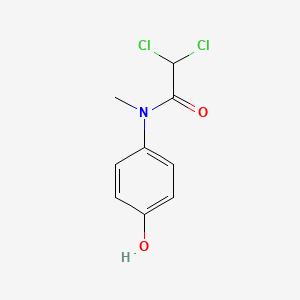
Diloxanide
Description
Properties
IUPAC Name |
2,2-dichloro-N-(4-hydroxyphenyl)-N-methylacetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C9H9Cl2NO2/c1-12(9(14)8(10)11)6-2-4-7(13)5-3-6/h2-5,8,13H,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GZZZSOOGQLOEOB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C1=CC=C(C=C1)O)C(=O)C(Cl)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C9H9Cl2NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0022939 | |
| Record name | Diloxanide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022939 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
234.08 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
579-38-4 | |
| Record name | Diloxanide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=579-38-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diloxanide [INN:BAN:DCF] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000579384 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diloxanide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08792 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diloxanide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022939 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diloxanide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.008.583 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DILOXANIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/89134SCM7M | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Pharmacological Mechanisms of Diloxanide Action
Elucidation of the Luminal Amebicidal Activity
The efficacy of diloxanide stems from its localized action within the intestinal lumen, where it encounters and targets E. histolytica patsnap.compatsnap.comksumsc.commims.comsmarthealer.pkiipseries.org. This luminal activity is crucial for reducing the parasitic load and preventing further invasion of the intestinal wall patsnap.com.
This compound directly destroys the trophozoite forms of Entamoeba histolytica, which are the active, motile, and disease-causing stages of the parasite nih.gove-lactancia.orgksumsc.comsmarthealer.pkdrugbank.comksumsc.com. This direct amoebicidal effect leads to the inhibition of the parasite's growth and replication, ultimately resulting in its demise patsnap.compatsnap.com.
Beyond its direct effects on trophozoites, this compound also plays a significant role in blocking their conversion into the more resistant and transmissible cyst forms nih.gove-lactancia.orgnih.govresearchgate.netdrugbank.com. It is also effective against the cyst form of the parasite patsnap.comsmarthealer.pk. This interference with the parasite's life cycle is particularly important for preventing the spread of infection and reducing the likelihood of disease recurrence patsnap.comsmarthealer.pkksumsc.commsdmanuals.com.
Direct Amoebicidal Effects on Entamoeba histolytica Trophozoites
Molecular and Biochemical Targets
While the precise biochemical interactions of this compound with amoebic cells are not fully elucidated, current research points to several hypothesized molecular and biochemical targets patsnap.com.
A prominent hypothesis suggests that this compound interferes with the protein synthesis pathways within Entamoeba histolytica patsnap.compatsnap.comdrugbank.comsmarthealer.pkiipseries.orgnih.govresearchgate.netdrugbank.com. This disruption is thought to inhibit the translation of mRNA, thereby halting the production of essential proteins required for the parasite's survival and leading to its death iipseries.org. This proposed mechanism is supported by the observed structural similarity between this compound and chloramphenicol, an antibacterial agent known for its protein synthesis inhibitory action nih.govresearchgate.net.
Some studies propose that this compound exerts its effects by interfering with the DNA synthesis of the parasite ijpsjournal.com. This disruption of DNA replication would impede the parasite's ability to proliferate, ultimately leading to its death ijpsjournal.com.
This compound is believed to broadly disrupt the metabolism of Entamoeba histolytica trophozoites patsnap.comnih.gov. This includes interfering with essential metabolic processes and enzymes that are critical for the parasite's survival and propagation patsnap.com.
Research Findings on Parasite Clearance Rate this compound has demonstrated significant efficacy in clearing Entamoeba histolytica from the intestinal lumen.
| Parasite Clearance Rate | Reference |
| 81-96% | nih.govresearchgate.net |
Proposed Disruption of Parasite DNA Synthesis
Prodrug Activation and Active Metabolite Formation
This compound is administered as its furoate ester, this compound furoate, which serves as a prodrug. patsnap.comresearchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.comebi.ac.uknih.gov This prodrug formulation is crucial for its therapeutic efficacy, as it undergoes a specific transformation within the body to yield the pharmacologically active compound, this compound. patsnap.comresearchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com
Upon oral administration, this compound furoate is subjected to hydrolysis within the gastrointestinal tract. patsnap.comresearchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com This enzymatic cleavage, primarily facilitated by esterases present in the intestines, results in the release of the active drug, this compound, and furoic acid. researchgate.netdrugbank.comdrugbank.come-lactancia.orgpharmaoffer.comwikipedia.org The slow absorption of this compound furoate from the gastrointestinal tract ensures that an adequate concentration of the medication is maintained in the intestinal lumen for an extended period, allowing the active this compound to exert its therapeutic effects directly at the site of amoebic infection. patsnap.comdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com
Following its formation in the gastrointestinal tract, a portion of the active this compound is absorbed into the systemic circulation. patsnap.com The absorbed this compound then undergoes extensive Phase II metabolism, primarily through glucuronidation. patsnap.comresearchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.comwikipedia.org This process, which largely occurs in the liver, involves the conjugation of this compound with glucuronic acid, forming a glucuronide conjugate. patsnap.comresearchgate.nete-lactancia.org
The glucuronide metabolite of this compound is considered pharmacologically inactive. e-lactancia.org Research findings indicate that in the systemic circulation, approximately 99% of this compound is present as its glucuronide metabolite, with only about 1% remaining as free, unconjugated this compound. researchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com This extensive and rapid glucuronidation, coupled with the subsequent renal excretion of the glucuronide metabolite (approximately 90% of the dose), significantly limits the systemic exposure to the active this compound. patsnap.comresearchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com This metabolic profile contributes to this compound's luminal amebicidal specificity, ensuring that a substantial concentration of the active drug remains within the intestinal lumen to combat Entamoeba histolytica trophozoites.
Table 1: Distribution of this compound and its Metabolite in Systemic Circulation
| Compound | Percentage in Systemic Circulation |
| This compound (free form) | ~1% researchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com |
| This compound Glucuronide | ~99% researchgate.netdrugbank.comdrugbank.comnih.govnih.gove-lactancia.orgpharmaoffer.com |
Preclinical and Clinical Efficacy Studies
In Vitro Investigations of Antiamoebic Potency
In vitro studies have demonstrated the direct antiamoebic activity of diloxanide against Entamoeba histolytica. This compound, the active form of this compound furoate, exhibited an inhibitory concentration 50% (IC50) of 9.1 µg/ml against wild-type clone A organisms of E. histolytica medchemexpress.com. For an emetine-resistant mutant clone C2 of E. histolytica, the IC50 was found to be 16.6 µg/ml, indicating a higher concentration required for inhibition in resistant strains medchemexpress.com.
This compound furoate has also shown potent activity against various strains of E. histolytica with specific minimum inhibitory concentration (MIC) values. For the BYso strain (grown with bacteria), the MIC was 2.5 µg/ml. Similarly, for the SFL3 strain (grown with Chrithidia sp.), the MIC was 2.5 µg/ml, and for the SFL3 strain (grown axenically), the MIC was 1.95 µg/ml e-lactancia.org. Notably, this compound furoate has been shown to have minimal or no in vitro effect on other intestinal protozoan parasites, such as Dientamoeba fragilis and Blastocystis hominis, highlighting its selective activity against E. histolytica.
