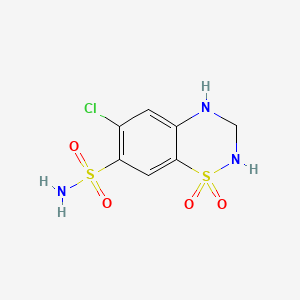
Hydrochlorothiazide
Description
Hydrochlorothiazide (HCTZ) is a thiazide diuretic widely used in managing hypertension and edema. It inhibits sodium-chloride symporters in the distal convoluted tubule, promoting natriuresis and diuresis. With a half-life of 6–15 hours, HCTZ is typically administered once daily. Its efficacy in lowering blood pressure (BP) is well-documented, particularly in combination with renin-angiotensin system (RAS) blockers like valsartan or angiotensin II receptor blockers (ARBs) . However, HCTZ is associated with electrolyte imbalances, notably hypokalemia, which necessitates monitoring or combination therapy with potassium-sparing agents .
Properties
IUPAC Name |
6-chloro-1,1-dioxo-3,4-dihydro-2H-1λ6,2,4-benzothiadiazine-7-sulfonamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JZUFKLXOESDKRF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1NC2=CC(=C(C=C2S(=O)(=O)N1)S(=O)(=O)N)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C7H8ClN3O4S2 | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | hydrochlorothiazide | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Hydrochlorothiazide | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2020713 | |
| Record name | Hydrochlorothiazide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2020713 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
297.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Crystals or white powder. (NTP, 1992), Solid | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>44.7 [ug/mL] (The mean of the results at pH 7.4), less than 0.1 mg/mL at 72.5 °F (NTP, 1992), In water, 722 mg/L at 25 °C, Soluble in ethanol at approximately 750 g/L; soluble in acetone, dilute ammonia; freely soluble in sodium hydroxide solution, n-butylamine, dimethylformamide; sparingly soluble in alcohol; insoluble in ether, chloroform, dilute mineral acids, Soluble in sodium hydroxide solution, Freely soluble in sodium hydroxide solution, in n-butylamine and in dimethylformamide; sparingly soluble in methanol; insoluble in dilute mineral acids, 0.722 mg/mL at 25 °C | |
| Record name | SID855646 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Density |
1.693 g/cu cm | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White, or practically white crystalline powder, White to off-white crystalline powder | |
CAS No. |
58-93-5 | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=58-93-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Hydrochlorothiazide [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000058935 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | hydrochlorothiazide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757059 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | hydrochlorothiazide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=53477 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Hydrochlorothiazide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2020713 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Hydrochlorothiazide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.367 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0J48LPH2TH | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
523 to 527 °F (NTP, 1992), 266-268, 273-275 °C, 274 °C | |
| Record name | HYDROCHLOROTHIAZIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20489 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Hydrochlorothiazide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00999 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Hydrochlorothiazide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3096 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Hydrochlorothiazide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001928 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Preparation Methods
Conventional Synthesis Routes
Two-Step Process via 4-Amino-6-chloro-1,3-Benzenedisulfonamide
The most widely documented method involves a two-step synthesis starting from 4-amino-6-chloro-1,3-benzenedisulfonamide (CAS 2736-25-4). This intermediate reacts with paraformaldehyde in the presence of an inorganic acid, typically sulfuric acid, in alcoholic solvents.
Step 1: Cyclocondensation Reaction
- Reactants : 4-Amino-6-chloro-1,3-benzenedisulfonamide, paraformaldehyde (3 equivalents), and sulfuric acid (20% aqueous solution).
- Solvent : Methanol, ethanol, or isopropyl alcohol (methanol preferred for higher yield).
- Conditions : Reflux at 65–70°C for 6–10 hours, with periodic paraformaldehyde additions to drive the reaction.
- Mechanism : Acid-catalyzed cyclization forms the benzothiadiazine ring system.
Step 2: Purification via Solvent Manipulation
- Dissolution : Crude product is dissolved in acetone or aqueous acetone.
- Decolorization : Activated carbon treatment at reflux removes impurities.
