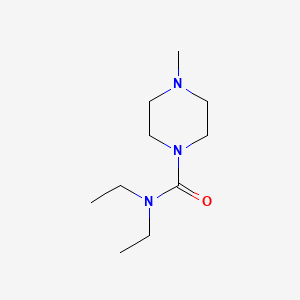
Diethylcarbamazine
Description
Historical Context of Diethylcarbamazine Discovery and Development
The discovery of this compound in 1947 by Yellapragada Subbarow marked a significant breakthrough in the treatment of filarial diseases wikipedia.orgnih.gov. Initial studies, such as those by Hewitt et al. in 1947, demonstrated its effectiveness as a filaricide in animal models like cotton rats infected with Litomosoides carinii and dogs with Dirofilaria immitis rsc.orgnih.gov. Following these promising results, DEC was first utilized for treating human filariasis in 1947 rsc.org. Its efficacy against microfilariae of various human filarial species, including Wuchereria bancrofti, Brugia malayi, Onchocerca volvulus, and Loa loa, was subsequently established rsc.org. The drug rapidly gained popularity as an oral chemotherapeutic agent due to its chemical stability jmaa.co.uk. Early research in Japan in the 1950s further explored its use and established various dosage regimens for administration in endemic areas nih.gov.
Current Significance in Global Health Initiatives
This compound remains an important and effective antifilarial drug, particularly recognized for its efficacy against all stages of the filarial life cycle, although its mode of action is not fully described researchgate.netpatsnap.com. It is a well-established anthelmintic primarily used to treat filarial infections causing diseases such as lymphatic filariasis, loiasis, and tropical pulmonary eosinophilia patsnap.com. DEC has been a crucial component of public health strategies, especially in regions where these infections are endemic patsnap.com.
The World Health Organization (WHO) launched the Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000, with DEC being a key drug in the mass drug administration (MDA) programs aimed at interrupting transmission nih.govmedrxiv.org. Pharmaceutical companies have committed to supplying DEC for free to the WHO for use in these programs, benefiting millions of people in at-risk communities eisai.com. The GPELF has achieved significant scale-up, providing billions of treatments and preventing millions of cases of LF disease plos.org.
Evolution of Research Paradigms for this compound
The understanding of how this compound exerts its effects has evolved over time, shifting from an initial focus on host-mediated responses to the recognition of direct effects on the parasite.
Historically, this compound was primarily understood to act by stimulating the host immune system rsc.orgjmaa.co.ukresearchgate.netplos.org. Early hypotheses suggested an indirect, host-mediated mode of action where DEC was thought to alter host arachidonic acid and nitric oxide metabolic pathways, leading to the immobilization and sequestration of microfilariae drugbank.comresearchgate.netplos.orgcambridge.org. Studies indicated that DEC's activity against Brugia malayi microfilariae was dependent on host inducible nitric oxide synthase (iNOS) and nitric oxide drugbank.comresearchgate.net. It was proposed that DEC sensitized microfilariae to the host's immune response, particularly facilitating phagocytosis drugbank.compatsnap.com. This host-mediated model helped explain the observed rapid clearance of microfilariae in vivo despite a lack of significant in vitro effect in some early studies cambridge.org.
However, more recent research has provided evidence for direct effects of DEC on filarial nematodes researchgate.netplos.orgnih.gov. Studies have demonstrated that low concentrations of DEC can have a direct impact on parasites like Brugia malayi, affecting their motility researchgate.net. Research has shown that DEC can increase the activation of Transient Receptor Potential (TRP) channels and calcium-dependent SLOw poke potassium channels (SLO-1) in the somatic muscle cells of B. malayi, resulting in temporary spastic paralysis researchgate.netnih.gov. Furthermore, direct effects on Ascaris suum have been observed, where DEC increases the activation of SLO-1 K+ currents plos.orgnih.gov. Studies have also indicated that DEC can affect the muscular activity of adult worms, potentially leading to paralysis and death patsnap.com. Research using in vitro and in vivo studies on W. bancrofti microfilariae has shown a direct mechanism of action involving apoptosis and organelle damage rsc.org. The nematode intestine has also been identified as a site of action for DEC, affecting TRP channels and Ca2+ signaling researchgate.net.
The evolving understanding of DEC's mechanism has led to shifting perspectives on its anti-filarial action. While the host-mediated effects involving immune modulation and alterations in arachidonic acid metabolism remain relevant, the discovery of direct effects on parasite ion channels and musculature has added another layer of complexity to its pharmacological profile drugbank.comresearchgate.netplos.orgcambridge.orgnih.gov.
Research findings have detailed DEC's interference with cyclooxygenase and lipoxygenase pathways, reducing the production of various prostaglandins and leukotrienes in the host drugbank.comresearchgate.net. It has also been shown to inhibit nuclear transcription factor kappa B (NF-κB) activation, a key regulator of proinflammatory genes researchgate.net.
The recognition of both host-mediated and direct parasite effects suggests a multifaceted mechanism of action for DEC, which is still not fully understood patsnap.complos.org. This evolving research paradigm highlights the complexity of anthelmintic action and the potential for drugs to exert effects through multiple pathways.
Research continues to explore the precise molecular targets of DEC within the parasite and the interplay between its direct and indirect effects. For instance, studies are investigating the concentration-response relationships of DEC on the motility of different stages of Brugia parasites and characterizing its effects on SLO-1 K channels nih.gov. The potential for synergistic effects when combined with other anthelmintics, such as emodepside, which also affects SLO-1 K+ channels, is also being explored plos.orgnih.gov.
The ongoing research into DEC's mechanism of action is crucial for optimizing its use in filariasis elimination programs and for understanding potential mechanisms of parasite resistance seq.es.
Data Table: Proposed Mechanisms of Action of this compound
| Mechanism Category | Proposed Actions | Filarial Target (if specified) | Source(s) |
| Host-Mediated | Alters host arachidonic acid metabolism (inhibits cyclooxygenase, lipoxygenase) | Microfilariae (sequestration) | drugbank.comresearchgate.netplos.orgcambridge.org |
| Host-Mediated | Alters host nitric oxide metabolic pathways (dependent on host iNOS) | Microfilariae (immobilization) | drugbank.comresearchgate.netplos.org |
| Host-Mediated | Inhibits NF-κB activation | Host (reduces inflammation) | researchgate.net |
| Host-Mediated | Sensitizes microfilariae to host immune response (facilitates phagocytosis) | Microfilariae | drugbank.compatsnap.com |
| Host-Mediated | Results in vasoconstriction and amplified endothelial adhesion | Microfilariae | cambridge.org |
| Direct Parasite | Opens Transient Receptor Potential (TRP) channels | Brugia malayi muscle cells | researchgate.netnih.gov |
| Direct Parasite | Activates calcium-dependent SLOw poke potassium channels (SLO-1) | Brugia malayi muscle cells, Ascaris suum | researchgate.netplos.orgnih.govnih.gov |
| Direct Parasite | Affects muscular activity | Adult worms | patsnap.com |
| Direct Parasite | Induces apoptosis and organelle damage | Wuchereria bancrofti microfilariae | rsc.orgnih.gov |
| Direct Parasite | Inhibits parasite eicosanoid metabolism (blocks cyclooxygenase pathway) | Microfilariae | seq.esnih.gov |
| Direct Parasite | Affects TRP channels and Ca2+ signaling in the intestine | Ascaris suum intestine | researchgate.net |
Properties
IUPAC Name |
N,N-diethyl-4-methylpiperazine-1-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H21N3O/c1-4-12(5-2)10(14)13-8-6-11(3)7-9-13/h4-9H2,1-3H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RCKMWOKWVGPNJF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)C(=O)N1CCN(CC1)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H21N3O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
1642-54-2 (citrate (1:1)), 5348-97-0 (mono-hydrochloride) | |
| Record name | Diethylcarbamazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000090891 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID1022928 | |
| Record name | Diethylcarbamazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022928 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
199.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.36e+02 g/L | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
90-89-1 | |
| Record name | Diethylcarbamazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=90-89-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diethylcarbamazine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000090891 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | 1-Piperazinecarboxamide, N,N-diethyl-4-methyl- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Diethylcarbamazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022928 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diethylcarbamazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.001.840 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DIETHYLCARBAMAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/V867Q8X3ZD | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
150-155, 48 °C | |
| Record name | Diethylcarbamazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00711 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diethylcarbamazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014849 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanisms of Action of Diethylcarbamazine
Interference with Parasite Physiology and Metabolism
Diethylcarbamazine is understood to interact with the arachidonic acid metabolic pathway, which plays a significant role in its mechanism of action, particularly against microfilariae. drugbank.commedex.com.bdhmdb.caresearchgate.netdrugbank.com
Arachidonic Acid Metabolism Inhibition in Microfilariae
This compound acts as an inhibitor of arachidonic acid metabolism in microfilariae. wikipedia.orgnih.gov This interference is thought to make the microfilariae more susceptible to attack by the host's innate immune system, although it does not necessarily kill the parasites directly. wikipedia.org Studies have confirmed the important role of the arachidonic acid metabolic pathway in this compound's in vivo mechanism of action. drugbank.comhmdb.caresearchgate.netdrugbank.com
This compound affects the 5-lipoxygenase pathway. drugbank.comresearchgate.netdrugbank.com Research indicates that this compound can interrupt this pathway distal to the formation of 5-hydroperoxy-eicosatetraenoic acid (5-HPETE) from arachidonic acid by 5-lipoxygenase. oup.com This interference contributes to the reduction in the production of certain eicosanoids. capes.gov.br
In addition to its effects on the 5-lipoxygenase pathway, this compound also targets the cyclooxygenase pathway and specifically COX-1. drugbank.comhmdb.caresearchgate.netdrugbank.comnih.gov Studies have shown that this compound's activity against Brugia malayi microfilariae is dependent on the cyclooxygenase pathway. drugbank.commedex.com.bdhmdb.caresearchgate.netdrugbank.comnih.gov Furthermore, experiments have demonstrated that treatment with this compound can result in a reduction in the amount of COX-1 protein in peritoneal exudate cells. researchgate.netnih.gov
This compound interferes with both cyclooxygenase and lipoxygenase pathways, leading to a reduction in the production of various eicosanoids, including thromboxane, prostacyclin, prostaglandins, and leukotrienes. capes.gov.brresearchgate.netwikem.orgnih.gov This reduction in inflammatory mediators is considered part of its anti-inflammatory properties. researchgate.netnih.gov Studies have shown that this compound can decrease the release of prostacyclin, prostaglandin E2, and thromboxane B2 from endothelial monolayers. nih.gov
Data from in vitro studies on bovine pulmonary arterial endothelium monolayers exposed to 2.5 µM this compound showed significant reductions in the release of cyclooxygenase pathway products:
| Eicosanoid | Reduction in Release | Statistical Significance |
| Prostacyclin | 78% | P < 0.001 |
| Prostaglandin E2 | 57% | P = 0.05 |
| Thromboxane B2 | 75% | P < 0.05 |
These findings suggest that the effects of this compound on host endothelial and parasite eicosanoid production may contribute to its mechanism of lowering microfilariae levels in circulation. nih.gov
Cyclooxygenase Pathway and COX-1
Direct Effects on Nematode Parasites
While initially thought to act primarily through host-mediated effects, recent studies have demonstrated that this compound also exerts direct effects on nematode parasites. researchgate.netnih.govnih.govproquest.comcambridge.org These direct effects contribute to the inhibition of parasite motility. researchgate.net
Low concentrations of this compound have been shown to have a direct effect on Brugia malayi parasites by opening Transient Receptor Potential (TRP) channels. researchgate.netnih.govasm.orgnih.govresearchgate.netresearchgate.netaminer.cnresearchgate.net This activation of TRP channels promotes calcium entry into the parasite muscle cells. asm.orgresearchgate.netresearchgate.net The influx of calcium can subsequently activate calcium-dependent SLOw poke potassium channels (SLO-1), which are the putative targets of other anthelmintics like emodepside. nih.govasm.orgnih.govresearchgate.netresearchgate.net The opening of these channels in somatic muscle cells can lead to a temporary spastic paralysis of the parasite. researchgate.netnih.gov Specifically, this compound has been shown to interact with the TRPC ortholog receptor TRP-2 to facilitate calcium entry into Brugia muscle cells. asm.orgresearchgate.netresearchgate.net
Studies investigating the effects of this compound on single Brugia muscle cells using calcium-imaging techniques have provided evidence for this mechanism. asm.orgresearchgate.net The direct effect of this compound on opening TRP channels, including TRP-2, is proposed as a mechanism for the rapid onset of its action. nih.gov
| Compound | Effect on Brugia Muscle Cells | Mechanism |
| This compound | Promotes Ca²⁺ entry | Activates Transient Receptor Potential (TRP) channels, including TRP-2 asm.orgresearchgate.netresearchgate.net |
The synergistic action observed when this compound is combined with emodepside on nematode motility and muscle membrane potentials may be explained by the combined effects on SLO-1 K⁺ channels, involving this compound's effects on arachidonic acid and nitric oxide pathways in the parasite, as well as emodepside's direct effects on SLO-1 K⁺ channels. nih.gov
Activation of Calcium-Dependent SLOw poke Potassium Channels (SLO-1)
Recent studies have revealed a direct effect of this compound on filarial parasites, specifically involving the activation of Transient Receptor Potential (TRP) channels in the parasite's muscle cells. nih.govnih.govresearchgate.netasm.org This activation, particularly of the TRPC ortholog TRP-2, promotes the entry of calcium ions into the muscle cells. nih.govnih.govresearchgate.netasm.orgresearchgate.net The increased intracellular calcium concentration, in turn, activates calcium-dependent SLOw poke potassium channels (SLO-1). nih.govnih.govresearchgate.netasm.orgresearchgate.netjensenlab.org Activation of SLO-1 potassium channels leads to hyperpolarization of the muscle cell membrane, reducing muscle excitability and resulting in temporary spastic paralysis of the parasite. patsnap.comnih.govresearchgate.netjensenlab.orgpfizer.com This direct action on ion channels contributes to the rapid onset of DEC's effects observed in vivo. nih.gov
Impact on Parasite Motility and Viability
This compound has been shown to directly inhibit the motility of both microfilariae and adult filarial worms. nih.govafricaresearchconnects.com Studies on Onchocerca volvulus microfilariae, for instance, demonstrated a concentration-dependent decrease in motility and survival when exposed to DEC in vitro. africaresearchconnects.com The initial effect observed was a reduction in motility, followed by death associated with a loss of darkly refractile nuclei. africaresearchconnects.com This suggests that DEC has both an immobilizing and a lethal action on microfilariae at concentrations achievable during treatment. africaresearchconnects.com While some studies historically suggested DEC primarily acted via the host immune system, recent findings confirm a direct inhibitory effect on parasite motility. nih.govafricaresearchconnects.com
Disruption of Adult Worm Muscular Activity
In addition to its effects on microfilariae, this compound has been observed to affect the muscular activity of adult filarial worms. patsnap.com This disruption can lead to paralysis and ultimately the death of the parasites. patsnap.com Although the mode of action on adult worms is less extensively documented than on microfilariae, there is evidence suggesting that DEC is macrofilaricidal, with degenerating adult worms observed in lymph nodes post-treatment. nafdac.gov.ngwho.int However, the macrofilaricidal effects can be inconsistent. nafdac.gov.ngwho.int The activation of TRP and SLO-1 channels discussed earlier is also relevant to the disruption of adult worm muscle function, contributing to paralysis. nih.govnih.govresearchgate.netasm.org
Impact on Reproductive Capabilities of Female Worms
