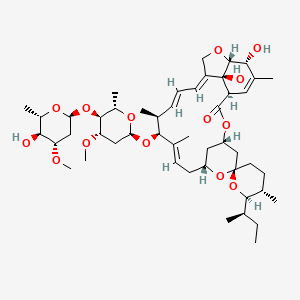
Ivermectin B1a
Description
Historical Context of Avermectin and Ivermectin Discovery
The narrative of Ivermectin B1a begins with the discovery of the avermectin family of compounds, a class of macrocyclic lactones with remarkable anthelmintic and insecticidal activities. This discovery was the result of a collaborative effort between research institutions in Japan and the United States.
Isolation of Streptomyces avermitilis and Avermectin Identification
The journey commenced with the isolation of a novel strain of actinomycete, Streptomyces avermitilis, from a soil sample collected in Japan in 1978. wikipedia.orgnih.gov This microorganism was found to produce a substance with potent activity against the nematode Nematospiroides dubius in mice during screening programs. wikipedia.orgacs.org The active compounds were subsequently isolated and identified as a family of eight closely related macrocyclic lactones, which were named avermectins. wikipedia.orgacs.org These compounds were found to be active against a wide range of nematodes and arthropods. acs.org
Derivation of this compound as a Semisynthetic Product from Avermectin B1
Avermectin B1, a mixture primarily composed of avermectin B1a and a minor component avermectin B1b, was identified as one of the most potent naturally occurring avermectins. wikipedia.orgwikipedia.org To enhance its pharmaceutical properties, avermectin B1 underwent chemical modification. wikipedia.org Selective hydrogenation of the double bond at the 22,23 position of avermectin B1 yielded 22,23-dihydroavermectin B1, known as ivermectin. wikipedia.orgnih.gov Ivermectin is a semisynthetic derivative of avermectin B1 and is typically composed of at least 80% 22,23-dihydroavermectin B1a (this compound) and up to 20% 22,23-dihydroavermectin B1b (Ivermectin B1b). wikipedia.orgdrugbank.comnih.govnih.govchemsrc.com This chemical transformation resulted in a compound with improved efficacy and safety profiles. nih.gov
The derivation process can be summarized as the selective reduction of a specific double bond in the macrocyclic lactone structure of avermectin B1. This semisynthesis was crucial in creating the highly effective antiparasitic agent known as ivermectin.
Recognition and Impact on Scientific Research: Nobel Prize in Physiology or Medicine
The profound impact of the discovery of avermectins and the subsequent derivation of ivermectin on global health and scientific research was recognized with the awarding of the 2015 Nobel Prize in Physiology or Medicine. acs.orgdrugbank.comresearchgate.netnobelprize.orgbmj.comwho.intki.seaoa.org Half of the prize was jointly awarded to William C. Campbell and Satoshi Ōmura for their discoveries concerning a novel therapy against infections caused by roundworm parasites. nobelprize.orgbmj.comaoa.org Their work led to the discovery of avermectin, from which the highly effective derivative ivermectin was developed. nobelprize.orgbmj.com This recognition underscored the significance of natural product discovery and chemical modification in addressing major global health challenges posed by parasitic diseases. nobelprize.orgwho.int
Scope and Significance of this compound in Chemical and Biological Research
Beyond its historical context, this compound holds significant standing in contemporary chemical and biological research. Its unique chemical structure and potent bioactivity have driven extensive investigations into its mechanisms of action and potential applications beyond its initial uses.
Research Trajectories Beyond Initial Anthelmintic Applications
While initially celebrated for its broad-spectrum anthelmintic and insecticidal properties, research into this compound and ivermectin has expanded significantly. Investigations have explored its potential in treating a wider range of parasitic infections and have also ventured into non-parasitic applications. nih.gov Emerging literature suggests potential roles in treating inflammatory conditions, viral infections, and certain cancers, although these areas require further research. nih.gov The exploration of these diverse research trajectories highlights the multifaceted nature of this compound's bioactivity.
Focus on Molecular and Cellular Mechanisms Underlying Bioactivity
A key area of research focuses on elucidating the molecular and cellular mechanisms through which this compound exerts its biological effects. The primary mechanism involves binding to glutamate-gated chloride channels, which are crucial to the nerve and muscle cells of invertebrates. wikipedia.orgdrugbank.comnih.gov This binding increases the permeability of the cell membrane to chloride ions, leading to hyperpolarization and subsequent paralysis and death of the parasite. wikipedia.orgdrugbank.comnih.gov
Research has also explored other potential mechanisms. Ivermectin is believed to act as an agonist of the neurotransmitter gamma-aminobutyric acid (GABA), disrupting GABA-mediated neurosynaptic transmission in the central nervous system of susceptible organisms. drugbank.comnih.gov Furthermore, studies have investigated its interactions with mammalian targets, such as tubulin, suggesting potential anti-mitotic activity and relevance in cancer research by promoting tubulin polymerization. mdpi.com
| Mechanism | Description | Primary Target Organisms |
|---|---|---|
| Glutamate-Gated Chloride Channel Binding | Increases cell membrane permeability to chloride ions, causing hyperpolarization, paralysis, and death. | Invertebrates (nematodes, arthropods) wikipedia.orgdrugbank.comnih.gov |
| GABA Agonism | Disrupts GABA-mediated neurosynaptic transmission. | Invertebrates drugbank.comnih.gov |
| Tubulin Interaction | Potential to bind and stabilize tubulin, affecting microtubule dynamics. | Mammalian cells (under investigation) mdpi.com |
These ongoing investigations into the molecular and cellular interactions of this compound are crucial for understanding its diverse biological effects and exploring its full therapeutic potential.
Emerging Research Avenues in Chemical Biology and Drug Discovery
Beyond its established antiparasitic actions, this compound and its parent compound, ivermectin, are being actively investigated in various emerging research avenues within chemical biology and drug discovery. These studies explore its potential against a broader spectrum of biological targets and diseases, moving beyond its traditional use against invertebrates. researchgate.netnih.govrovedar.com
One significant area of research focuses on the antiviral properties of ivermectin, including studies against SARS-CoV-2. caymanchem.comnih.govresearcher.liferesearchgate.netnih.gov Research suggests that ivermectin may inhibit the replication of certain viruses by interfering with host cell processes, such as the nuclear transport of viral proteins mediated by importin α/β1 heterodimers. nih.govresearchgate.netnih.govscielo.org.mxscielo.org.mx Computational studies have indicated that this compound and B1b may bind to the ARM2-ARM4 domains of human importin α isoforms, potentially sharing binding sites with SARS-CoV-2 proteins like the nucleocapsid (N) and ORF6 proteins. scielo.org.mxscielo.org.mx
The potential anticancer effects of ivermectin and its derivatives, including this compound, are also under investigation. nih.govrovedar.commdpi.comfrontiersin.orgafjbs.com Studies have explored its ability to inhibit the proliferation of various cancer cell lines, including colorectal cancer cells. mdpi.comfrontiersin.org Proposed mechanisms involve the induction of apoptosis and the modulation of cellular signaling pathways such as Akt/mTOR and WNT-TCF pathways. nih.govrovedar.comfrontiersin.orgresearchgate.net Research has also examined the interaction of avermectin compounds, including this compound, with tubulin, a key component of the cytoskeleton, suggesting a potential mechanism for anticancer activity by affecting microtubule dynamics. mdpi.com Molecular docking studies have indicated favorable binding affinities between this compound and β-tubulin.
Furthermore, research is exploring the potential antibacterial properties of ivermectin and its derivatives. nih.govnih.govmdpi.com Studies have investigated the activity of ivermectin and modified derivatives against bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), examining their effects on bacterial cell walls, membranes, and DNA binding. mdpi.com
The study of photoreactive this compound derivatives represents another avenue in chemical biology research. researcher.lifenih.gov These derivatives are synthesized to act as probes for identifying the amino acid residues that interact with ivermectin in target proteins, such as glutamate-gated chloride channels (GluCls) in invertebrates. nih.gov This research contributes to a deeper understanding of the molecular interactions underlying ivermectin's activity. nih.gov
Research also delves into the pharmacokinetics of this compound, utilizing methods like Liquid Chromatography-Mass Spectrometry (LC-MS/MS) to study its behavior in biological systems. researcher.liferesearchgate.net These studies are crucial for understanding absorption, distribution, metabolism, and excretion, which can inform the potential repurposing of the compound. drugbank.comresearcher.liferesearchgate.net
The exploration of this compound in these diverse areas highlights its significance as a subject of ongoing academic research, revealing potential applications beyond its well-established role as an antiparasitic agent.
Key Research Findings on this compound in Emerging Avenues
| Research Area | Key Finding | Relevant Target(s) | Reference(s) |
| Antiviral Activity | Inhibition of SARS-CoV-2 replication in vitro. caymanchem.comnih.govresearcher.liferesearchgate.netnih.gov Interference with nuclear transport. nih.govresearchgate.netnih.govscielo.org.mxscielo.org.mx | Importin α/β1 heterodimer, SARS-CoV-2 proteins (N, ORF6) | caymanchem.comnih.govresearcher.liferesearchgate.netnih.govscielo.org.mxscielo.org.mx |
| Anticancer Potential | Inhibition of cancer cell proliferation (e.g., colorectal cancer cells). mdpi.comfrontiersin.org Induction of apoptosis. nih.govrovedar.commdpi.comfrontiersin.orgresearchgate.net Modulation of signaling pathways. nih.govrovedar.comfrontiersin.orgresearchgate.net Interaction with tubulin. mdpi.com | Akt/mTOR, WNT-TCF pathways, Tubulin | nih.govrovedar.commdpi.comfrontiersin.orgresearchgate.net |
| Antibacterial Activity | Activity against certain bacteria (e.g., MRSA). mdpi.com Effects on cell wall, membrane, and DNA binding. mdpi.com | Bacterial cell components, Genomic DNA | mdpi.com |
| Chemical Biology Probes | Synthesis of photoreactive derivatives for target identification in GluCls. researcher.lifenih.gov | Glutamate-gated chloride channels (GluCls) | researcher.lifenih.gov |
Properties
Key on ui mechanism of action |
Ivermectin binds selectively and with high affinity to glutamate-gated chloride ion channels in invertebrate muscle and nerve cells of the microfilaria. This binding causes an increase in the permeability of the cell membrane to chloride ions and results in hyperpolarization of the cell, leading to paralysis and death of the parasite. Ivermectin also is believed to act as an agonist of the neurotransmitter gamma-aminobutyric acid (GABA), thereby disrupting GABA-mediated central nervous system (CNS) neurosynaptic transmission. Ivermectin may also impair normal intrauterine development of _O. volvulus_ microfilariae and may inhibit their release from the uteri of gravid female worms. |
|---|---|
CAS No. |
70288-86-7 |
Molecular Formula |
C48H74O14 |
Molecular Weight |
875.1 g/mol |
IUPAC Name |
(1S,4S,5'S,6R,6'R,8R,10E,12S,13S,14E,16E,20R,21R,24S)-6'-[(2S)-butan-2-yl]-21,24-dihydroxy-12-[(2R,4S,5S,6S)-5-[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-4-methoxy-6-methyloxan-2-yl]oxy-5',11,13,22-tetramethylspiro[3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6,2'-oxane]-2-one |
InChI |
InChI=1S/C48H74O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38/h12-15,19,25-26,28,30-31,33-45,49-50,52H,11,16-18,20-24H2,1-10H3/b13-12+,27-15+,32-14+/t25-,26-,28-,30-,31-,33+,34-,35+,36-,37-,38-,39-,40+,41-,42-,43+,44-,45+,47+,48+/m0/s1 |
InChI Key |
AZSNMRSAGSSBNP-TYECJXEWSA-N |
SMILES |
CCC(C)C1C(CCC2(O1)CC3CC(O2)CC=C(C(C(C=CC=C4COC5C4(C(C=C(C5O)C)C(=O)O3)O)C)OC6CC(C(C(O6)C)OC7CC(C(C(O7)C)O)OC)OC)C)C |
Isomeric SMILES |
CC[C@H](C)[C@@H]1[C@H](CC[C@@]2(O1)C[C@@H]3C[C@H](O2)C/C=C(/[C@H]([C@H](/C=C/C=C/4\CO[C@H]5[C@@]4([C@H](C=C([C@H]5O)C)C(=O)O3)O)C)O[C@H]6C[C@@H]([C@H]([C@@H](O6)C)O[C@H]7C[C@@H]([C@H]([C@@H](O7)C)O)OC)OC)\C)C |
Canonical SMILES |
CCC(C)C1C(CCC2(O1)CC3CC(O2)CC=C(C(C(C=CC=C4COC5C4(C(C=C(C5O)C)C(=O)O3)O)C)OC6CC(C(C(O6)C)OC7CC(C(C(O7)C)O)OC)OC)C)C |
Appearance |
Solid powder |
Other CAS No. |
71827-03-7 70288-86-7 |
Pictograms |
Acute Toxic; Health Hazard; Environmental Hazard |
Purity |
>90% (or refer to the Certificate of Analysis) |
shelf_life |
>2 years if stored properly |
solubility |
Insoluble |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
MK-933; Ivermectin; Ivomec; L 64047; 1 Mectizan; MK 933; MK-0933; Noromectin; Pandex. |
Origin of Product |
United States |
Molecular and Cellular Mechanisms of Action
Interaction with Glutamate-Gated Chloride Channels (GluClRs)
Glutamate-gated chloride channels are inhibitory receptors found exclusively in invertebrates and belong to the pentameric Cys-loop receptor family of ligand-gated ion channels. frontiersin.orguq.edu.auuq.edu.aumcgill.canih.gov Ivermectin B1a targets these channels with high potency. frontiersin.orgresearchgate.netuq.edu.auuq.edu.auresearchgate.net
Selective Binding and High Affinity to Invertebrate GluClRs in Nerve and Muscle Cells
This compound binds selectively and with high affinity to glutamate-gated chloride channels present in the nerve and muscle cells of invertebrates. nih.govdrugbank.comfrontiersin.orgpatsnap.comresearchgate.netuq.edu.auuq.edu.auoup.comresearchgate.netresearchgate.net This selective binding is a key factor in its effectiveness against parasites while exhibiting lower toxicity to mammals, which either lack these channels or have them in locations protected by the blood-brain barrier. patsnap.com High-affinity binding sites for ivermectin have been identified in membrane preparations from various invertebrates, including Haemonchus contortus and Caenorhabditis elegans, with dissociation constants (KD) in the nanomolar to picomolar range. researchgate.net
Induction of Chloride Ion Influx and Cellular Hyperpolarization