**Table 1: In Vitro Antiamoebic Potency of this compound and this compound Furoate against *Entamoeba histolytica***
| Compound | E. histolytica Strain/Condition | Metric | Value (µg/ml) | Reference |
| This compound | Wild-type clone A | IC50 | 9.1 | medchemexpress.com |
| This compound | Emetine-resistant clone C2 | IC50 | 16.6 | medchemexpress.com |
| This compound Furoate | BYso (grown with bacteria) | MIC | 2.5 | e-lactancia.org |
| This compound Furoate | SFL3 (grown with Chrithidia sp.) | MIC | 2.5 | e-lactancia.org |
| This compound Furoate | SFL3 (grown axenically) | MIC | 1.95 | e-lactancia.org |
In Vivo Efficacy in Animal Models
Preclinical evaluations in animal models have provided substantial evidence for the in vivo efficacy of this compound furoate in treating amoebiasis.
Studies conducted in weanling rats demonstrated a clear dose-response relationship for this compound furoate in clearing amoebic infections. Oral administration of this compound furoate for three days resulted in varying cure rates depending on the dose. At a dose of 200 mg/kg, 100% of the treated rats achieved a cure. Lower doses also showed significant efficacy: 85% of rats were cured at 150 mg/kg, 77% at 100 mg/kg, and 44.4% at 75 mg/kg escholarship.orgasm.org. The effective dose 50% (ED50) for this compound furoate in this rat model was determined to be 77.9 mg/kg escholarship.orgasm.org.
Table 2: Dose-Response Relationship of this compound Furoate in Weanling Rats
| Dose (mg/kg/day) | Duration (days) | Cure Rate (%) | Reference |
| 200 | 3 | 100 | escholarship.orgasm.org |
| 150 | 3 | 85 | escholarship.orgasm.org |
| 100 | 3 | 77 | escholarship.orgasm.org |
| 75 | 3 | 44.4 | escholarship.orgasm.org |
| ED50 | 77.9 mg/kg | escholarship.orgasm.org |
Beyond parasite clearance, in vivo studies also assessed the resolution of amoebic lesions. In the weanling rat model, treatment with this compound furoate at a dose of 200 mg/kg resulted in the complete absence of amoebic lesions in the caecum, indicating effective resolution of the pathological manifestations of the infection escholarship.orgasm.org.
Preclinical evaluations of this compound furoate for potential embryotoxic and teratogenic effects have been conducted in animal models. Studies in New Zealand white rabbits, administered this compound furoate via oral intubation at doses of 120 or 300 mg per kg of body weight per day from the first to the twenty-ninth day of pregnancy, revealed no embryotoxic or teratogenic effects. Similar findings were observed in rats that received the same doses from the first to the twentieth day of pregnancy. Furthermore, some animals given a lower dose of this compound furoate until term demonstrated normal parturition, survival, and development of their offspring.
Assessment of Amoebic Lesion Resolution
Clinical Efficacy in Amoebiasis Cohorts
Clinical studies have consistently supported the effectiveness of this compound, particularly in cases of asymptomatic intestinal amoebiasis.
This compound furoate is considered a drug of choice for treating asymptomatic intestinal amoebiasis, especially in individuals who are passing Entamoeba histolytica cysts. Clinical data indicate that this compound furoate achieves a high clinical cure rate, often exceeding 90% in asymptomatic carriers. Parasite clearance rates in clinical settings have been reported to range from 81% to 96% medchemexpress.com. In specific studies, the parasitological cure rate (PCR) for this compound furoate was reported as 93% in one cohort of men and 88% in another. While this compound furoate is effective in eradicating intestinal infection and preventing reinfection, its efficacy is generally lower in cases of invasive intestinal amebiasis, and it demonstrates little to no activity outside the intestinal lumen, making it ineffective for extraintestinal amebiasis.
Table 3: Clinical Efficacy of this compound Furoate in Asymptomatic Intestinal Amoebiasis
| Outcome | Range/Value (%) | Reference |
| Clinical Cure Rate | >90 | |
| Parasite Clearance Rate | 81–96 | medchemexpress.com |
| Parasitological Cure Rate | 93 (Study 1) | |
| Parasitological Cure Rate | 88 (Study 2) |
Drug Resistance and Relapse Mechanisms
Emergence of Clinical Resistance to Diloxanide and Related Compounds
The direct emergence of clinical resistance specifically to this compound in Entamoeba histolytica is not extensively documented or widely demonstrated in clinical settings oup.com. However, laboratory studies have shown that E. histolytica mutants can develop resistance through selection effects oup.com. The broader context of amebicidal drug resistance reveals concerns regarding related compounds. For instance, resistance to metronidazole, a commonly used tissue amebicide, has been observed clinically and in laboratory settings for E. histolytica, as well as other anaerobic protozoa like Giardia duodenalis and Trichomonas vaginalis nih.gov. Reports of metronidazole treatment failure and variations in drug sensitivity suggest that E. histolytica may exhibit reduced susceptibility to medication researchgate.net. Furthermore, a recent study from India indicated a rising inhibitory concentration of nitroimidazoles against E. histolytica wjgnet.com. The potential for Entamoeba histolytica to develop resistance to various antiparasitic drugs, including this compound furoate, is an active area of investigation smarthealer.pk.
Molecular Basis of Parasite Resistance Development
The precise molecular basis underlying resistance development to this compound remains largely unelucidated, partly because its exact mechanism of action is not fully understood patsnap.comnih.gov. This compound is believed to disrupt protein synthesis and mitochondrial function in E. histolytica trophozoites, leading to parasite death smarthealer.pkpatsnap.com. Any resistance mechanisms would likely involve alterations in these cellular processes or drug uptake/metabolism pathways.
For related nitroimidazole compounds like metronidazole, more defined molecular mechanisms of resistance have been identified, particularly in Trichomonas vaginalis and Giardia duodenalis. These include:
Bypass Metabolism: The development of alternative metabolic pathways that circumvent the drug's action nih.gov.
Alternative Oxidoreductases: The presence of enzymes that can bypass the drug's activation pathway nih.gov.
Reduced Drug Activation: Resistance to metronidazole can be associated with the nim gene, which encodes a nitroimidazole reductase enzyme. The presence of the nimE gene has been observed in some recurrent amebic liver abscess patients, suggesting a role in resistance wjgnet.com. This enzyme is involved in activating metronidazole to its cytotoxic radical form; thus, alterations could lead to decreased drug susceptibility nih.gov.
While these specific molecular mechanisms are primarily described for nitroimidazoles, the general principles of protozoal drug resistance often involve genetic factors such as mutations in genes encoding drug targets or efflux pumps mdpi.com.
Strategies to Mitigate Drug Resistance in this compound Therapies
To combat the emergence of drug resistance and enhance treatment efficacy, several strategies are employed and investigated in the context of this compound therapies:
Combination Regimens: this compound is frequently used in combination with systemic amebicides, such as metronidazole or tinidazole smarthealer.pkresearchgate.net. This approach targets both the luminal (cyst and trophozoite forms) and systemic (invasive trophozoite forms) stages of E. histolytica, ensuring more complete parasite eradication and reducing the likelihood of resistance development or relapse smarthealer.pkresearchgate.net. Combination therapy has been shown to prevent disease recurrence researchgate.net.