This compound also impacts the reproductive capabilities of female filarial worms, contributing to a reduction in microfilarial loads. patsnap.com Studies have shown that DEC can reduce the total number of intrauterine embryos, particularly ova, indicating an inhibition of early embryogenesis. kln.ac.lk By limiting the production and release of microfilariae from female worms, DEC indirectly reduces the transmission potential of the parasite. patsnap.com
Host-Mediated Immunomodulatory Mechanisms
Beyond its direct effects on the parasite, this compound is also understood to exert significant effects by modulating the host immune response. drugbank.comannapurnapharmacy.compatsnap.comnafdac.gov.ngafricaresearchconnects.comnih.govresearchgate.netcambridge.org This host-mediated action is considered crucial for the effective clearance of microfilariae. patsnap.comnafdac.gov.ng
Sensitization of Microfilariae to Phagocytosis
A primary host-mediated mechanism involves the sensitization of microfilariae to phagocytosis by host immune cells. drugbank.comannapurnapharmacy.compatsnap.comCurrent time information in Zagorje ob Savi, SI.nih.gov this compound is thought to alter the surface properties of microfilariae, making them more recognizable and susceptible to uptake and destruction by phagocytes, such as macrophages and granulocytes. patsnap.comnafdac.gov.ngcambridge.org This process is believed to involve changes in the parasite's arachidonic acid metabolism, which can lead to amplified endothelial adhesion and enhanced adherence of host platelets and granulocytes to the microfilariae. nafdac.gov.ngwho.intcambridge.org The rapid sequestration of microfilariae in organs like the liver and spleen, where they are subsequently phagocytosed, is a hallmark of DEC treatment. jsparasitol.org Inducible nitric oxide synthase (iNOS) and nitric oxide have been shown to be essential for this rapid sequestration. drugbank.comnafdac.gov.ngwho.intplos.org
Alteration of Host Arachidonic Acid Metabolism
Host arachidonic acid metabolism plays a crucial role in inflammatory responses, leading to the production of various lipid mediators, including prostaglandins and leukotrienes. This compound has been shown to interfere with this pathway, contributing to its anti-inflammatory properties and its effects on parasite clearance. wikipedia.orgwikipedia.orgnih.gov
Inhibition of Lipoxygenase (LOX) and Cyclooxygenase (COX) Enzymes
Studies have demonstrated that DEC interferes with both the cyclooxygenase (COX) and lipoxygenase (LOX) pathways, which are central to the metabolism of arachidonic acid. wikipedia.orgwikipedia.orgnih.govuwm.edu.pl This interference leads to a reduction in the production of various eicosanoids, such as prostaglandins, thromboxanes, and leukotrienes. wikipedia.orguwm.edu.pl Specifically, DEC has been identified as an inhibitor of enzymes in the 5-lipoxygenase pathway. wikipedia.org Experimental evidence supports the interaction of DEC with the host cyclooxygenase pathway for prostaglandin synthesis. wikipedia.org
Reduction of Prostaglandin D2 (PGD2) Production
The inhibition of COX and LOX enzymes by this compound results in the suppression of prostaglandin production, including Prostaglandin D2 (PGD2). wikipedia.orguni.lu PGD2 is a major prostaglandin produced by mast cells and is involved in various physiological and pathological processes, including inflammation and allergic responses. wikipedia.orgctdbase.org Reduced production of PGD2 due to DEC's action on arachidonic acid metabolism can influence immune cell responses. uni.lu
Modulation of Nitric Oxide Pathways
This compound's activity is also linked to the modulation of host nitric oxide pathways. Nitric oxide (NO) is a signaling molecule involved in numerous physiological processes, including immune responses and vasodilation. wikipedia.orgctdbase.orgiiab.meguidetopharmacology.org
Dependence on Inducible Nitric Oxide Synthase (iNOS)
Research indicates that the efficacy of this compound, particularly its activity against microfilariae, is dependent on host inducible nitric oxide synthase (iNOS) and nitric oxide. wikipedia.orgiiab.mefishersci.canih.govitb.ac.iduni.lu Studies using iNOS knockout mice have shown that DEC's activity against Brugia malayi microfilariae is abolished in the absence of iNOS, suggesting a critical role for this enzyme in the drug's mechanism of action. wikipedia.orgfishersci.canih.govuni.lu Inducible nitric oxide synthase produces large amounts of NO as a defense mechanism and is synthesized by various cell types in response to cytokines. wikipedia.org
Inhibition of Nuclear Transcription Factor Kappa B (NF-κB) Activation
Recent studies have indicated that this compound inhibits the activation of nuclear transcription factor Kappa B (NF-κB). wikipedia.orguwm.edu.pl NF-κB is a key regulator of genes involved in inflammatory and immune responses. wikipedia.orgyoutube.com
Impact on Leukocyte Chemotaxis and Granulocyte Degranulation
This compound has been shown to block several steps in both the cyclooxygenase and lipoxygenase pathways, which are involved in the production of inflammatory mediators. This interference includes the inhibition of leukocyte chemotaxis and granulocyte degranulation axonmedchem.comresearchgate.net. By affecting these processes, DEC can modulate the recruitment and activation of immune cells at the site of infection or inflammation. Studies suggest that DEC's influence on arachidonic acid metabolism contributes to its anti-inflammatory properties researchgate.net.
Enhancement of Platelet and Granulocyte Adhesion and Cytotoxic Activity
Evidence suggests that this compound enhances the adherence and cytotoxic activity of host platelets and granulocytes, particularly towards microfilariae researchgate.netnih.govcambridge.orgcolab.ws. This effect is thought to be mediated, in part, by the drug's alteration of arachidonic acid metabolism in both the microfilariae and host endothelial cells nih.govcambridge.org. The resulting changes can lead to amplified endothelial adhesion, contributing to the immobilization of microfilarial parasites nih.govcambridge.org. Enhanced adherence of human neutrophils and eosinophils to tissue culture plastic after incubation with this compound citrate has been observed, with eosinophils showing greater sensitivity researchgate.net. This direct stimulation of host effector cell adherence may partially explain DEC's therapeutic action in vivo researchgate.net. This enhancement of adherence and cytotoxicity by host platelets and granulocytes represents an activation of the innate, non-specific immune system nih.govcambridge.org.
Influence on Eosinophilopoiesis and Eosinophil Accumulation
Research findings related to DEC's influence on eosinophils are summarized in the table below:
| Effect of DEC | Observation | Model/Mechanism | Source |
| Suppression of eosinophil lineage | Reduced eosinophil counts in lungs and blood. | iNOS/CD95L-dependent mechanism | researchgate.netnih.gov |
| Suppression of eosinophilopoiesis | Inhibits IL-5-dependent eosinophilopoiesis in bone marrow. | Direct suppression, iNOS/CD95L dependence | researchgate.netnih.govatsjournals.org |
| Prevention of eosinophil accumulation | Prevents pulmonary eosinophil accumulation in allergic inflammation models. | Impact on hematopoietic response | nih.govatsjournals.org |
| Effect on skin eosinophils | Significantly decreased in a skin allergy model. | Observed in trimellitic anhydride-induced allergy | apjai-journal.org |
Enhancement of Antibody Production and Cytokine Response
This compound has been reported to have immunomodulatory properties, including the enhancement of antibody production and cytokine responses tandfonline.comnih.govnih.gov. Studies in mice challenged with antigens have shown that DEC treatment can enhance cytokine production and antibody production tandfonline.comnih.govnih.gov. Specifically, low-dose DEC treatment enhanced cytokine production in response to a thymus-dependent antigen (tetanus toxoid) and antibody production in response to a thymus-independent antigen (lipopolysaccharide) tandfonline.comnih.gov. These effects suggest a stimulating immunomodulatory role for DEC, influencing both cellular and humoral immune responses tandfonline.comnih.gov. The enhancement of antibody production and cytokine response may contribute to the host's ability to clear parasites nih.gov.
Pharmacokinetic and Pharmacodynamic Research of Diethylcarbamazine
Absorption and Bioavailability Studies
Diethylcarbamazine is readily absorbed following oral administration. who.intdrugbank.com Studies indicate that its bioavailability ranges between 80% and 85%. who.int Following a single oral dose of one this compound tablet in healthy volunteers in a fed state, the mean peak plasma concentration (Cmax) was reported as 598 ± 84 ng/ml, with a corresponding area under the curve (AUC) of 7950 ± 1660 ng.h/ml. who.int The mean time to reach peak plasma concentration (Tmax) was approximately 2.25 ± 1.17 hours. who.int Another study reported a Tmax of 1 to 2 hours and a peak plasma concentration of 80-200 ng/mL following a 50 mg dose. medscape.com The absorption is almost complete via the oral route, with maximum concentrations typically reached 2–3 hours post-administration in a fed state. who.intwho.int this compound tablets are preferably administered after meals. who.intwho.int
Metabolism and Metabolite Identification
This compound is partially metabolized, primarily to this compound N-oxide. drugbank.commedscape.com In rats and monkeys, 10-20% of an intravenous dose is excreted unchanged in the urine within the first 3 hours. who.int Metabolites that are eliminated more slowly include N-ethyl-4-methyl-1-piperazine-carboxamide (MEC) and its N-oxides, 4-methyl-piperazine-carboxamide, and N,N-diethyl-1-piperazine-carboxamide. who.int In vivo, most metabolites, particularly the N-oxides, demonstrate activity against microfilariae, and the N-oxides also show activity against adult worms and infective larvae. who.int While this compound is rapidly metabolized in the liver, producing this compound-N-oxide as a metabolite, this metabolite is considered inactive in some contexts. nih.gov Studies in rats have identified unchanged drug and two metabolites in urine after oral administration of [14C]this compound: an N'-de-ethylation product and the N4-oxide. tandfonline.com The N-oxide has been identified as a major metabolite in rats, and its proportion excreted in urine appears independent of the administered dose. tandfonline.com
Elimination and Half-Life Determination
This compound is primarily eliminated through renal clearance, which accounts for approximately 50% of the total plasma clearance. who.int The remaining clearance occurs via metabolism, leading to the formation of metabolites that are also cleared renally. who.int Urinary excretion, and consequently the plasma half-life, is influenced by urinary pH. who.int About 5% of a dose is excreted in the feces. who.int The reported plasma half-life of this compound is approximately 8 hours. drugbank.commedscape.comnih.gov Other sources suggest a plasma half-life generally ranging from 6 to 12 hours. who.intwho.int In a study involving healthy volunteers, the elimination half-life was approximately 12 hours. who.int In rats, a mean elimination half-life of around 1.5 hours was observed after oral administration of 200 mg/Kg. researchgate.net Elimination half-life is prolonged and AUC is increased in alkaline urine, which may necessitate dose reductions in patients with diets that promote urinary alkalinization. aap.org
Pharmacokinetic Variability and Covariates
High inter-individual variability (IIV) in the pharmacokinetics of anti-filarial drugs, including this compound, has been reported in mass drug administration (MDA) campaigns. unmc.edu Addressing this variability is important for optimizing dosing to maximize efficacy and limit toxicities. unmc.edu Plasma levels of DEC have shown relatively little variation among individuals compared to other co-administered drugs like albendazole and ivermectin, which is likely due to DEC's good bioavailability. nih.gov
Influence of Body Weight and Gender
Body weight and gender have been identified as significant covariates influencing the pharmacokinetics of this compound, particularly affecting the volume of distribution (V/F). researchgate.netnih.govnih.govresearchgate.netasm.org Population pharmacokinetic modeling has indicated that body weight significantly impacts drug exposure in both male and female populations. nih.govresearchgate.netasm.org One study observed a correlation between body weight and gender over the volume of distribution. researchgate.netnih.gov Model-based simulations have demonstrated that body weight and sex affect drug exposure parameters, including Cmax and AUC. unmc.edu While DEC dosing is often dependent on body weight, intersubject variations in pharmacokinetic parameters could be related to the degree of dose adjustment based on weight. researchgate.netnih.gov The Cmax for DEC was found to be higher in female participants compared to male participants in one study. nih.govplos.org
Impact of Infection Status
Studies have investigated the impact of infection status on this compound pharmacokinetics. In an open-label cohort study involving adults infected with Wuchereria bancrofti and uninfected individuals, moderate to heavy W. bancrofti infection did not significantly affect the pharmacokinetic parameters for DEC following a single co-administered dose with albendazole and ivermectin, compared to uninfected individuals. researchgate.netplos.orgresearchgate.net There was no significant difference in AUC0-inf or Cmax between LF-infected and uninfected participants. plos.orgresearchgate.net The inclusion of infection status as a covariate in population pharmacokinetic models did not appear to improve data fitting in one study. nih.gov
Population Pharmacokinetic Modeling
Population pharmacokinetic (PopPK) modeling is a valuable tool for understanding the pharmacokinetic behavior of this compound and identifying sources of variability. nih.gov PopPK models for DEC have been developed using software such as Phoenix NLME. researchgate.netnih.govnih.govresearchgate.netasm.org These models typically employ a one-compartment linear pharmacokinetic model with first-order absorption and an absorption lag time (Tlag) to describe the data. researchgate.netnih.govnih.govresearchgate.netasm.org Covariates considered in model building have included age, gender, body weight, infection status, creatinine clearance (CLCR), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels. researchgate.netnih.govnih.govresearchgate.netasm.org
A final population pharmacokinetic model adequately described the pharmacokinetics of DEC in studied populations. nih.govresearchgate.netasm.org This modeling has confirmed the significant influence of body weight and gender on the volume of distribution of DEC. nih.gov Model-based simulations have further indicated the significant impact of body weight on exposure in both sexes. nih.govresearchgate.netasm.org These analyses can support the development of drug-drug interaction models for DEC when coadministered with other agents in disease control programs. nih.govasm.org The developed models can be used for future studies to optimize drug dosing and contribute to lymphatic filariasis eradication goals. unmc.edu
Drug-Drug Interactions at the Pharmacokinetic Level
Drug interactions involving this compound Citrate are considered relatively rare, although they can occur. patsnap.com DEC may interact with other medications that influence the immune system or share similar metabolic pathways. patsnap.com
Studies have investigated the pharmacokinetic interactions of DEC when co-administered with other drugs commonly used in mass drug administration (MDA) programs for neglected tropical diseases. For instance, a study examining the co-administration of DEC (6 mg/kg) and albendazole (400 mg) in healthy volunteers found that this combination did not significantly alter the pharmacokinetic profile of either drug. who.intresearchgate.net This suggests that co-administration of DEC and albendazole is unlikely to result in adverse pharmacokinetic interactions that would preclude their combined use for lymphatic filariasis control. researchgate.net
Further research has explored the pharmacokinetic interactions in triple-drug therapies. A study investigating the co-administration of ivermectin, this compound, and albendazole (IDA) in adults with and without Wuchereria bancrofti infection in Côte d'Ivoire found no significant differences in the pharmacokinetics of ivermectin, DEC, or albendazole based on infection status. plos.org The geometric mean ratios for Cmax, AUC0–t, and AUC0–∞ for all three drugs were within the conventional acceptance range of 80–125%, indicating a lack of clinically relevant pharmacokinetic drug interactions. plos.orgplos.org
Another study assessed the pharmacokinetic and safety profile of co-administering azithromycin with the IDA regimen. This open-label, randomized study in adult volunteers found no significant drug-drug interactions between the study arms. researchgate.net The geometric mean ratios for the pharmacokinetic parameters (Cmax, AUC0-t, and AUC0-∞) for ivermectin, DEC, albendazole sulfoxide, and azithromycin were within the 80-125% range. researchgate.net
However, it is worth noting that while some studies suggest a lack of significant pharmacokinetic interactions with commonly co-administered anthelmintics, DEC has been listed as having moderate interactions with at least 71 different drugs and mild interactions with at least 101 different drugs in one database. medicinenet.com The clinical significance and pharmacokinetic mechanisms of these potential interactions would require further investigation.