Binding of this compound to GluClRs causes a significant increase in the permeability of the cell membrane to chloride ions. nih.govwikipedia.orgdrugbank.compatsnap.comsemanticscholar.orgnih.govresearchgate.net This influx of negatively charged chloride ions leads to hyperpolarization of the nerve or muscle cell membrane. nih.govdrugbank.compatsnap.comsemanticscholar.orgnih.govresearchgate.net The hyperpolarization inhibits neuronal excitation and disrupts normal synaptic transmission, ultimately resulting in paralysis and death of the parasite. nih.govdrugbank.comfrontiersin.orgpatsnap.comresearchgate.netuq.edu.auuq.edu.ausemanticscholar.org
Structural Basis of GluClR Binding: Insights from X-ray Crystallography and Molecular Modeling
X-ray crystallography has provided significant insights into the structural basis of this compound binding to GluClRs. The crystal structure of the C. elegans α GluClR in complex with ivermectin revealed that the drug binds within the transmembrane domain of the receptor. nih.govfrontiersin.orgresearchgate.netuq.edu.auresearchgate.net The binding site is located in a cleft at the interface between adjacent subunits of the pentameric channel. frontiersin.orgresearchgate.netuq.edu.auresearchgate.netnih.gov Molecular modeling studies further support this location and have helped to elucidate the specific interactions between this compound and residues within the binding pocket. nih.govnih.govresearchgate.net This binding tends to push apart the membrane-spanning regions of adjacent subunits, contributing to channel opening. researchgate.net
Allosteric Modulation and Conformational Dynamics of GluClRs
This compound acts as a positive allosteric modulator of GluClRs. nih.govfrontiersin.orgresearchgate.netuq.edu.auplos.org It binds to a site distinct from the orthosteric glutamate binding site, yet influences the channel's conformation and activity. nih.govfrontiersin.orgresearchgate.netuq.edu.auresearchgate.netplos.org Binding of this compound stabilizes an open or a "wide-open" channel conformation, leading to sustained chloride influx. nih.govfrontiersin.orgresearchgate.netuq.edu.auresearchgate.netnih.govplos.org Molecular dynamics simulations have visualized the conformational changes induced by ivermectin binding, showing that it can induce a global conformational change that propagates from the transmembrane domain to the neurotransmitter binding site, potentially explaining how it potentiates glutamate-gated currents. nih.govfrontiersin.orgresearchgate.netuq.edu.auplos.org This allosteric modulation is characterized by slow activation and often irreversible or very slowly reversible effects compared to the rapid and reversible gating induced by the endogenous ligand glutamate. frontiersin.orgresearchgate.net
Modulation of Gamma-Aminobutyric Acid (GABA) Receptors
In addition to GluClRs, this compound is also known to interact with GABA receptors, another class of inhibitory ligand-gated chloride channels belonging to the Cys-loop family. nih.govwikipedia.orgdrugbank.combiosensis.comfrontiersin.orgpatsnap.comoup.comsemanticscholar.orgmcgill.caresearchgate.netresearchgate.netnih.govnih.govnih.govresearchgate.net
Agonistic Activity on Invertebrate GABA Receptors
This compound is believed to act as an agonist or positive allosteric modulator of invertebrate GABA receptors. nih.govdrugbank.compatsnap.comoup.comresearchgate.netresearchgate.net By binding to these receptors, this compound can enhance the inhibitory effects of GABA, further contributing to the disruption of neurotransmission and paralysis in susceptible organisms. drugbank.compatsnap.comresearchgate.net Studies in various invertebrate species, including Ascaris suum and Drosophila, have demonstrated the interaction of ivermectin with GABA receptors, although the specific effects (agonism, potentiation, or even inhibition depending on the receptor subtype and concentration) can vary. oup.comnih.govresearchgate.net This modulation of GABA receptors complements the primary action on GluClRs in mediating the antiparasitic effects of this compound. drugbank.compatsnap.comresearchgate.net
Disruption of GABA-Mediated Neurosynaptic Transmission in Parasitic Systems
Ivermectin is understood to function as an agonist of the neurotransmitter gamma-aminobutyric acid (GABA), thereby interfering with GABA-mediated neurosynaptic transmission within the central nervous system of parasites. drugbank.comnih.gov This interaction contributes to the disruption of neuronal signaling. In parasitic nematodes like Ascaris, avermectin B1a (the major component of ivermectin) has been shown to affect interneurons and inhibitory motoneurons, leading to paralysis. uq.edu.au Studies in Caenorhabditis elegans muscle cells indicate that ivermectin inhibits GABA receptors. plos.orgplos.org The mechanism involves the opening of GABA-controlled chloride ion channels in the muscle membrane, which reduces membrane resistance and increases inward conductance, ultimately resulting in paralysis in arthropods. nih.gov
Comparative Analysis with GluClR Interactions and Concentration-Dependent Effects
A pivotal mechanism of action for this compound involves its selective binding with high affinity to glutamate-gated chloride channels (GluClRs). These channels are predominantly found in the nerve and muscle cells of invertebrates and are absent in mammals, contributing significantly to the drug's selective toxicity against parasites. drugbank.comnih.govuq.edu.aupatsnap.comfrontiersin.orgfrontiersin.org Binding of ivermectin to GluClRs at nanomolar concentrations leads to an increase in the permeability of the cell membrane to chloride ions. drugbank.comnih.govpatsnap.comaopwiki.org This influx of chloride ions causes hyperpolarization of the cell membrane, inhibiting neuronal transmission and resulting in paralysis and eventual death of the parasite. drugbank.comnih.govpatsnap.comaopwiki.org
The interaction with GluClRs exhibits concentration-dependent effects. At low concentrations, such as 5 nM for GluCl, ivermectin can act as an allosteric modulator, enhancing the binding of glutamate. nih.gov At higher concentrations, around 140 nM for GluCl activation, it functions as a direct channel activator. nih.gov The binding site for ivermectin in GluClRs is located within the transmembrane domain, in a cleft formed at the interface between adjacent subunits. nih.govuq.edu.aufrontiersin.org Structural studies, including X-ray crystallography of ivermectin complexed with C. elegans α GluClR, have provided insights into these binding interactions. uq.edu.aufrontiersin.org
Interactions with Other Ligand-Gated Ion Channels
Beyond its primary targets, this compound has been shown to interact with other ligand-gated ion channels, although typically at higher concentrations than those required for potent GluClR activation. These interactions include modulation of Glycine receptors (GlyRs), Nicotinic Acetylcholine Receptors (nAChRs), and P2X4 cation channels. nih.govuq.edu.aufrontiersin.orgtandfonline.com
Glycine Receptor (GlyR) Modulation
This compound interacts with Glycine receptors (GlyRs), which are inhibitory chloride channels found in vertebrates. At micromolar concentrations (with EC50 values generally between 1 and 5 μM depending on the GlyR subunit composition), ivermectin directly activates these receptors. nih.govuq.edu.aufrontiersin.orgpsu.edu Interestingly, at lower concentrations, approximately 30 nM, ivermectin can potentiate currents elicited by subsaturating concentrations of glycine. nih.govpsu.edunih.gov Research suggests that ivermectin activates the GlyR through a mechanism distinct from the orthosteric glycine binding site. psu.edunih.gov The binding site for ivermectin on GlyRs is located within the transmembrane domains at the interface of adjacent subunits, similar to its binding site on GluClRs. nih.gov
Nicotinic Acetylcholine Receptor (nAChR) Interactions
Ivermectin also interacts with nicotinic acetylcholine receptors (nAChRs), which are excitatory cation channels. At higher, micromolar concentrations, it can activate or modulate certain vertebrate Cys-loop receptors, including nicotinic receptors. uq.edu.aufrontiersin.org Specifically, ivermectin acts as a positive allosteric modulator of the alpha-7 neuronal nicotinic acetylcholine receptor (α7-nAChR). biosensis.comcellsignal.cnconicet.gov.arnih.govmdpi.com While ivermectin alone does not typically activate nAChRs, it can significantly potentiate acetylcholine-induced currents at concentrations around 30 μM. nih.gov Studies involving mutated and chimeric nAChR subunits indicate that the transmembrane region of the receptor plays a critical role in mediating this allosteric modulation by ivermectin. nih.gov
P2X4 Cation Channel Activation
Ivermectin has been identified as a potentiator of the P2X4 receptor, an ATP-gated cation channel. nih.govuq.edu.aufrontiersin.orgtandfonline.comfrontiersin.org Unlike its action on GluClRs and GlyRs, ivermectin does not directly activate P2X4 channels in the absence of ATP. nih.govuq.edu.aufrontiersin.org However, it enhances the amplitude of ATP-induced currents with an EC50 of approximately 0.25 μM. nih.govfrontiersin.org Evidence suggests that ivermectin interacts with the transmembrane domains of the P2X4 receptor. uq.edu.aunih.govfrontiersin.orgresearchgate.net Some research indicates the presence of at least two distinct binding sites for ivermectin on the P2X4 receptor, exhibiting different affinities and contributing to effects such as increased maximal current and slowed channel deactivation. nih.govfrontiersin.orgresearchgate.net
pH-Gated Chloride Channel Interactions
In addition to the aforementioned channels, ivermectin has been shown to interact with pH-gated chloride channels, particularly in insects. researchgate.net These channels are also members of the ligand-gated ion channel superfamily. Ivermectin has demonstrated the ability to potentiate currents mediated by pH-gated chloride channels. researchgate.net
Histamine-Gated Chloride Channel Modulation
This compound is known to modulate the activity of ligand-gated chloride channels, particularly those found in invertebrates. A primary target is the glutamate-gated chloride channel (GluClR), which is exclusively present in protostome invertebrates nih.govfrontiersin.orgresearchgate.netdrugbank.com. This compound activates these channels at nanomolar concentrations, leading to an increase in chloride ion permeability across the cell membrane nih.govdrugbank.comaopwiki.org. This influx of chloride ions results in hyperpolarization of neuronal and muscle cells, disrupting normal signal transmission and leading to paralysis and death in parasites drugbank.comaopwiki.org.
Structural studies, including X-ray crystallography of ivermectin complexed with C. elegans α GluClR, have shown that ivermectin binds within the transmembrane domain at the interface between adjacent subunits nih.govfrontiersin.orgresearchgate.net. This binding induces a conformational change that facilitates the opening of the channel pore nih.govfrontiersin.orgresearchgate.net. While the interaction with GluClRs is a well-established anthelmintic mechanism, this compound can also modulate other Cys-loop receptors, such as GABA type-A and glycine receptors, at higher, micromolar concentrations nih.govfrontiersin.orgresearchgate.net. Histamine-gated chloride channels (HisCls), found in arthropod photoreceptors, are also subject to modulation by this compound, although the detailed mechanism may vary researchgate.net.
Non-Ion Channel Molecular Interactions
Beyond its well-characterized effects on ion channels, this compound engages in several non-ion channel molecular interactions that contribute to its broader biological activities.
Tubulin Binding and Microtubule Dynamics Modulation
Research indicates that this compound interacts directly with tubulin, a key component of the eukaryotic cytoskeleton, and modulates microtubule dynamics longdom.orgnih.govneu.edu.trresearchgate.net. Studies, including molecular docking analyses, suggest that this compound can bind to tubulin, potentially at sites similar to those targeted by known microtubule-stabilizing agents like Taxol longdom.orgresearchgate.net. This interaction can influence the polymerization and stability of microtubules nih.govneu.edu.tr.
Molecular docking studies have provided insights into the binding affinity of this compound to tubulin. Table 1 presents representative data on the binding affinity of this compound compared to other compounds.
| Compound | Binding Affinity (Kcal/mol) | Target Protein |
| This compound | -18.0 | Tubulin |
| Taxol | -17.4 | Tubulin |
| Selamectin | -9.1 | Tubulin |
| Doramectin | -8.9 | Tubulin |
Table 1: Binding Affinities of Compounds to Tubulin based on Molecular Docking Studies longdom.org
These findings suggest that this compound may act as a microtubule stabilizer, potentially impeding cellular processes that rely on dynamic microtubule assembly and disassembly longdom.orgnih.gov.
Impact on Cellular Proliferation Pathways in Model Systems
In various model systems, including different cancer cell lines, this compound has demonstrated the ability to inhibit cellular proliferation longdom.orgnih.govembopress.orgfrontiersin.orgresearchgate.netdovepress.com. This anti-proliferative effect is often associated with the induction of cell cycle arrest, observed in both G0/G1 and S phases, and the promotion of apoptosis (programmed cell death) frontiersin.orgresearchgate.net.
The modulation of several key cellular signaling pathways is implicated in these effects. This compound has been shown to influence pathways such as the WNT-TCF, Hippo, and Akt/mTOR pathways frontiersin.orgresearchgate.netdovepress.com. Additionally, it can increase the production of reactive oxygen species (ROS), contributing to its impact on cell viability frontiersin.orgresearchgate.net. These multifaceted interactions with proliferation pathways highlight the complex cellular responses to this compound in model systems.
Farnesoid X Receptor (FXR) Partial Agonism
Studies have identified Avermectin B1a as a partial agonist of the Farnesoid X Receptor (FXR), a nuclear receptor that plays a crucial role in regulating bile acid homeostasis, lipid metabolism, and inflammation researchgate.netnih.govmdpi.comdntb.gov.ua. In cell-based assays utilizing an FXR ligand-binding domain FRET sensor, Avermectin B1a was shown to activate the receptor researchgate.netnih.gov. This activation led to increased mRNA expression of known FXR target genes, such as OSTα and OSTβ, in model cell lines like Huh7 cells researchgate.netnih.gov. While characterized as a weak and partial agonist, this interaction suggests a potential influence of this compound on FXR-mediated transcriptional regulation.