Diagnostic Improvements: Enhanced diagnostic tools are crucial for distinguishing pathogenic E. histolytica from non-pathogenic E. dispar, which can help in targeted treatment and reduce unnecessary drug exposure that might contribute to resistance researchgate.net.
Identification of New Therapeutic Targets: Ongoing research aims to understand the molecular mechanisms and virulence factors of E. histolytica to identify novel targets for drug development researchgate.netresearchgate.net. Strategies include improving the potency of existing amebicides, repurposing approved drugs, and drug rediscovery researchgate.net.
Factors Contributing to Relapse Rates in Monotherapy and Combination Regimens
Relapse in amoebiasis treatment can occur due to several factors, particularly when this compound is used as monotherapy or when treatment regimens are incomplete.
Luminal Action of this compound: this compound is a luminal amebicide, meaning its primary action is confined to the intestinal lumen smarthealer.pknih.govwikipedia.org. It is highly effective against the cyst form of E. histolytica, which is responsible for transmission and recurrence smarthealer.pkpatsnap.com. However, this compound has no significant activity outside the intestinal lumen researchgate.netwikipedia.org. If invasive trophozoites are present in tissues (e.g., liver, brain), this compound alone will not eradicate them, leading to potential relapse from extra-intestinal foci or persistent intestinal infection if not all trophozoites are cleared wjgnet.comresearchgate.net.
Incomplete Treatment Courses: Adherence to the full prescribed course of treatment is essential. Incomplete treatment can lead to the survival of residual parasites, which can then proliferate and cause a relapse of the infection patsnap.com.
Lack of Luminal Agent Post-Systemic Treatment: For symptomatic amoebiasis or amebic liver abscess, systemic amebicides like metronidazole or tinidazole are used to treat invasive forms. However, these drugs may not fully clear luminal cysts. Failure to follow up systemic treatment with a luminal agent like this compound furoate can result in relapse, as parasites can persist in the colon wjgnet.comdroracle.ai.
Monotherapy vs. Combination Therapy: Studies indicate that this compound monotherapy may be associated with high relapse rates. In contrast, combination therapies, where this compound is used alongside a tissue-active amebicide, show no relapse, highlighting the importance of comprehensive treatment that targets both luminal and systemic forms of the parasite researchgate.net.
Table 1: Comparative Aspects of this compound Furoate and Metronidazole in Amoebiasis Treatment
| Feature | This compound Furoate | Metronidazole | References |
| Primary Target | Luminal trophozoites & cysts | Invasive trophozoites (luminal & extra-intestinal) | researchgate.netsmarthealer.pk |
| Site of Action | Intestinal lumen | Systemic (tissue-penetrating) | smarthealer.pkwikipedia.org |
| Effectiveness | High (81–96% parasite clearance rate) | High (90–95% cure rate) | researchgate.net |
| Relapse Rates | High when used as monotherapy; No relapse in combination therapy | No relapse (when followed by luminal agent) | researchgate.netwjgnet.com |
| Role in Treatment | Second-line for asymptomatic carriers; Follow-up to systemic amebicides for symptomatic cases | First-line for symptomatic intestinal amebiasis or amebic liver abscess | wikipedia.orgdroracle.ai |
Chemical Synthesis and Structural Modifications
Established Synthetic Methodologies for Diloxanide Furoate
The synthesis of this compound furoate often involves the selective introduction of a hydroxyl group onto an aromatic ring, a process that demands high control over reaction conditions.
A significant advancement in the synthesis of this compound furoate involves selenium-catalyzed para-hydroxylation of N-aryl-hydroxamic acids. This method has been successfully applied for gram-scale production of this compound furoate researchgate.netresearchgate.netnih.govd-nb.info. Mechanistically, this redox-neutral transformation proceeds via an N-O bond cleavage followed by a selenium-induced wikipedia.orgwikidata.org-rearrangement, ultimately yielding para-hydroxyaniline derivatives researchgate.netresearchgate.netnih.govd-nb.info. Specifically, dichloroacetyl hydroxamic acid serves as a precursor, undergoing selenium-catalysis to produce the corresponding para-dichloroacetyl aminophenol nih.govd-nb.info. This approach offers an unconventional and efficient route to para-aminophenols, which are essential intermediates in the synthesis of this compound furoate nih.gov.
While organo-photocatalysis and transition metal catalysis have been explored for directed hydroxylation of substituted anilides to synthesize α-aminophenol derivatives, including other drug molecules like paracetamol and practolol, specific established methodologies for the synthesis of this compound furoate using these approaches are not explicitly detailed in the provided search results researchgate.net. The selenium-catalyzed method is directly and specifically linked to this compound furoate synthesis, focusing on para-hydroxylation, whereas the organo-photocatalysis often describes ortho-hydroxylation researchgate.net.
The selenium-catalyzed para-hydroxylation method demonstrates excellent regioselectivity, ensuring that the hydroxylation occurs at the desired para-position of the N-aryl-hydroxamic acid precursor researchgate.netresearchgate.netnih.govd-nb.info. This selectivity is crucial for the precise synthesis of this compound furoate. Furthermore, the method exhibits broad functional group compatibility, tolerating a diverse range of functionalities, including ester, bromo, and iodo groups within the substrate researchgate.netresearchgate.netnih.govd-nb.info. This high tolerance allows for the facile late-stage functionalization of complex biologically active molecules, making it a versatile synthetic tool researchgate.net.
Organo-Photocatalysis and Transition Metal Catalysis for Directed Hydroxylation
Structure-Activity Relationship (SAR) Investigations of this compound Derivatives
Understanding the SAR of this compound is fundamental to elucidating its mechanism of action and optimizing its therapeutic profile.
Impact of Dichloroacetamide Moiety on Antiprotozoal Activity
The antiprotozoal activity of this compound is understood to be linked to its dichloroacetamide moiety, which bears structural similarity to chloramphenicol nih.govresearchgate.netresearchgate.net. This structural resemblance is thought to contribute to its mechanism of action. This compound primarily acts against the trophozoites of Entamoeba histolytica, inhibiting their protein synthesis nih.govresearchgate.netresearchgate.netdrugbank.com. By blocking protein synthesis, this compound prevents the conversion of trophozoites into more virulent and invasive cyst forms, thereby exerting its antiamoebic effect nih.govresearchgate.net. Although the exact mechanism of action is not fully elucidated, the inhibition of protein synthesis is a widely accepted hypothesis drugbank.compharmaoffer.com.
Role of the Furoate Ester in Prodrug Properties and Efficacy
This compound furoate functions as a prodrug, meaning it is administered in an inactive form and then metabolized into the active compound, this compound, within the body researchgate.netdrugbank.compharmaoffer.comnih.govcemm.at. Upon reaching the gastrointestinal tract, the furoate ester undergoes hydrolysis, releasing the active this compound researchgate.netdrugbank.compharmaoffer.comcemm.at. This prodrug strategy is advantageous as it allows for a high luminal concentration of the active drug, contributing to its greater efficacy in managing asymptomatic amoebiasis compared to other agents like metronidazole researchgate.net. The bioavailability of this compound (in its parental form) is reported to be 90%, although this compound furoate itself is slowly absorbed from the gastrointestinal tract drugbank.compharmaoffer.com.