Research into the metabolic pathways of DEC is ongoing. One study investigated the effects of rifampicin (a potent inducer of hepatic cytochrome P450 enzymes) and ketoconazole (an inhibitor of hepatic cytochrome P450 enzymes) on the pharmacokinetics of a single oral dose of DEC in healthy volunteers. The results showed that neither rifampicin nor ketoconazole significantly altered the mean pharmacokinetic parameters of DEC, including Cmax, AUC0-48, AUC0−∝, t1/2, tmax, ka, ke, Vd/F, and Cl/F. psu.edu This study suggested that it was not possible to definitively conclude whether DEC is metabolized via cytochrome P450 enzymes based on these results and indicated that P-glycoprotein (P-gp) does not appear to play a significant role in DEC metabolism. psu.edu
Alkalinization of urine can affect DEC excretion. Studies have shown that the plasma elimination half-life and total urinary excretion of unchanged DEC are significantly less in patients receiving sodium bicarbonate compared to a control group. psu.edu
Pharmacodynamic Correlates of Efficacy
The mechanism of action of this compound is multifaceted and not entirely understood, but it is thought to primarily involve the disruption of the parasitic worms' biological processes. patsnap.com DEC is believed to sensitize microfilariae to the host's immune response, particularly facilitating the action of phagocytes, which aids in the clearance of microfilariae from the bloodstream. patsnap.commedex.com.bd This immune modulation is considered a key aspect of its mechanism. patsnap.com
One proposed mechanism involves DEC altering arachidonic acid metabolism in microfilariae and host endothelial cells. who.intcambridge.org These changes may lead to vasoconstriction and amplified endothelial adhesion, resulting in the immobilization of microfilarial parasites and enhanced adherence and cytotoxic activity by host platelets and granulocytes. who.intcambridge.org This suggests an activation of the innate, non-specific immune system. who.intcambridge.org Studies have shown that DEC's activity against Brugia malayi microfilariae is dependent on inducible nitric-oxide synthase and the cyclooxygenase pathway, confirming the importance of the arachidonic acid metabolic pathway in its in vivo mechanism of action. medex.com.bdwho.int
Beyond its effects on microfilariae, DEC has also been observed to affect the muscular activity of adult worms, potentially leading to paralysis and death. patsnap.com While the mode of action on adult worms is less documented, there is evidence suggesting a macrofilaricidal effect, although it can be inconsistent. who.int Degenerating adult worms have been observed in lymph nodes after treatment. who.int A single dose of DEC at 6 mg/kg body weight is estimated to inactivate 50% to 80% of adult worms. nih.gov
Pharmacodynamic effects of DEC include a decrease of microfilariae in the blood. who.int This reduction in blood microfilariae is a standard measure of efficacy in lymphatic filariasis. who.int Studies have shown that a single dose of DEC can be as effective as a 12-day treatment regimen in clearing microfilariae from the blood. who.int The improved efficacy of two-drug regimens, such as DEC combined with albendazole, in reducing microfilaraemia in endemic populations supports their use in MDA programs. who.int
In the context of loiasis, while successful treatment with DEC has been reported, understanding the long-term success and factors for treatment failure is ongoing. psu.edu Antifilarial IgG antibody levels have been explored as a marker, but their utility in discriminating between individuals likely to be cured or experience relapse has been limited. psu.edu
Research into the efficacy of DEC extends beyond filarial infections. A study evaluating DEC for the treatment of allergic rhinitis showed statistically significant improvement in various parameters, including symptoms, absolute eosinophil count, serum total IgE, and skin prick tests, suggesting its potential effectiveness in this condition. researchgate.netnih.gov DEC's anti-inflammatory properties, potentially resulting from interference with arachidonic acid metabolism, may contribute to its effects in allergic conditions. researchgate.net
Clinical Efficacy Research and Outcomes
Efficacy in Lymphatic Filariasis
Diethylcarbamazine (DEC), introduced in 1947, has demonstrated significant efficacy in the treatment of human lymphatic filariasis, primarily caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori. jst.go.jpwikipedia.orgoup.comnih.gov Research has focused on optimizing its use and understanding its impact on the parasite and disease transmission. jst.go.jp
Microfilaricidal Efficacy
DEC is recognized as an effective microfilaricidal agent against the lymphatic-dwelling filariae. oup.comnih.gov Studies have consistently shown that DEC treatment leads to substantial reductions in the prevalence and density of microfilariae (mf) in the blood. nih.govnih.govoup.com For instance, mass treatment with DEC-medicated salt has resulted in large reductions in microfilariae prevalence, ranging from 43% to 100%, in studies with high coverage levels. nih.gov Significant decreases in microfilariae density have also been observed. nih.gov A study using DEC-medicated salt for one year reported a 99.5% reduction in mf density. nih.gov Another study in Haiti showed a 95% reduction in mf prevalence after one year of treatment. nih.gov
Adulticidal Efficacy
While primarily known for its microfilaricidal effects, research indicates that DEC can also damage or kill adult filarial worms, although this effect can be inconsistent. jst.go.jpoup.comnih.govijdvl.comwho.int Studies suggest that conventional dosages of DEC can effectively kill adult worms in many patients infected with W. bancrofti, B. malayi, and B. timori. oup.comnih.gov Ultrasonography studies have shown that even single doses of DEC can kill adult worms when they are susceptible. ijdvl.comoup.com However, a significant proportion of adult W. bancrofti may not be susceptible to DEC. oup.com Histological evidence also supports an adulticidal effect of low-dose DEC in bancroftian filariasis. oup.com
Impact on Disease Transmission
The sustained reduction of microfilariae levels by DEC is crucial for preventing the transmission of lymphatic filariasis. ijdvl.com By significantly decreasing the number of circulating microfilariae, which are the stage transmitted by mosquitoes, DEC treatment helps to interrupt the parasite's life cycle. nih.govnih.gov Studies have shown that mass administration of DEC can lead to substantial reductions in vector infection and infectivity rates. nih.govoup.comnih.gov For example, ten rounds of mass DEC administration in south India resulted in a 91% reduction in vector infection rates and an 89% reduction in vector infectivity rates. oup.comnih.gov These reductions in microfilaremia and vector infection contribute significantly to the interruption of transmission. nih.govoup.com
Effectiveness of Single-Dose Regimens
Single-dose regimens of DEC have been investigated for their effectiveness, particularly in mass drug administration (MDA) programs. jst.go.jpnih.govwho.intresearchgate.netnih.gov Studies have indicated that a single dose of DEC can be as effective as multi-dose regimens in clearing microfilariae from the blood. ijdvl.comwho.int Annual single-dose treatments with DEC have been reported to be effective in reducing microfilariae rate and density, making them applicable for large-scale control campaigns. researchgate.net Research in Brazil demonstrated a marked reduction in mf, circulating filarial antigen (CFA), and antifilarial antibodies in individuals infected with W. bancrofti after repeated rounds of annual single-dose DEC. nih.gov A meta-analysis across studies also suggested that a single dose is as effective as a 12-day treatment regimen for microfilariae clearance. who.int
Efficacy of Spaced-Dose and Low-Dose Regimens
Spaced-dose and low-dose DEC regimens have also been explored for their efficacy. jst.go.jpoup.com Studies suggest that spaced doses of DEC (weekly or monthly) may be more effective than the same total dosage given in consecutive daily doses for killing adult worms. oup.comnih.gov Chronic administration of low-dose DEC, such as through medicated salt, has been shown to effectively control filariasis caused by W. bancrofti or B. malayi. oup.comnih.govnih.gov This approach has demonstrated large and consistent reductions in microfilariae prevalence and density in endemic communities. nih.gov
Combination Chemotherapy Studies
Combination chemotherapy involving DEC and other antifilarial drugs has been a significant area of research to enhance efficacy and accelerate elimination efforts. jst.go.jpoup.comoup.comajtmh.orgnih.govoup.comwustl.edumdpi.comrjpbr.complos.orgmedrxiv.orgplos.orgplos.orgwustl.eduoup.comnih.gov Combinations of DEC with albendazole (ALB) or ivermectin (IVM), or a triple combination of IVM, DEC, and ALB (IDA), have been studied. jst.go.jpoup.comoup.comajtmh.orgnih.govoup.comwustl.edumdpi.comrjpbr.complos.orgmedrxiv.orgplos.orgplos.orgwustl.eduoup.comnih.gov
Studies have indicated that drug combinations containing DEC are highly effective against microfilarial prevalence and intensity compared to single drugs or other combinations. nih.gov Combined treatment with DEC plus ALB has shown significant reductions in microfilarial intensity. nih.gov
More recently, the triple-drug combination (IDA) has demonstrated superior efficacy in clearing microfilariae compared to the two-drug regimen of DEC and ALB. ajtmh.orgoup.comwustl.eduplos.orgmedrxiv.orgplos.orgplos.orgoup.comnih.gov Clinical trials have shown that a single dose of IDA is significantly more effective for clearing W. bancrofti blood microfilariae than single or two annual doses of DEC/ALB. nih.gov For example, one study reported that 96% of participants treated with IDA were amicrofilaremic at 24 months, compared to 56% after a single dose of DEC/ALB and 75% after two annual doses of DEC/ALB. nih.gov Another study showed that one year after a single round of MDA, IDA cleared microfilariae in 96.3% of infected participants, compared to 83.6% in the DA arm. plos.org In Haiti, MDA with IDA was significantly more effective for clearing microfilariae compared to the standard DA regimen. plos.org
While IDA shows enhanced microfilaricidal efficacy, the impact on clearing filarial antigenemia (suggesting adult worm burden) can be less pronounced and similar between combination regimens. plos.orgplos.orgwustl.edunih.gov
Here is a summary of some research findings on the efficacy of DEC-containing regimens:
This compound with Albendazole
The combination of this compound and albendazole has been a widely used two-drug regimen for mass drug administration (MDA) programs aimed at eliminating lymphatic filariasis (LF). Clinical trials have investigated the efficacy of this combination in clearing microfilariae (Mf) from the blood of infected individuals. Studies have shown that while effective, this dual therapy may not achieve the same sustained clearance rates as newer triple-drug regimens in some settings. For instance, clinical trials in Papua New Guinea (PNG) demonstrated that a single dose of the triple-drug combination (IDA) was more effective than the DEC plus albendazole (DA) combination for achieving sustained clearance of Wuchereria bancrofti Mf. oup.com In a study in Côte d'Ivoire, IDA was more effective for clearing W. bancrofti Mf and killing adult worms than ivermectin plus albendazole (IA), but the Mf clearance effect was not as long-lasting as seen in studies in PNG or Indonesia. oup.com However, a single treatment with IDA was found to be equivalent to two annual treatments with IA in that setting. oup.com
A community-based cluster-randomized trial in a moderately endemic, treatment-naive area in PNG compared the safety and efficacy of IDA and DA for LF. The study found that IDA was more effective for clearing microfilariae compared to DA. plos.org Similarly, a study in Haiti showed that significantly more participants who were Mf positive at baseline became Mf negative one year after treatment with IDA (94.4%) compared to after DA (75.9%). plos.org
| Study Location | Regimen | Outcome Measured | Key Finding | Citation |
| Papua New Guinea | IDA vs. DA | Mf Clearance (sustained) | IDA more effective than DA. | oup.com |
| Côte d'Ivoire | IDA vs. IA | Mf Clearance | IDA more effective than IA, but effect not as long-lasting as in PNG/Indonesia. | oup.com |
| Côte d'Ivoire | IDA vs. IA | Adult Worm Killing | IDA more effective than IA. | oup.com |
| Côte d'Ivoire | Single IDA vs. Two annual IA | Mf Clearance | Single IDA equivalent to two annual IA. | oup.com |
| Papua New Guinea | IDA vs. DA | Mf Clearance | IDA more effective than DA in a community-based trial. | plos.org |
| Haiti | IDA vs. DA | Mf Clearance (1 year) | Significantly more Mf negative after IDA (94.4%) vs DA (75.9%). | plos.org |
Triple-Drug Therapy (this compound, Albendazole, Ivermectin)
The triple-drug combination of ivermectin, this compound, and albendazole (IDA) has emerged as a significantly more effective regimen for accelerating the elimination of lymphatic filariasis in areas not co-endemic for onchocerciasis or loiasis. oup.comwustl.edu Clinical trials have demonstrated the superiority of a single dose of IDA compared to the two-drug combinations (DEC plus albendazole or ivermectin plus albendazole) for clearing larval filarial parasites from the blood of infected persons. oup.com
Studies in heavily infected individuals in PNG showed that IDA was more effective than the DEC and albendazole combination for achieving sustained clearance of Wuchereria bancrofti Mf, with 96% of IDA recipients being Mf negative three years after a single dose. oup.com Follow-up studies indicated that most individuals in this trial remained amicrofilaremic nearly five years after a single dose of IDA. oup.com Similar results, albeit with shorter follow-up periods, were observed in a clinical trial of IDA in individuals with Brugia infection. oup.com
A large multicenter community study involving over 26,000 people in five countries (India, Papua New Guinea, Haiti, Indonesia, and Fiji) provided evidence supporting the use of IDA for LF elimination programs. wustl.edu This study contributed to the World Health Organization's endorsement of IDA for use in LF elimination programs. wustl.edu A systematic review and meta-analysis concluded that triple therapy caused more clearance of microfilaria in the blood compared to dual therapy 12 months after drug administration. nih.gov Another systematic review highlighted that IDA reduces microfilaria formation more than the DEC and albendazole combination. warmadewa.ac.id
| Study/Analysis | Regimen | Outcome Measured | Key Finding | Citation |
| Clinical Trials (PNG) | Single-dose IDA vs. Single-dose DA | Sustained Mf Clearance | IDA more effective; 96% Mf negative at 3 years with IDA. | oup.com |
| Clinical Trials (PNG) | Single-dose IDA | Long-term Mf Clearance | Most individuals remained amicrofilaremic nearly 5 years post-treatment. | oup.com |
| Clinical Trial (Côte d'Ivoire) | Single-dose IDA vs. Single-dose IA | Mf Clearance (12 months) | 71% Mf negative after IDA vs. 26% after IA. | oup.com |
| Multicenter Community Study | IDA vs. DA | Efficacy | Supported the use of IDA for LF elimination. | wustl.edu |
| Systematic Review & Meta-analysis | Triple Therapy vs. Dual Therapy | Mf Clearance (12 months) | Triple therapy caused more clearance of microfilaria. | nih.gov |
| Systematic Review | IDA vs. DEC + Albendazole | Microfilaria Formation | IDA reduces microfilaria formation more than DEC + Albendazole. | warmadewa.ac.id |
Efficacy in Loiasis
This compound is considered the gold standard for the curative treatment of loiasis, a filarial infection caused by Loa loa. loaloa.org It exhibits activity against both the adult worms (macrofilaricidal) and the microfilariae (microfilaricidal). loaloa.org DEC is thought to act by inducing paralysis in the macro- and microfilariae. loaloa.org
Studies have reported successful treatment of loiasis with DEC. psu.edunih.gov In a long-term follow-up study of expatriate visitors to endemic regions, using a strict definition of successful treatment, 38% of patients appeared to be cured after one course of therapy, and an additional 16% were cured after two courses. psu.edunih.govoup.com However, some patients continued to be symptomatic despite receiving multiple courses of treatment. psu.edunih.govoup.com The resolution of signs and symptoms and the demonstration of necrotic adult parasites in biopsies from treated individuals suggest that DEC is effective in killing adult parasites. psu.edu Experimental studies in animals infected with L. loa also found dead and moribund adult worms only in DEC-treated animals, although complete cure was not achieved in all treated animals. psu.edu
Despite its activity, DEC monotherapy may not always be successful, and multiple courses may be required to achieve clinical and parasitological cure. plos.org A retrospective study found that only 50% of patients treated with DEC alone achieved a parasitological cure, while another study reported a cure rate of 73%. plos.org A long-term follow-up study in non-endemic countries showed an efficacy of only 38% after a single treatment course. plos.org Combinations of DEC with ivermectin or albendazole did not improve the proportion of parasitological cure in one study. plos.org
Research has also explored DEC as a chemoprophylactic agent for loiasis. A randomized, double-blind, placebo-controlled trial involving temporary residents in endemic areas demonstrated that weekly oral DEC significantly reduced the incidence of clinical disease and the rate of seroconversion for antifilarial IgG antibody compared to placebo. nih.gov