Potential Interactions with Potassium Channels
Beyond its primary targets, there is evidence suggesting potential interactions of this compound with potassium channels. Specifically, ivermectin has been reported to activate G-protein-gated inwardly rectifying K+ channels (GIRK channels) nih.gov. While the full extent and implications of this interaction for this compound are still under investigation, it indicates a broader spectrum of ion channel modulation beyond the chloride channels.
Inhibition of Importin α/β Pathway
This compound acts as a specific inhibitor of the importin α/β-mediated nuclear import pathway biosensis.commedchemexpress.comfocusbiomolecules.comnih.govresearchgate.netnih.gov. This pathway is essential for the translocation of numerous proteins from the cytoplasm into the nucleus, a process critical for various cellular functions, including signal transduction, gene expression, and viral replication nih.govresearchgate.netnih.gov. By inhibiting the importin α/β heterodimer, this compound can disrupt the nuclear localization of proteins that rely on this transport mechanism nih.govnih.gov. This inhibition has been shown to impact the replication of certain viruses that depend on importin α/β for the nuclear entry of their proteins nih.govresearchgate.netnih.gov.
This compound is a major component of the antiparasitic drug ivermectin, a member of the avermectin family of macrocyclic lactones wikipedia.orgbiosensis.commedchemexpress.com. Ivermectin itself is typically composed of at least 80% this compound and not more than 20% ivermectin B1b wikipedia.orgmedchemexpress.com. Preclinical research on this compound focuses on understanding its mechanisms of action and potential biological effects through various in vitro methodologies and model systems. These studies are crucial for elucidating its interactions with molecular targets, particularly ligand-gated ion channels, and investigating other cellular effects.
Preclinical Research Methodologies and Model Systems
In Vitro Cellular and Biochemical Assays A variety of in vitro assays are employed to study the effects of Ivermectin B1a at the cellular and biochemical levels, including recombinant receptor expression systems, electrophysiological techniques, radioligand binding assays, spectroscopic techniques, fluorescence-based assays, and tubulin polymerization assays.
Recombinant Receptor Expression Systems (e.g., Xenopus laevis oocytes, COS-1 cells) Recombinant receptor expression systems, such as Xenopus laevis oocytes and COS-1 cells, are widely used to study the function and pharmacology of specific receptors targeted by this compound. Xenopus laevis oocytes, for instance, are valuable for expressing exogenous ion channels and receptors, allowing for the characterization of drug effects using electrophysiologyoup.commdpi.comnih.govmcgill.ca. Studies using Xenopus laevis oocytes have shown that this compound derivatives can activate homomeric glutamate-gated chloride channels (GluCls) expressed in these cellsnih.gov. Functional expression in Xenopus laevis oocytes and subsequent electrophysiological assays have demonstrated that GluCls from organisms like the carmine spider mite are sensitive to ivermectinoup.com. Furthermore, studies on Xenopus laevis oocytes injected with RNA from C. elegans have shown that ivermectin can induce inward membrane currents associated with increased chloride ion entrymdpi.com. Preapplication of ivermectin in the micromolar range has also been reported to enhance acetylcholine-evoked currents of neuronal nicotinic acetylcholine receptors reconstituted in Xenopus laevis oocytesresearchgate.net. Research expressing Cooperia oncophora GluCl subunits in Xenopus laevis oocytes has shown that mutations in these subunits can affect sensitivity to ivermectinmcgill.ca.
COS-1 cells have also been utilized to express recombinant receptors. Photoreactive this compound derivatives have been synthesized and tested on GluCls expressed in COS-1 cells, demonstrating low- or subnanomolar affinity for these channels nih.gov. COS-7 cells have been used in studies investigating the binding of [3H]ivermectin to recombinant GluCl subunits researchgate.net.
Electrophysiological Techniques (e.g., Whole-Cell Voltage Clamp, Patch Clamp) Electrophysiological techniques, including whole-cell voltage clamp and patch clamp, are fundamental for measuring the ion flow through channels and receptors modulated by this compound. Whole-cell voltage clamp allows for the recording of macroscopic currents from an entire cell, providing information on the overall activity of expressed channels or native receptorsmcgill.cafrontiersin.orgplos.orgresearchgate.net. Studies using whole-cell voltage clamp on Xenopus laevis oocytes expressing GluCls have characterized the effects of ivermectin on channel activation and sensitivitynih.govmcgill.ca. Whole-cell patch-clamp recordings from transfected cells, such as HEK293T cells expressing human P2X4 receptor channels, have been used to demonstrate that ivermectin can facilitate purinergic responsesresearchgate.net. In the context of GABA receptors, whole-cell recording has shown that ivermectin can induce both potentiation of reversible GABA-gated currents and an irreversibly-activated current componentfrontiersin.org.
Patch clamp techniques, including outside-out and inside-out configurations, enable the study of single-channel activity nih.gov. Patch-clamp studies on Ascaris suum muscle membranes have examined the effects of dihydroavermectin B1a (this compound) on chloride channels, revealing that while high concentrations did not open characteristic GABA channels, low concentrations could directly activate chloride channels after a delay nih.govannualreviews.org. Patch-clamp recordings have also been used to evaluate the functional properties of hybrid compounds with potential affinity for the Ivermectin binding site of GABA receptors researchgate.net. Studies investigating the effects of ivermectin on SARS-CoV-2 E protein channels expressed in HEK293 cells have also employed patch-clamp electrophysiology researchgate.net.
Radioligand Binding Assays (e.g., [3H]-GABA binding) Radioligand binding assays are used to quantify the binding affinity and density of receptors for this compound or its related compounds. These assays typically involve incubating tissue membranes or cells with a radiolabeled ligand, such as [3H]-ivermectin or [3H]-GABA, and measuring the amount of bound radioactivity. Studies using [3H]-ivermectin binding to membranes from Haemonchus contortus larvae and recombinant GluCl subunits expressed in COS-7 cells have determined high-affinity binding sitesresearchgate.net. The affinity of [3H]ivermectin binding to H. contortus larval membranes was reported to be 70 ± 7 pM, while binding to recombinant HcGluClα subunits showed a Kd of 26 ± 12 pM and to Hcgbr-2B subunits a Kd of 70 ± 16 pMresearchgate.net.
Radioligand binding studies using [3H]avermectin B1a in rat brain membranes have shown specific high-affinity binding that is reversible and partially dependent on chloride ions nih.gov. This binding was partially inhibited by GABA receptor agonists and influenced by the presence of chloride ions, suggesting an association with the GABA-benzodiazepine receptor-chloride ion channel complex nih.gov. Competition studies using [3H]-GABA binding activity in extracts from Trichinella spiralis muscle larvae have indicated that ivermectin can act as a competitive inhibitor, suggesting the involvement of GABA receptors in its mechanism of action in nematodes nih.gov.
Spectroscopic Techniques (e.g., UV-Vis Spectrophotometry for Degradation) Spectroscopic techniques, particularly UV-Vis spectrophotometry, are employed to study the chemical properties of this compound, including its stability and degradation under various conditions. UV-Vis spectrophotometry measures the absorption of light by a substance at different wavelengths, which can be used to monitor changes in concentration or chemical structure. This technique has been used to evaluate the photostability of ivermectin when exposed to UV-A and UV-C radiationsciforum.netmdpi.comscribd.com. Studies have shown that exposure to UV radiation causes significant alterations in the chemical structure of ivermectin, evidenced by changes in absorption spectrasciforum.netmdpi.comscribd.com. UV-Vis spectrophotometry has also been used to track the photodegradation of ivermectin in different aqueous environments, such as seawatersciforum.netmdpi.com. Additionally, UV-Vis spectrophotometry can be used in method development for the quantification of ivermectinhjhs.co.in.
Data on the photostability of this compound under UV-Vis spectrophotometry:
| Condition | Observation | Reference |
|---|---|---|
| UVA and UVC light | Significant alterations in chemical structure | sciforum.netmdpi.com |
| UV radiation | Changes in absorption spectra | sciforum.netmdpi.com |
Tubulin Polymerization Assays Tubulin polymerization assays are biochemical tests used to assess the ability of a compound to promote or inhibit the assembly of tubulin monomers into microtubules. These assays typically measure changes in turbidity or fluorescence as tubulin polymerizes in the presence of the test compound. Studies have investigated the effect of Avermectin B1a on tubulin polymerization, particularly in the context of its potential anti-cancer propertiesdntb.gov.uanih.govresearchgate.netnih.govneu.edu.tr. Research using in vitro tubulin polymerization assays has shown that Avermectin B1a can promote tubulin polymerizationdntb.gov.uanih.govresearchgate.netnih.govneu.edu.tr. For example, at a concentration of 30 µM, Avermectin B1a was found to enhance tubulin polymerization, similar to the effect of paclitaxel, a known microtubule-stabilizing agentdntb.gov.uanih.govresearchgate.netnih.gov. This effect on tubulin polymerization is suggested as a potential mechanism underlying the observed anti-proliferative effects of Avermectin B1a in certain cancer cell linesdntb.gov.uanih.govnih.gov.
Data on Avermectin B1a's effect on tubulin polymerization:
| Compound | Concentration | Effect on Tubulin Polymerization | Reference |
|---|---|---|---|
| Avermectin B1a | 30 µM | Promotes polymerization | dntb.gov.uanih.govnih.gov |
| Paclitaxel | 10 µM | Promotes polymerization (control) | dntb.gov.uanih.govresearchgate.net |
Cell Viability and Clonogenicity Assays in Cancer Cell Lines
Research has demonstrated that this compound, as a major component of ivermectin, can inhibit the proliferation and reduce the viability of various cancer cell lines. Assays such as CCK-8 and MTT are commonly employed to quantify these effects. For instance, ivermectin dose-dependently inhibited the growth of colorectal cancer cell lines SW480 and SW1116, as measured by CCK-8 assay. This inhibition was observed to be both concentration- and time-dependent. Avermectin B1a specifically showed anti-proliferative activity against the HCT-116 colon cancer cell line with an IC50 value of 30 µM after 24 hours of treatment using the MTT assay. At this concentration, a significant decrease in cell viability was noted.
Beyond simply reducing viability, this compound impacts the ability of cancer cells to form colonies, a measure of clonogenicity. Studies have shown that ivermectin can decrease the clonogenic capacity in several cancer cell lines, including MDA-MB-231 and MCF-7 breast cancer cells, and SKOV-3 ovarian cancer cells. While some cell lines like DU145 prostate cancer cells showed resistance, others like HT-1080, MDA-MB-468, MDA-MB-231, and SKOV-3 exhibited reduced colony formation upon ivermectin treatment. This effect on clonogenicity suggests an impact on the self-renewal potential of cancer cells, including cancer stem-like cells. Ivermectin has been observed to preferentially inhibit the viability and clonogenic capacity of cancer stem-like cell populations compared to parental cells in models like SKOV-3 and MDA-MB-231.
The mechanisms underlying these effects involve the induction of cell cycle arrest and apoptosis. Ivermectin has been shown to induce cell cycle arrest at the G0/G1 phase in some cancer cells, such as glioma cells, and at the S phase in others, like cholangiocarcinoma cells and colorectal cancer cells (SW480 and SW1116). This arrest is associated with the modulation of proteins involved in cell cycle control. Apoptosis, or programmed cell death, is another key mechanism. Ivermectin dose-dependently promoted apoptosis in colorectal cancer SW480 and SW1116 cells, increasing Caspase-3/7 activity and upregulating proapoptotic proteins like Bax while downregulating antiapoptotic Bcl-2. Avermectin B1a specifically induced apoptosis in HCT-116 cells, with a notable increase in the apoptotic rate after treatment. The induction of apoptosis by ivermectin can occur through various pathways, including ROS-mediated mitochondrial apoptosis and caspase-dependent mechanisms.
Furthermore, this compound's effects on cancer cells can involve the modulation of signaling pathways critical for cancer progression. These include the Akt/mTOR, WNT-TCF, and Hippo pathways. Ivermectin has been shown to inhibit the WNT-TCF pathway, which is crucial for the growth and self-renewal of some cancer cells, including colon cancer stem cells. It can also affect tubulin polymerization, suggesting a role as a microtubule-targeting agent in some cancer cell lines like HCT-116.
Here is a summary of findings from cell viability and clonogenicity assays:
| Cancer Cell Line (Source) | Assay Type | Observed Effect (Ivermectin/Avermectin B1a) | Key Findings | Citations |
| SW480, SW1116 (Colorectal Cancer) | CCK-8 | Dose-dependent inhibition of viability | Inhibited proliferation, induced apoptosis (increased Caspase-3/7, Bax; decreased Bcl-2), increased ROS, induced S phase arrest. | |
| HCT-116 (Colon Cancer) | MTT, Flow Cytometry (Apoptosis) | Anti-proliferative, induced apoptosis | IC50 of 30 µM (24h), promoted tubulin polymerization, diminished migration. | |
| MCF-7, MDA-MB-231, MDA-MB-468 (Breast) | Cell Viability, Clonogenicity | Reduced viability and clonogenicity | Among the most sensitive cell lines tested, induced G0-G1 arrest, synergistic with chemotherapy. Preferential inhibition of CSCs. | |
| SKOV-3 (Ovarian Cancer) | Cell Viability, Clonogenicity | Reduced viability and clonogenicity | Among the most sensitive cell lines tested. Reduces viability and colony formation in cancer stem-like populations. | |
| DU145 (Prostate Cancer) | Cell Viability, Clonogenicity | Less sensitive/resistant | Showed resistance compared to other cell lines. | |
| HT-1080, MDA-MB-468, MDA-MB-231, SKOV-3 | Clonogenicity | Reduced colony formation | Differential effects on clonogenic capacity across various cell lines. | |
| DLD1, Ls174T (Colon Cancer), CC14, CC36 | Clonogenic Spheroid Assay | Inhibited spheroid formation | Diminished frequency of clonal spheroids, suggesting effects on cancer stem cell self-renewal. Induced apoptosis (increased activated Caspase3). | |
| MNNG, MG63, U2OS (Osteosarcoma) | CCK-8, Colony Formation | Dose-dependent inhibition of proliferation | IC50 values ranged from 4.194 to 6.506 µM (48h). Inhibited migration and invasion. Induced apoptosis and autophagy. | |
| Nasopharyngeal Carcinoma cells | Cytotoxicity | Cytotoxic effect | Mechanism related to reduced PAK1 kinase activity inhibiting MAPK pathway. | |
| H1299 (Lung Cancer) | Proliferation Assay | Inhibited proliferation | Inhibited via inhibition of YAP1 activity. | |
| MKN1, SH-10-TC (Gastric Cancer) | Proliferation Assay | Inhibited proliferation | Sensitivity depended on YAP1 expression. | |
| C6, U251 (Glioma) | Cell Cycle Analysis | Induced G0/G1 arrest | Blocked cell cycle at G0/G1 phase. | |
| Cholangiocarcinoma cells | Cell Cycle Analysis | Induced S phase arrest | Caused cell arrest at the S phase. | |
| LUAD cells | Colony Formation, Viability | Impeded colony formation and viability | Inhibited proliferation, caused apoptosis, enhanced autophagy flux. Inhibited PAK1 expression. | |
| Dalton's lymphoma cells (T-cell lymphoma) | Viability, Flow Cytometry | Dose-dependent decrease in viability | IC50 of 10.55 µg/mL (24h). Induced G0-G1 arrest and mitochondrial-dependent apoptosis. |
These findings highlight the broad spectrum of anti-cancer effects of ivermectin, largely attributed to its B1a component, across various cancer types through mechanisms involving reduced cell viability, inhibited proliferation, impaired clonogenicity, cell cycle arrest, and induction of apoptosis.Preclinical research into this compound's effects extensively utilizes invertebrate model systems, particularly nematodes and arthropods, due to their susceptibility to the compound and their relevance as targets for antiparasitic agents. These models provide valuable insights into the mechanisms of action and efficacy of this compound.