- Acidification : Filtrate pH is adjusted to <3.0 using sulfuric acid to precipitate the product.
- Isolation : Hot filtration at 45–60°C minimizes residual solvent, yielding >99.9% pure Hydrochlorothiazide with 86% overall yield.
Key Advantages :
Aqueous Phase Synthesis
A modified approach eliminates organic solvents, enhancing safety and environmental compatibility:
- Reactants : 4-Amino-6-chloro-1,3-benzenedisulfonamide and formaldehyde (molar ratio 1:0.3–0.4).
- Conditions : Reaction at 90–100°C for 0.5–1.5 hours in water.
- Purification : Post-reaction cooling to 10–20°C, decolorization with activated carbon, and pH adjustment to 5.5–7.0 using sodium hydroxide.
Comparison with Alcoholic Synthesis :
| Parameter | Alcoholic Method | Aqueous Method |
|---|---|---|
| Solvent | Methanol | Water |
| Reaction Time | 6–10 hours | 0.5–1.5 hours |
| Purity | >99.9% | Not explicitly reported |
| Environmental Impact | Higher (organic waste) | Lower |
Advanced Formulation Techniques
Nanoparticle Preparation via Antisolvent Precipitation
To address this compound’s poor solubility (BCS Class IV), nanoparticle formulations have been developed:
Critical Factors in Synthesis and Stability
Industrial-Scale Considerations
Cost and Availability of Starting Materials
4-Amino-6-chloro-1,3-benzenedisulfonamide is commercially available but can be synthesized via the Novello process (US 2,809,194), ensuring supply chain resilience.
Waste Management
Aqueous methods generate less hazardous waste, aligning with green chemistry principles.
Chemical Reactions Analysis
Degradation Reactions
Hydrochlorothiazide undergoes degradation reactions that can produce potentially harmful byproducts. A significant finding is the formation of nitrosamines when this compound is exposed to nitrosating agents. Specifically, the N-nitrosamine derivative of this compound (NO-HCTZ) has been shown to be unstable at physiological pH, decomposing rapidly to release formaldehyde and other byproducts such as thiatriazine and primary amines .
Table 1: Key Degradation Products of this compound
| Degradation Product | Description | Stability at pH 7 |
|---|---|---|
| Formaldehyde | A toxic aldehyde formed from NO-HCTZ | Highly unstable |
| Thiatriazine | A cyclic compound resulting from degradation | Stable |
| Primary Amine | Resulting amine from degradation | Varies |
Maillard Reaction with Lactose
This compound can also participate in Maillard reactions, particularly when mixed with lactose in pharmaceutical formulations. This reaction occurs between reducing sugars and amino compounds, leading to the formation of complex products that may affect drug stability and efficacy.
Table 2: Kinetic Parameters of HCTZ-Lactose Interaction
| pH Condition | Activation Energy (kJ/mol) | Reaction Rate Constant (k) |
|---|---|---|
| Basic (pH 13.1) | 82.43 | Determined via HPLC |
| Neutral (pH 7.1) | 100.28 | Determined via HPLC |
The activation energies indicate that the Maillard reaction is significantly affected by pH levels, suggesting that controlling pH can mitigate undesirable reactions during drug formulation .
Proposed Mechanism of NO-HCTZ Decomposition
The degradation mechanism of NO-HCTZ involves several steps where the compound loses nitrogen gas and forms formaldehyde as a major byproduct:
This reaction pathway illustrates how minor modifications in environmental conditions (such as pH) can lead to significant changes in product formation .
Kinetic Studies
Kinetic studies utilizing High Performance Liquid Chromatography (HPLC) have been employed to analyze the rates of these reactions under varying conditions. The first-order kinetics observed in both basic and neutral conditions highlight the predictable nature of these reactions, allowing for better control in pharmaceutical applications .
Scientific Research Applications
FDA-Approved Indications
Hydrochlorothiazide is primarily indicated for:
- Hypertension : It is used as a first-line treatment for essential hypertension, either alone or in combination with other antihypertensive agents. Studies have shown that thiazide diuretics, including this compound, are effective in lowering blood pressure, particularly in certain populations such as Black patients .
