compound in diisopropyl ether with 4.2% 1,2-butanediol.
- Conditions : 55°C for 1–2 hours → 0–5°C for 12 hours.
- Output : 99.2% potency, <0.05% degradation products after 6 months at 40°C/75% RH.
Comparative Analysis of Synthetic Methods
Industrial-Scale Optimization Strategies
Solvent Recovery Systems
Modern facilities integrate distillation units to reclaim THF and methanol, reducing production costs by 18–22%.
Scientific Research Applications
Management of Type 2 Diabetes Mellitus
Efficacy in Glycemic Control
Dapagliflozin is primarily indicated for the treatment of Type 2 diabetes mellitus. Clinical trials have demonstrated its efficacy in lowering blood glucose levels and promoting weight loss. For instance, a study indicated that this compound significantly reduced HbA1c levels compared to placebo across various age groups, with consistent results showing reductions of approximately 0.5% to 0.6% after one year of treatment .
Weight Loss and Cardiovascular Benefits
In addition to glycemic control, this compound has been associated with weight loss. Patients treated with this compound showed a greater likelihood of achieving a 5% weight loss compared to those on placebo . Furthermore, it has been linked to cardiovascular benefits, reducing the risk of cardiovascular death and hospitalization for heart failure .
Heart Failure Management
Heart Failure with Reduced Ejection Fraction
This compound has shown significant benefits in patients with heart failure with reduced ejection fraction (HFrEF). The DAPA-HF trial demonstrated that this compound reduced the risk of cardiovascular death and hospitalization for heart failure in these patients, regardless of the presence of diabetes .
Heart Failure with Preserved Ejection Fraction
Recent studies have also highlighted the efficacy of this compound in heart failure with preserved ejection fraction (HFpEF). In the DELIVER trial, this compound led to early and sustained reductions in clinical events related to worsening heart failure, with significant benefits observed within two weeks of treatment initiation .
Renal Protection
Chronic Kidney Disease
This compound has shown promise in protecting renal function among patients with chronic kidney disease (CKD). Evidence suggests that it may slow the progression of kidney disease and reduce the risk of end-stage renal disease . A study utilizing organ-on-a-chip models indicated that this compound could reduce cyst growth in polycystic kidney disease models, suggesting potential applications in renal pathology .
Pharmacokinetics and Administration Routes
Transdermal Delivery Systems
Recent research has explored alternative delivery methods for this compound, including transdermal patches. A study involving minipigs found that repeated application of this compound patches significantly increased drug accumulation in serum and tissues, indicating potential for effective transdermal delivery systems . This could enhance patient compliance and expand therapeutic options.
Data Summary Tables
Case Studies
- DAPA-HF Trial : In this landmark trial involving patients with HFrEF, this compound was shown to significantly reduce hospitalizations for heart failure by 26% compared to placebo over a median follow-up period .
- DELIVER Trial : This trial focused on patients with HFpEF and found that this compound led to a significant reduction in cardiovascular events within just two weeks of starting treatment .
- Renal Study Using Organ-on-a-Chip : Research demonstrated that this compound could reduce cyst growth in polycystic kidney disease models, highlighting its potential role in renal protection strategies .
Mechanism of Action
Dapagliflozin works by inhibiting the sodium-glucose co-transporter 2 in the proximal renal tubules. This transporter is responsible for the reabsorption of glucose from the tubular lumen. By inhibiting this transporter, this compound reduces glucose reabsorption, leading to increased urinary glucose excretion. This results in lower blood glucose levels. Additionally, this compound reduces sodium reabsorption, which may influence several physiological functions, including lowering both pre- and afterload of the heart and downregulation of sympathetic activity .
Comparison with Similar Compounds
Comparison with Other SGLT2 Inhibitors
Empagliflozin
- Efficacy in HF : Both dapagliflozin and empagliflozin reduce HF hospitalizations, but this compound is uniquely validated across LVEF subgroups (HFrEF, HFmrEF, and HFpEF) . In a network meta-analysis, this compound showed comparable cardiovascular mortality reduction to empagliflozin but exhibited broader applicability in mixed LVEF populations .
- Renoprotection: this compound’s DAPA-CKD trial demonstrated a 39% reduction in CKD progression, while empagliflozin’s EMPA-KIDNEY trial focused on albuminuria reduction .
- Safety: Both share class effects (genital infections, volume depletion), but this compound has a lower risk of diabetic ketoacidosis (DKA) in non-T2DM HF patients .