In Vivo and Ex Vivo Invertebrate Model Systems
Ivermectin, with this compound as its main component, is a potent antiparasitic agent widely used against nematodes and arthropods. Its efficacy in these organisms is primarily mediated through its interaction with glutamate-gated chloride channels (GluCls), which are crucial for the function of invertebrate nerve and muscle cells and are absent in vertebrates at therapeutic concentrations.
Nematode Models (e.g., Caenorhabditis elegans, Haemonchus contortus, Ascaris suum, Cooperia oncophora)
Nematode models, both free-living and parasitic, have been instrumental in understanding this compound's anthelmintic properties.
Caenorhabditis elegans: This free-living nematode is a widely used model organism due to its well-characterized genetics and nervous system. Studies in C. elegans have shown that ivermectin binds specifically to GluCls, leading to increased chloride ion influx, hyperpolarization of cell membranes, and subsequent paralysis and death of the worm. The avr-15 gene in C. elegans encodes a chloride channel subunit that mediates sensitivity to ivermectin and is involved in inhibitory glutamatergic neurotransmission. While ivermectin is known to target GluCls in pharyngeal muscle, leading to inhibited pumping and larval arrest, research also indicates it can inhibit C. elegans muscle GABA and L-AChR receptors, contributing to its pleiotropic effects. C. elegans is also used for anthelmintic drug screening and studying drug-drug interactions, demonstrating a clear dose effect for ivermectin on worm movement.
Haemonchus contortus: This parasitic nematode, a major issue in livestock, is a key model for studying anthelmintic resistance. In vitro studies using muscle transducer systems have evaluated the effects of macrocyclic lactones, including this compound, on H. contortus muscle contraction and motility. Resistance in H. contortus isolates has been linked to altered responses to this compound and Ivermectin B1b, suggesting both components are involved in the mode of action and the development of resistance. While GluCls are considered primary targets, recent research indicates that ivermectin can also bind to H. contortus tubulins, affecting microtubule stability at micromolar concentrations, which could lead to mitotic arrest. Studies on the metabolic fate of ivermectin in H. contortus have shown that the worm's enzymatic system is unable to metabolize the compound, suggesting that biotransformation does not contribute to ivermectin resistance in this species.
Ascaris suum: This large parasitic nematode of swine has been used for electrophysiological studies to understand the effects of this compound on neuromuscular transmission. This compound has been shown to affect chloride channels in A. suum muscle. While it failed to open characteristic GABA channels at high concentrations, it depressed GABA-activated currents. At lower concentrations, this compound produced opening of smaller chloride channels with long open times, suggesting a site of action within the membrane's lipid phase. Studies using electrophysiological preparations of A. suum pharyngeal muscle have revealed the presence of glutamate-gated receptors sensitive to avermectin analogues, where glutamate and ivermectin cause hyperpolarization and increased chloride conductance. In vivo studies in pigs have demonstrated the efficacy of ivermectin against A. suum infections, with significant reductions in fecal egg counts observed after treatment.
Cooperia oncophora: This gastrointestinal nematode of cattle is another important model for evaluating this compound's anthelmintic efficacy. Studies have shown that avermectin B1a (a precursor to ivermectin) is highly effective against C. oncophora in experimentally infected calves, achieving significant worm burden reductions. Efficacy studies with ivermectin formulations in cattle have also confirmed its effectiveness against C. oncophora, demonstrating persistent control for a period after treatment. Research into ivermectin resistance in C. oncophora has investigated genetic variability in glutamate-gated chloride channel genes.
Arthropod Models (e.g., Bombyx mori, Locusta migratoria, Drosophila melanogaster)
Arthropod models are used to study the insecticidal and acaricidal activities of this compound. Ivermectin targets glutamate-gated chloride channels in arthropods, similar to nematodes, leading to paralysis and death.
Bombyx mori: While not explicitly detailed in the provided snippets regarding this compound's specific effects on Bombyx mori, avermectins are known for their insecticidal properties and are used against various insect pests. Research on avermectins in lepidopteran insects like B. mori would likely focus on their neurotoxic effects mediated by GluCls.
Locusta migratoria: Studies on the desert locust, Locusta migratoria, have contributed to understanding the effects of dihydroavermectin B1a (ivermectin) on insect muscle. Specific locust muscles that were not sensitive to GABA responded to dihydroavermectin with an increase in membrane Cl- conductance. This highlights the distinct target of ivermectin (GluCls) compared to GABA receptors in certain arthropod tissues.
Drosophila melanogaster: The fruit fly Drosophila melanogaster is a valuable genetic model for studying the molecular targets of ivermectin in arthropods. Research in Drosophila has confirmed that ivermectin acts as a positive allosteric modulator on histamine-gated Cl- channels, in addition to its primary action on GluCls. This indicates that ivermectin can interact with multiple types of ligand-gated chloride channels in arthropods.
Studies on Parasite Neurotransmission and Muscular Contractility
A core area of research involves investigating how this compound affects neurotransmission and muscular contractility in parasites. The binding of this compound to GluCls on nematode neurons and pharyngeal muscle cells causes an irreversible opening of these channels, leading to a sustained influx of chloride ions. This results in hyperpolarization or depolarization of the cell membrane, effectively blocking neurotransmission and causing paralysis.
In Ascaris suum, electrophysiological studies have shown that ivermectin affects chloride conductance in muscle cells and pharyngeal muscle, impacting their electrical activity and contractility. The effect on pharyngeal pumping is particularly important for the anthelmintic action, as it inhibits feeding.
In Haemonchus contortus, in vitro muscle transducer systems allow for the measurement of the impact of this compound on muscle contraction, providing insights into the mechanisms of paralysis and resistance.
While the primary target in nematodes is GluCls, studies also explore effects on other ion channels. For example, ivermectin has inhibitory effects on γ-aminobutyric acid (GABA) channel conductances in A. suum at certain concentrations, although it doesn't act as a direct GABA agonist.
Assessment of Anthelmintic and Insecticidal Activity in Model Organisms
The efficacy of this compound as an anthelmintic and insecticide is directly assessed in these model organisms through various methods.
In nematode models, efficacy is often measured by the reduction in worm burden or fecal egg counts in infected hosts (in vivo studies) or by assessing motility and survival in vitro. Studies in cattle infected with Cooperia oncophora, Haemonchus contortus, and Ascaris suum have demonstrated high efficacy of avermectin B1a and ivermectin in reducing parasite loads. The faecal egg count reduction test (FECRT) is a common method to evaluate anthelmintic efficacy and detect resistance in parasitic nematodes like Haemonchus contortus.
In arthropod models, insecticidal activity is assessed by observing paralysis, mortality, and effects on behavior and reproduction. While specific data on Bombyx mori, Locusta migratoria, and Drosophila melanogaster regarding quantitative insecticidal activity of this compound were not extensively detailed in the provided snippets, the known mechanism of action on arthropod GluCls underlies its potent insecticidal effects against a wide range of species. The impact on motility and nervous system function in models like Locusta migratoria and the interaction with ion channels in Drosophila melanogaster are key aspects of these assessments.
Advanced Structural and Computational Approaches
Structural studies of this compound involve determining its three-dimensional arrangement, which is essential for understanding how it interacts with proteins and other biomolecules. Ivermectin is a macrocyclic lactone disaccharide. This compound, the major component of ivermectin, has a specific chemical structure (C48H74O14) characterized by a 16-membered macrocyclic ring, a spiroketal, and a disaccharide moiety. The key structural difference between this compound and Ivermectin B1b lies in the substituent at the C-25 position: this compound has a sec-butyl group, while Ivermectin B1b has an isopropyl group. Crystal structures of this compound have been determined, providing precise atomic coordinates for structural analysis.
Computational approaches, particularly molecular docking and molecular dynamics simulations, are extensively used to predict and analyze the binding of this compound to its target proteins.
Molecular Docking: This technique predicts the preferred orientation and binding affinity of a ligand (this compound) to a receptor protein. Studies have focused on docking this compound to its primary targets, the glutamate-gated chloride channels (GluCls), as well as other potential targets. Molecular docking to GluCls has helped elucidate the binding site of ivermectin, which is located in the transmembrane domain at the interface of adjacent subunits, making contacts with the M1, M2, and M3 transmembrane helices and the M2-M3 loop. These interactions are crucial for the allosteric modulation and opening of the channel. Molecular docking studies have also explored the interaction of this compound with other proteins, including tubulin, where it is predicted to bind to the Taxol-binding site on β-tubulin with high affinity, suggesting a potential mechanism for its anti-mitotic effects. Computational studies have also investigated the binding of ivermectin (including this compound) to various SARS-CoV-2 related proteins and host factors like importin α isoforms, suggesting potential alternative mechanisms of action.
Molecular Dynamics Simulations: These simulations provide dynamic insights into the stability of the ligand-protein complex and the conformational changes that occur upon binding. Molecular dynamics simulations complement docking studies by simulating the movement and interactions of the this compound-protein complex over time, allowing researchers to assess the stability of predicted binding poses and understand the dynamic nature of the interaction. These simulations can reveal how this compound binding affects the conformation of the target protein, such as the proposed structural changes induced in SARS-CoV-2 proteins like the 3CL protease and HR2 domain upon ivermectin binding. Molecular dynamics has been used to validate docking results and assess the stability of ivermectin binding to targets like importin α/β1 heterodimer.
These advanced techniques provide a detailed molecular understanding of how this compound interacts with its targets, explaining its potent anthelmintic and insecticidal activities through the modulation of GluCls and exploring its potential interactions with other biological molecules. The integration of structural data from techniques like X-ray crystallography with computational predictions from docking and dynamics simulations offers a powerful approach to dissecting the molecular basis of this compound's biological effects.
Here is a summary of key findings from structural and computational studies:
| Method | Target Protein/Channel | Key Findings | Citations |
| X-ray Crystallography | C. elegans α GluClR | Determined the binding site of ivermectin in the transmembrane domain at subunit interfaces, showing contacts with M1, M2, M3, and the M2-M3 loop. Identified potential hydrogen bonds. | |
| Molecular Docking | Glutamate-gated Chloride Channels (GluCls) | Predicted binding poses and affinities, supporting the allosteric modulation mechanism. Identified key residues involved in binding. | |
| Molecular Docking | β-tubulin | Predicted high binding affinity for this compound at the Taxol-binding site, suggesting a potential anti-mitotic mechanism. | |
| Molecular Docking | Human importin α isoforms | Showed favorable binding affinities, suggesting interference with nuclear import pathways. | |
| Molecular Docking | SARS-CoV-2 proteins (e.g., 3CL protease, helicase, NSP13) | Predicted binding affinities and potential inhibitory effects, suggesting interactions with viral replication machinery. Differential affinities for B1a and B1b observed. | , , , , , , |
| Molecular Dynamics Simulation | Human importin α/β1 heterodimer | Assessed the stability of the ivermectin-importin complex and the dynamic nature of the interaction. | |
| Molecular Dynamics Simulation | SARS-CoV-2 proteins (e.g., 3CL protease, HR2 domain) | Simulated conformational changes induced by ivermectin binding, suggesting potential mechanisms of viral protein inhibition. Differential behavior of B1a and B1b noted. | , |
These studies underscore the importance of advanced structural and computational methods in elucidating the molecular interactions of this compound and its diverse biological activities.
X-ray Crystallography of Ivermectin-Receptor Complexes
X-ray crystallography is a powerful technique used to determine the three-dimensional structure of molecules, including protein-ligand complexes, at atomic resolution. This method has been instrumental in visualizing how this compound binds to its target receptors.