- Edema : The drug is also prescribed to manage edema associated with various conditions such as:
Off-Label Uses
This compound has several off-label applications, including:
- Nephrogenic Diabetes Insipidus : It can help reduce urine output in patients with this condition by promoting sodium reabsorption .
- Calcium Nephrolithiasis Prevention : this compound may reduce urinary calcium excretion, thus preventing kidney stones .
Hypertension Management
A significant study compared the efficacy of this compound with another thiazide-like diuretic, chlortalidone (CHL), in older adults with hypertension. The results indicated comparable outcomes in terms of cardiovascular events between the two medications, suggesting that this compound remains a viable option for hypertension management .
Risk Assessment Studies
Recent research has raised concerns about long-term use of this compound and its association with skin cancer risks. A nested case-control study found an increased risk of squamous cell carcinoma and basal cell carcinoma among users of this compound, particularly at higher cumulative doses . The findings suggest a need for careful monitoring of patients on long-term this compound therapy.
Table 1: this compound Indications and Dosing
| Condition | FDA Approval | Typical Dose Range (mg/day) |
|---|---|---|
| Hypertension | Yes | 12.5 - 50 |
| Edema (various causes) | Yes | 25 - 100 |
| Nephrogenic Diabetes Insipidus | Off-label | 25 - 50 |
| Calcium Nephrolithiasis | Off-label | 25 - 100 |
Table 2: Clinical Study Outcomes on this compound
| Study Reference | Population Size | Duration (years) | Primary Outcome | Result |
|---|---|---|---|---|
| Diuretic Comparison Project | 13,523 | 2.4 | Composite cardiovascular events | Comparable rates (10% vs 10.4%) |
| Skin Cancer Risk Study | Varies | N/A | Risk of skin cancers | Increased risk observed |
Mechanism of Action
Hydrochlorothiazide exerts its effects by inhibiting the sodium-chloride symporter in the distal convoluted tubules of the kidneys. This inhibition prevents the reabsorption of sodium and chloride ions, leading to increased excretion of these ions along with water. The resulting diuretic effect reduces blood volume and decreases peripheral vascular resistance, thereby lowering blood pressure . Additionally, this compound’s action on ion transport can influence electrolyte balance and renal function .
Comparison with Similar Compounds
Thiazide Diuretics: Chlorthalidone vs. Hydrochlorothiazide
Chlorthalidone, another thiazide-like diuretic, shares structural similarities with HCTZ but differs pharmacokinetically. It has a longer half-life (40–60 hours) and larger volume of distribution due to extensive partitioning into red blood cells, enabling once-daily dosing .
Key Differences:
Loop Diuretics: Furosemide vs. This compound
Furosemide, a loop diuretic, acts on the ascending loop of Henle, causing potent natriuresis. While HCTZ and furosemide 25–40 mg twice daily show similar hypotensive effects, furosemide induces greater urinary output and shorter duration of action (half-life: 1–2 hours) . Hypokalemia is less frequent with furosemide, though both drugs reduce serum potassium .
Clinical Implications:
Combination Therapies: RAS Blockers + HCTZ
Combining HCTZ with RAS blockers (e.g., valsartan, telmisartan, benazepril) enhances efficacy:
- Valsartan/HCTZ: Reduces systolic BP (SBP) by 20–25 mmHg and diastolic BP (DBP) by 10–15 mmHg, outperforming monotherapy. Hypokalemia incidence drops from 4.5% (HCTZ alone) to 2.1% (combination) due to valsartan’s potassium-sparing effect .
- Telmisartan/HCTZ : Superior to HCTZ alone in mild-to-moderate hypertension, with 24-hour ambulatory BP control .
Efficacy in Resistant Hypertension:
- 60–70% of patients unresponsive to monotherapy achieve BP control with RAS blocker/HCTZ combinations .