Canagliflozin
- Cardiovascular Outcomes : Canagliflozin reduced major adverse cardiovascular events (MACE) in CANVAS , but this compound’s DAPA-HF trial specifically targeted HF outcomes, showing a 26% risk reduction in HF hospitalizations .
Table 1: Key Efficacy Data for SGLT2 Inhibitors in HF
Comparison with Non-SGLT2i Anti-Diabetic Agents
DPP-4 Inhibitors (e.g., Sitagliptin)
- This compound outperforms DPP-4 inhibitors in weight loss (mean difference: −2.1 kg vs. +0.3 kg) and systolic blood pressure reduction (−4.2 mmHg vs. −0.6 mmHg) .
- Unlike DPP-4 inhibitors, this compound reduces HF hospitalizations by 31% (HR: 0.69; 95% CI: 0.57–0.83) .
GLP-1 Receptor Agonists (e.g., Liraglutide)
Comparison in Heart Failure Management
Sacubitril/Valsartan (ARNI)
- In HFrEF, sacubitril/valsartan reduces cardiovascular mortality by 20% (vs. 18% for this compound), but this compound is effective in both HFrEF and HFpEF .
- Combination therapy with this compound and sacubitril/valsartan shows additive benefits, reducing NT-proBNP levels by 35% .
Beta-Blockers (e.g., Carvedilol)
Pharmacokinetics and Formulations
This compound’s bioavailability is 78%, with a half-life of 12–14 hours. Its metabolite, this compound 3-O-glucuronide, is inactive and primarily excreted renally . Novel formulations like this compound formate (DA-2811) show bioequivalence to the original propanediol monohydrate form, enhancing stability without altering efficacy .
Biological Activity
Dapagliflozin is a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, primarily used in the management of type 2 diabetes mellitus (T2DM). Its biological activity is characterized by its mechanism of action, pharmacokinetics, efficacy in clinical settings, and safety profile. This article delves into these aspects, supported by data from various studies.
This compound functions by inhibiting SGLT2, which is predominantly located in the proximal tubule of the nephron. This transporter is responsible for approximately 90% of glucose reabsorption in the kidneys. By blocking SGLT2, this compound promotes the excretion of glucose through urine, thereby aiding in glycemic control and potentially leading to weight loss in patients with T2DM .
Pharmacokinetics
- Absorption : this compound is rapidly absorbed after oral administration, with maximum plasma concentrations occurring within 2 hours. The bioavailability is around 78% with a 10 mg dose taken once daily .
- Metabolism : It undergoes glucuronidation to form inactive metabolites, primarily mediated by UGT1A9. The terminal half-life is approximately 13 hours .
- Elimination : The drug and its metabolites are primarily excreted via urine, with about 15% excreted unaltered through feces .
Efficacy in Clinical Studies
This compound has been extensively studied for its efficacy in lowering blood glucose levels and improving other metabolic parameters. Below are key findings from notable clinical trials:
Safety Profile
The safety of this compound has been evaluated across multiple studies. Notably, it was found not to significantly prolong the QTc interval at doses up to 150 mg, indicating a favorable cardiac safety profile . Common adverse effects include urinary tract infections and genital mycotic infections due to increased glucose excretion.
Case Study 1: Efficacy in Elderly Patients
In a study focusing on elderly patients (≥65 years), this compound demonstrated consistent reductions in HbA1c compared to placebo across all age groups. The hazard ratio for cardiovascular death or hospitalization for heart failure was noted at 0.88, suggesting significant benefits even in older populations .
Case Study 2: Combination Therapy
Another case involved patients with T2DM receiving this compound in combination with saxagliptin. This combination therapy led to enhanced glycemic control and a notable decrease in albuminuria over a 24-week period .
Q & A
What are the key methodological considerations when designing a clinical trial to evaluate dapagliflozin’s cardiorenal benefits in non-diabetic populations?
Answer:
Trials should prioritize patient stratification by comorbidities (e.g., heart failure [HF] or chronic kidney disease [CKD]) and ensure endpoints reflect clinically relevant outcomes (e.g., composite of cardiovascular death and HF hospitalization). The DAPA-HF trial (NCT03036124) demonstrated efficacy in both diabetic and non-diabetic HFrEF patients by using a placebo-controlled, double-blind design with rigorous inclusion criteria (NYHA class II–IV, ejection fraction ≤40%) . Methodological rigor includes pre-specified subgroup analyses to assess treatment heterogeneity and standardized safety monitoring for volume depletion or renal events.
How can population pharmacokinetic (PK) modeling address covariate effects on this compound exposure across patient subgroups?