A key application of X-ray crystallography in this compound research has been the structural determination of its complex with the C. elegans α GluClR. This structure revealed that ivermectin binds within the transmembrane domain of the channel, specifically in a cleft located at the interface between adjacent subunits. researchgate.netfrontiersin.org The binding site involves contacts with the M1 and M3 membrane-spanning domains of adjacent subunits and the M2-M3 loop. researchgate.net This structural information provides a visual basis for understanding how this compound allosterically modulates these channels, promoting an open-pore conformation. frontiersin.orgtiho-hannover.de The structure also initially identified potential hydrogen bonds linking ivermectin to specific transmembrane residues, such as L218, S260, and T285 in the C. elegans α GluClR. frontiersin.org
Beyond GluClRs, X-ray crystallography has also been used to characterize the crystal structures of ivermectin itself, including new pseudopolymorphs. mdpi.com These studies provide detailed information about the molecular conformation of this compound in the solid state, which can be compared with computationally modeled structures. mdpi.com Furthermore, crystallography has been employed to investigate the binding pattern of ivermectin to other potential targets, such as the Farnesoid X receptor (FXR), indicating a unique atomic-level interaction pattern. google.com
Table 1: Selected this compound-Related Crystal Structures
| Target Receptor/Molecule | Organism | PDB ID (if available) | Key Binding Site Location | Relevant Findings | Source |
| α GluClR | C. elegans | 3RI5 (Ivermectin complex) | Transmembrane domain, subunit interface | Binding cleft between M1/M3, contacts with M2-M3 loop, stabilizes open state. researchgate.netfrontiersin.org | researchgate.netfrontiersin.org |
| Ivermectin Pseudopolymorphs | N/A | N/A | Crystal lattice | Detailed molecular conformation in solid state. mdpi.com | mdpi.com |
| Farnesoid X Receptor (FXR) | Mammalian | N/A | Specific binding pattern | Unique atomic-level binding pattern. google.com | google.com |
Molecular Docking and Dynamics Simulations (e.g., with Tubulin, GluClRs)
Molecular docking and dynamics simulations are computational techniques used to predict the preferred orientation and binding affinity of a ligand (like this compound) to a receptor and to simulate the dynamic behavior of the complex over time. These methods are valuable for exploring potential interactions and conformational changes that may occur upon binding.
Molecular docking studies have been extensively used to investigate the interaction of this compound with various target proteins. For instance, studies have explored the binding of this compound to tubulin, a key component of the cytoskeleton. Research using computational analysis, including the CCDC GOLD suite and the ChemPLP and ASP scoring functions, has investigated the semi-flexible molecular docking of this compound to the taxane sites of A. thaliana and H. contortus β1-tubulin. ukma.edu.ua These studies predicted that this compound binding could cause M-loop stabilization in tubulin, similar to other microtubule-stabilizing agents. ukma.edu.uanih.gov Molecular dynamics simulations further confirmed the stability of these this compound-tubulin complexes. ukma.edu.uanih.gov Docking analysis has indicated that this compound can exhibit high binding affinity for β-tubulin, in some cases higher than that of reference compounds like Taxol. longdom.org
Molecular docking and dynamics simulations have also been applied to study this compound interactions with Cys-loop receptors, complementing crystallographic data. These simulations can provide insights into the dynamic nature of the binding event and the subsequent conformational changes that lead to channel activation. frontiersin.org
Furthermore, computational studies have explored the binding of this compound to proteins associated with SARS-CoV-2, such as importin α isoforms and the SARS-CoV-2 spike protein (S1 and S2 subunits). scielo.org.mxnih.govnih.gov Molecular docking methods were used to analyze binding affinities and identify active residues involved in interactions, such as hydrogen bonds and Pi-Sigma interactions with importin α isoforms. scielo.org.mx Molecular dynamics simulations were employed to assess the stability of these complexes. nih.govresearchgate.net These studies have suggested differential binding behaviors of this compound and its homolog Ivermectin B1b with viral and host structures. nih.govoutbreak.info
Table 2: Examples of Molecular Docking and Dynamics Simulation Studies with this compound
| Target Protein/Receptor | Model System(s) | Methodologies Used | Key Findings | Source |
| β-tubulin | A. thaliana, H. contortus | Molecular Docking, Molecular Dynamics | Predicted binding to taxane site, M-loop stabilization, high binding affinity. ukma.edu.uanih.govlongdom.org | ukma.edu.uanih.govlongdom.org |
| GluClRs | Invertebrate | Molecular Docking, Molecular Dynamics | Insights into dynamic binding and conformational changes leading to activation. frontiersin.org | frontiersin.org |
| Importin α isoforms | Human | Molecular Docking, Molecular Dynamics | Analysis of binding affinities, identification of interacting residues (hydrogen bonds, Pi-Sigma). scielo.org.mxresearchgate.net | scielo.org.mxresearchgate.net |
| SARS-CoV-2 Spike Protein | SARS-CoV-2 | Molecular Docking, Molecular Dynamics | Binding to S1 and S2 subunits, differential binding compared to Ivermectin B1b, complex stability. nih.gov | nih.gov |
| SARS-CoV-2 Main Protease | SARS-CoV-2 | Molecular Docking, Blind Docking | High binding affinity. researchgate.netresearchgate.net | researchgate.netresearchgate.net |
Site-Directed Mutagenesis for Binding Site Elucidation
Site-directed mutagenesis is a technique used to alter specific amino acid residues in a protein to understand their role in protein function, including ligand binding. By mutating residues suspected to be part of the binding site for this compound and observing the effect on binding affinity or channel activity, researchers can confirm the involvement of these residues.
Site-directed mutagenesis experiments have been crucial in probing the binding site for ivermectin in Cys-loop receptors, including GluClRs and glycine receptors (GlyRs). frontiersin.orgnih.gov These studies have helped to confirm and refine the understanding of the binding site initially suggested by crystallographic data. For example, while crystallography of the C. elegans GluClR suggested hydrogen bonds with specific residues, mutagenesis studies on other ivermectin-sensitive anionic Cys-loop receptors, such as the α1 GlyR, have raised questions about whether these specific hydrogen bonds are universally essential for high ivermectin potency. frontiersin.org
Mutagenesis studies have also been used to investigate the structural determinants for this compound activation of other ion channels, such as G-protein-gated inwardly rectifying K+ channels. researchgate.net Furthermore, mutations in GluClRs have been shown to reduce ivermectin sensitivity, providing a molecular basis for ivermectin resistance observed in parasites and insects. nih.gov Studies involving mutations at specific positions in GABAA receptor subunits have provided insights into how ivermectin binding at different subunit interfaces can lead to distinct effects, such as potentiation of GABA-induced current or irreversible channel activation. nih.gov
Quantum Chemistry Analysis of Molecular Interactions
Quantum chemistry analysis, often employing methods like Density Functional Theory (DFT), provides a theoretical framework to study the electronic structure and properties of molecules and to calculate the energies and nature of molecular interactions. This approach can complement experimental and classical simulation techniques by offering detailed insights into the forces driving this compound binding and the resulting effects on its targets.
DFT calculations have been used in conjunction with X-ray crystallography to provide structure-property information for ivermectin. researchgate.net These calculations can help to understand the electronic distribution within the this compound molecule and how it might interact with the electrostatic environment of a binding site. Theoretical evaluations using DFT have also been performed to compare with X-ray structural parameters, confirming the agreement between theoretical and experimental geometries of ivermectin-related compounds. researchgate.net
Quantum chemistry methods can also be applied to analyze the nature of non-covalent interactions, such as hydrogen bonds and Pi-Pi interactions, between this compound and residues in its binding sites. scielo.org.mxresearchgate.net This level of detail is important for understanding the forces that stabilize the ligand-receptor complex and contribute to binding affinity. Studies investigating the interaction of ivermectin homologs with proteins have utilized computational chemistry perspectives, including analyses of induced disturbances influenced by factors like hydrophobicity. nih.govoutbreak.info
Mechanisms of Ivermectin B1a Resistance
Genetic and Molecular Basis of Resistance
The development of resistance to Ivermectin B1a is often linked to genetic alterations that affect the drug's target sites or influence its interaction with the organism.
Identification of Mutations in Glutamate-Gated Chloride Channel Subunits (e.g., GluCla3, GluClb)
Mutations within the genes encoding glutamate-gated chloride channel subunits, particularly GluCla3 and GluClb, have been identified as key contributors to ivermectin resistance in various nematode species mcgill.canih.govmcgill.ca. Studies in Cooperia oncophora, a parasitic nematode of cattle, have shown that ivermectin-resistant isolates possess amino acid differences in both GluCla3 and GluClb subunits compared to susceptible strains mcgill.canih.gov. For instance, mutations in the resistant GluCla3 subunit of C. oncophora resulted in a significant decrease in sensitivity to both glutamate and ivermectin when expressed in Xenopus laevis oocytes mcgill.canih.gov. Specifically, a single amino acid substitution, L256F, in the GluCla3 subunit was found to account for the reduced response to agonists mcgill.ca. Similarly, mutations in the GluClb subunit of resistant C. oncophora abolished glutamate sensitivity mcgill.canih.gov.
In the nematode Haemonchus contortus, a leucine to phenylalanine mutation at position 256 (L256F) in the GluClalpha3B subunit increased the EC50 for L-glutamate and reduced the Hill number, while also increasing the Kd for radiolabeled ivermectin binding nih.gov. This suggests that this mutation affects both glutamate activation and ivermectin binding nih.gov.
Research in the model nematode Caenorhabditis elegans has also highlighted the importance of GluCl subunit genes (avr-14, avr-15, glc-1) in ivermectin resistance cabidigitallibrary.orgresearchgate.netnih.gov. High levels of resistance in C. elegans have been shown to require simultaneous mutations in multiple alpha-subunit GluCl genes cabidigitallibrary.orgresearchgate.net. A naturally occurring four amino-acid deletion in the ligand-binding domain of GLC-1, an alpha-subunit, has been shown to confer resistance to avermectins in C. elegans nih.gov.
Analysis of Autosomal, Recessive, and Polygenic Resistance Patterns in Pest Species
The inheritance of ivermectin resistance has been studied in various pest species, revealing different genetic patterns. In several arthropods, including the Colorado potato beetle, house fly, and two-spotted spider mite, resistance to avermectins (including this compound) is typically described as autosomal, recessive, and polygenic researchgate.netannualreviews.orgexeter.ac.ukcapes.gov.br. Polygenic resistance indicates that multiple genes contribute to the resistance phenotype, making it a complex trait influenced by variations at several genetic loci massey.ac.nzscielo.brannualreviews.orgresearchgate.net.
In some cases, resistance may not be strictly recessive or polygenic. For example, resistance to T. colubriformis has been reported to be inherited as an incompletely dominant trait massey.ac.nz. The polygenic nature of resistance, while potentially slowing the initial development of high-level resistance compared to monogenic traits, means that resistance can be readily selected for under continuous drug pressure annualreviews.org. Studies in Haemonchus contortus and Onchocerca volvulus also suggest that ivermectin resistance can be polygenic, involving multiple genes including those encoding GluCl subunits and transporters scielo.brresearchgate.netplos.org.
Biochemical Mechanisms of Resistance
Beyond alterations at the drug target site, biochemical processes within the organism can also contribute to this compound resistance by affecting the drug's concentration or availability at its target.
Enhanced Xenobiotic Metabolism (e.g., Oxidative Metabolism)
Increased metabolic detoxification of this compound is a significant biochemical resistance mechanism nih.govresearchgate.netannualreviews.orgresearchgate.netresearchgate.netmyspecies.info. The drug undergoes metabolism primarily in the liver in mammals, and similar processes occur in target invertebrates nih.govnih.govdrugbank.comwho.int. Oxidative metabolism, largely mediated by cytochrome P450 (CYP) enzymes, plays a key role in breaking down ivermectin into less active or inactive metabolites nih.govresearchgate.netannualreviews.orgresearchgate.netmyspecies.infowho.int.
Specific CYP enzymes, such as CYP3A4, CYP3A5, and CYP2C9, have been identified as being involved in the metabolism of ivermectin through reactions like C-hydroxylation and O-demethylation nih.govresearchgate.net. In the body louse, Pediculus humanus capitis, CYP6CJ1 has been implicated in the oxidative metabolism and/or sequestration of ivermectin myspecies.info. Enhanced expression or activity of these metabolic enzymes in resistant individuals can lead to a more rapid breakdown of this compound, reducing its effective concentration at the target site researchgate.netannualreviews.orgmyspecies.info.
Altered Drug Penetration and Transport
Changes in the ability of this compound to penetrate tissues and be transported within the organism can also contribute to resistance researchgate.netannualreviews.org. Reduced drug penetration through the cuticle or gut wall can limit the amount of drug reaching the target neurons and muscle cells researchgate.netannualreviews.org.
Furthermore, the involvement of ATP-binding cassette (ABC) transporters, particularly P-glycoprotein (P-gp), in the efflux of ivermectin has been widely recognized as a mechanism of reduced drug accumulation at target sites nih.govdovepress.comresearchgate.netscielo.brannualreviews.orgresearchgate.netmyspecies.infodrugbank.comresearchgate.netuu.nl. P-gp is an efflux pump that can transport various substrates, including ivermectin, out of cells researchgate.net. Overexpression or increased activity of P-gp in resistant parasites can lead to enhanced efflux of this compound, thereby lowering its intracellular concentration and reducing its efficacy nih.govresearchgate.netscielo.brannualreviews.orguu.nl. Studies have shown that ivermectin is a substrate for and can be inhibited by P-gp nih.govresearchgate.netresearchgate.net. Other ABC transporters like MRP1, MRP2, and BCRP1 have also been noted in relation to ivermectin interactions nih.govresearchgate.net.
Resistance mechanisms can involve a combination of these genetic and biochemical factors, making the study and management of this compound resistance a complex undertaking.