Potassium-Sparing Diuretics: Amiloride/HCTZ vs. HCTZ Alone
Adding amiloride (a potassium-sparing diuretic) to HCTZ mitigates hypokalemia. In patients over 65, HCTZ + amiloride reduces serum potassium by 0.46 baseline standard deviations (bSD) versus 0.19 bSD with atenolol, while improving sodium excretion .
Beta-Blockers: Propranolol vs. This compound
HCTZ demonstrates racial variability in efficacy:
- Black Patients: Greater BP reduction with HCTZ than propranolol.
- White Patients: Propranolol shows better response, though still less effective than HCTZ .
Adverse Effects:
- HCTZ: Higher incidence of hypokalemia and biochemical abnormalities.
- Propranolol: Fewer electrolyte issues but linked to weight gain .
Herbal Compounds: Alisol A vs. This compound
Alisol A, a terpenoid from Alisma plantago-aquatica, exhibits diuretic effects comparable to HCTZ in rats, increasing Na⁺ and K⁺ excretion.
Data Tables
Table 1: Pharmacokinetic Comparison
| Drug | Half-Life | Volume of Distribution | Key Feature |
|---|---|---|---|
| This compound | 6–15 hours | Moderate | Renal excretion |
| Chlorthalidone | 40–60 hours | High (RBC binding) | Once-daily dosing |
| Furosemide | 1–2 hours | Low | Rapid diuresis, short-acting |
Biological Activity
Hydrochlorothiazide (HCTZ) is a thiazide diuretic commonly used to manage hypertension and edema associated with various medical conditions. Its biological activity primarily involves the inhibition of sodium and chloride reabsorption in the distal convoluted tubules of the kidneys, which leads to increased urine output and decreased blood pressure. This article provides a comprehensive overview of HCTZ's biological activity, including its mechanism of action, pharmacokinetics, clinical implications, and associated risks.
HCTZ exerts its diuretic effect by inhibiting the sodium-chloride symporter (SLC12A3) in the distal convoluted tubule. This inhibition prevents sodium reabsorption, leading to increased sodium and water excretion. The overall impact is a reduction in blood volume and blood pressure. In detail:
- Inhibition of Sodium Reabsorption : HCTZ blocks the reabsorption of approximately 5-10% of filtered sodium, which increases the osmotic load in the nephron, promoting diuresis .
- Alteration of Electrolyte Balance : The inhibition of sodium reabsorption also affects potassium levels, often leading to hypokalemia (low potassium levels) as potassium is secreted in exchange for sodium .
Pharmacokinetics
The pharmacokinetic profile of HCTZ includes:
- Absorption : HCTZ is 65-75% bioavailable when taken orally, with peak plasma concentrations occurring 1-5 hours post-administration .
- Distribution : The volume of distribution varies widely (0.83-4.19 L/kg), and it is approximately 40-68% protein-bound in plasma .
- Metabolism : HCTZ is not significantly metabolized; it is excreted unchanged in urine .
- Half-Life : The elimination half-life ranges from 5.6 to 14.8 hours .
Clinical Implications
HCTZ is primarily indicated for:
- Hypertension Management : It effectively lowers blood pressure and is often used as a first-line treatment for hypertension .
- Edema Treatment : HCTZ is utilized in managing edema associated with heart failure, liver cirrhosis, nephrotic syndrome, and corticosteroid therapy .
Table 1: Clinical Indications for this compound
| Condition | Indication |
|---|---|
| Hypertension | First-line treatment |
| Congestive heart failure | Edema management |
| Hepatic cirrhosis | Edema management |
| Nephrotic syndrome | Edema management |
| Corticosteroid therapy | Edema management |
Risks and Side Effects
Despite its therapeutic benefits, HCTZ has been associated with several adverse effects:
- Electrolyte Imbalance : Commonly leads to hypokalemia, hyperuricemia (risk for gout), and dyslipidemia (increased cholesterol levels) .
- Hyperglycemia : There is an association with increased fasting blood glucose levels, particularly in patients with pre-existing diabetes .
- Skin Cancer Risk : Some studies suggest a potential link between long-term HCTZ use and an increased risk of non-melanoma skin cancer, although findings are not conclusive .