Molecular Pharmacology and Pharmacodynamics Research
Quantitative Analysis of Parasite Load Reduction Dynamics
Diloxanide demonstrates significant efficacy in reducing the parasite load of Entamoeba histolytica within the intestinal lumen. Studies have reported a parasite clearance rate ranging from 81% to 96%. Quantitative analyses in animal models further illustrate this reduction. For instance, research conducted in weanling rats infected with E. histolytica showed a dose-dependent cure rate following administration of this compound furoate.
Table 1: this compound Furoate Efficacy in Reducing E. histolytica Infection in Weanling Rats
| This compound Furoate Dose (mg/kg/day) | Cure Rate (%) |
| 200 | 100 |
| 150 | 85 |
| 100 | 77 |
| 75 | 44.4 |
The effective dose 50 (ED50) for this compound furoate in this rat model was determined to be 77.9 mg/kg. These findings underscore the compound's capacity to effectively reduce and eliminate parasitic burden in the gut.
Correlation of Luminal Concentration with Therapeutic Outcomes
A key pharmacodynamic characteristic of this compound furoate is its slow absorption from the gastrointestinal tract. wikipedia.org This property is crucial as it ensures that a high and sustained concentration of the active compound, this compound, remains within the intestinal lumen for an extended period. wikipedia.org The effectiveness of this compound is directly correlated with these elevated concentrations in the intestinal fluids, where E. histolytica resides. As a luminal amebicide, this compound's therapeutic action is primarily confined to the intestinal tract, effectively targeting trophozoites present there and preventing further invasion of the intestinal wall. nih.govuni.luwikipedia.orgwikidata.org While the parent compound, this compound, can be rapidly absorbed (bioavailability of approximately 90%), the absorbed fraction is extensively conjugated with glucuronic acid, forming an inactive metabolite. Only a minor fraction (approximately 1%) of free this compound circulates systemically, reinforcing its localized luminal activity. nih.govuni.lu
Insights into Differential Activity Against Trophozoite and Cyst Stages
This compound exhibits activity against both the trophozoite and cyst forms of Entamoeba histolytica. It is known to destroy the active trophozoites of E. histolytica. nih.govuni.luwikipedia.org Furthermore, a significant aspect of its action is its ability to block the conversion of trophozoites into the more virulent and invasive cyst forms. This dual activity is particularly important because the cyst stage is responsible for the transmission of amoebiasis and the recurrence of infection. wikidata.org this compound is considered an excellent luminal amebicide specifically for the cyst stage. It is important to note that this compound's activity is largely restricted to the intestinal lumen, meaning it has little to no activity against invasive or extraintestinal forms of amebiasis, which are typically treated with tissue-active amebicides like metronidazole. In contrast to this compound, metronidazole primarily targets trophozoites in both intestinal and tissue locations but does not effectively eradicate cysts from the intestines.
Pharmacokinetic Profiles and Biotransformation Pathways
Gastrointestinal Hydrolysis of Diloxanide Furoate
This compound furoate functions as a prodrug, meaning it is an inactive compound that undergoes metabolic conversion within the body to yield the active pharmacological agent. Upon oral administration, this compound furoate undergoes extensive hydrolysis within the gastrointestinal tract, specifically in the intestinal lumen. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netpatsnap.comnih.govmims.com This hydrolysis reaction cleaves the furoate ester, releasing the active compound, this compound, and furoic acid. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netpatsnap.comnih.govmims.com This conversion is a critical step, as it allows the active this compound to exert its therapeutic effects directly within the intestines, the primary site of amoebic infection. patsnap.com The majority of this hydrolysis occurs before the compound is absorbed into the systemic circulation. e-lactancia.org
Systemic Absorption and Distribution Characteristics
Following its hydrolysis in the gastrointestinal tract, this compound demonstrates specific absorption characteristics. While this compound furoate itself is slowly absorbed from the gastrointestinal tract, the resulting parent compound, this compound, is rapidly absorbed. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.gov The bioavailability of this compound in its parental form is approximately 90%. drugbank.comdrugbank.comnih.gove-lactancia.orgnih.govwikipedia.org Despite this significant absorption, a substantial portion of the drug remains within the intestinal lumen, which is crucial for its localized therapeutic action against Entamoeba histolytica. patsnap.com
A summary of key absorption and distribution parameters is provided in the table below:
| Parameter | Value | Source |
| Bioavailability (this compound) | ~90% | drugbank.comdrugbank.comnih.gove-lactancia.orgnih.govwikipedia.org |
| Systemic this compound (Free) | 1% | drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.gov |
| Systemic this compound (Glucuronide) | 99% | drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.gov |
| Time to Peak Concentration (Tmax) | ~2 hours | e-lactancia.orgresearchgate.net |
| Duration of Action | ~6 hours | e-lactancia.orgresearchgate.net |
Metabolic Fate: Glucuronidation and Inactivation Processes
Following its absorption, this compound undergoes extensive biotransformation, primarily through glucuronidation. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netpatsnap.comnih.govwikipedia.org This metabolic process, considered a Phase II reaction, largely occurs in the liver. researchgate.net During glucuronidation, this compound is conjugated with glucuronic acid, forming a glucuronide metabolite. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netpatsnap.comnih.govwikipedia.org This glucuronide conjugate is pharmacologically inactive. e-lactancia.orgresearchgate.net The high percentage of this compound existing as its glucuronide metabolite (99%) in the systemic circulation underscores the efficiency of this inactivation pathway. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.gov
Elimination Pathways and Excretion Dynamics
The elimination of this compound and its metabolites from the body occurs predominantly via renal excretion. Approximately 90% of the administered dose of this compound is rapidly excreted in the urine, primarily in the form of its inactive glucuronide metabolite. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.govwikipedia.org The remaining portion, about 10% of the dose, is excreted in the feces as unchanged this compound. drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.govmims.comwikipedia.org The elimination half-life of this compound is reported to be 3 hours. drugbank.comnih.govwikipedia.org
A summary of elimination parameters is presented in the table below:
| Parameter | Value | Source |
| Primary Elimination Route | Renal (Urine) | drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.govwikipedia.org |
| Excretion in Urine | ~90% (as glucuronide) | drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.govwikipedia.org |
| Excretion in Feces | ~10% (as this compound) | drugbank.comdrugbank.comnih.gove-lactancia.orgresearchgate.netnih.govmims.comwikipedia.org |
| Elimination Half-Life | 3 hours | drugbank.comnih.govwikipedia.org |
Toxicological Investigations and Safety Profiles
Mechanistic Studies of Diloxanide's Toxicity Profile
The precise mechanism by which this compound might exert toxicity in mammalian systems is not extensively documented in current literature. Its primary therapeutic action against Entamoeba histolytica trophozoites is believed to involve interference with protein synthesis, leading to the parasite's demise researchgate.netijpsjournal.compatsnap.com. This antiparasitic mechanism is thought to be related to the drug's dichloroacetamide group, which bears structural similarities to chloramphenicol researchgate.net. This compound furoate, the prodrug form, undergoes hydrolysis in the gastrointestinal tract to release the active compound, this compound wikipedia.orge-lactancia.orgdrugbank.com. The active this compound is then rapidly absorbed but is extensively conjugated with glucuronic acid, with approximately 99% circulating as the inactive glucuronide metabolite e-lactancia.orgdrugbank.comdrugbank.com. This rapid and extensive first-pass metabolism and subsequent excretion significantly limit systemic exposure to the active compound, thereby mitigating potential systemic toxicity wikipedia.orge-lactancia.org. While protein synthesis inhibition is its mode of action against the parasite, specific mechanistic studies detailing direct toxicity to mammalian cells at clinically relevant concentrations are not prominently reported, suggesting that its pharmacokinetic profile largely dictates its favorable systemic safety.