| Study/Context | Treatment Regimen | Outcome Measured | Key Finding | Citation |
| Treatment of Loiasis (General) | DEC | Macro- and Microfilaricidal Activity | Considered gold standard; active against adult worms and microfilariae. | loaloa.org |
| Long-term Follow-up (Expatriates) | DEC (one course) | Cure Rate | 38% appeared cured after one course. | psu.edunih.govoup.com |
| Long-term Follow-up (Expatriates) | DEC (two courses) | Cure Rate (additional) | Additional 16% cured after two courses. | psu.edunih.govoup.com |
| Experimental Study (L. loa in drills) | DEC | Adult Worm Viability | Dead and moribund adult worms found in treated animals. | psu.edu |
| Retrospective Study (TropNet) | DEC alone | Parasitological Cure Rate | Only 50% achieved cure in one study; 73% in another; 38% in long-term follow-up in non-endemic areas. | plos.org |
| Retrospective Study (TropNet) | DEC + IVM or ALB | Parasitological Cure Rate | Combinations did not improve cure rate compared to DEC alone. | plos.org |
| Chemoprophylaxis Trial (Temporary Residents) | Weekly Oral DEC | Incidence of Clinical Disease & Seroconversion | Significantly reduced clinical disease and seroconversion compared to placebo. | nih.gov |
Efficacy in Tropical Pulmonary Eosinophilia
Tropical Pulmonary Eosinophilia (TPE) is a clinical syndrome characterized by paroxysmal cough, dyspnea, wheezing, eosinophilia, elevated serum IgE, and pulmonary infiltrates, often occurring in individuals from filarial endemic regions. nih.govijcdas.commja.com.au this compound is the treatment of choice for TPE and is known for its dramatic effect on the clinical features of the condition. nih.gov
Studies have consistently shown a good response to DEC treatment in most patients with TPE. nih.gov Clinical criteria, including residence or travel history to a filarial endemic region, characteristic respiratory symptoms, eosinophilia, elevated IgE and filarial antibodies, and pulmonary infiltrations, along with a positive response to DEC, are used for diagnosis. nih.govijcdas.com
Research indicates that DEC is active against both microfilariae and adult worms, contributing to the rapid alleviation of symptoms. nih.gov In a case series, all but one patient treated with DEC responded and reported resolution of presenting symptoms. oup.com Another study reported marked symptomatic improvement in almost all patients following a three-week course of DEC. ersnet.org Symptoms decreased rapidly, and eosinophil counts significantly reduced after treatment. mja.com.auersnet.org
Despite the generally good clinical response, mild interstitial lung disease may persist in some patients even after treatment. nih.gov Studies have also indicated a relapse rate of approximately 20% within 5 years. nih.gov
| Study/Context | Treatment Regimen | Outcome Measured | Key Finding | Citation |
| Treatment of TPE (General) | DEC | Clinical Features | Dramatic response observed. | nih.gov |
| Case Series (Nonendemic Setting) | DEC | Symptom Resolution | All but one patient responded with symptom resolution. | oup.com |
| Retrospective Study (Bihar, India) | DEC (3 weeks) | Symptomatic Improvement | Marked improvement in almost all patients (96% clinical response). | ersnet.org |
| Case Report (Australia) | DEC (14 days) | Symptom Decrease & Eosinophil Count | Symptoms decreased rapidly, eosinophil count nearly normal by 4 weeks. | mja.com.au |
| Long-term Outcome | DEC | Residual Disease & Relapse | Mild interstitial lung disease may persist; ~20% relapse rate in 5 years. | nih.gov |
Research on Efficacy in Onchocerciasis (with caveats for adverse reactions)
Research has explored the efficacy of this compound in the treatment of onchocerciasis (Onchocerca volvulus infection), but its use is significantly limited by severe adverse reactions. psu.eduird.frfrontiersin.org DEC treatment in onchocerciasis leads to the destruction of microfilariae in the subcutaneous tissues. nih.govajtmh.org
Early controlled clinical trials compared oral and topical DEC for onchocerciasis. In one trial, oral DEC quickly reduced the number of microfilariae per skin snip to 2% of initial levels, while topical DEC reduced them to 20%. nih.gov However, these treatments were accompanied by common side-effects, which were more frequent with topical DEC. nih.gov
The destruction of microfilariae by DEC in individuals with onchocerciasis frequently triggers a complex of severe allergic reactions known as the Mazzotti reaction. ird.frfrontiersin.orgajtmh.org These reactions can be severe and even life-threatening, particularly in heavily infected individuals. frontiersin.orgwho.int Due to the propensity to induce these adverse drug reactions, DEC at regular doses used for other filarial infections is clearly contraindicated in onchocerciasis. unilag.ng
Studies evaluating topical application of DEC to limit severe reactions had mixed results, with some finding almost complete clearance of microfilariae from the skin in lightly infected patients, while others reported that adverse effects were even more marked with topical application than oral treatment in heavily infected patients. ird.fr Further studies showed that even at very low doses, DEC still induced serious reactions, and microfilarial counts were not significantly altered despite some clinical evidence of microfilariae killing. who.int
The severe side-effects and the risk of aggravating ocular lesions have rendered DEC largely redundant in the treatment of onchocerciasis, especially for mass treatment programs. frontiersin.orgwho.int While some studies explored strategies like pretreatment with ivermectin to reduce microfilarial counts before administering IDA (which includes DEC) for onchocerciasis, the primary concern regarding DEC's direct use in onchocerciasis due to the Mazzotti reaction remains. plos.org
| Study/Context | Treatment Regimen | Outcome Measured | Key Finding | Citation |
| Controlled Clinical Trial | Oral DEC | Skin Microfilariae Reduction | Reduced counts to 2% of initial levels. | nih.gov |
| Controlled Clinical Trial | Topical DEC | Skin Microfilariae Reduction | Reduced counts to 20% of initial levels. | nih.gov |
| General Context (Onchocerciasis Treatment) | DEC | Microfilariae Destruction | Destroys microfilariae in subcutaneous tissues. | ajtmh.org |
| General Context (Onchocerciasis Treatment) | DEC | Adverse Reactions (Mazzotti) | Frequently accompanied by severe allergic reactions, limiting usefulness and contraindicated at regular doses in co-endemic areas. | ird.frfrontiersin.orgajtmh.orgwho.intunilag.ng |
| Topical Application Studies | Topical DEC | Microfilariae Clearance & AEs | Mixed results; some clearance but adverse effects potentially more marked than oral in heavily infected; serious reactions even at low doses. | ird.frwho.int |
Exploratory Research for Novel Therapeutic Applications
Beyond its established uses in treating filarial infections, exploratory research has investigated novel therapeutic applications for this compound, leveraging its pharmacological properties.
Allergic Rhinitis
Exploratory research has suggested this compound as a potential drug for the treatment of allergic rhinitis. nih.govnih.govresearchgate.netjcdronline.org Studies have evaluated its effectiveness in managing the symptoms of allergic rhinitis, particularly in patients with associated eosinophilia. jcdronline.org
A double-blind randomized controlled trial compared the efficacy of this compound with a combination of montelukast and levocetirizine in patients with allergic rhinitis. The study found statistically significant improvement in various parameters, including symptoms, absolute eosinophil count, serum total IgE, and response in skin prick tests, in both treatment groups. nih.govnih.govresearchgate.net Notably, the improvement was reported to be better with this compound compared to the montelukast and levocetirizine combination, and the effects were sustained for three months in the this compound group. nih.govnih.govresearchgate.net
Another study specifically investigated the role of this compound in managing allergic rhinitis with eosinophilia. This descriptive study found that a significant proportion of the study population with allergic rhinitis exhibited eosinophilia. jcdronline.org Treatment with this compound demonstrated utility in reducing symptom scores in these patients and significantly decreased eosinophil scores in both blood and nasal tissue. jcdronline.org These findings suggest its potential as an adjunctive treatment for allergic rhinitis with eosinophilia. jcdronline.org
This compound is thought to exert its effect in allergic rhinitis through various pharmacological actions, including being a potent leukotriene inhibitor and decreasing eosinophil synthesis and numbers, which may reduce the response to allergens. nih.gov
| Study/Context | Treatment Regimen | Outcome Measured | Key Finding | Citation |
| Double-Blind Randomized Controlled Trial | This compound vs. Montelukast + Levocetirizine | Symptoms, Eosinophil Count, IgE, Skin Prick Test | Statistically significant improvement in both groups; better improvement with DEC, sustained for 3 months. | nih.govnih.govresearchgate.net |
| Descriptive Study (Allergic Rhinitis w/ Eosinophilia) | This compound | Symptom Control, Blood & Nasal Eosinophilia | Reduced symptom scores and significantly decreased eosinophil counts in blood and nasal tissue in patients with eosinophilia. | jcdronline.org |
| Proposed Mechanism of Action | This compound | Leukotriene Inhibition, Eosinophil Reduction | Potent leukotriene inhibitor; decreases eosinophil synthesis and numbers, potentially reducing allergen response. | nih.gov |
Acute Lung Inflammation Models
Studies utilizing experimental models of acute lung inflammation have provided evidence for the anti-inflammatory effects of this compound. In a mouse model of acute inflammation induced by carrageenan, DEC administration led to reductions in lung injury, polymorphonuclear cell (PMN) migration, nitric oxide (NO) production, and the release of proinflammatory cytokines and COX-2. researchgate.netnih.gov These findings suggest that DEC may be a potential therapeutic agent for acute lung inflammation. nih.gov
Further research in mice demonstrated that DEC attenuates lipopolysaccharide (LPS)-induced acute lung injury by promoting the apoptosis of inflammatory cells. researchgate.net This pro-apoptotic activity was associated with the inactivation of nuclear factor kappa-B (NF-κB). researchgate.net DEC treatment reversed histological and ultrastructural changes, reduced intense cell infiltration and pulmonary edema, and decreased levels of myeloperoxidase (MPO) and NO in LPS-induced lung injury. researchgate.net The study indicated that DEC increased the expression of pro-apoptotic proteins in both intrinsic (Bax, cytochrome c, and caspase-9) and extrinsic (Fas, FADD, and caspase-8) apoptotic pathways, while reducing the expression of the anti-apoptotic protein Bcl-2. researchgate.net These results suggest that DEC may accelerate the resolution of inflammation by stimulating apoptosis of inflammatory cells, highlighting its potential as a pharmacological treatment for acute lung injury. researchgate.net
In a murine model of asthma sensitized with ovalbumin, DEC demonstrated an anti-allergic effect by decreasing cellular infiltration, cytokine levels (IL-4 and IL-5), IgE, eosinophil peroxidase (EPO), and eotaxin2. semanticscholar.org This suggests a role for DEC in blocking pulmonary eosinophilic inflammation. nih.govsemanticscholar.org
Data from studies on acute lung inflammation models:
| Model Type | Inflammatory Stimulus | Key Findings | Source |
| Mouse Model of Acute Inflammation | Carrageenan | Reduced lung injury, PMN migration, NO production, proinflammatory cytokines, and COX-2. Inhibited NF-κB activation. | researchgate.netnih.gov |
| Mouse Model of Acute Lung Injury | LPS | Attenuated lung injury, reduced cell infiltration, pulmonary edema, MPO, and NO. Promoted inflammatory cell apoptosis via NF-κB inactivation. | researchgate.net |
| Murine Model of Asthma | Ovalbumin | Decreased cellular infiltration, IL-4, IL-5, IgE, EPO, and eotaxin2. Blocked pulmonary eosinophilic inflammation. | semanticscholar.org |
Potential Role in Other Inflammatory Conditions
Beyond lung inflammation, the anti-inflammatory properties of this compound have been investigated in other experimental models. Research indicates that DEC interferes with the arachidonic acid metabolism, affecting lipoxygenase (LOX) and cyclooxygenase (COX) enzymes. researchgate.net This interference can lead to a reduction in the production of various inflammatory mediators, including thromboxane, prostacyclin, prostaglandins, and leukotrienes. researchgate.net
Studies have shown that DEC can inhibit nuclear transcription factor kappa B (NF-κB) activation, a key regulator of proinflammatory genes such such as TNF-α, IL-1β, inducible nitric oxide synthase (iNOS), and COX-2. researchgate.net This inhibitory effect on NF-κB has been observed in different experimental models of inflammation. researchgate.net
In models of hepatic inflammation and fibrosis induced by carbon tetrachloride (CCl4), DEC treatment has been shown to reduce chronic inflammation and fibrosis by decreasing the expression of inflammatory markers such as IL-1β, COX-2, NF-κB, interferon-γ, and TGF-β. nih.gov DEC treatment in these models led to a reduction in inflammatory infiltrates, liver necrosis, and fibrosis. nih.gov
In an experimental model of alcoholic liver disease, DEC was found to be effective at attenuating proinflammatory cytokines, oxidative stress, and necrosis, suggesting a potential therapeutic use in liver inflammation. researchgate.net Additionally, DEC has shown a role in reducing hepatic cell damage in malnourished mice. researchgate.net
In an isoproterenol-induced acute myocardial infarction (AMI) rat model, DEC treatment significantly suppressed inflammation, iNOS, TGF-β1, COX-2, and PARP protein expression. spandidos-publications.com This suggests a protective effect of DEC in inhibiting NF-κB activation through the PARP pathway in this model. spandidos-publications.com
Potential Role in COVID-19 (Repurposing Studies)
Given its established anti-inflammatory and immunomodulatory activities, this compound has been proposed as a potential repurposed drug for the treatment of COVID-19, particularly focusing on its potential role in addressing COVID-19-related pulmonary fibrosis. scienceopen.comnih.gov
The hypothesis for repurposing DEC for COVID-19 is based on several factors, including its inhibitory effects on arachidonic acid metabolism, which can influence prostaglandin production, and its demonstrated anti-inflammatory actions in animal models of lung inflammation. arxiv.orgresearchgate.net It is postulated that the immunologic effect and enhancement of antibody production by DEC, potentially through the inhibition of LOX and COX enzymes, could contribute to an anti-COVID-19 effect. researchgate.netfrontiersin.org
Furthermore, the anti-fibrotic activity of this compound observed in other inflammatory models makes it a potential candidate for treating the pulmonary fibrosis that can occur as a consequence of COVID-19. scienceopen.comnih.gov While the potential of DEC for COVID-19 treatment has been hypothesized and supported by its known mechanisms and effects in inflammatory models, experimental and clinical studies are needed to assess this possible application. scienceopen.comnih.gov
Data related to the potential role of DEC in COVID-19 (Repurposing Studies):
| Area of Potential Benefit in COVID-19 | Proposed Mechanism(s) | Supporting Evidence (from other models) | Current Status | Source |
| Lung Inflammation | Inhibition of arachidonic acid metabolism, LOX and COX enzymes, NF-κB inactivation. | Reduced lung injury, inflammatory cell infiltration, cytokine production in acute lung inflammation models. researchgate.netnih.govresearchgate.net | Hypothesis for repurposing; requires specific COVID-19 studies. | arxiv.orgresearchgate.net |
| Pulmonary Fibrosis | Anti-fibrotic activity. | Reduced fibrosis in models of liver injury. nih.gov | Potential candidate; requires experimental and clinical assessment in the context of COVID-19. | scienceopen.comnih.gov |
| Immune Modulation | Enhancement of antibody production, influence on cytokine responses. | Enhanced antibody production and cytokine response in animal models. arxiv.org | Postulated mechanism for anti-COVID-19 effect; requires further investigation. | researchgate.netfrontiersin.org |
Drug Resistance and Susceptibility Studies
Observed Reductions in Diethylcarbamazine Susceptibility
Evidence suggests that the susceptibility of filarial nematodes to this compound may not always be 100% seq.es. While confirmed reports of resistance in Wuchereria bancrofti have not been widely provided, there is growing evidence of reduced susceptibility in filarial nematodes seq.es. Detailed studies on DEC resistance are challenging due to a limited understanding of its precise mechanism of action seq.es. However, the persistence of parasites after treatment over an extended period can serve as parasitological evidence of anthelmintic drug resistance seq.es. Studies involving DEC-medicated salt, while generally showing significant reductions in microfilariae prevalence, have also indicated that transmission can continue, suggesting the presence of an infection reservoir nih.gov. For instance, one study reported an 85% reduction in microfilariae prevalence after six months of DEC-medicated salt intervention, but this reduction decreased to 32% after eight years, highlighting potential recrudescence or ongoing transmission due to less susceptible parasites nih.gov.
