Donepezil
Description
Donepezil is a reversible acetylcholinesterase (AChE) inhibitor widely prescribed for the treatment of Alzheimer’s disease (AD). It functions by enhancing cholinergic neurotransmission through the inhibition of AChE, which hydrolyzes acetylcholine (ACh) in synaptic clefts. This compound exhibits dual binding to both the catalytic anionic site (CAS) and peripheral anionic site (PAS) of AChE, stabilizing the enzyme structure and reducing ACh turnover . Molecular dynamics (MD) simulations demonstrate its stable binding, with root-mean-square deviation (RMSD) values as low as 0.4 Å during simulations, indicating minimal structural fluctuations . Its pharmacokinetic profile includes high oral bioavailability and a long half-life (~70 hours), enabling once-daily dosing .
Properties
IUPAC Name |
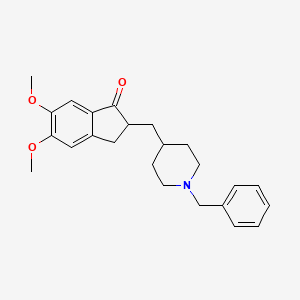
2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ADEBPBSSDYVVLD-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C2C(=C1)CC(C2=O)CC3CCN(CC3)CC4=CC=CC=C4)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C24H29NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
120011-70-3 | |
| Record name | Donepezil [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0120014064 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8048317 | |
| Record name | Donepezil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048317 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
379.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Donepezil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005041 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
31mg/mL | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
120014-06-4 | |
| Record name | Donepezil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=120014-06-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Donepezil [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0120014064 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Donepezil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8048317 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | DONEPEZIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/8SSC91326P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Donepezil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7743 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Donepezil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005041 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
223-227 | |
| Record name | Donepezil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00843 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of Donepezil typically involves the aldol condensation of benzylpiperidine-carboxyaldehyde with dimethoxyindanone, utilizing the Wittig reaction . This is followed by a dehydration step and catalytic reduction of the exocyclic double bond to yield the desired product .
Industrial Production Methods: Industrial production of this compound involves optimizing the synthetic route for large-scale manufacturing. This includes ensuring the efficiency, cost-effectiveness, and scalability of the process. Eco-friendly strategies are also being explored to minimize the environmental impact of the production process .
Chemical Reactions Analysis
Types of Reactions: Donepezil undergoes various chemical reactions, including oxidation, reduction, and substitution.
Common Reagents and Conditions:
Oxidation: this compound can be oxidized using mild oxidants like Chloramine-T in an acidic medium.
Reduction: Catalytic reduction is used in the synthesis process to reduce the exocyclic double bond.
Major Products: The major products formed from these reactions include various this compound analogs and derivatives, which are explored for their potential therapeutic benefits .
Scientific Research Applications
Mechanism of Action
Donepezil selectively and reversibly inhibits the acetylcholinesterase enzyme, which normally breaks down acetylcholine . By inhibiting this enzyme, this compound increases the levels of acetylcholine in the brain, enhancing cholinergic transmission and alleviating the symptoms of Alzheimer’s dementia . The main molecular targets are the acetylcholinesterase enzyme and the cholinergic pathways in the brain .
Comparison with Similar Compounds
Structural and Binding Affinity Comparisons
Donepezil’s indanone and benzylpiperidine moieties are critical for its AChE inhibition. Derivatives and analogs have been designed to enhance potency or dual-target activity:
Key Findings :
- Dual-binding derivatives (e.g., Compound B) outperform this compound by targeting both CAS and PAS, with subnanomolar IC50 values .
- BuChE-selective hybrids (14g/14h) demonstrate superior inhibition of butyrylcholinesterase (BuChE), a secondary target in late-stage AD .
- Indole derivatives (e.g., IIId) achieve higher AChE inhibition through additional hydrogen bonding with Phe288 .
Pharmacokinetic and Formulation Comparisons
Key Findings :
- Transdermal delivery systems (TDS) provide steady-state plasma concentrations with 98% bioequivalence to oral doses, minimizing fluctuations and adverse effects .
- Extended-release tablets (23 mg) achieve higher Cmax and prolonged Tmax, suitable for patients requiring dose escalation .
In Vivo and Clinical Efficacy
- Neuroinflammation modulation : this compound reduces hippocampal TNF-α and IL-1β levels in AD mice, comparable to traditional therapies like Shenghui Decoction (SHD) .
- Cognitive improvement : In Aβ1-42-induced AD models, this compound restores synaptic plasticity and neurogenesis, paralleling the effects of rosemary-derived compounds (rosmarinic/ursolic acids) .
Biological Activity
Donepezil is a reversible inhibitor of acetylcholinesterase (AChE) primarily used in the management of Alzheimer's disease (AD). Its biological activity encompasses a range of neuroprotective effects, cognitive enhancement, and modulation of neurotransmitter systems. This article reviews the biological activity of this compound, supported by data tables, case studies, and detailed research findings.