Preclinical Safety Assessments (e.g., Reproductive and Developmental Toxicity Studies)
Preclinical studies have been conducted to evaluate the reproductive and developmental safety of this compound furoate. These assessments are critical for understanding potential risks during gestation and early development.
Table 1: Preclinical Reproductive and Developmental Toxicity Studies of this compound Furoate
| Study Type | Species | Dose (mg/kg/day) | Gestation Period | Findings | Citation |
| Reproductive/Developmental Toxicity | New Zealand White Rabbit | 120 or 300 | Day 1-29 of pregnancy | No embryotoxic or teratogenic effects observed | e-lactancia.org |
| Reproductive/Developmental Toxicity | Rat | 120 or 300 | Day 1-20 of pregnancy | No embryotoxic or teratogenic effects observed | e-lactancia.org |
As detailed in Table 1, studies in New Zealand white rabbits, administered oral this compound furoate at doses of 120 or 300 mg/kg/day from day 1 to 29 of pregnancy, demonstrated no embryotoxic or teratogenic effects e-lactancia.org. Similar results were observed in rats given the same doses from day 1 to 20 of pregnancy e-lactancia.org. These preclinical findings are instrumental in informing clinical recommendations regarding the use of this compound during pregnancy, often suggesting its administration after the first trimester wikipedia.orge-lactancia.org.
Comparative Toxicology with Other Antiamoebic Agents
Table 2: Comparative Toxicological Characteristics of Antiamoebic Agents
| Agent | Primary Action Site | Key Toxicological/Pharmacokinetic Factor | Mechanism of Action (Antiparasitic) | Citation |
| This compound | Intestinal Lumen | Extensive glucuronidation, limiting systemic exposure of active form; rapid excretion | Interferes with protein synthesis in trophozoites | researchgate.netpatsnap.comwikipedia.orge-lactancia.orgdrugbank.comdrugbank.com |
| Metronidazole | Systemic (Intestinal & Extraintestinal) | Systemic absorption; formation of reactive nitroso radicals | Inhibits nucleic acid synthesis, disrupts DNA | wikipedia.orgmims.comdigitaloceanspaces.com |
| Tinidazole | Systemic (Intestinal & Extraintestinal) | Systemic absorption; slower metabolism than metronidazole | Damages DNA strands or inhibits DNA synthesis | mims.comdigitaloceanspaces.com |
| Paromomycin | Intestinal Lumen | Poor systemic absorption; potential for systemic toxicity with impaired GI integrity | Inhibits bacterial protein synthesis (30S ribosomal subunit) | wikipedia.orgfishersci.nlmims.com |
As shown in Table 2, this compound furoate is predominantly a luminal amebicide, meaning its therapeutic effects are largely confined to the intestinal lumen, where it directly targets trophozoites patsnap.comdigitaloceanspaces.com. This localized action is a significant factor contributing to its generally mild systemic impact wikipedia.org. In contrast, nitroimidazole derivatives such as metronidazole and tinidazole are systemically active agents that penetrate tissues, making them effective against both intestinal and extraintestinal forms of amoebiasis wikipedia.orgmims.comdigitaloceanspaces.com. Their mechanism involves the formation of reactive nitroso radicals that disrupt microbial DNA wikipedia.orgmims.com. While effective, metronidazole is associated with a broader range of systemic considerations, and tinidazole, although structurally similar, is often reported to be better tolerated digitaloceanspaces.com. Paromomycin, another luminal amebicide, functions by inhibiting bacterial protein synthesis fishersci.nlmims.com. Its poor systemic absorption also contributes to its localized action and generally favorable systemic safety profile, though concerns regarding ototoxicity and nephrotoxicity exist if significant systemic absorption occurs, particularly in patients with compromised gastrointestinal integrity wikipedia.orgfishersci.nlmims.commims.com. Older agents like emetine are rarely used due to their pronounced toxicity . This comparative analysis highlights that this compound's limited systemic exposure to the active compound and its primary luminal activity contribute to its generally favorable tolerability profile compared to systemically active agents that may exhibit more widespread toxicities wikipedia.orgdigitaloceanspaces.com.
Factors Influencing Tolerability in Clinical Settings
Several key factors contribute to the observed tolerability of this compound in clinical practice:
Pharmacokinetic Profile: this compound furoate, the administered prodrug, undergoes significant hydrolysis in the intestinal lumen to yield this compound and furoic acid e-lactancia.org. The active this compound is then rapidly absorbed, but critically, it undergoes extensive glucuronidation in the liver e-lactancia.orgdrugbank.comdrugbank.com. Approximately 99% of this compound in the systemic circulation exists as the inactive glucuronide conjugate, with only about 1% remaining as free, active this compound e-lactancia.orgdrugbank.comdrugbank.com. This rapid and extensive first-pass metabolism substantially limits systemic exposure to the active compound, thereby minimizing potential systemic toxicity e-lactancia.org.
Luminal Action: As a luminal amebicide, this compound primarily exerts its therapeutic effects within the gastrointestinal tract, where the target parasites reside patsnap.comwikipedia.org. Its limited systemic tissue penetration means that systemic toxicological effects are less likely compared to drugs that achieve high concentrations in various body tissues digitaloceanspaces.com.
Clinical Observations: Clinical experience and studies have generally indicated that this compound is a well-tolerated medication, both when administered alone and in combination with other antiamoebic agents like metronidazole researchgate.net. The mildness of its effects is consistent with its pharmacokinetic and pharmacodynamic characteristics wikipedia.org.