Mechanisms of this compound Resistance
The exact mechanisms by which filarial parasites develop resistance to this compound are not yet fully understood, partly due to the limited knowledge of DEC's mode of action seq.es. Historically, DEC was thought to primarily act on the host immune system, rather than directly on the parasite nih.govresearchgate.net. However, recent research indicates that low concentrations of DEC can have direct effects on Brugia malayi parasites, causing rapid, temporary spastic paralysis by opening Transient Receptor Potential (TRP) channels, including TRP-2 subunits, in their muscle cells nih.govasm.org. This direct action leads to calcium entry and activation of calcium-dependent SLO-1 potassium channels nih.govasm.org. Recovery from this temporary paralysis is consistent with the inactivation of TRP channels nih.gov.
While the direct mechanisms of resistance are still under investigation, insights from resistance to other anthelmintics like benzimidazoles and ivermectin in nematodes suggest potential avenues. For instance, resistance to benzimidazoles is often associated with specific mutations in the gene encoding β-tubulin seq.esnih.gov.
Role of Genetic Variation in Parasite Populations
Genetic variation within parasite populations can significantly influence drug efficacy and contribute to the development and spread of drug resistance seq.esnih.gov. Genetic diversity can arise from the mixing of genetically different populations, and drug pressure from anthelmintic treatments can also play a significant role in increasing this diversity by selecting for resistant alleles seq.es. Studies using techniques like random amplified polymorphic DNA (RAPD) have shown the existence of genetic variability in W. bancrofti populations seq.es. Higher genetic variability has been detected in densely populated, urban areas, consistent with the expectation that higher transmission rates and larger parasite population sizes may increase genetic diversity nih.gov. The introduction of new drugs or widespread use of existing ones can lead to the selection of alleles encoding resistance, impacting the drug's efficacy against the parasite seq.es.
Single Nucleotide Polymorphisms (SNPs)
Single nucleotide polymorphisms (SNPs) are variations in a single nucleotide base within a DNA sequence nih.gov. While research on specific SNPs directly conferring this compound resistance in filarial nematodes is limited, studies on resistance to other anthelmintics highlight the potential role of SNPs in drug resistance mechanisms nih.govresearchgate.net. For example, specific mutations, which are essentially SNPs, in the β-tubulin gene have been associated with benzimidazole resistance in nematodes seq.es. Research on SNPs in other contexts, such as in drug-resistant genes in Plasmodium falciparum, indicates that recombination and selection can play important roles in shaping the evolution of drug resistance plos.org. Further research is needed to identify specific SNPs in filarial nematodes that may be associated with reduced susceptibility or resistance to this compound.
Strategies to Mitigate Resistance
Addressing the potential for this compound resistance requires proactive strategies to preserve the effectiveness of available treatments. A review of resistance mechanisms to anthelmintics is considered necessary to optimize treatment for human lymphatic filariasis seq.es.
Combination Therapies to Overcome Resistance
Combination therapy is a key strategy to mitigate the development of drug resistance and improve treatment efficacy nih.govwikipedia.orgfishersci.sefabad.org.trresearchgate.netasm.org. By using multiple drugs with different mechanisms of action, the likelihood of parasites being resistant to all components of the combination simultaneously is reduced. The Global Programme to Eliminate Lymphatic Filariasis (GPELF) currently recommends annual treatment of entire communities with combinations of drugs, such as this compound and albendazole, or ivermectin and albendazole, depending on the co-endemicity of onchocerciasis seq.esmdpi.comwustl.edu.
Recent studies have explored the efficacy of triple-drug therapy involving ivermectin, this compound, and albendazole (IDA) oup.comwustl.edumedrxiv.org. A single dose of this combination has shown superior efficacy in clearing W. bancrofti microfilariae compared to two-drug regimens oup.comwustl.edu. Mathematical models suggest that drug regimens with better activity against microfilariae, like the triple therapy, could significantly improve the chances for eliminating LF oup.com. Furthermore, research indicates potential synergistic effects between this compound and other anthelmintics like emodepside, which targets SLO-1 K+ channels in nematodes asm.orgasm.org. Combinations of DEC and emodepside have shown synergistic effects on muscle membrane potentials and long-term muscle paralysis in Brugia malayi, suggesting their potential in combination therapies to enhance efficacy and potentially overcome resistance asm.orgasm.org.
Data Table: Microfilariae Prevalence Reduction with DEC-Medicated Salt
| Intervention Duration | Follow-up Period | Initial Microfilariae Prevalence Reduction | Reduction After Follow-up |
| 6 months (DEC-medicated salt) | 8 years | 85% | 32% |
Data derived from a study on DEC-medicated salt nih.gov.
Data Table: Microfilariae Clearance with Different Regimens
| Regimen | Microfilariae Clearance at 12 Months (approx.) |
| This compound + Albendazole (single dose) | 25% oup.com |
| Ivermectin + this compound + Albendazole (single dose) | Complete clearance for at least one year (in a pilot study) wustl.edu |
Synergistic Drug Combinations (e.g., this compound and Emodepside)
Research into synergistic drug combinations aims to enhance the efficacy of this compound and potentially overcome reduced susceptibility. Studies have explored the combined effects of this compound and emodepside, an anthelmintic with a distinct mode of action involving the activation of nematode SLO-1 K+ channels. plos.orgnih.gov
Mechanism of Synergy: Investigations in Ascaris suum and Brugia malayi have shed light on the potential mechanisms underlying the synergistic effects of this compound and emodepside.
this compound has been shown to have direct effects on nematode parasites, increasing the activation of SLO-1 K+ currents and potentiating the effects of emodepside in Ascaris suum. plos.orgnih.gov This synergy on membrane potential and SLO-1 K+ current could involve this compound's effects on parasite arachidonic acid and nitric oxide pathways, in addition to emodepside's direct action on SLO-1 channels. plos.org
In Brugia malayi muscles, this compound elicits Ca2+ signals through TRP-2 channels. nih.govasm.org These Ca2+ signals are potentiated by emodepside, which activates SLO-1 Ca2+-activated K+ channels, the putative target of emodepside. nih.govnih.govasm.org The combination of this compound and emodepside leads to a larger Ca2+ signal compared to either compound alone. nih.govasm.org
Observed Effects: The synergistic relationship between emodepside and this compound has been observed to result in the potentiation of flaccid paralysis in Brugia, which is dependent on TRP-2 channels. nih.govresearchgate.net This combination also increases membrane potential hyperpolarization in the muscles of Ascaris suum. researchgate.net
These findings suggest the potential for combining this compound and emodepside as an anthelmintic treatment, predicted to be synergistic. plos.orgnih.gov While emodepside shows effects against filarial nematodes and is being investigated for river blindness, its in vivo potency against some filarial parasites like Brugia is limited, highlighting the potential benefit of combination therapy. nih.gov
Development of Resistance Markers
The development of drug resistance in human helminths is a growing concern, particularly with large-scale drug administration programs. seq.es While confirmed reports of resistance to DEC in Wuchereria bancrofti are lacking, there is growing evidence of resistance to other anthelmintics like ivermectin and benzimidazole. seq.es
Identifying molecular markers for anthelmintic resistance is crucial for early detection and monitoring. For benzimidazole resistance in many nematodes, specific mutations in the gene encoding β-tubulin, particularly substituting tyrosine for phenylalanine at position 167 or 200, have been associated with resistance. seq.es However, for this compound, detailed studies of resistance are challenging due to a less clear understanding of its mechanism of action. seq.es The molecular basis of potential DEC resistance is not yet known, and markers are not currently available. who.int Further studies are required to assess the development of drug resistance under continuous low-dose DEC administration. nih.gov
Adverse Reactions and Safety Profile Research
Mazzotti Reaction: Pathophysiology and Management
The Mazzotti reaction is a complex, acute inflammatory response triggered by the rapid death of microfilariae following the administration of microfilaricidal drugs like diethylcarbamazine. taylorandfrancis.comcapes.gov.brtandfonline.com This reaction was first described in 1948 and is a significant side effect of treatment for Onchocerca volvulus infection. taylorandfrancis.comwikipedia.org The severity of the Mazzotti reaction can range from mild to severe and, in rare cases, can be life-threatening. wikipedia.orgontosight.ai
The clinical features typically manifest within seven days of treatment initiation. wikipedia.orgnih.gov Management of the Mazzotti reaction primarily involves supportive care to alleviate symptoms. ontosight.ai Anti-inflammatory medications, such as corticosteroids and antihistamines, may be administered in some cases. ontosight.ai Close monitoring of patients during the initial phase of treatment is crucial for prompt identification and management of adverse reactions. ontosight.ai
Immunological Mechanisms Underlying the Mazzotti Reaction
The Mazzotti reaction is fundamentally an immunological response to the antigens released from dying microfilariae. capes.gov.brresearchgate.netaltmeyers.org When microfilariae are damaged or killed by DEC, parasitic antigens are released into the host organism, triggering an inflammatory immune reaction. researchgate.netaltmeyers.org
The rapid killing of microfilariae by DEC leads to the release of a large amount of parasitic antigens. researchgate.netaltmeyers.org These antigens provoke an exuberant inflammatory response in the host. altmeyers.org This inflammatory reaction can be localized or generalized. researchgate.netug.edu.gh The clinical features of the reaction are thought to be due to the effects of mediators secreted from inflammatory cells at the sites where microfilariae are being destroyed. researchgate.netug.edu.gh
Eosinophils play a prominent role in the immunological response associated with the Mazzotti reaction. taylorandfrancis.comcapes.gov.brnih.gov Eosinophil degranulation, with the subsequent release of inflammatory mediators into the tissues and peripheral blood, is a significant feature observed in patients experiencing post-treatment reactions. capes.gov.brnih.govasm.org This degranulation occurs with a time course generally consistent with what would be required of an initiator of such reactions. capes.gov.brnih.gov Evidence suggests eosinophil attachment to and eosinophil-mediated degradation of dying filarial parasites in biopsy material from humans undergoing the Mazzotti reaction. taylorandfrancis.com Studies have shown increased levels of eosinophil-associated proteins like Eosinophil Derived Neurotoxin (EDN) and Major Basic Protein (MBP) which peak within the first few days of treatment, coinciding with the infiltration and degranulation of eosinophils in the affected tissues, such as the skin. asm.orgfrontiersin.org
While eosinophil degranulation is prominent, the reaction does not appear to primarily require the generation of circulating immune complexes or systemic complement activation, even in generalized reactions. researchgate.netug.edu.gh Other inflammatory mediators and pathways, such as kinins, prostaglandins, leukotrienes, and platelet-derived factors, may also be involved, but current evidence does not strongly implicate them as primary initiators of the response. capes.gov.brnih.gov
Cytokines play a crucial role in regulating the immune response during parasitic infections. parasite-journal.org In the context of the Mazzotti reaction, specific cytokines are implicated in the inflammatory cascade. Serum levels of Interleukin-5 (IL-5), a cytokine known to induce eosinophilia, have been observed to rise sharply after DEC treatment, preceding the increase in eosinophil levels. taylorandfrancis.com This suggests a temporal relationship between IL-5 and the subsequent development of eosinophilia seen in these patients. taylorandfrancis.com The mechanism by which IL-5 is selectively induced to high levels is not fully clear but may involve the presentation of antigens from dead and dying parasites to T cells, skewing the cytokine response towards a high IL-5 production. taylorandfrancis.com IL-5 has been detected in the Mazzotti reaction that can follow treatment of onchocerciasis. nih.gov Other cytokines examined, such as IL-3 and GM-CSF, were not consistently detectable in the serum before or after treatment in some studies. taylorandfrancis.com Pro-inflammatory cytokines like TNF-α and IL-6 are also involved in inflammatory processes and immune responses in parasitic diseases, although their specific initiating role in the Mazzotti reaction requires further clarification. nih.govexplorationpub.com
Eosinophil Degranulation and Inflammatory Mediators
Clinical Manifestations and Severity
The clinical manifestations of the Mazzotti reaction can vary in severity and typically appear within hours to days after DEC administration. researchgate.netaltmeyers.org Common symptoms include a severe pruritic skin rash, fever, headache, muscle and joint pain (arthralgia), lymphadenopathy (swollen lymph nodes), tachycardia (rapid heart rate), hypotension (low blood pressure), edema (swelling), and abdominal pain. taylorandfrancis.comcapes.gov.brwikipedia.orgontosight.aialtmeyers.orgnih.gov Ocular involvement can also occur, with inflammatory reactions in the anterior and posterior segments of the eye. who.intajtmh.org In severe cases, complications such as bronchospasm, thrombocytopenia, and even anaphylaxis have been reported. ontosight.ai While nearly all patients treated for onchocerciasis with microfilaricides may experience some form of Mazzotti reaction, most events are transient and self-limiting. tandfonline.com Only a small percentage of individuals report moderate to severe reactions. taylorandfrancis.com
Risk Factors (e.g., Microfilarial Load, Co-infection with Onchocerciasis)
The occurrence and intensity of the Mazzotti reaction are strongly correlated with the intensity of the filarial infection, specifically the microfilarial load. capes.gov.brwikipedia.orgaltmeyers.orgnih.gov Patients with higher microfilarial loads are more likely to experience more severe reactions. taylorandfrancis.comtandfonline.com Studies have definitively shown a correlation between the severity of the Mazzotti reaction, including symptoms like hypotension, fever, adenitis, and pruritus, and the intensity of infection as determined by skin snip quantification. nih.govajtmh.org Peripheral blood eosinopenia and neutrophilia also correlate with infection intensity. nih.govajtmh.org
Co-infection with other filarial parasites, such as Loa loa, can also increase the risk of severe adverse reactions, including potentially life-threatening encephalopathy, particularly when using medications like ivermectin in areas where loiasis is endemic. tandfonline.comnih.gov While DEC is not the preferred treatment for onchocerciasis in co-endemic areas due to the risk of severe Mazzotti reactions and exacerbation of ocular pathology, the principle of higher parasite load correlating with increased reaction severity is relevant across microfilaricidal treatments. gre.ac.uk
Common Adverse Effects and Their Mechanisms
Common adverse effects associated with this compound administration are frequently observed and are often a result of the body's inflammatory response to the 죽어가는 (dying) microfilariae. These reactions are typically mild to moderate in severity and transient in nature. nih.gov