Chemical Synthesis and Derivatization of Ivermectin B1a
Biosynthetic Pathways of Avermectins
Avermectins, the precursors to ivermectin, are a series of eight closely related pentacyclic lactones. nih.govpnas.org Their biosynthesis is a complex process primarily carried out by the soil bacterium Streptomyces avermitilis. wikipedia.orgresearchgate.netbrieflands.commdpi.com
Role of Streptomyces avermitilis Fermentation in Avermectin Production
Streptomyces avermitilis is the key microorganism responsible for the industrial production of avermectins through fermentation. wikipedia.orgbrieflands.commdpi.com This bacterium produces avermectin as a secondary metabolite. brieflands.commdpi.com The fermentation process involves cultivating S. avermitilis in a suitable growth medium containing carbon sources, such as soluble corn starch, and other nutrients. brieflands.com Optimized fermentation conditions, including temperature, pH, and incubation time, are crucial for maximizing avermectin yield. brieflands.com For instance, studies have shown that specific media compositions and parameters like a fermentation period of 5-10 days at temperatures around 28-31°C and a pH of 7.0 can lead to increased avermectin production. brieflands.com Different strains of S. avermitilis and fermentation methods, including submerged and solid-state fermentation, have been explored to enhance production efficiency. brieflands.cominnovareacademics.in
Here is a table summarizing some reported fermentation conditions for avermectin production:
| Fermentation Method | Strain/Details | Substrate/Medium Components | Optimized Conditions | Reported Avermectin Yield | Source |
| Submerged | S. avermitilis 41445 | Soluble corn starch, yeast extract, KCl, CaCO₃, MgSO₄ | 10 days, 31°C, pH 7.0, 10% inoculum | ~17 mg/L (Avermectin B1b) | brieflands.com |
| Submerged | S. avermitilis (gene replacement) | Not specified | 5 days, 28°C, pH 7.0 ± 0.2, 10% inoculum | ~310 mg/L (Avermectin B1a) | brieflands.com |
| Solid State | S. avermitilis NRRL 8165 (ATCC 31267) | Sorghum seeds (optimized) | 15 days, 28°C, 105% initial moisture, 20% inoculum, 8% sucrose, 5% soyameal | 5.8 mg/g dry substrate | innovareacademics.in |
| Submerged | S. avermitilis NRRL 8165 (ATCC 31267) | Not specified | Not specified | 0.175 mg/mL | innovareacademics.in |
| Submerged | S. avermitilis AVE-T27 (ivermectin-producing) | Not specified | Not specified | 3450 ± 65 μg/ml (Ivermectin B1a) | nih.gov |
| Submerged | S. avermitilis NA-108 (parental) | Not specified | Not specified | 3577 ± 26 μg/ml (Total Avermectins) | nih.gov |
Polyketide Synthase (PKS) Involvement and Domain Swap Strategies for Analog Generation
The polyketide backbone of avermectins is synthesized by a type I modular polyketide synthase (PKS) system. nih.govnih.govnih.gov This system involves large, multifunctional polypeptides containing multiple modules, each responsible for catalyzing a specific step in chain elongation. nih.govnih.gov The avermectin PKS in S. avermitilis is encoded by a gene cluster containing four large open reading frames (ORFs): aveA1, aveA2, aveA3, and aveA4. nih.gov These genes collectively encode 12 sets of enzyme activities organized into modules. nih.gov
The PKS utilizes acyl units derived from precursors like isobutyryl-CoA or 2-methylbutyryl-CoA, which originate from valine and isoleucine, respectively, to build the polyketide chain, leading to the production of the 'a' and 'b' components of avermectins. pnas.orgnih.gov
Domain swap strategies involving PKS modules have been employed to generate novel avermectin analogs. nih.govnih.govresearchgate.net By replacing specific domains or modules within the avermectin PKS with those from other polyketide synthases, researchers can alter the substrate specificity or introduce new functional groups, leading to the biosynthesis of modified polyketides. nih.govnih.govresearchgate.netcore.ac.uk For example, swapping the loading module of the avermectin PKS with that from the milbemycin PKS has resulted in the production of new avermectin derivatives with altered substituents at the C25 position. nih.gov Similarly, replacing dehydratase (DH) and ketoreductase (KR) domains in the avermectin PKS with domains from other PKS systems has been explored to modify the reduction state of the polyketide chain. nih.govresearchgate.net These strategies highlight the potential of combinatorial biosynthesis for creating structural diversity within the avermectin family. nih.govresearchgate.net
Semisynthetic Approaches to this compound
This compound is primarily produced semisynthetically from its precursor, avermectin B1a, which is obtained through fermentation of Streptomyces avermitilis. wikipedia.org The semisynthetic route involves a selective chemical modification of the avermectin B1a structure. wikipedia.org
Selective Hydrogenation of Avermectin B1a Precursors
The key step in the semisynthesis of this compound is the selective hydrogenation of the double bond located at the C22-C23 position of avermectin B1a. wikipedia.orgresearchgate.netsums.ac.ir Avermectin B1 is a mixture of two homologous compounds, avermectin B1a and avermectin B1b, with avermectin B1a being the major component (typically >80%). wikipedia.org Selective hydrogenation of this mixture yields ivermectin, which is a mixture of 22,23-dihydroavermectin B1a and 22,23-dihydroavermectin B1b, with the B1a component also being predominant. wikipedia.org This reaction specifically reduces the double bond at C22-C23 while leaving other double bonds in the molecule intact. sums.ac.ir
Catalytic Systems for Hydrogenation (e.g., RhCl₃•H₂O, Wilkinson's Catalyst)
The selective hydrogenation of avermectin B1a is typically carried out using specific catalytic systems. researchgate.netsums.ac.irresearchgate.netbocsci.com Rhodium-based catalysts are commonly employed for this transformation. researchgate.netsums.ac.irresearchgate.netbocsci.com
Wilkinson's catalyst, chlorotris(triphenylphosphine)rhodium(I) [RhCl(PPh₃)₃], is a well-known homogeneous catalyst used for the hydrogenation of alkenes and is effective in the semisynthesis of ivermectin. researchgate.netsums.ac.irresearchgate.netwikipedia.orgbyjus.comonlytrainings.com The mechanism of hydrogenation using Wilkinson's catalyst involves oxidative addition of hydrogen, coordination of the alkene, migratory insertion, and reductive elimination. byjus.comonlytrainings.com
Another catalytic system reported for the hydrogenation of avermectins to ivermectin involves RhCl₃•H₂O in the presence of triphenylphosphine. researchgate.net This system also facilitates the selective reduction of the C22-C23 double bond. researchgate.net The choice of catalyst and reaction conditions, such as solvent and hydrogen pressure, can influence the efficiency and selectivity of the hydrogenation process. researchgate.net
Here is a table illustrating an example of hydrogenation conditions:
| Reactant | Catalyst | Co-catalyst/Ligand | Solvent | Hydrogen Pressure | Temperature | Reaction Time | Product Yield (this compound/B1b) | Source |
| Avermectin B1a/B1b mixture | Not explicitly named (Rhodium catalyst) | Tris-(mexylphenyl)phosphine | Acetone:Cyclohexane (2:1) | 5 bar | 88°C | 4 hours | 89.9% | |
| Avermectin B1a/B1b mixture | Not explicitly named (Rhodium catalyst) | Not specified | Not specified | 20 bar | 88°C | 10 hours | 86% (B1a), 4% (B1b) | |
| Avermectin B1a/B1b mixture | Wilkinson's catalyst [RhCl(PPh₃)₃] | Not specified | Toluene | Not specified | Not specified | Not specified | Not specified | sums.ac.ir |
| Avermectin | RhCl₃•H₂O | Triphenylphosphine | Not specified | Not specified | Not specified | Not specified | Not specified | researchgate.net |
Total Chemical Synthesis Strategies
While ivermectin is primarily produced via semisynthesis from microbiologically derived avermectins, total chemical synthesis of avermectin B1a has also been achieved by academic research groups. researchgate.netbocsci.comrsc.orgnih.govacs.orgacs.org Total synthesis approaches are often complex due to the intricate pentacyclic structure of avermectin B1a, which includes a 16-membered macrocyclic lactone, a spiroketal, and a hexahydrobenzofuran segment. bocsci.comrsc.org
Various strategies have been developed for the total synthesis of avermectin B1a, often involving the convergent coupling of smaller, elaborately constructed fragments representing different parts of the molecule. bocsci.comrsc.org Key challenges in total synthesis include the stereocontrolled formation of multiple chiral centers, the construction of the macrocyclic ring, and the formation of the spiroketal and hexahydrobenzofuran systems. bocsci.comrsc.org
Reported total synthesis schemes have featured key steps such as:
Introduction of specific carbon fragments through reactions like selective ring opening of epoxides and reactions with stabilized anions. rsc.org
Coupling of 'northern' and 'southern' fragments of the molecule using reactions like deconjugative vinyl sulphone anion sequences or Wittig reactions. bocsci.comrsc.org
Formation of the macrocyclic lactone ring through macrolactonization reactions. bocsci.comrsc.org
Introduction of the oleandrosyl-oleandrose disaccharide moiety through glycosylation reactions. bocsci.comrsc.org
One reported total synthesis highlighted the use of a vinylogous Mukaiyama aldol reaction, an intramolecular aldol reaction to form the octahydrobenzofuran, protection strategies for labile functional groups, regioselective double bond installation, diastereoselective construction of molecular segments, and an efficient macrolactonization. bocsci.com While total synthesis provides a complete chemical route to avermectin B1a, these methods are generally not economically viable for large-scale industrial production compared to the fermentation-based semisynthetic route. researchgate.net
Historical Perspectives and Early Total Synthesis Approaches
The discovery of avermectins, the parent compounds of ivermectin, originated from the isolation of a novel soil bacterium, Streptomyces avermitilis, by Satoshi Omura and his co-workers at the Kitasato Institute in Tokyo in the 1970s. researchgate.netbocsci.comresearchgate.netnih.gov Samples of this bacterium were sent to Merck & Co., Inc., where William Campbell and his team identified potent antiparasitic activity in the fermentation broths. researchgate.netnih.govacs.org This collaborative effort led to the isolation and identification of the avermectins. nih.gov
Early approaches to the synthesis of avermectins and subsequently ivermectin were driven by their potent biological activities and complex structures. researchgate.netbocsci.com The unique pentacyclic architecture of the avermectin family attracted significant interest from synthetic organic chemists. bocsci.com While the primary source of ivermectin remains the semi-synthetic hydrogenation of avermectin B1 (a mixture of B1a and B1b) obtained from fermentation, total synthesis has been explored by academic research groups. researchgate.netresearchgate.net These early total synthesis strategies aimed to construct the intricate macrocyclic lactone core and append the disaccharide moiety. rsc.orgorkg.org
Recent Advancements in Efficient and Straightforward Total Synthesis
More recent efforts in the total synthesis of avermectin B1a have focused on developing more efficient and straightforward routes compared to earlier approaches. researchgate.netbocsci.comnih.gov These advancements often involve innovative chemical reactions and strategies to assemble the complex molecular architecture. For instance, one revisited total synthesis of avermectin B1a highlighted key steps such as a vinylogous Mukaiyama aldol reaction, an intramolecular aldol reaction to form the octahydrobenzofuran, and an E-selective Wittig reaction to connect molecular segments. bocsci.com Another approach involved a convergent synthesis utilizing selective ring opening of an epoxide and coupling of distinct molecular fragments. rsc.org These recent syntheses contribute to a deeper understanding of the chemical reactivity and construction of such complex natural products.
Economic Viability Considerations for Industrial Production
The industrial production of ivermectin primarily relies on the fermentation of Streptomyces avermitilis to produce avermectin B1, followed by a chemical hydrogenation step to yield ivermectin (the 22,23-dihydro derivative). researchgate.netresearchgate.netpsu.edugoogle.comprocurementresource.com This semi-synthetic route is generally considered more economically viable for large-scale production compared to total chemical synthesis from basic precursors. The cost of producing ivermectin is relatively low, with estimates for a treatment course being quite low when considering the cost of the active pharmaceutical ingredient (API), excipients, formulation, tax, and profit. medrxiv.orgdrugpatentwatch.com
Rational Design and Synthesis of this compound Analogues
The rational design and synthesis of this compound analogues are pursued to explore structure-activity relationships, improve pharmacokinetic properties, enhance bioactivity against specific targets, or overcome resistance issues. This involves making targeted modifications to the this compound structure.
Modifications at Specific Positions (e.g., C-5, C-13, C-25, Sugar Moiety)
Modifications at various positions of the this compound molecule have been investigated. The disaccharide substituent at C-13 and the hydroxyl group at C-5 are considered potentially significant structural determinants of anthelmintic and insecticidal activity. researchgate.net The bisoleandrosyl group at C-13 is lipophilic and is often a target for chemical modification, as it is not considered crucial for biological activity. nih.gov The C-4 position has also been a focus for modification to alter properties like solubility and stability without necessarily affecting potency. nih.gov
Specific modifications explored include alterations at C-13, such as the introduction of an amide moiety, which can lead to derivatives with altered biological activities. researchgate.net The sugar moiety itself can be modified or removed; for example, acid hydrolysis can cleave the glycosidic bond, yielding the aglycone. Enzymatic glycosylation of avermectin B1a at the 4''-position of the sugar moiety has been shown to enhance anti-nematodal activity. nih.gov Modifications at the C-25 position are notable because this is where this compound and B1b differ. researchgate.net Designed biosynthesis has been used to generate 25-methyl and 25-ethyl ivermectin derivatives with enhanced insecticidal activity. nih.govd-nb.info
Photoreactive Derivatives for Binding Site Probing
Photoreactive derivatives of this compound have been synthesized to investigate its binding site on target proteins, particularly glutamate-gated chloride channels (GluCls), which are key targets in invertebrates. nih.govresearchgate.netresearchgate.net By incorporating a photoreactive group into the this compound structure, researchers can induce covalent cross-linking between the derivative and amino acid residues at the binding site upon irradiation with light. nih.gov This technique, known as photoaffinity labeling, helps to identify the specific residues that interact with the drug. Studies have synthesized this compound derivatives with photoreactive substitutions at positions like C-13, demonstrating their high affinity for GluCls and their utility as probes for chemically labeling the macrolide-binding site. nih.govresearchgate.net
Hybrid Compounds with Enhanced Bioactivity or Specific Target Interactions
The creation of hybrid compounds involves combining the this compound structure, or parts of it, with other pharmacophores to generate molecules with potentially enhanced bioactivity or novel target interactions. This strategy aims to leverage the known efficacy of ivermectin while introducing new properties or expanding the spectrum of activity. Research has explored the synthesis of ivermectin derivatives conjugated with dipeptides or carbohydrates to improve water solubility, facilitating biochemical studies. isuct.ru
Furthermore, hybrid molecules have been designed by appending selected pharmacophores to the ivermectin macrolide, sometimes retaining the sugar moieties. acs.org These hybrid compounds have been evaluated for various activities, such as antiplasmodial effects, with some demonstrating enhanced activity compared to the parent ivermectin molecule. researchgate.netacs.org Designed biosynthesis approaches have also led to hybrid compounds sharing structural features of both avermectins and milbemycins, resulting in enhanced insecticidal activity. nih.govd-nb.info These efforts highlight the potential of creating novel chemical entities based on the ivermectin scaffold.