Drug Interactions and Combination Therapies
Pharmacodynamic Interactions with Nitroimidazole Derivatives (e.g., Metronidazole, Tinidazole)
Diloxanide primarily functions as a luminal amoebicide, exerting its effects directly within the intestinal lumen by targeting the trophozoites and cysts of Entamoeba histolytica. Its mechanism is believed to involve the disruption of protein synthesis in E. histolytica trophozoites, thereby inhibiting their growth and replication and preventing their conversion into more virulent, invasive cyst forms. patsnap.compatsnap.comsmarthealer.pkresearchgate.netnih.govzynapte.comdrugbank.comdrugbank.comrwandafda.gov.rw
In contrast, nitroimidazole derivatives like metronidazole and tinidazole act as tissue amoebicides. They are effective against the invasive forms of amoebiasis, targeting trophozoites present in the intestinal walls or extraintestinal tissues such as the liver. The mechanism of action for metronidazole and tinidazole involves their reduction intracellularly within the microorganism, leading to the formation of cytotoxic free radicals that damage DNA or inhibit DNA synthesis. zynapte.comrwandafda.gov.rwwikipedia.orgmims.commims.comzeelabpharmacy.comlongdom.orgpharaohacademy.commedscape.com
The co-administration of this compound with nitroimidazole derivatives creates a synergistic pharmacodynamic interaction. This combination provides a dual-action approach, effectively targeting both the luminal and invasive tissue forms of the parasite. This comprehensive eradication strategy is beneficial for treating various stages of amoebiasis, from asymptomatic cyst passage to acute amoebic dysentery and extraintestinal manifestations. patsnap.compatsnap.comsmarthealer.pknih.govdrugbank.comdrugbank.comzeelabpharmacy.comlongdom.orgpharaohacademy.commedscape.comtaylorandfrancis.comdroracle.ai
Pharmacokinetic Interactions with Other Co-administered Medications
This compound furoate, the administered form of the drug, is a prodrug that undergoes hydrolysis in the gastrointestinal tract to release the active compound, this compound. patsnap.compatsnap.comzynapte.comdrugbank.comdrugbank.comrwandafda.gov.rwwikipedia.orgnih.gove-lactancia.org A significant portion of the active this compound remains in the intestine to exert its therapeutic effects, while a smaller fraction is absorbed systemically. The absorbed this compound is predominantly conjugated with glucuronic acid in the liver and subsequently excreted via urine. patsnap.comzynapte.comdrugbank.comdrugbank.comwikipedia.orge-lactancia.orgfda.gov.ph
Metronidazole is characterized by rapid and nearly complete absorption from the gastrointestinal tract. It is widely distributed throughout body tissues and fluids, metabolized in the liver primarily by the CYP3A4 isoenzyme, and primarily excreted in the urine. zynapte.commims.commims.com Similarly, tinidazole is rapidly and almost completely absorbed, metabolized in the liver (also via the CYP3A4 isoenzyme), and excreted through both urine and feces. zynapte.commims.com
While some sources indicate no known clinically significant drug interactions for this compound furoate fda.gov.phmycme.comdruginfosys.com, others suggest that this compound may interact with certain antibiotics, antimalarials, and other drugs metabolized through the liver, though comprehensive interaction studies are limited. patsnap.com When co-administered with tinidazole, potential interactions may arise in patients with underlying conditions such as kidney diseases, liver diseases, peripheral neuropathy, epilepsy, blood disorders, and G-6 phosphate dehydrogenase deficiency. apollopharmacy.in Furthermore, metronidazole can potentiate the effects of oral anticoagulants, leading to prolonged prothrombin time. mims.commims.com Enzyme inducers like phenytoin and phenobarbital can increase the metabolism of metronidazole, thereby reducing its half-life. rwandafda.gov.rwmims.commims.comksumsc.com
Rationale and Efficacy of this compound-Based Combination Regimens
The primary rationale for combining this compound with nitroimidazole derivatives stems from their complementary mechanisms of action and sites of activity. This compound effectively eliminates the cyst and trophozoite forms of E. histolytica within the intestinal lumen, addressing the source of infection and preventing transmission. patsnap.compatsnap.comsmarthealer.pknih.govzynapte.comdrugbank.comdrugbank.comzeelabpharmacy.compharaohacademy.commedscape.comtaylorandfrancis.comdroracle.aiwikipedia.orgnih.gov Concurrently, nitroimidazoles target the invasive trophozoites in the intestinal wall and extraintestinal sites, resolving acute symptoms and complications of invasive amoebiasis. zynapte.comrwandafda.gov.rwwikipedia.orgmims.commims.comzeelabpharmacy.comlongdom.orgpharaohacademy.commedscape.comwikipedia.org This comprehensive approach ensures the eradication of the parasite from both luminal and systemic locations, which is critical for successful treatment and prevention of recurrence. patsnap.compatsnap.comzeelabpharmacy.commedscape.comdroracle.ai
Clinical studies have demonstrated the high efficacy of this compound-based combination regimens. For instance, a study evaluating a combined this compound furoate-metronidazole preparation in patients with amoebiasis and giardiasis reported a 100% parasitic clearance rate in both groups. Abdominal pain was completely relieved in 91% of amoebiasis patients and 84% of giardiasis patients. researchgate.net Another study observed a 98% parasitological cure rate for amoebiasis and a 92% rate for giardiasis after a five-day treatment course with metronidazole and this compound. annalskemu.org Combination therapy is generally considered more effective in reducing parasitological failure compared to metronidazole monotherapy. medscape.comtaylorandfrancis.com this compound furoate is frequently used sequentially after treatment with metronidazole or tinidazole to eliminate any residual intestinal amoebae and prevent relapse. medscape.comtaylorandfrancis.comdroracle.aiwikipedia.org
Table 1: Efficacy of this compound-Based Combination Regimens
| Combination Regimen | Condition Treated | Parasitological Cure Rate | Clinical Cure Rate | Reference |
| This compound Furoate + Metronidazole | Amoebiasis | 100% | 91% (abdominal pain relief) | researchgate.net |
| This compound Furoate + Metronidazole | Giardiasis | 100% | 84% (abdominal pain relief) | researchgate.net |
| Metronidazole + this compound | Amoebiasis | 98% | 98% | annalskemu.org |
| Metronidazole + this compound | Giardiasis | 92% | 98% | annalskemu.org |
Impact of Combination Therapies on Resistance Development and Relapse Prevention
The use of combination therapies involving this compound plays a crucial role in both mitigating the development of drug resistance and preventing disease relapse.
Relapse Prevention: this compound furoate is particularly vital for preventing relapse, especially following initial treatment with tissue-acting amoebicides like metronidazole or tinidazole. Its luminal activity ensures the elimination of any remaining cysts in the intestine, which are responsible for both disease transmission and recurrence. patsnap.comnih.govzeelabpharmacy.commedscape.comtaylorandfrancis.comdroracle.aiwikipedia.org Studies have indicated high relapse rates when this compound is used as monotherapy, whereas combination therapy has been associated with no observed relapse. researchgate.net This highlights the importance of this compound in a sequential or concurrent regimen to achieve complete parasitic clearance and significantly reduce the chances of recurrence. zeelabpharmacy.com
Advanced Methodologies in Diloxanide Research
Spectrophotometric and Chromatographic Analytical Techniques
A variety of spectrophotometric and chromatographic methods have been developed for the assay and degradation product analysis of Diloxanide furoate, often in combination with other drugs. These techniques offer high sensitivity, selectivity, and accuracy for quantitative determination and stability assessment.
Spectrophotometric Methods: Several spectrophotometric approaches are employed for this compound furoate analysis. Direct spectrophotometry involves measuring the absorbance of this compound furoate, for instance, at 260 nm, with a reported molar absorptivity of 22970 L/mol cm scribd.com. Another method utilizes the reaction of this compound furoate with potassium permanganate in the presence of sodium hydroxide, yielding a bluish-green species measurable at 610 nm, showing linearity in the range of 2.5-20 µg/mL researchgate.netnih.gov.
Chemometric-assisted spectrophotometry, including Principal Component Regression (PCR) and Partial Least Squares (PLS), has been successfully applied for the simultaneous determination of this compound furoate and metronidazole, even in the presence of this compound furoate's alkaline degradates. These methods utilize absorption spectra in the 225-320 nm range nih.govfue.edu.eg. Derivative spectrophotometry, specifically second (D2) and third (D3) derivatives, has been developed to quantify this compound furoate in the presence of its degradation product, this compound, by measuring peak amplitudes at specific wavelengths (e.g., 260 nm and 270 nm for D2 and D3, respectively) tandfonline.com. Furthermore, the double divisor ratio spectra derivative (DDRSD) method has been exploited for the quantification of this compound furoate and metronidazole in the presence of this compound furoate degradation products (furoic acid and 4-hydroxy-N-methyl), demonstrating linearity in the range of 2–25 µg/mL for this compound furoate pharaohacademy.compharaohacademy.compharaohacademy.com.