This compound enhances cholinergic neurotransmission by inhibiting AChE, thereby increasing the concentration of acetylcholine in synaptic clefts. This action is crucial in alleviating cognitive deficits associated with AD. Additionally, this compound may exert neuroprotective effects through the following mechanisms:
- Protection Against Glutamate Toxicity : this compound has been shown to protect neurons from excitotoxic damage induced by excessive glutamate levels .
- Neurotrophic Mechanisms Activation : It may promote neurotrophic factors that support neuronal survival and function .
- Reduction of Cortico-Hippocampal Atrophy : Studies indicate that this compound can reduce brain atrophy in AD patients .
Efficacy in Clinical Trials
Numerous clinical trials have demonstrated the efficacy of this compound in improving cognitive function and overall clinical status in patients with mild to moderate AD. Key findings from various studies include:
- In a 24-week double-blind study , this compound significantly improved cognition as measured by the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog) and Clinical Dementia Rating Scale (CDR) compared to placebo .
- A 144-week open-label extension study showed sustained cognitive benefits with this compound treatment, where 88% of participants increased their dosage to 10 mg/day after 6 weeks .
Summary of Clinical Trial Results
| Study Type | Duration | Participants | Dosage | Key Findings |
|---|---|---|---|---|
| Double-blind, placebo-controlled | 24 weeks | 473 | 5 mg or 10 mg/day | Significant improvement in cognition (ADAS-Cog scores) |
| Open-label extension | 144 weeks | 763 | Up to 10 mg/day | Sustained cognitive benefits; majority on higher dosage |
| Early-stage AD trial | 24 weeks | 153 | 5 mg or 10 mg/day | Improvements in MMSE scores; well-tolerated |
Adverse Effects
While generally well-tolerated, this compound can cause side effects, predominantly cholinergic in nature. Common adverse events include:
- Diarrhea
- Nausea
- Vomiting
- Insomnia
These side effects are typically mild to moderate and transient .
Case Studies
Several case studies have illustrated the practical application of this compound. For instance:
- A case-control study involving patients treated with this compound showed significant improvements in daily living activities and cognitive assessments compared to untreated controls .
- Another study highlighted that early initiation of this compound therapy resulted in better cognitive outcomes over time compared to delayed treatment .
Research Findings on Structural Modifications
Recent research has focused on modifying the chemical structure of this compound to enhance its biological activity. For example:
- New derivatives have been synthesized that exhibit improved AChE inhibition with IC50 values as low as 0.36 nM , indicating a stronger affinity for the target enzyme .
- Some modified compounds also demonstrated neuroprotective properties against oxidative stress and amyloid-beta aggregation, suggesting potential for broader therapeutic applications beyond AD .
Q & A
Q. How can in vitro–in vivo correlations (IVIVC) be established for this compound formulations?
- Methodological Answer : Use Design of Experiments (DoE) to model formulation variables. For example, varying lactose (72.5–80%), HPMC 100 cps (0–15.075%), and HPMC 4000 cps (7.525–15.1%) revealed that 1/V_max (inverse of dissolution rate) ranged from 1.326–1.538 h⁻¹, enabling Level A IVIVC . Validate models using Akaike information criterion (AIC) and predict in vivo absorption profiles via convolution methods.
Q. What mechanisms underlie this compound’s variable effects on brain network dynamics in non-AD conditions?
- Methodological Answer : Investigate this compound’s modulation of critical brain dynamics via EEG or fMRI. A study using permutation entropy analysis found that this compound (unlike Atomoxetine) did not alter scaling exponents (α) in healthy subjects, suggesting its effects are context-dependent and dose-sensitive . Pair neurophysiological measures with acetylcholinesterase activity assays to dissociate central vs. peripheral effects.
Q. How can neurogenesis assays be integrated into preclinical studies of this compound?
- Methodological Answer : Use BrdU/NeuN co-labeling in hippocampal dentate gyrus sections. In traumatic brain injury models, this compound (2 mg/kg/day for 2 weeks) increased neural progenitor proliferation by 40–60% compared to controls, validated via one-way ANOVA and Fisher’s LSD post-hoc tests . Include Morris water maze tests to correlate neurogenesis with functional recovery.
Data Contradiction Analysis
Q. Why do some studies report minimal this compound efficacy in advanced AD despite higher-dose formulations?
- Methodological Answer : Stratify patients by baseline Mini-Mental State Examination (MMSE) scores. A post-hoc analysis found that this compound 23 mg/day outperformed 10 mg/day only in patients with MMSE ≤16, suggesting efficacy is stage-specific . Use mixed-effects models to account for disease progression heterogeneity.
Q. How to address discrepancies in this compound’s impact on peripheral vs. central cholinergic activity?
- Methodological Answer : Combine red blood cell acetylcholinesterase inhibition assays (peripheral marker) with CSF acetylcholine measurements. While this compound’s EC50 for enzyme inhibition is 15.6 ng/mL , its limited blood-brain barrier penetration (~10–15%) may explain weaker central effects at lower doses .
Methodological Tables
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