Structural-Activity Relationship Studies of Derivatives
Structural-activity relationship (SAR) studies of this compound derivatives aim to understand how modifications to its chemical structure affect its biological activity. Research indicates that certain structural features are crucial for its antiparasitic activity. For example, the presence of two sugar moieties at position 13 is considered essential for biological activity. gpatindia.com The reduction of the 22,23 position of avermectins to form ivermectin is reported to show a better safety index while retaining potency. gpatindia.comrsc.org The aliphatic nature of the ring containing the 5-OH/OMe group is also necessary for antiparasitic activity; replacement with an aromatic ring can lead to a loss of activity. gpatindia.com
Modifications at the 4''-O position of the oleandrosyl moiety have been explored, with some 4'-O-substituted derivatives retaining biological activity. gpatindia.com Conversely, a 5-O-substituted analogue has been reported to have no biological activity, suggesting the importance of a hydroxy or methoxy group at the 5-position for antiparasitic activity. gpatindia.com
Studies on ivermectin analogues like abamectin, doramectin, eprinomectin, and emamectin, which share the macrocyclic lactone backbone but differ in functional groups, have provided insights into SAR related to their interactions with target proteins such as glutamate-gated chloride channels (GluCls), glycine receptors (GlyRs), and farnesoid X receptors (FXRs). nih.gov For instance, doramectin, with a larger cyclohexyl group in the spiroketal moiety compared to ivermectin, shows different modulation effects on certain receptors while having similar effects on others. nih.gov
Research has also focused on creating hybrid molecules. For example, ivermectin hybrids targeting Plasmodium parasites have been synthesized and evaluated for activity against hepatic and erythrocytic stages. miguelprudencio.com These studies have shown that molecular modification of ivermectin can lead to compounds with enhanced activity compared to the parent compound or its aglycone, highlighting the potential of SAR studies in developing more potent derivatives. miguelprudencio.com
Synthesis of Ivermectin Analogues with Specific Functional Groups (e.g., NSAIDs)
The synthesis of ivermectin analogues with specific functional groups, such as conjugation with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), has been investigated to potentially enhance or broaden the therapeutic profile. One approach involves synthesizing ester derivatives at the 4''-hydroxyl group of the oleandrosyl moiety of ivermectin. iajpr.comzenodo.org
A reported method for synthesizing such analogues involves the Steglich Esterification reaction between carboxyl-containing NSAIDs and the 4''-hydroxyl group of ivermectin. iajpr.comzenodo.org This reaction typically utilizes dicyclohexylcarbodiimide (DCC) as a coupling agent and 4-dimethylaminopyridine (DMAP) as a catalyst. iajpr.comzenodo.org
Studies have synthesized a series of such ester derivatives using various potent NSAIDs. iajpr.com The chemical structures of these synthesized compounds are characterized using techniques such as Infrared (IR) spectroscopy and ¹H Nuclear Magnetic Resonance (NMR). iajpr.com While the primary aim of such synthesis is often to explore altered or improved biological activities, like anthelmintic activity in the case of NSAID conjugates, the chemical procedures focus on forming the ester linkage between the hydroxyl group on the ivermectin sugar moiety and the carboxyl group of the NSAID. iajpr.comzenodo.org
This type of derivatization demonstrates the potential for chemically linking ivermectin to other pharmacologically active molecules to create novel compounds with potentially synergistic or expanded effects.
Environmental Fate and Degradation of Ivermectin B1a
Environmental Mobility and Sorption Characteristics
Ivermectin B1a exhibits limited mobility in the environment, primarily due to its strong tendency to bind to soil and sediment particles. nih.govpublications.gc.canih.gov This sorption process is a key factor controlling its transport and bioavailability. researchgate.net
Soil Immobility and Sorption Coefficients (Koc Values)
This compound is considered immobile in soil. nih.gov Studies have reported high organic carbon-normalized sorption coefficients (Koc) for ivermectin, indicating a strong affinity for organic matter in soils and sediments. core.ac.ukresearchgate.netnih.gov Reported Koc values for ivermectin range significantly, from approximately 4,000 to 25,800 L/kg, depending on the soil type and experimental conditions. nih.govcore.ac.ukresearchgate.net A study using three European soils (Madrid, York, and artificial soil) found Koc values between 4.00 x 10³ and 2.58 x 10⁴ L/kg. researchgate.net Another study reported Koc values of 12,600–15,700 for ivermectin. core.ac.uk These high Koc values are indicative of strong sorption, which limits the movement of this compound through soil profiles. nih.govoup.com
Sorption and Desorption Kinetics in Different Soil Types
The sorption of this compound to soil is often described as extensive and essentially irreversible. researchgate.net Studies have investigated the sorption and desorption behavior in various soil types. researchgate.netresearchgate.net Equilibrium distribution in soil has been reported to be reached relatively quickly, within 16 to 48 hours. oup.com The sorption and desorption data can often be well fitted using Freundlich isotherms. researchgate.net The strength of sorption can vary depending on soil properties, including organic carbon content. conicet.gov.arresearchgate.net Research suggests that interactions between the hydroxyl pharmacophore of this compound and polar functional groups in humic acid play a significant role in the sorption mechanism, potentially involving hydrogen bonding. researchgate.net While hydrophobic partitioning contributes to binding, specific interactions, possibly involving the formation of complexes with immobile, inorganic soil matter, may also be important, particularly given the lower lipophilicity of ivermectin compared to some related compounds like abamectin. researchgate.netresearchgate.net
Data on sorption coefficients in different soils:
| Soil Type | Kd (L/kg) | Koc (L/kg) | Reference |
| Clay Loam | 227 | 14,800 | oup.com |
| Silty Loam | 333 | 15,700 | oup.com |
| Various Soils | 57-396 | 4,000 - 25,800 | researchgate.net |
| Mixed Artificial Soil | 180 | 5002 (log Koc 3.7) | researchgate.netresearchgate.net |
Degradation Pathways in Environmental Matrices
This compound undergoes degradation in the environment through various pathways, including photodegradation and biotransformation. nih.gov These processes lead to the formation of less bioactive compounds. nih.gov
Photodegradation in Aquatic and Terrestrial Environments
Photodegradation is a significant degradation pathway for this compound, occurring rapidly in both water and as thin films on surfaces when exposed to light. nih.govnih.gov In water, photodegradation half-lives can be as short as 0.5 days or less in the summer. nih.gov On surfaces, the half-life can be less than 1 day. nih.gov Photodegradation has been observed to occur primarily at a wavelength of 350 nm. mdpi.comresearchgate.net
Influence of UV Radiation (UVA, UVC)
Exposure to UV radiation, including both UVA and UVC, causes significant alterations in the chemical structure of ivermectin. mdpi.comresearchgate.netsciforum.net Studies using UV-Vis spectrophotometry have shown changes in the absorption spectra of ivermectin solutions upon irradiation with UVA and UVC lamps. mdpi.comresearchgate.netsciforum.net Computational calculations suggest that the polarization and lability of certain bonds within the this compound molecule make them susceptible to photoinduced dissociation and attack by free radicals, contributing to the degradation pathways. mdpi.com The rate of photodegradation can be influenced by the environmental matrix; for instance, degradation in seawater has been observed to be favored compared to river water, suggesting that the composition of the water influences its stability. mdpi.com The presence of organic matter in water can also absorb UV radiation, potentially decreasing the rate of photodegradation. sciforum.net Photodegradation of ivermectin produces geometric isomers of its C8-C9 and C10-C11 olefins. google.com
Interactive Data Table: Photodegradation Half-lives
| Environment | Condition | Half-life (t½) | Reference |
| Water | Summer | ≤ 0.5 days | nih.gov |
| Thin films/Surfaces | - | < 1 day | nih.gov |
| Open Water | Summer (clear skies) | 12 hours | publications.gc.ca |
| Open Water | Winter (clear skies) | 39 hours | publications.gc.ca |
Role of Environmental Factors (e.g., Water Composition, Turbidity)
The photodegradation of this compound in aqueous solutions is influenced by the composition of the water. Studies have shown that the presence of species in the water can affect the rate of degradation. For instance, photodegradation appears to be favored in seawater compared to river water, suggesting that water composition plays a role in its stability. mdpi.comsciforum.netscribd.com Dissolved organic matter, such as humic and fulvic acids, can compete with this compound for light absorption, potentially reducing the efficiency of its photodegradation. mdpi.comsciforum.net Turbidity in water can also inhibit degradation by creating "shadow zones" that reduce the exposure of the compound to direct sunlight. mdpi.comsciforum.net
Identification of Photodegradation Products and Mechanisms (e.g., Free Radical Generation)
This compound is susceptible to photodegradation when exposed to light, particularly UVA-Vis radiation. mdpi.comsciforum.netresearchgate.net Photodegradation has been observed to occur primarily at a wavelength of 350 nm. mdpi.comsciforum.net Exposure to UVA and UVC radiation can cause significant alterations in the chemical structure of ivermectin. sciforum.netresearchgate.net Computational calculations have been used to deduce potential photoproducts formed during irradiation, aiding in the understanding of degradation mechanisms. mdpi.comsciforum.net The photodegradation of this compound can involve photoinduced mechanisms leading to the generation of free radicals, including singlet oxygen (¹O₂), superoxide anion (.O₂⁻), and hydroxyl radical (.OH). mdpi.comsciforum.netscribd.comresearchgate.netsciforum.net These free radicals can interact with the ivermectin molecule, contributing to its breakdown. The polarization of certain bonds within the this compound molecule makes them susceptible to photoinduced dissociation and attack by hydroxyl radicals, corresponding to bonds that are broken during degradation. sciforum.net
Aerobic Degradation in Soil
This compound undergoes aerobic degradation in soil, leading to the formation of less bioactive compounds. nih.govnih.govnih.gov Its degradation rate in soil can vary depending on factors such as soil type, sorption capacity, temperature, and oxygen availability. usda.govresearchgate.net
The degradation rates of this compound have been studied in both soil and soil/feces mixtures. In soil, the half-life of avermectin B1a can range from 2 to 8 weeks under aerobic conditions. nih.govnih.gov When present in soil/feces mixtures, which is a common route of entry into the environment due to animal excretion, the degradation half-life for ivermectin can range from 7 to 14 days in summer temperatures. nih.govnih.govpublications.gc.causda.govresearchgate.netresearchgate.net However, persistence can be much longer at lower temperatures, potentially ranging from 91 to 217 days in winter. usda.govresearchgate.net Studies on manure composting have shown a more rapid decrease in ivermectin concentration, with a half-life of around 3.6 days under aerobic, self-heating conditions. cornell.edu In contrast, studies on feces/soil mixtures stored indoors at room temperature have shown slower degradation, with half-lives between 93 and 240 days. cornell.edu
Here is a summary of aerobic degradation rates:
| Matrix | Conditions | Half-life (approximate) | Source |
| Soil | Aerobic | 2-8 weeks | nih.govnih.gov |
| Soil/Feces Mixtures | Aerobic, Summer Temperatures | 7-14 days | nih.govnih.govpublications.gc.causda.govresearchgate.netresearchgate.net |
| Soil/Feces Mixtures | Aerobic, Winter Temperatures | 91-217 days | usda.govresearchgate.net |
| Manure Composting | Aerobic, Self-heating | 3.6 days | cornell.edu |
| Feces/Soil Mixtures | Aerobic, Indoor Room Temperature | 93-240 days | cornell.edu |
| Soil (Sandy, 19.3°C) | Aerobic dissipation (DT50) | 15.5 days | researchgate.net |
| Soil (Clay, 19.3°C) | Aerobic dissipation (DT50) | 11.5 days | researchgate.net |
Aerobic degradation of this compound in soil results in the formation of metabolites that are less bioactive than the parent compound. nih.govnih.govnih.gov The transformation of the drug in soil has been shown to produce more polar by-products of ivermectin. nih.gov These polar by-products are reported to be less toxic than the parent drug to certain organisms, such as daphnids. nih.gov Two major metabolites of ivermectin identified in the liver of cattle and swine are 24-hydroxymethyl-H₂B₁a and 3'-O-desmethyl-H₂B₁a, although their formation specifically during aerobic soil degradation is not explicitly detailed in the search results. nih.gov
Degradation Rates in Soil and Soil/Feces Mixtures
Biotransformation by Microorganisms
Biotransformation by microorganisms plays a role in the degradation of this compound in the environment. nih.govresearchgate.net Ivermectin is a derivative of avermectin B1, which is a fermentation product of the soil-dwelling actinomycete Streptomyces avermitilis. core.ac.uk While Streptomyces avermitilis is known to produce avermectins and ivermectin, other microorganisms can also be involved in the breakdown of this compound in the environment. mdpi.comresearchgate.net For instance, Saccharopolyspora erythraea has been shown to glycosylate avermectins and ivermectins. asm.org Aerobic biodegradation in soils under suitable conditions is a known process for macrocyclic lactones like ivermectin. cornell.edu The metabolism of ivermectin has been studied in mammals, identifying cytochrome P450 enzymes as principal metabolizing enzymes, but microbial biotransformation in the environment involves different pathways and organisms. upce.cz
Photocatalytic Degradation in Aqueous Solutions
Photocatalytic degradation has been evaluated as a method for degrading ivermectin in aqueous solutions. Studies using UVC irradiation and titanium dioxide (TiO₂) in suspension have demonstrated efficient degradation. researchgate.net Photocatalysis with UVC and TiO₂ resulted in the degradation of 98% of ivermectin (at a concentration of 500 µg L⁻¹) in water within 600 seconds. researchgate.net This process can lead to the complete elimination of toxicity to organisms like Daphnia similis after a relatively short exposure time (e.g., 10 minutes). researchgate.net
Environmental Persistence and Accumulation
This compound exhibits persistence in various environmental compartments, particularly in soil and animal feces. The degradation half-lives of ivermectin in soil/feces mixtures have been studied under different conditions. Under laboratory conditions and in outdoor settings during winter and summer, these half-lives indicate that ivermectin can remain bound to soil for a considerable duration. nih.govpublications.gc.ca Dissipation half-lives in soil can vary significantly, ranging from 16 to 1520 days, influenced by factors such as soil type, sorption capacity, temperature, and oxygen availability. researchgate.net In mixtures of soil and manure or feces, ivermectin can be very persistent, with reported half-lives ranging from 7 to 217 days. researchgate.netresearchgate.net Some studies indicate that ivermectin can persist in dung for extended periods, potentially up to several months or even over a year in certain environments like reindeer pastures. publications.gc.camdpi.comresearchgate.net