Reported common adverse effects include headache, dizziness, nausea, vomiting, abdominal pain, loss of appetite, fatigue, muscle pain, joint pain, fever, skin rash, itching, and swollen lymph nodes. patsnap.compatsnap.commedicinenet.comhumanitas.netrxlist.com Gastrointestinal disturbances such as nausea, vomiting, and diarrhea are common. patsnap.compatsnap.com Headache, dizziness, and fatigue are also frequently reported. nih.govpatsnap.compatsnap.com Skin reactions, including rashes and itching, are also common. patsnap.compatsnap.comhumanitas.net
The mechanism underlying these common adverse effects is believed to involve the host immune system's reaction to antigens released by the 죽어가는 (dying) parasites. medicinenet.comwho.int this compound is thought to sensitize microfilariae to phagocytosis, facilitating their clearance by the host's immune cells. patsnap.commedicinenet.comhumanitas.netdrugbank.com This immune activation leads to an inflammatory response, manifesting as the various symptoms observed. The intensity of these reactions can be related to the intensity of the infection. medicinenet.com
In individuals without circulating microfilariae, adverse reactions at recommended dosages may include nausea, vomiting, abdominal pain, diarrhea, loss of appetite, muscle pain, dizziness, drowsiness, fatigue, and headache, typically starting within one to two hours and lasting for several hours. who.int In patients with circulating microfilariae, adverse reactions can be more common and severe, particularly with a high parasite burden. who.int
Severe Adverse Effects (e.g., Encephalopathy in Loa Loa Infections)
While most adverse effects are mild, severe reactions can occur, particularly in patients with high microfilarial loads. patsnap.com One of the most serious potential adverse effects of this compound treatment is encephalopathy, which is predominantly associated with Loa loa infections and high levels of Loa loa microfilaremia. who.intnih.govnih.govmsf.orgajtmh.org
Severe post-treatment reactions, including fatal encephalopathy, occur almost exclusively in patients with high microfilarial loads of Loa loa. nih.gov The association between DEC and severe post-treatment reactions, including encephalopathy, in patients with high levels of Loa loa microfilaremia was first described in 1948. nih.gov Cases of encephalitis following DEC treatment have been observed in patients with Loa loa filariasis, with some cases resulting in fatal outcomes or severe sequelae. nih.govajtmh.org This complication can occur even with strict therapeutic precautions, including low initial doses and gradual dose increases. nih.govajtmh.org
The risk of fatal encephalopathy or other severe adverse neurologic events is directly related to the microfilarial load of Loa loa. nih.govcdc.gov Available data suggest that the risk of fatal encephalopathy in patients treated with DEC with microfilarial loads below 8,000 microfilariae per mL approaches zero. cdc.gov Some experts suggest a more conservative threshold of 2,500 microfilariae per mL for initiating treatment. cdc.gov
Other severe reactions can include intense itching, swelling, and systemic symptoms like fever and hypotension, sometimes referred to as a severe inflammatory response or Mazzotti reaction, which is believed to be an immune response to dying microfilariae. patsnap.com
Safety in Mass Drug Administration Programs
Mass Drug Administration (MDA) programs utilizing this compound, often in combination with other anthelmintics like albendazole, are a cornerstone strategy for the elimination of lymphatic filariasis. scielo.brplos.orgmdpi.combmj.com Assessing the safety profile of DEC in these large-scale community-based programs is critical for their success and community adherence. scielo.brnih.gov
Studies conducted in the context of MDA programs have evaluated the incidence, type, and severity of adverse events following DEC administration, often in combination with albendazole. nih.govscielo.brplos.orgmdpi.combmj.comresearchgate.net While adverse reactions are common, they are frequently mild to moderate and transient. nih.govresearchgate.net
A large-scale prospective active safety surveillance study in Kenya involving over 10,000 participants receiving single-dose DEC and albendazole reported a cumulative incidence of adverse events of 16.2% over a seven-day follow-up period. nih.govresearchgate.net Of the reported events, 87.3% were graded as mild, 12.4% as moderate, and 0.3% as severe. nih.govresearchgate.net The most common adverse events in this study were dizziness, headache, loss of appetite, fever, and drowsiness. nih.govresearchgate.net These events typically resolved within 72 hours. nih.govresearchgate.net
Another study in Brazil assessing adverse drug reactions following the first round of mass treatment with DEC in two communities found that the prevalence of adverse drug reactions varied between the communities and was influenced by factors such as dosage adjustment based on weight-for-age and sex. scielo.br Common symptoms reported included drowsiness, nausea, headache, dizziness, and abdominal pain, which were considered related to the chemical toxicity of the drug and the death of microfilariae. scielo.br
Research comparing dual therapy (DEC and albendazole) with triple therapy (ivermectin, DEC, and albendazole) in MDA programs has indicated that while both regimens are generally safe and well-tolerated, the incidence of experiencing one or more adverse events might be higher with the triple therapy. bmj.comwustl.edu However, the adverse events were typically systemic, mild to moderate, and transient. bmj.com
Factors influencing the occurrence of adverse events in MDA programs can include age, sex, taking concurrent medications, and the type of meal consumed before administration. nih.govscielo.brmdpi.combmj.comresearchgate.net Studies have sometimes observed a higher incidence of adverse events in females compared to males. scielo.brmdpi.com
The frequency and severity of adverse reactions are significant factors influencing compliance with MDA programs. scielo.brnih.gov Therefore, active adverse reaction surveillance systems are warranted to minimize their impact on compliance. nih.gov
Here is a data table summarizing common adverse events reported in MDA studies:
| Adverse Event | Incidence (Example from Kenya Study nih.govresearchgate.net) | Severity (Kenya Study nih.govresearchgate.net) |
| Dizziness | 5.9% | Mild to Moderate |
| Headache | 5.6% | Mild to Moderate (Severe in 0.3%) |
| Loss of Appetite | 3.3% | Mild to Moderate |
| Fever | 2.9% | Mild to Moderate |
| Drowsiness | 2.6% | Mild to Moderate |
| Nausea | Common patsnap.compatsnap.commedicinenet.comhumanitas.netscielo.br | Mild to Moderate |
| Abdominal Pain | Common patsnap.compatsnap.commedicinenet.comscielo.br | Mild to Moderate |
| Myalgia (Muscle Pain) | Common patsnap.commedicinenet.com | Mild to Moderate |
| Arthralgia (Joint Pain) | Common patsnap.commedicinenet.com | Mild to Moderate |
| Skin Rash | Common patsnap.compatsnap.comhumanitas.net | Mild to Moderate |
| Itching | Common patsnap.compatsnap.comhumanitas.net | Mild to Moderate |
| Swollen Lymph Nodes | Common humanitas.net | Mild to Moderate |
Note: Incidence rates can vary between studies and populations.
Drug Interactions Influencing Adverse Effects
Drug interactions can potentially influence the adverse effect profile of this compound. While comprehensive data on all possible interactions are extensive, some interactions have been noted.
This compound has been reported to have moderate interactions with a significant number of other drugs. medicinenet.comrxlist.com Mild interactions with an even larger number of drugs have also been noted. medicinenet.comrxlist.com
Specific interactions mentioned in available data include the potential for this compound to increase the risk or severity of adverse effects when combined with certain medications such as Acetylcholine, Amifampridine, Bethanechol, and Nicotine. drugbank.com It may also increase the neuromuscular blocking activities of Aclidinium and Moxisylyte, and increase the bradycardic activities of several beta-blockers including Alprenolol, Nadolol, Nebivolol, Betaxolol, Bisoprolol, and Celiprolol. drugbank.com
Conversely, the therapeutic efficacy of some drugs may be decreased when used in combination with this compound, including Amantadine, Amitriptyline, Amobarbital, Amoxapine, Biperiden, Brompheniramine, Buclizine, Chlorphenoxamine, Chlorpromazine, Chlorprothixene, and Nicardipine. drugbank.com
It is crucial for healthcare providers to be aware of potential drug interactions when prescribing this compound and to consider a patient's concurrent medications to mitigate the risk of increased adverse effects or reduced efficacy of other treatments.
Research Methodologies and Analytical Techniques for Diethylcarbamazine
Clinical Trial Design and Implementation
Clinical trials are essential for evaluating the efficacy and safety of DEC in humans. Various designs are utilized depending on the research question.
Double-Blind Studies
Double-blind studies are frequently employed in DEC research to prevent bias from both participants and researchers knowing the assigned treatment. In this design, neither the patient nor the investigator is aware of whether the patient is receiving DEC or the control. A double-blind, placebo-controlled study in Tanzania evaluated DEC for the treatment of hydrocoele. nih.gov Another double-blind trial in Tahiti compared the efficacy and tolerability of single doses of ivermectin and DEC for treating Wuchereria bancrofti carriers. nih.gov A randomized double-blind clinical trial in Thailand investigated a single dose of doxycycline in combination with DEC for bancroftian filariasis. thaiscience.info
Bioequivalence Studies
Bioequivalence studies are conducted to determine if different formulations or generic versions of DEC deliver the same amount of the active compound to the bloodstream over the same period as a reference product. These studies are crucial for ensuring that different products are interchangeable. Guidance for the design of bioequivalence studies for DEC recommends a single-dose cross-over design, typically conducted in the fed state as DEC is preferably administered with food. who.intwho.int A minimum sample size of 14 subjects is recommended, with intensive blood sampling in the initial hours to characterize the maximum concentration (Cmax) of DEC. who.intwho.int A washout period of 7 days is considered sufficient based on DEC's elimination half-life. who.intwho.int LC-MS/MS analytical methodology is indicated as suitable for measuring DEC in human plasma for these studies. who.intwho.int
In Vitro and In Vivo Parasite Studies
Research on DEC also involves studying its effects directly on parasites, both in laboratory settings (in vitro) and within living organisms (in vivo). These studies help to understand the mechanism of action and efficacy against specific filarial species. While the provided search results primarily focus on clinical trials and analytical methods, the context of DEC as an anthelmintic drug used for treating filariasis implies the necessity of such studies to evaluate its direct impact on parasites like Wuchereria bancrofti, Brugia malayi, and Brugia timori. nih.gov
Immunological Assays
Immunological assays are used to measure the host's immune response in the context of filarial infections and DEC treatment. These assays can help assess the impact of DEC on the interaction between the host immune system and the parasite. For instance, studies have utilized immunological assays to investigate antibodies that cross-react with DEC and Wuchereria bancrofti microfilariae. nih.gov Enzyme-Linked Immunosorbent Assay (ELISA) is one method used in such immunological studies related to DEC. nih.govnih.gov Serological evaluations, including techniques like two-site immuno-radiometric assay (IRMA) and Western blotting, have been used to measure changes in filarial antigen levels in response to DEC therapy, providing an indirect measure of adult worm populations. ajtmh.org
Analytical Methods for Diethylcarbamazine Quantification
Accurate quantification of DEC in various samples is vital for pharmacokinetic studies, bioequivalence trials, and quality control of pharmaceutical formulations and medicated products. Several analytical methods have been developed for this purpose.
High-Performance Liquid Chromatography (HPLC) is a widely used method for the estimation of DEC. HPLC methods have been developed for analyzing DEC in medicated salt samples, utilizing a C8 column and UV detection at 210 nm. nih.govresearchgate.netnih.gov The mobile phase typically involves a mixture of acetonitrile and a phosphate buffer. nih.govresearchgate.net RP-HPLC methods have also been developed for the simultaneous estimation of DEC with other drugs in tablet formulations, often using a C18 column and UV detection at wavelengths around 210-224 nm. nih.govsemanticscholar.orginnovareacademics.in
Liquid Chromatography-Mass Spectrometry (LC-MS) and tandem Mass Spectrometry (LC-MS/MS) offer high sensitivity and selectivity for DEC quantification, particularly in biological fluids like human plasma. nih.govwpmucdn.comresearchgate.netnamibian-studies.com LC-MS/MS methods have been developed for the simultaneous determination of DEC and other anti-filarial drugs and their metabolites in plasma, employing reversed-phase columns and gradient elution with mobile phases containing formic acid in methanol and water. nih.govresearchgate.net These methods often utilize electrospray ionization in positive multiple reaction monitoring mode. researchgate.net LC-MS/MS is considered a sensitive and selective method suitable for pharmacokinetic studies and therapeutic drug monitoring. nih.govresearchgate.netresearchgate.net
Gas Chromatography (GC) methods, including those with alkali flame ionization detection, have also been reported for the determination of DEC in human plasma, offering sensitivity for pharmacokinetic studies. researchgate.net
In addition to chromatographic methods, titrimetric and spectrophotometric methods have been proposed for the determination of DEC citrate in bulk drug and formulations, often utilizing reactions involving iodate and iodide. scielo.brscielo.br A low-tech titrimetric method has been developed specifically for the analysis of DEC citrate in medicated salt, suitable for settings with limited equipment. nih.govresearchgate.net
The linearity and sensitivity of these methods vary depending on the technique and specific parameters used. For instance, HPLC methods have shown linearity over various concentration ranges, and LC-MS/MS methods have demonstrated low limits of quantification in the nanogram per milliliter range. semanticscholar.orginnovareacademics.inresearchgate.netnamibian-studies.com
Here is a table summarizing some analytical methods for this compound quantification:
| Method | Sample Matrix | Detection Method | Column Type (if applicable) | Key Mobile Phase Components (if applicable) | Limit of Quantification (Example) |
| HPLC | Medicated Salt | UV (210 nm) | C8 | Acetonitrile, Phosphate Buffer | 0.5 µg/ml nih.gov |
| RP-HPLC | Tablet Formulation | UV (224 nm) | C18 | Phosphate Buffer, Acetonitrile | 100 ng/ml semanticscholar.org |
| RP-HPLC | Tablet Formulation | UV (210 nm) | Phenyl-hexyl | Triethylamine Buffer, Acetonitrile | Not specified innovareacademics.in |
| LC-MS/MS | Human Plasma | Tandem Mass Spectrometry | C18 | Formic Acid in Methanol/Water | 1 ng/mL researchgate.net, 11.41 ng/ml namibian-studies.com |
| LC-MS | Human Plasma | Mass Spectrometry | Fusion-RP | Formic Acid, Acetonitrile | 4 ng/ml researchgate.net |
| GC | Human Plasma | Flame Ionization | Capillary (e.g., AT-35) | Not specified | 100 ng/ml researchgate.net |
| Titrimetry | Medicated Salt | Titration | Not applicable | Not applicable | Not specified nih.govresearchgate.net |
| Spectrophotometry | Bulk Drug, Formulations | Spectrophotometry | Not applicable | Not applicable | 2.5-50 µg/mL (Method B), 2.5-30 µg/mL (Method C) scielo.brscielo.br |
High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) is a widely used technique for the analysis of this compound in various samples, including pharmaceutical formulations and medicated salt. Several RP-HPLC methods have been developed and validated for the estimation of DEC.