Chromatographic Methods: High-Performance Liquid Chromatography (HPLC) is extensively used for the simultaneous estimation of this compound furoate with co-formulated drugs and for stability-indicating assays.
RP-HPLC for Simultaneous Estimation :
For this compound furoate and metronidazole, methods include using a C18 analytical column with a mobile phase of acetonitrile-0.05 M dibasic potassium phosphate (25:75, v/v) at pH 4, detected at 254 nm nih.gov. Another RP-HPLC method uses a Shimadzu C18 column with acetonitrile and 0.1% v/v Glacial Acetic acid (40:60) as the mobile phase, detected at 273 nm, showing linearity for this compound furoate from 15.625–500 µg/mL ijnrd.org. A different method employed a C8 column with methanol:acetonitrile:0.05 M phosphate buffer (pH 4.0) in a 45:25:30 v/v ratio, detecting at 277 nm, with retention times of 6.42 min for this compound furoate .
For this compound furoate and ornidazole, an RP-HPLC method utilizes a Waters Symmetry C18 column with triethylammonium phosphate buffer (pH 2.3):acetonitrile (40:60 v/v) as the mobile phase, detected at 270 nm. This compound furoate typically elutes at 4.31 min and exhibits linearity in the range of 46.87-140.62 µg/mL indexcopernicus.com.
For this compound furoate and tinidazole, a method uses a mobile phase of acetonitrile, methanol, and 0.2 M potassium dihydrogen phosphate (pH 5) in a 2:3:2 ratio on an SS Wakosil-II C-18 column, detected at 282 nm, with linearity from 10-70 µg/mL for this compound furoate researchgate.net.
Stability-Indicating HPLC : HPLC methods are crucial for monitoring this compound furoate degradation products under various stress conditions (e.g., alkaline hydrolysis, photodegradation), ensuring the method's specificity and stability ijnrd.orgebi.ac.uknih.govresearchgate.net.
High-Performance Thin-Layer Chromatography (HPTLC) and Thin-Layer Chromatography (TLC)-Densitometry are also employed for this compound furoate analysis. TLC-densitometry has been developed for the separation and determination of this compound furoate, metronidazole, and its impurity (4-nitroimidazole) on silica gel 60 F254 plates, using ethyl acetate/acetone/hexane/ammonia solution (9.5:0.5:0.3:0.3, by volume) as the mobile phase, with UV scanning at 276 nm researchgate.net. An HPTLC method for simultaneous determination of this compound furoate and tinidazole uses silica gel 60F254 plates and dichloromethane-methanol (9.6:0.25, v/v) as the mobile phase, with UV detection at 280 nm. This compound furoate showed an Rf of 0.45 and linearity from 40-500 µg/mL scispace.com. TLC procedures have also been developed for the fractionation and identification of this compound furoate's alkaline hydrolysis products ebi.ac.uknih.gov.
| Analytical Technique | Application | Key Parameters / Findings for this compound Furoate | Reference |
|---|---|---|---|
| Chemometric-assisted Spectrophotometry (PCR, PLS) | Simultaneous determination with metronidazole, presence of degradates | Absorption spectra: 225-320 nm | nih.govfue.edu.eg |
| Direct Spectrophotometry | Assay in dosage forms | Reaction with KMnO4/NaOH, measurement at 610 nm; Linearity: 2.5-20 µg/mL; Molar absorptivity: 1.1 x 10^4 L/mol cm | researchgate.netnih.gov |
| Derivative Spectrophotometry (D2, D3) | Determination in presence of degradation product (this compound) | D2 at 260 nm, D3 at 270 nm in 0.1 N HCl | tandfonline.com |
| Double Divisor Ratio Spectra Derivative (DDRSD) | Quantification with metronidazole, presence of degradation products (FUR, DEG) | Linearity: 2–25 µg/mL | pharaohacademy.compharaohacademy.compharaohacademy.com |
| RP-HPLC (with Metronidazole) | Simultaneous estimation, stability-indicating assay | C18 column; Mobile phase: Acetonitrile:0.05 M dibasic potassium phosphate (25:75, v/v) pH 4; Detection: 254 nm nih.gov; Retention time: 8.498 min ijnrd.org; Linearity: 15.625–500 µg/mL ijnrd.org | nih.govijnrd.org |
| RP-HPLC (with Ornidazole) | Simultaneous quantitative estimation | Waters Symmetry C18 column; Mobile phase: Triethylammonium phosphate buffer (pH 2.3):acetonitrile (40:60 v/v); Detection: 270 nm; Retention time: 4.31 min; Linearity: 46.87-140.62 µg/mL | indexcopernicus.com |
| RP-HPLC (with Tinidazole) | Simultaneous quantification | SS Wakosil-II C-18 column; Mobile phase: Acetonitrile, methanol, 0.2 M potassium dihydrogen phosphate (pH 5) (2:3:2); Detection: 282 nm; Linearity: 10-70 µg/mL | researchgate.net |
| TLC-Densitometry | Separation and determination with metronidazole and impurity | Silica gel 60 F254 plates; Mobile phase: Ethyl acetate/acetone/hexane/ammonia solution (9.5:0.5:0.3:0.3, by volume); UV-scan: 276 nm | researchgate.net |
| HPTLC | Simultaneous determination with tinidazole | Silica gel 60F254 plates; Mobile phase: Dichloromethane-methanol (9.6:0.25, v/v); UV-detection: 280 nm; Retention factor (Rf): 0.45; Linearity: 40-500 µg/mL | scispace.com |
Application of Advanced Spectroscopic and Spectrometric Methods for Structural Elucidation of Metabolites and Degradants
Advanced spectroscopic and spectrometric methods play a critical role in the structural elucidation of this compound furoate's metabolites and degradation products, providing detailed information about their chemical structures.
Mass Spectrometry (MS): Mass spectrometry, particularly High-Resolution Mass Spectrometry (HRMS), is a powerful tool for identifying and characterizing degradation products and metabolites. Studies have utilized MS, often in conjunction with chromatographic techniques like HPLC and TLC, to identify the hydrolysis products of this compound furoate. Key degradation products identified from alkaline hydrolysis of this compound furoate include furoic acid, this compound, and methylfuroate ebi.ac.uknih.gov. HRMS instruments, such as the Orbitrap Q Exactive Plus, offer high resolution, accuracy, and sensitivity, enabling the identification of previously undetected metabolites and the rapid determination of their chemical structures. This capability is vital for understanding metabolic pathways and identifying "soft spots" in compounds jubilantbiosys.com. Liquid Chromatography-Mass Spectrometry (LC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS) are generally applied for profiling and structural elucidation of various metabolites and compounds ipk-gatersleben.de.
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy provides invaluable insights into the atomic-level structure of compounds. Proton NMR (1H-NMR) spectra of this compound furoate have been obtained using high-field instruments (e.g., 300, 400, or 500 MHz). Advanced NMR techniques such as Distortionless Enhancement by Polarization Transfer (DEPT), Correlation Spectroscopy (COSY), and Heteronuclear Correlation (HETCOR) spectra are employed to assign resonance bands and confirm the connectivity of atoms within the molecule scribd.com. These techniques are fundamental for confirming the structure of the parent drug and elucidating the structures of any newly formed degradants or metabolites.