The strong binding affinity of ivermectin to organic carbon (high Koc) contributes to its tendency to accumulate in soil and sediments rather than remaining in the water column. nih.govnih.govwikidata.orgpublications.gc.cacsic.es This strong sorption to soil particles has been confirmed through laboratory and field research. nih.gov
Assessment of Environmental Translocation Potential
The high adsorption coefficient (Koc) of this compound, indicating its strong binding to organic carbon, suggests that it is immobile in soil. nih.govwikidata.orgcsic.esresearchgate.net This strong binding capacity generally limits its potential for translocation through leaching into groundwater. publications.gc.ca
While runoff and groundwater seepage are not considered major sources of contamination due to these properties, some translocation can occur. publications.gc.ca Ivermectin has been detected in drainage and runoff waters, particularly following initial rainfall events after the deposition of treated animal dung or application of manure. researchgate.netresearchgate.net Soil erosion is also identified as a mechanism by which ivermectin bound to soil particles can be transported from fields into adjacent water bodies. nih.govpublications.gc.caresearchgate.net
Absence of Significant Bioconcentration in Aquatic Organisms
Despite having a high octanol/water partition coefficient (Kow), which might suggest a potential for bioconcentration in the fatty tissues of aquatic species, studies have shown that this compound does not strongly bioconcentrate in aquatic organisms like fish. nih.govoup.comoup.comnih.gov
Experimental studies on bluegill sunfish and sturgeon have measured bioconcentration factors (BCF) that were significantly lower than predicted based on the Kow value. oup.comoup.comnih.gov For instance, in bluegill sunfish, the observed BCF for whole fish was 56 L/kg, while the calculated BCF based on aqueous solubility was substantially higher. oup.com Similarly, estimated BCF values for avermectin B1a in sturgeon muscle were around 41-42 L/kg. nih.gov This lower-than-expected bioconcentration is hypothesized to be partly due to the large molecular size of ivermectin, which may limit its ability to cross biological membranes, resulting in an unusually low uptake rate. publications.gc.caoup.com Furthermore, studies indicate rapid depuration of the compound from fish tissues. oup.comnih.gov Consequently, this compound is not expected to biomagnify in the food chain. oup.comnih.gov
The following table summarizes some bioconcentration data for this compound and related compounds in aquatic organisms:
| Organism | Compound | Matrix | Bioconcentration Factor (BCF) (L/kg) | Depuration Half-life (days) | Source |
| Bluegill Sunfish | Avermectin B1a | Whole Fish | 56 | 3.3 | oup.com |
| Bluegill Sunfish | Avermectin B1a | Fillet | 28 | - | oup.com |
| Bluegill Sunfish | Avermectin B1a | Viscera | 84 | - | oup.com |
| Sturgeon | Avermectin B1a | Muscle | 42 (low conc.), 41 (high conc.) | 4.95 (low conc.), 4.33 (high conc.) | nih.gov |
| Bluegill Sunfish | Emamectin Benzoate | Whole Fish | 80 | ~1.4 (90% clearance in 14d) | acs.org |
| Bluegill Sunfish | Emamectin Benzoate | Fillet | 30 | ~1.4 (90% clearance in 14d) | acs.org |
| Bluegill Sunfish | Emamectin Benzoate | Viscera | 116 | ~1.4 (90% clearance in 14d) | acs.org |
Impact of Residues in Feces on Non-Target Organisms
A significant proportion of administered ivermectin (estimated between 80% and 98%) is excreted unmetabolized in the feces of treated animals. publications.gc.camdpi.comresearchgate.netacs.orgresearchgate.netunileon.es These residues in feces pose a notable environmental risk to non-target organisms, particularly the invertebrate communities that inhabit and rely on dung for their lifecycle, such as dung beetles and flies. researchgate.netnih.govresearchgate.netmdpi.comresearchgate.netkorea.ac.krnih.govconicet.gov.arscienceopen.comcambridge.orgbiorxiv.org
Future Research Directions and Applications of Ivermectin B1a Research
Elucidation of Novel Molecular Targets and Pathways
While Ivermectin B1a is well-known for its high-affinity binding to glutamate-gated chloride channels (GluCls) in invertebrates, leading to paralysis and death of parasites, research is actively exploring additional molecular targets and pathways modulated by the compound. Studies have indicated that ivermectin, which is primarily composed of this compound and Ivermectin B1b, can also interact with other ligand-gated ion channels and receptors, including Cys-loop receptors and P2X4 receptors. nih.gov More recently, it has been revealed that ivermectin activates G-protein-gated inwardly rectifying K+ (GIRK) channels, particularly subtype 2 (GIRK2), highlighting a novel target where the interaction occurs at the interface between the transmembrane and intracellular domains. nih.gov
Beyond ion channels, investigations are exploring the potential of ivermectin and its components to interact with mammalian proteins and pathways, particularly in the context of its observed antiviral and potential anticancer activities. Molecular docking studies have suggested that ivermectin can bind to key proteins of SARS-CoV-2, such as the nucleocapsid (N) and ORF6 proteins, by interacting with the same active sites on human importin α isoforms, suggesting a potential mechanism for its antiviral effects by blocking nuclear transport of viral proteins. scielo.org.mx, researchgate.net Furthermore, research into the anticancer properties of avermectin B1a has shown it can induce apoptosis and inhibit migration in cancer cell lines, potentially by promoting tubulin polymerization, similar to the mechanism of action of some established anticancer drugs. mdpi.com, longdom.org Future research aims to fully elucidate the specific binding sites and downstream signaling pathways involved in these interactions, potentially uncovering new therapeutic applications for this compound or its derivatives.
Development of Advanced Preclinical Models for Mechanism Study
Understanding the intricate mechanisms of this compound requires sophisticated preclinical models that can accurately mimic the biological systems of target organisms and hosts. While traditional animal models have been instrumental, there is a growing need for advanced in vitro and in silico models that offer higher throughput, better control over experimental conditions, and reduced reliance on animal testing.
Advanced in vitro models, such as organoids and organ-on-a-chip systems, are being developed to provide more physiologically relevant platforms for studying the interaction of this compound with specific tissues and cell types, including parasite nervous and muscle cells, as well as mammalian cells relevant to potential off-target effects or novel applications. mdpi.com These models can facilitate detailed investigations into the compound's effects at the cellular and subcellular levels, including ion channel gating, protein interactions, and downstream signaling events.
In silico approaches, including molecular docking, molecular dynamics simulations, and quantitative structure-activity relationship (QSAR) modeling, play a crucial role in predicting potential molecular targets, understanding binding affinities, and designing novel derivatives with tailored properties. scielo.org.mx, longdom.org, researchgate.net The integration of these computational methods with experimental data from advanced in vitro models is essential for accelerating the discovery and characterization of this compound's molecular mechanisms.
Innovative Strategies for Combating Resistance
The development of resistance in target parasites poses a significant threat to the long-term efficacy of ivermectin. Research into the mechanisms of resistance is crucial for developing strategies to overcome this challenge. Studies have identified several potential mechanisms of ivermectin resistance in ectoparasites and nematodes, including mutations in GluCl genes, changes in the expression of ABC transporters (efflux pumps), and the upregulation of detoxification enzymes like cytochrome P450s. nih.gov, researchgate.net, researchgate.net
Future research is focused on several innovative strategies to combat resistance. This includes the identification of novel drug targets that are not subject to existing resistance mechanisms. Additionally, research is exploring the potential of combination therapies, where this compound is used in conjunction with other compounds that can either enhance its activity or interfere with resistance mechanisms, such as efflux pump inhibitors. researcher.life, researchgate.net The development of new this compound derivatives with altered structures that are not recognized by resistance mechanisms or have improved activity against resistant strains is also a key area of research. researcher.life, researchgate.net Furthermore, genetic and molecular studies aimed at understanding the evolution and spread of resistance genes in parasite populations are vital for informing resistance management strategies.
Exploration of New Synthetic Methodologies for Derivatives with Tailored Bioactivities
The macrocyclic lactone structure of this compound provides a scaffold for the synthesis of numerous derivatives with potentially altered or enhanced bioactivities. Research is actively exploring new synthetic methodologies to create libraries of this compound derivatives for evaluation against a range of targets, including resistant parasites, viruses, and cancer cells. researcher.life, researchgate.net
Innovative synthetic approaches, such as click chemistry and other modular strategies, are being employed to introduce various functional groups and structural modifications at specific positions on the this compound molecule. researchgate.net These modifications can influence properties such as target affinity, pharmacokinetic profile, metabolic stability, and the ability to overcome resistance mechanisms. For example, research has involved the synthesis of photoreactive this compound derivatives to facilitate the identification of binding sites on GluCls. researcher.life, nih.gov The goal is to design and synthesize derivatives with improved potency, broader spectrum of activity, reduced potential for resistance development, or novel therapeutic applications beyond antiparasitic uses.
Enhanced Environmental Risk Assessment and Mitigation Strategies for Environmental Release
Given the widespread use of ivermectin in veterinary medicine, concerns exist regarding its potential impact on non-target organisms and ecosystems following environmental release through animal waste. Ivermectin is known to be strongly sorbed to soil and sediments due to its low water solubility and high affinity for organic matter. core.ac.uk, oup.com, researchgate.net While it can degrade in the environment, its persistence and potential toxicity to a range of invertebrates, including dung-dwelling organisms and aquatic invertebrates, have been documented. core.ac.uk, oup.com, researchgate.net, nih.gov, wildlifehealthaustralia.com.au
Future research is focused on enhancing environmental risk assessment methodologies to better predict the fate and effects of this compound in various environmental compartments. This includes developing more refined models for estimating predicted environmental concentrations (PECs) in soil, water, and sediment, taking into account different routes of exposure and environmental conditions. researchgate.net, nih.gov Research is also needed to assess the chronic and sublethal effects of this compound on sensitive non-target species and to understand the potential for bioaccumulation and biomagnification in food webs. researchgate.net
Furthermore, research is exploring mitigation strategies to reduce the environmental impact of this compound. This could involve developing new formulations with altered environmental properties, implementing improved waste management practices on farms, or exploring alternative control methods for parasites that minimize environmental release of the compound. wildlifehealthaustralia.com.au Understanding the degradation pathways of this compound in different environmental matrices is also crucial for developing bioremediation or other treatment strategies. core.ac.uk, mdpi.com
Q & A
Q. How can researchers reliably identify and quantify Ivermectin B1a in mixed samples (e.g., commercial ivermectin formulations)?
Methodological Answer:
- Use reversed-phase HPLC with UV detection (e.g., C18 column, mobile phase: acetonitrile/water gradient). Quantify based on retention times and peak areas calibrated against certified reference standards (e.g., 22,23-dihydroavermectin B1a vs. B1b) .
- Validate the method using parameters such as limit of detection (LOD: ~0.1 µg/mL), limit of quantification (LOQ: ~0.5 µg/mL), and recovery rates (≥95%) .
Q. What is the primary mechanism of action of this compound in parasitic infections?
Methodological Answer:
Q. What analytical methods are suitable for distinguishing this compound from structurally similar analogs (e.g., avermectin B1a)?
Methodological Answer:
- Employ high-resolution mass spectrometry (HRMS) to differentiate based on molecular weight (B1a: 875.09 Da vs. avermectin B1a: 873.1 Da) .
- Use NMR spectroscopy (e.g., -NMR) to resolve structural differences at the C25 position (sec-butyl vs. iso-propyl substituents) .
Advanced Research Questions
Q. How should researchers design experiments to evaluate this compound’s efficacy in vivo versus in vitro models?
Methodological Answer:
- In vitro: Use cell-based assays (e.g., HEK293 cells expressing GluCl) to measure EC values. Include controls for nonspecific cytotoxicity (e.g., LDH release assays) .
- In vivo: Optimize dosage using pharmacokinetic modeling (e.g., compartmental analysis) to account for metabolic differences between species. For murine models, typical doses range from 0.2–2 mg/kg .
Q. How can contradictory data on this compound’s antiviral activity (e.g., against SARS-CoV-2) be resolved?
Methodological Answer:
- Conduct dose-response meta-analysis of existing studies, stratifying by experimental design (e.g., cell type, viral load, treatment duration). Use sensitivity analysis to identify confounding variables (e.g., purity of B1a vs. B1b mixtures) .
- Replicate key studies under standardized conditions, adhering to Good Laboratory Practice (GLP) guidelines for assay validation .
Q. What methodologies are appropriate for studying synergistic effects between this compound and other antiparasitics?
Methodological Answer:
Q. How can degradation products of this compound be characterized under varying storage conditions?
Methodological Answer:
- Perform forced degradation studies (e.g., exposure to heat, light, or acidic/alkaline conditions). Identify degradation products via LC-MS/MS and compare toxicity profiles using in silico tools (e.g., Toxtree) .
- Establish stability-indicating methods with specificity for degradation markers (e.g., 8,9-Z isomer) .
Q. What pharmacokinetic modeling approaches are recommended for optimizing this compound dosing in clinical trials?
Methodological Answer:
- Use non-linear mixed-effects modeling (NONMEM) to analyze population PK data. Incorporate covariates such as body weight, hepatic function, and co-administered drugs .
- Validate models using bootstrap resampling and visual predictive checks (VPCs) .
Methodological Considerations
- Reproducibility: Ensure raw data (e.g., chromatograms, electrophysiological traces) are archived in open-access repositories with metadata compliant with FAIR principles .
- Ethical Compliance: For human studies, adhere to protocols outlined in (e.g., informed consent, IRB approval) and ICH guidelines for clinical trial design .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