One method for determining DEC in medicated salt utilizes a Phenomenex C8 column with a mobile phase composed of acetonitrile and phosphate buffer (20 mM KH₂PO₄, adjusted to pH 3.2 with 10% ortho-phosphoric acid) in a 1:9 ratio, at a flow rate of 1.5 ml/min. UV detection is performed at 210 nm. This method demonstrated a coefficient of variation below 10% in the range of 1-25 µg/ml and a minimum detectable level of 0.5 µg/ml. nih.govresearchgate.net Analysis of medicated salt samples using this method showed varying DEC content depending on the preparation method. nih.govresearchgate.net
Another RP-HPLC method for the simultaneous estimation of this compound citrate and cetirizine hydrochloride in formulations employs a Phenomenex C18 column with a mobile phase of acetonitrile and mixed phosphate buffer (KH₂PO₄ and K₂HPO₄, adjusted to pH 3.0 with 10% orthophosphoric acid) in a 60:40 ratio, at a flow rate of 1 ml/min. UV detection is carried out at 220 nm, which is an isosbestic point for both compounds. The method showed linearity in the range of 24-144 µg/ml for this compound citrate with a correlation coefficient of 0.998.
A stability-indicating RP-HPLC method for the simultaneous estimation of this compound citrate, guaiphenesin, and chlorpheniramine maleate in tablet dosage forms uses a luna phenyl-hexyl column with an isocratic mobile phase of 0.1% triethylamine buffer (pH adjusted to 2.5 with orthophosphoric acid) and acetonitrile (50:50 v/v) at a flow rate of 1.0 ml/min and UV detection at 210 nm. The retention time for this compound citrate was found to be 7.76 min. innovareacademics.in The method was linear in the range of 1-15 µg/ml for this compound citrate with a correlation coefficient of 0.999. innovareacademics.in
HPLC methods are also suitable for pharmacokinetics studies and can be scaled for preparative separation to isolate impurities. sielc.com For LC-MS compatible applications, the mobile phase typically uses formic acid instead of phosphoric acid. sielc.com
Mass Spectrometry (LC-MS/MS)
Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) is a sensitive and selective technique used for the quantification of this compound, particularly in biological matrices like human plasma, for clinical pharmacokinetic studies. researchgate.netresearchgate.netnih.govnih.govwpmucdn.com
A rapid and sensitive LC-MS/MS method has been developed and validated for the simultaneous quantitation of this compound, albendazole, and albendazole metabolites in human plasma. researchgate.netnih.govnih.gov This method utilizes solid phase extraction for sample preparation and employs a reversed-phase column (Acquity UPLC® BEH C18) with gradient elution using mobile phases containing formic acid. researchgate.netnih.govnih.gov Analytes are monitored using MS/MS with an electrospray ionization source in positive multiple reaction monitoring mode. researchgate.netnih.govnih.gov The method demonstrated linearity for DEC over a concentration range of 1-2000 ng/mL with a correlation coefficient (r²) of 0.998 or better. researchgate.netnih.gov The lower limit of quantitation (LLOQ) for DEC was reported as 1 ng/mL in one study. researchgate.netnih.gov Another LC-MS method reported an LLOQ of 4 ng/mL for DEC in human plasma with a linear range of 4–2200 ng/mL. researchgate.netwpmucdn.com This LC-MS assay requires a smaller plasma sample volume (0.25 mL) compared to some previous methods. wpmucdn.com
LC-MS/MS methods offer high sensitivity and specificity, making them valuable for accurately determining drug concentrations in complex biological samples and for drug-drug interaction studies. researchgate.netnih.gov
Spectrophotometric Methods (e.g., UV, Folin-Ciocalteu Reagent)
Spectrophotometric methods, including UV spectroscopy and methods utilizing reagents like Folin-Ciocalteu, have been developed for the determination of this compound in bulk drug and pharmaceutical formulations. scielo.brfabad.org.trscielo.brresearchgate.net
UV spectrophotometry can be used to determine DEC by measuring absorbance in acidic solutions. For example, methods measuring absorbance at 210 nm in 0.1M HCl or at 209 nm in 0.1M H₂SO₄ have been described. researchgate.net However, these UV methods may have higher lower limits of quantitation (LLOQ) compared to chromatographic techniques. researchgate.net
Methods employing the Folin-Ciocalteu (F-C) reagent are based on the formation of a blue chromogen due to the reduction of tungstate and/or molybdate in the reagent by DEC in an alkaline medium. researchgate.netfabad.org.tr This colored species has an absorption maximum at 760 nm. researchgate.netfabad.org.tr The absorbance is linearly dependent on the concentration of DEC, obeying Beer's law over a specific concentration range (e.g., 10-100 μg mL⁻¹). researchgate.netfabad.org.tr This method has been successfully applied to the determination of DEC in bulk powder, tablets, and syrup. fabad.org.tr
Other spectrophotometric methods involve reactions with reagents like iodate and iodide mixture, where the liberated iodine is measured spectrophotometrically at 370 nm or as the iodine-starch complex at 570 nm. scielo.brscielo.br These methods show linear calibration curves over specific concentration ranges. scielo.brscielo.br Extractive spectrophotometric methods based on the formation of ion-pair complexes between DEC and acidic dyes like Tropaeolin 000 have also been developed, with absorbance measured at 490 nm. researchgate.netresearchgate.net
Spectrophotometric methods are often considered simple and rapid for the quantification of DEC. researchgate.netfabad.org.tr
Gas Chromatography (GC)
Gas Chromatography (GC) has been used for the analysis of this compound, particularly for its determination in human plasma for pharmacokinetic studies. researchgate.netscielo.brnih.gov
A sensitive and selective GC method using flame ionization detection has been developed for DEC in human plasma. nih.gov This method involves solid phase extraction of DEC and an internal standard (1-diethylcarbamyl-4-ethyl piperazine HCl) from plasma. nih.gov Separation is achieved on a capillary column (e.g., Heliflex® AT-35). nih.gov The assay was linear in the concentration range of 100-2000 ng/ml for DEC in human plasma, demonstrating good precision and accuracy. nih.gov The retention times for DEC and the internal standard were approximately 5.5 and 7.28 minutes, respectively. nih.gov
While GC methods have been utilized, the analysis of DEC in plasma by GC can be complicated by the presence of its metabolite, DEC-N-oxide, which can be converted to a substance that coelutes with DEC under certain GC conditions. researchgate.net
Proton Magnetic Resonance (PMR) Spectroscopy
Proton Magnetic Resonance (PMR) spectroscopy, also known as ¹H NMR, has been employed for the quantitative determination of this compound in standard mixtures and tablet formulations. scielo.brtandfonline.comtandfonline.com
A PMR procedure using deuterium oxide as a solvent and maleic acid as an internal standard has been developed. tandfonline.com The method is based on integrating the characteristic peaks of DEC and the internal standard in the PMR spectrum. tandfonline.com This procedure has been reported as simple, specific, and accurate for the quantitative analysis of this compound citrate. tandfonline.comtandfonline.com The average recovery of pure DEC in standard mixtures using this method was reported as 100.03 ± 0.11% w/w. tandfonline.comtandfonline.com
Low-Tech Analytical Methods for Field Use (e.g., Back-Titration)
In settings where access to advanced analytical instrumentation is limited, low-tech methods are valuable for the analysis of this compound, particularly in formulations like medicated salt used in mass drug administration programs. nih.govplos.orgresearchgate.net
A simple titration-based assay, specifically a back-titration procedure, has been developed for determining this compound citrate concentrations in medicated salt. nih.govplos.orgresearchgate.net This method capitalizes on the acidic nature of this compound citrate. nih.govplos.orgresearchgate.net A known excess of a strong base (e.g., sodium hydroxide) is added to a sample containing DEC citrate, reacting completely with the acidic protons. nih.govplos.org The remaining excess base is then back-titrated with a standard acid (e.g., HCl) using a common indicator like phenolphthalein to determine the neutralization point. nih.govplos.org
Direct titration of DEC citrate with a base is not analytically useful due to the range of pKa values in the citrate component, which results in an unclear endpoint. nih.govplos.org The back-titration method provides a clear endpoint and can be carried out with basic laboratory equipment such as a balance, volumetric glassware, and burets, without the need for electrical power. nih.govplos.orgresearchgate.net This makes it suitable for quality control and field analysis in resource-limited settings. nih.govplos.org Comparison studies have been conducted to evaluate this titration method against HPLC. researchgate.net
Population Pharmacokinetic Modeling Techniques
Population pharmacokinetic (PK) modeling techniques are applied to study the pharmacokinetics of this compound in different populations, such as individuals infected with lymphatic filariasis and healthy individuals. asm.orgnih.govresearchgate.netresearchgate.net These techniques aim to describe the typical pharmacokinetic behavior of the drug in a population and identify factors (covariates) that contribute to variability in drug exposure. asm.orgnih.gov
Nonlinear mixed-effect modeling (NLME) is a common approach used for population PK analysis of DEC. asm.orgresearchgate.net Software like Phoenix NLME 8.0 has been utilized for building these models. asm.orgnih.govresearchgate.net
Studies have developed one-compartment linear pharmacokinetic models with first-order absorption and an absorption lag time to describe DEC concentration-time data. asm.orgnih.gov Covariates investigated for their influence on DEC pharmacokinetics include age, gender, body weight, infection status, creatinine clearance, and liver enzyme levels (AST and ALT). asm.orgnih.govresearchgate.netresearchgate.net
Research findings from population PK modeling of DEC have indicated that body weight and gender are significant covariates influencing the volume of distribution (V/F) of DEC. asm.orgnih.govresearchgate.netresearchgate.net Model-based simulations have shown that body weight significantly impacts DEC exposure in both male and female populations. asm.orgnih.govresearchgate.netresearchgate.net These analyses are valuable for understanding inter-subject variability in DEC pharmacokinetics and can support the development of drug-drug interaction models and potentially inform dosing strategies, although dosage information itself is outside the scope here. asm.orgnih.govresearchgate.net
Global Health Impact and Control Programs
Role of Diethylcarbamazine in Lymphatic Filariasis Elimination Programs
This compound has played a significant role in lymphatic filariasis control since the late 1940s. nih.gov It is highly effective at killing the microfilariae (Mf), the larval stage of the parasite found in the blood, and repeated doses over time can also kill adult worms. nih.gov The World Health Organization (WHO) Global Programme to Eliminate Lymphatic Filariasis (GPELF), established in 2000, adopted strategies centered on preventive chemotherapy, primarily utilizing mass drug administration (MDA) with antifilarial medications like DEC to interrupt transmission. dovepress.complos.orgmdpi.com
Mass Drug Administration (MDA) Strategies
Mass Drug Administration is a key strategy in LF elimination programs, aiming to reduce parasite density in infected individuals and lower infection prevalence in communities to levels where transmission is no longer sustainable. dovepress.com DEC is a primary drug used in MDA, often in combination with other antifilarial drugs. dovepress.comasm.org
The GPELF initially recommended single-dose, two-drug regimens administered annually. dovepress.com Outside of areas co-endemic for onchocerciasis or loiasis, the recommended combination typically includes this compound citrate and albendazole (DA). dovepress.commdpi.comnih.govplos.org In areas co-endemic for onchocerciasis, ivermectin and albendazole (IA) are used. mdpi.comnih.gov For areas co-endemic with loiasis, albendazole is administered twice yearly. nih.gov
More recently, a triple-drug therapy combining ivermectin, this compound, and albendazole (IDA) has shown superior efficacy in clearing microfilariae compared to the DA regimen in clinical trials. plos.orgplos.orgajtmh.orgoup.comnih.govmedrxiv.org The WHO endorsed the use of IDA in specific settings outside of onchocerciasis-endemic areas to accelerate LF elimination. nih.govmedrxiv.orgplos.orgplos.org Studies have demonstrated that IDA can achieve sustained microfilariae clearance for up to three years after a single dose. asm.orgplos.org
Research findings highlight the impact of repeated rounds of MDA with DEC and albendazole. For instance, a study in Papua New Guinea showed that three rounds of MDA decreased microfilaremia rates significantly. plos.org
Table 1: Impact of MDA with DEC + Albendazole on Microfilaremia and Antigenemia Rates in Papua New Guinea plos.org
| Metric | Pre-MDA | After 3 Rounds MDA | Percentage Decrease |
| Microfilaremia Rate | 18.6% | 1.3% | 94% |
| Filarial Antigenemia Rate | 47.5% | 17.1% | 64% |
Another study in Egypt demonstrated that annual MDA with DEC and albendazole significantly reduced prevalence rates of microfilaremia and circulating filarial antigenemia, as well as mosquito infection rates, suggesting that elimination was likely after five rounds of MDA. nih.gov
Table 2: Reduction in Infection and Transmission Indicators After MDA in Egypt nih.gov
| Indicator | Giza (Pre-MDA) | Giza (Post-MDA) | Qalubyia (Pre-MDA) | Qalubyia (Post-MDA) |
| Microfilaremia Prevalence | 11.5% | 1.2% | 3.1% | 0% |
| Circulating Filarial Antigenemia Prevalence | 19.0% | 4.8% | 13.6% | 3.1% |
| Mosquito Infection Rate | 3.07% | 0.19% | 4.37% | 0% |
This compound-Medicated Salt Programs
In addition to tablet-based MDA, the administration of DEC through medicated salt has been explored and implemented as a community-based control measure for lymphatic filariasis. nih.govajtmh.orgnih.gov This strategy involves adding low concentrations of DEC to common salt consumed by the population over several months to a year. nih.govplos.org
DEC-medicated salt played a significant role in China's filariasis control program and has shown success in trials conducted in countries like India, Brazil, and Tanzania. ajtmh.orgnih.govajtmh.org Studies have indicated that DEC-fortified salt is effective in significantly reducing microfilaremia prevalence and density. nih.gov For instance, in China, fortified salt with varying DEC concentrations administered over 4-6 months resulted in substantial reductions in Mf prevalence and density one year post-intervention. nih.gov A study in the Andaman Islands, India, showed that the combination of annual MDA-DA and DEC-fortified salt led to a greater reduction in Mf prevalence compared to MDA alone. nih.gov
The use of DEC-medicated salt is considered a simple, rapid, safe, inexpensive, efficient, prophylactic, and practical approach for filariasis elimination. nih.gov
Disease Burden Reduction and Disability-Adjusted Life Years (DALYs) Averted
The implementation of LF elimination programs, heavily reliant on MDA with drugs including DEC, has resulted in a considerable reduction in the global burden of the disease. The GPELF has distributed billions of treatments, significantly reducing the population at risk. mdpi.comwoundsinternational.comwho.int
Modeling suggests that scaling up MDA efforts can lead to millions of incremental DALYs averted over a multi-year horizon and can be a cost-effective intervention. bmj.complos.org The impact on DALYs averted is influenced by factors such as MDA coverage and baseline prevalence. lstmed.ac.uk
Challenges and Future Directions in Control Programs
Despite the significant progress made in LF elimination using DEC-based strategies, several challenges remain in achieving and sustaining elimination goals.