Infrared (IR) Spectroscopy: Infrared spectroscopy is utilized to identify functional groups present in this compound furoate and its degradation products. It is often used in conjunction with other techniques like TLC, HPLC, and MS to confirm the identity of hydrolysis products ebi.ac.uknih.gov.
| Compound | Degradation/Metabolite Type | Techniques Used for Elucidation | Key Findings | Reference |
|---|---|---|---|---|
| This compound Furoate | Parent Drug | 1H-NMR (300, 400, 500 MHz, DEPT, COSY, HETCOR), UV-Vis, Fluorescence, Differential Scanning Calorimetry | Detailed spectral assignments, UV max at 260 nm, emission max at 335 nm | scribd.com |
| Furoic acid | Alkaline Hydrolysis Product | TLC, HPLC, IR, Mass Spectrometry | Identified as a hydrolysis product | ebi.ac.uknih.gov |
| This compound | Alkaline Hydrolysis Product, Active Metabolite (from prodrug this compound Furoate) | TLC, HPLC, IR, Mass Spectrometry | Identified as a hydrolysis product; also the active ingredient formed from this compound furoate hydrolysis in GI tract | ebi.ac.uknih.govdrugbank.com |
| Methylfuroate | Alkaline Hydrolysis Product | TLC, HPLC, IR, Mass Spectrometry | Identified as a hydrolysis product | ebi.ac.uknih.gov |
| General Metabolites/Degradants | Various | High-Resolution Mass Spectrometry (HRMS), LC-MS, GC-MS | Accurate metabolic profiling, identification of unknown metabolites and soft spots | jubilantbiosys.comipk-gatersleben.de |
In Vitro and In Silico Models for Mechanism of Action and SAR Studies
Understanding the mechanism of action (MOA) and structure-activity relationships (SAR) of this compound is crucial for drug development, though its exact MOA has remained largely elusive.
Mechanism of Action (MOA): Despite its established efficacy in treating intestinal amoebiasis, the precise mechanism of action of this compound furoate is largely unknown drugbank.comncats.ionih.govnih.gov. However, current understanding suggests that this compound acts against protein synthesis in Entamoeba histolytica trophozoites. This proposed mechanism is based on its structural similarity to chloramphenicol, particularly at the dichloroacetamide group. By inhibiting protein synthesis, this compound is thought to block the conversion of trophozoites into more virulent and invasive cyst forms nih.govresearchgate.netresearchgate.net. In vitro studies are fundamental in drug discovery for investigating drug effects on target organisms and understanding resistance mechanisms nih.govacs.org.
Structure-Activity Relationship (SAR) Studies: There is a recognized need to further explore the structure-activity relationship of this compound to optimize its efficacy and understand its interactions at a molecular level nih.govresearchgate.net. In silico models, such as Quantitative Structure-Activity Relationship (QSAR) models, are increasingly applied in drug discovery to predict various properties of compounds, including their ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) parameters, based on their chemical structures acs.orgnih.gov. These computational approaches quantify relationships between structural features and biological activities, aiding in the prediction of potential drug candidates and identifying structural alerts related to adverse outcomes like drug-induced liver injury (DILI) nih.gov. While specific detailed SAR studies for this compound are noted as an area requiring further investigation, the general application of in silico methodologies provides a framework for future research in this area nih.govresearchgate.netumassmed.eduresearchgate.netresearchgate.netcam.ac.ukgoogle.com.
| Area of Study | Methodology | Key Insights / Applications for this compound | Reference |
|---|---|---|---|
| Mechanism of Action (MOA) | In vitro studies, Structural similarity analysis | Largely unknown; proposed to inhibit protein synthesis in E. histolytica trophozoites by blocking conversion to cyst forms, due to structural similarity to chloramphenicol's dichloroacetamide group. | drugbank.comncats.ionih.govnih.govresearchgate.netresearchgate.net |
| Structure-Activity Relationship (SAR) | In silico models (e.g., QSAR) | Recognized need for further exploration. QSAR models are used to predict ADMET properties and potential toxicity based on chemical structure, aiding in drug candidate identification and optimization. | nih.govresearchgate.netacs.orgnih.govresearchgate.netresearchgate.net |
Q & A
Q. What are the key chemical and pharmacological properties of Diloxanide, and how do they inform experimental design?
this compound (C₉H₉Cl₂NO₂, MW 234.08) is a luminal amebicide used to treat asymptomatic intestinal amoebiasis. Its mechanism involves hydrolysis to the active metabolite this compound furoate, which directly inhibits Entamoeba histolytica cysts. Key properties include solubility in DMSO (33.33 mg/mL) and stability at -20°C (powder) or -80°C (solution). Researchers should prioritize purity verification via HPLC (retention time, peak symmetry) and confirm identity using CAS 579-38-4 reference standards .
Q. What standardized protocols exist for synthesizing this compound and ensuring chemical purity?
Synthesis involves amidation of dichloroacetyl chloride with N-methyl-4-hydroxyaniline. Post-synthesis, purity is validated using RP-HPLC with UV detection (λ = 210–254 nm). Parameters include:
Q. How is this compound quantified in combination therapies (e.g., with metronidazole), and what methodological challenges arise?
Simultaneous quantification in binary mixtures requires chromatographic separation (C18 column, mobile phase: methanol-phosphate buffer pH 3.5). Key challenges include:
- Peak interference : Excipients (e.g., microcrystalline cellulose) may co-elute; gradient elution or dual-wavelength detection (254 nm for this compound, 275 nm for metronidazole) mitigates this .
- Sample preparation : Sonication in methanol for 30 minutes ensures complete extraction .
Advanced Research Questions
Q. How can researchers design a stability-indicating HPLC method to quantify this compound degradation products under stress conditions?
Stress testing involves exposing this compound furoate to:
- Acid/alkali hydrolysis : 1M HCl/NaOH at 80°C for 6 hours.
- Oxidation : 3% H₂O₂ at 25°C for 24 hours.
- Photolysis : UV light (254 nm) for 48 hours . Method validation includes:
- Forced degradation : ≥10% degradation to confirm method robustness.
- Specificity : Baseline separation of degradation peaks (resolution > 2.0) .
Q. What experimental strategies resolve discrepancies between in vitro and in vivo efficacy data for this compound?
Discrepancies often stem from pharmacokinetic factors (e.g., poor luminal bioavailability). Solutions include:
- In vitro-in vivo correlation (IVIVC) : Simulate gastrointestinal pH/temperature in dissolution studies .
- Animal models : Use E. histolytica-infected hamsters to measure cyst reduction rates, adjusting dosages based on plasma concentration-time profiles .
- Metabolite tracking : LC-MS/MS to quantify active vs. inactive metabolites in fecal samples .
Q. How can researchers optimize this compound’s formulation to enhance stability in tropical climates?
Stability challenges (e.g., hydrolysis in high humidity) are addressed by:
Q. What analytical approaches detect emerging protozoal resistance to this compound, and how can they inform drug redesign?
Resistance mechanisms (e.g., altered drug efflux pumps) are identified via:
- Genomic sequencing : Compare E. histolytica isolates pre-/post-treatment for mutations in EhPgp1 .
- Efflux inhibition assays : Co-administration with verapamil (P-glycoprotein inhibitor) restores susceptibility .
- Structure-activity relationship (SAR) studies : Modify the dichloroacetamide moiety to reduce pump recognition .
Methodological Guidelines
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