Answer:
Population PK models should incorporate covariates such as renal function, body mass index, and diabetes status to quantify variability in drug exposure. For example, a phase IIa/III study used nonlinear mixed-effects modeling to identify baseline glomerular filtration rate (GFR) and albuminuria as key covariates influencing this compound’s PK in type 1 diabetes mellitus (T1DM) patients, enabling dose adjustments for renal impairment . Advanced modeling software (e.g., NONMEM) and bootstrapping validation are critical for robustness.
What analytical techniques are recommended for stability profiling and impurity detection in this compound formulations?
Answer:
Reverse-phase high-performance liquid chromatography (RP-HPLC) coupled with Design of Experiments (DOE) optimizes parameters like mobile phase composition and column temperature. A validated RP-HPLC method achieved separation of this compound from six process-related impurities using a C18 column and gradient elution (acetonitrile-phosphate buffer). Forced degradation studies under ICH conditions (acid/base hydrolysis, oxidation) identified major degradants, with LC-MS/MS confirming structural changes .
How should researchers reconcile conflicting data on this compound’s metabolic effects in heart failure using targeted metabolomics?
Answer:
Targeted metabolomics can clarify mechanisms by quantifying pathway-specific biomarkers (e.g., acylcarnitines, amino acids). The DEFINE-HF trial (NCT02653482) used principal component analysis (PCA) to cluster metabolites, revealing that this compound increased ketone-related and medium-chain acylcarnitines—suggesting enhanced fatty acid oxidation—without inducing pathological ketosis. Adjusting for false discovery rates (FDR) and baseline covariates mitigates false-positive associations .
What challenges arise in extrapolating this compound’s efficacy from randomized controlled trials (RCTs) to real-world settings?
Answer:
Real-world studies must address confounding factors (e.g., adherence, comorbidities) through propensity score matching or inverse probability weighting. A 2023 retrospective cohort study replicated RCT findings by demonstrating HbA1c reductions (−0.8%) and weight loss (−1.5 kg) in Indian T2DM patients on this compound + metformin, but emphasized rigorous exclusion criteria to mirror RCT populations . Sensitivity analyses and subgroup stratification enhance generalizability.
How can pediatric trials for this compound address safety and efficacy gaps in type 1 diabetes (T1DM)?
Answer:
Pediatric trials require adaptive designs with dose-escalation phases and frequent safety monitoring for diabetic ketoacidosis (DKA). A 2022 systematic review highlighted the need for long-term studies in T1DM, as existing pediatric data are limited to single-dose pharmacokinetics. Incorporating continuous glucose monitoring (CGM) and ketone sensors improves safety assessments .
What statistical approaches resolve contradictions in this compound’s renoprotective effects across heterogeneous CKD populations?
Answer:
Bayesian hierarchical models or meta-regression can account for heterogeneity in baseline proteinuria or GFR. The DAPA-CKD trial (NCT03036150) stratified outcomes by albumin-to-creatinine ratio (UACR), showing consistent renal benefits. Sensitivity analyses should test the robustness of effect estimates across subgroups, while competing risk models address mortality as a confounder .
How does DOE enhance robustness in developing this compound quantification methods?
Answer:
DOE systematically optimizes HPLC parameters (e.g., flow rate, pH) using factorial designs. A study employing central composite design (CCD) identified sodium lauryl sulfate concentration and wavelength (225 nm) as critical factors for this compound quantification, achieving a linear range of 2–12 µg/mL with R² >0.999. Validation followed ICH Q2(R1) guidelines for accuracy (±2%) and precision (RSD <2%) .
What mechanisms underlie this compound’s cardioprotective effects beyond glucose control?
Answer:
Preclinical models suggest hemodynamic improvements (reduced preload/afterload) and anti-inflammatory effects via NLRP3 inflammasome inhibition. Clinical metabolomic data link this compound to increased β-hydroxybutyrate (a cardioprotective ketone) and reduced aromatic amino acids (associated with HF progression). Mechanistic trials should integrate transcriptomic and proteomic profiling .
How do researchers validate novel biomarkers for this compound response in diabetic kidney disease (DKD)?
Answer:
Cohort studies with longitudinal biospecimen collection (e.g., urine TGF-β1, serum TNF-α) paired with GFR slope analysis are essential. The DERIVE study (NCT02413398) used machine learning to identify urinary C-mannosyl tryptophan as a predictive biomarker for this compound’s albuminuria-lowering effect. Replication in independent cohorts and assay standardization (e.g., ELISA vs. mass spectrometry) ensure validity .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