Sustaining Microfilarial Clearance
While DEC is effective at clearing microfilariae, sustaining this clearance over the long term can be challenging, particularly with single-dose regimens of DEC and albendazole. asm.org The reappearance of microfilariae after treatment necessitates repeated annual rounds of MDA, typically for at least 5 years, which is the approximate lifespan of adult worms. asm.org
The introduction of the triple-drug therapy (IDA) offers a potential solution to this challenge, as it has demonstrated the ability to sustain microfilarial clearance for longer periods after a single administration compared to the DA regimen. asm.orgplos.org However, persistent microfilaremia after MDA can still occur, often attributed to individuals not taking the medications as prescribed rather than drug failure. ajtmh.org
Ensuring High Coverage and Compliance
Achieving and maintaining high coverage and compliance rates in MDA programs are critical for interrupting transmission and achieving elimination. The recommended minimum epidemiological coverage for effective reduction of LF transmission is at least 65% of the total eligible population in an implementation unit. plos.orgwho.intnih.gov However, consistently reaching and sustaining this level can be difficult. journalofcomprehensivehealth.co.infrontiersin.orgplos.org
Factors influencing non-coverage and non-compliance include unawareness of MDA, absence from home, travel, refusal to take the drug, illness, and fear of side effects. journalofcomprehensivehealth.co.infrontiersin.orgplos.orginfontd.org Studies have shown variability in compliance rates, with some populations reporting high distribution but lower consumption of the drugs. journalofcomprehensivehealth.co.ininfontd.org
Efforts to improve coverage and compliance involve engaging communities in planning and implementation, addressing local concerns, ensuring drug distributors are trusted and well-trained, and providing clear information about the program. nih.govjournalofcomprehensivehealth.co.in Strategies to enhance administration and ensure directly observed treatment can also improve ingestion rates. ajtmh.orgnih.gov
Challenges also exist in monitoring and evaluating coverage accurately, as reported data may not always reflect actual drug ingestion. dovepress.comnih.gov Innovative approaches and improved surveillance systems are needed to identify and reach individuals who are missed during MDA rounds, as these individuals can serve as reservoirs of infection and pose a threat to elimination efforts. medrxiv.orgfrontiersin.org
Addressing Emerging Drug Resistance
This compound (DEC) has been a cornerstone in the treatment and control of lymphatic filariasis for many decades. Despite its widespread use since 1947, confirmed resistance to DEC in the primary target parasites, such as Wuchereria bancrofti and Brugia malayi, has not been widely reported nih.gov. However, the potential for drug resistance to emerge remains a significant concern, particularly with large-scale mass drug administration (MDA) programs implemented globally to eliminate lymphatic filariasis seq.esmdpi.com.
While widespread resistance to DEC has not been confirmed, it is recognized that susceptibility to the drug is not 100% in all cases of lymphatic filariasis treatment seq.esmdpi.comnih.gov. Some studies and clinical observations have indicated that a small percentage of treated patients may not respond to DEC despite multiple courses of therapy oup.com. The reasons for these treatment failures are not fully understood and could involve factors such as inadequate drug concentrations, inherent variability in parasite susceptibility, or the host's immune response oup.com.
Research into the mechanisms of action of DEC is ongoing, and a better understanding of how the drug affects parasites is crucial for anticipating and addressing potential resistance sciencedaily.com. While it was historically thought that DEC primarily acted by sensitizing microfilariae to the host's immune system, more recent research suggests direct effects on the parasites sciencedaily.comdrugbank.complos.org. Studies using Ascaris suum, a related nematode, have shown that DEC can directly affect voltage-activated potassium channels (SLO-1) in the parasite, suggesting a direct anthelmintic effect plos.orgnih.gov. This understanding of direct mechanisms could help in identifying potential pathways for resistance development.
The difficulty in demonstrating anthelmintic resistance in filarial parasites like W. bancrofti is partly due to the challenges in culturing these parasites in animal models and the lack of a free-living stage seq.es. Parasitological evidence of resistance often relies on demonstrating the persistence of parasites after treatment over an extended period seq.es.
Monitoring for emerging resistance is a critical component of elimination programs. While specific data tables detailing confirmed DEC resistance are scarce in the available literature, the ongoing surveillance within MDA programs for lymphatic filariasis implicitly serves to detect potential shifts in parasite susceptibility that could indicate emerging resistance. The Global Programme to Eliminate Lymphatic Filariasis (GPELF) utilizes strategies such as repeated annual doses of antifilarial drugs and transmission assessment surveys to monitor the impact of interventions plos.orgoup.com. Although these monitoring efforts are primarily aimed at assessing the reduction of infection prevalence and interruption of transmission, they would likely identify areas where drug efficacy might be declining.
The development of new diagnostic tools, such as antigen tests for adult worms, which can be performed at any time, offers potential for improved monitoring of infection prevalence and could potentially aid in detecting subtle changes in parasite populations that might correlate with reduced drug efficacy nih.gov.
The potential for resistance also underscores the importance of combination therapies. The co-administration of DEC with other antifilarial drugs, such as albendazole and ivermectin (IDA regimen), has shown increased effectiveness in clearing microfilariae compared to two-drug regimens, which could potentially mitigate the risk of resistance development by targeting parasites through multiple mechanisms plos.orgoup.com.
Further research is needed to fully understand the mechanisms of DEC action and to develop sensitive methods for detecting and monitoring potential drug resistance in filarial parasites nih.gov. Studies exploring the genetic diversity of filarial parasites and potential resistance-associated alleles are also important for proactive resistance management seq.es.
Future Research Directions for Diethylcarbamazine
Elucidating Remaining Gaps in Mechanism of Action
While DEC has been used for decades, its precise mechanism of action is not yet fully understood and remains partially unclear. researchgate.netnih.govaap.orgpatsnap.comnih.gov It is known to be remarkably effective at killing microfilariae and can also eventually kill many adult worms, though often after repeated doses over a long period. nih.gov Proposed mechanisms include sensitizing microfilariae to phagocytosis by the host immune system, interfering with parasite arachidonic acid metabolism, and affecting parasite muscle activity. researchgate.netaap.orgpatsnap.comsmolecule.com
Recent research has provided new insights, suggesting that DEC may have direct effects on parasites, in addition to host-mediated effects. nih.govplos.orgsciencedaily.com Studies have shown that DEC can increase the activation of voltage-activated potassium (SLO-1) currents in Ascaris suum and potentiate the effects of emodepside, another anthelmintic. nih.govplos.orgresearchgate.net Furthermore, DEC has been shown to interact with transient receptor potential (TRP) channels, specifically TRP-2 channels, in Brugia malayi muscle cells, promoting calcium entry. researchgate.netasm.org This calcium influx can activate SLO-1 channels, the putative target of emodepside, suggesting a potential synergistic effect when used in combination. researchgate.netasm.org
Development of Novel Formulations and Delivery Systems
Limitations such as DEC's short half-life and the need for repeated dosing have prompted research into improved delivery systems. smolecule.com Novel formulations are being explored to enhance drug efficacy, potentially reduce dosage, and improve patient compliance. smolecule.com
Research is focusing on developing sustained-release formulations and targeted delivery systems. smolecule.com Examples include the development of microparticles and nanocapsules designed to improve drug delivery to the lymphatic system, where adult filarial worms reside. smolecule.comasianpubs.org Studies have successfully fabricated microparticles of diethylcarbamazine citrate using materials like alginate and chitosan, demonstrating sustained drug release in vitro. asianpubs.org Medicated chewing gum containing this compound citrate has also been investigated as a novel buccal delivery system, showing promising in vitro release profiles and buccal absorption. longdom.org Enhanced intradermal delivery of antifilarial drug nanosuspensions using dissolving microneedles is another avenue being explored for targeted delivery. nih.gov
Future research in this area aims to optimize these novel formulations through further in vitro and in vivo evaluations to ensure increased efficacy and minimized side effects. asianpubs.org
Pharmacogenomics and Personalized Medicine Approaches
The response to anthelmintic treatment can vary among individuals. seq.es Pharmacogenomics, the study of how genetic variations influence drug response, holds potential for tailoring drug therapy to individual genetic profiles to enhance efficacy and safety. researchgate.netnih.gov
While the application of pharmacogenomics to DEC is not as extensively documented as for some other drugs, the principles of personalized medicine are relevant. Identifying genetic factors in the host that influence DEC metabolism, transport, or immune responses could help predict individual responses to treatment and identify those at higher risk of adverse reactions. researchgate.netnih.gov Similarly, understanding genetic variations in the parasites that might affect their susceptibility to DEC is crucial for predicting treatment outcomes and monitoring for potential drug resistance. seq.esscielo.br
Future research should explore the pharmacogenomic landscape related to DEC, both in the host and the parasite, to pave the way for more personalized and effective treatment strategies. researchgate.netnih.govki.seresearchgate.netki.se
Identification of New Molecular Targets
Although DEC is effective against microfilariae and has some effect on adult worms, there is a demand for novel macrofilaricidal drugs that effectively kill the long-lived adult parasites responsible for the pathology of lymphatic filariasis. nih.govbenthaminfo.commdpi.com Identifying new molecular targets in filarial parasites is a critical area of future research.
Genomic and proteomic approaches, combined with bioinformatics analysis and functional genomics data from model nematodes like Caenorhabditis elegans, are being used to identify potentially essential nematode genes and generate a pool of candidate drug targets. benthaminfo.comneb.cn For example, calumenin, a Ca2+ binding protein, has been identified as a novel and nematode-specific drug target due to its involvement in fertility and cuticle development. nih.govmdpi.com
Further research is needed to validate these candidate targets and explore their potential for the development of new antifilarial drugs, including those that could be used in combination with or as alternatives to DEC. nih.govbenthaminfo.commdpi.comneb.cnnih.gov
Repurposing this compound for Other Diseases
Beyond its primary use as an anthelmintic, DEC has demonstrated other pharmacological activities, including anti-inflammatory, immunomodulatory, and anti-fibrotic properties. nih.govnih.govtaylorandfrancis.com These properties suggest potential for repurposing DEC for the treatment of diseases other than filariasis. nih.govnih.govnih.govdoi.orgfrontiersin.org
Research indicates that DEC interferes with arachidonic acid metabolism and inhibits NF-κB activation, which are key regulators of inflammatory responses. researchgate.netnih.gov It has shown promise in attenuating lung injury in animal models. nih.gov The anti-fibrotic activity of DEC has led to suggestions for its potential use in treating conditions like COVID-19-related pulmonary fibrosis. nih.govnih.goveurekaselect.com
Future research should investigate the potential of DEC in treating various inflammatory and fibrotic conditions, as well as exploring other possible therapeutic applications based on its known pharmacological activities. nih.govnih.govnih.govdoi.orgfrontiersin.orgeurekaselect.com
Q & A
What validated analytical methods are recommended for quantifying diethylcarbamazine in pharmaceutical formulations and biological samples?
Basic
Reverse-phase high-performance liquid chromatography (RP-HPLC) is widely validated for quantifying this compound citrate in pharmaceutical formulations. The method demonstrates linearity in the range of 1–15 µg/ml with a regression equation of Y = 561,967x + 13,655 (r² = 0.999) and accuracy confirmed via recovery studies (98–102%) . For medicated salt in field settings, a low-cost titrimetric approach using back-titration with sodium hydroxide is recommended, achieving precision within ±5% .
How can researchers resolve contradictions in this compound's mechanism of action between direct antiparasitic effects and host-mediated immune responses?
Advanced
To reconcile these mechanisms, employ in vitro electrophysiological assays (e.g., voltage-clamp on Ascaris suum muscle cells) to study DEC’s direct modulation of SLO-1 potassium channels . Concurrently, use in vivo models (e.g., rat AMI or filarial infection) to assess host immune modulation, such as DEC’s inhibition of NF-κB activation or prostaglandin synthesis . Cross-validate findings by combining DEC with immune modulators (e.g., emodepside) to observe synergistic effects .
What are the key considerations when designing in vivo studies to evaluate this compound's cardioprotective effects?
Basic
Use male Wistar rats (n=6–12/group) with standardized weights (230–250 g) and housing conditions (23°C, 12h light/dark cycle). Include control groups (sham, DEC-alone), an AMI model group (isoproterenol-induced), and a treatment group (DEC 50 mg/kg/day for 12 days). Measure cardiac biomarkers (CK, LDH), oxidative stress markers (ROS), and inflammatory cytokines. Apply one-way ANOVA with Tukey’s post-hoc test for statistical analysis .
What methodologies are employed to assess the synergistic effects of this compound with other anthelmintics like emodepside?
Advanced
Use voltage-clamp techniques to measure SLO-1 potassium current activation in Ascaris suum. DEC (100 µM) shifts the voltage sensitivity (V50) from 7.66 ± 0.6 mV to 6.26 ± 0.6 mV (p < 0.001), and combined with emodepside (1 µM), V50 decreases further to 3.16 ± 1.1 mV (p < 0.01) . Pair these in vitro results with in vivo efficacy trials in filarial models, monitoring microfilarial clearance rates and parasite paralysis .
How do physiological factors such as urinary pH influence this compound's pharmacokinetics, and how should this be accounted for in study design?
Basic
Alkaline urine (pH 7.5–8) prolongs DEC’s elimination half-life, while acidification accelerates excretion. In pharmacokinetic studies, control dietary intake (e.g., vegetarian vs. non-vegetarian diets) and monitor urinary pH. Use crossover designs to compare DEC clearance under varying pH conditions .
What strategies can be implemented in clinical trial designs to evaluate the safety and efficacy of this compound in community-based mass drug administration programs?
Advanced
Adopt cluster randomization and collect data via tablet-based systems for real-time uploads. Include endpoints like filarial antigen test (FTS) scores, microfilarial (MF) prevalence, and adverse event rates. For example, in DA (DEC + albendazole) vs. IDA (ivermectin + DEC + albendazole) trials, MF prevalence in FTS-positive individuals decreased from 40% to 7.3% post-treatment .
What statistical approaches are recommended for analyzing data from studies comparing this compound's efficacy across different treatment groups?
Basic
Use one-way ANOVA for inter-group comparisons (e.g., cardiac biomarkers in AMI models) followed by Tukey’s test for pairwise analysis. For non-normal data, apply Kruskal-Wallis with Dunn’s correction. Report mean ± SD and significance thresholds (p < 0.05) .
How can researchers investigate the role of this compound in modulating oxidative stress and inflammatory pathways in parasitic infections?
Advanced
Quantify ROS production via fluorescence assays (e.g., DCFH-DA) in AMI models, where DEC reduces ROS by 50% (p < 0.01) . Measure NF-κB activation using Western blotting and cytokine levels (e.g., TNF-α, IL-6) via ELISA. Co-administer DEC with prostaglandin inhibitors (e.g., indomethacin) to explore feedback mechanisms .
What experimental models are used to predict and counteract potential anthelmintic resistance to this compound?
Advanced
Develop in vitro resistance models by exposing parasites (e.g., Brugia malayi) to sublethal DEC doses over multiple generations. Use genetic sequencing to identify resistance markers (e.g., SLO-1 mutations). Test DEC in combination with emodepside or albendazole to delay resistance, leveraging synergistic pathways .
How should researchers address variability in DEC efficacy across different filarial species (e.g., Wuchereria bancrofti vs. Loa loa)?
Advanced
Conduct species-specific in vitro microfilarial motility assays and compare DEC’s IC50 values. In clinical trials, stratify participants by infection type and monitor antigen clearance kinetics. For example, DEC achieves >90% microfilarial reduction in W. bancrofti but requires caution in Loa loa-endemic areas due to encephalopathy risks .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