Case Studies and Research Findings
Recent studies have provided insights into the efficacy and safety profile of HCTZ:
- A comparative study indicated that chlorthalidone may have greater antihypertensive efficacy than HCTZ at lower doses .
- In a large cohort study involving older adults, no significant differences in cardiovascular outcomes were observed between patients treated with HCTZ versus those on chlorthalidone .
Table 2: Comparative Efficacy of this compound vs Chlorthalidone
| Study Parameter | This compound | Chlorthalidone |
|---|---|---|
| Mean Systolic BP Reduction | Moderate | Greater |
| Incidence of Hypokalemia | Higher | Lower |
| Cardiovascular Events | Comparable | Lower in some studies |
Q & A
Basic Research Questions
Q. How can UV spectrophotometry be optimized for quantifying HCTZ in pharmaceutical formulations?
- Methodological Answer : Use derivative spectrophotometry to mitigate interference from co-formulated drugs or matrix components. For example, diffuse reflectance spectroscopy with p-dimethylaminocinnamaldehyde (PDAC) in acidic conditions (λ = 585 nm) achieves specificity after heating at 80°C for 8 minutes. Validate linearity (3.36×10⁻²–1.01×10⁻¹ mol/L, r = 0.998) and compare results with pharmacopeial methods . Accuracy can be assessed at 50%, 100%, and 150% concentration levels with recovery rates tabulated across triplicate runs .
Q. What are the standard validation parameters for HCTZ analytical methods, and how are they applied?
- Methodological Answer : Key parameters include:
- Accuracy : Test via spiked recovery experiments at three concentration levels (e.g., 50%, 100%, 150%) with ≤2% RSD .
- Linearity : Validate using ≥5 concentrations, ensuring correlation coefficients >0.995 .
- Specificity : Confirm via spectral comparison with USP reference standards or forced degradation studies .
Q. How can HCTZ be distinguished from co-formulated antihypertensives like angiotensin receptor blockers (ARBs)?
- Methodological Answer : Use tandem mass spectrometry (MS/MS) or SIMS-induced fragmentation patterns coupled with Principal Component Analysis (PCA) to isolate spectral signatures. For example, PCA differentiates HCTZ from candesartan cilexetil in combined formulations .
Advanced Research Questions
Q. How can chemometric approaches enhance HPLC method development for HCTZ combination therapies?
- Methodological Answer : Apply factorial design (e.g., 2³ full factorial) to optimize mobile phase composition (methanol content, pH, temperature) and response variables (retention factors, resolution). Derringer’s desirability function can balance competing objectives, such as achieving baseline separation of HCTZ and irbesartan while minimizing run time .
Q. What experimental designs improve HCTZ dissolution in poorly soluble formulations?
- Methodological Answer : Use Box-Behnken designs to optimize spray-drying parameters (outlet temperature, atomization pressure, drug load). For instance, polyvinylpyrrolidone and colloidal silicon dioxide carriers enhance solubility by reducing particle size to 45–59 µm and improving dissolution rates by >50% compared to pure HCTZ .
Q. How do genetic polymorphisms influence HCTZ efficacy in hypertension management?
- Methodological Answer : Integrate metabolomic and genomic data to identify markers like PRKAG2 (rs2727563), DCC (rs12604940), and EPHX2 (rs13262930). A genetic response score (GRS) combining these alleles explains 11–12% of blood pressure variability. Carriers of 6 favorable alleles show ∆SBP/∆DBP reductions of −16.3/−10.4 mmHg vs. −1.5/−1.2 mmHg in low-GRS patients .
Q. How should conflicting clinical trial data on HCTZ cardiovascular outcomes be reconciled?
- Methodological Answer : Conduct meta-analyses stratified by trial design and patient subgroups. For example, while ALLHAT found HCTZ superior to ACE inhibitors in reducing heart failure (HF) risk (RR = 1.19), MIDAS reported no difference in carotid intimal-medial thickness progression but higher vascular events with calcium channel blockers. Consider confounders like baseline BP, comorbidities, and adherence .
Q. What bioanalytical challenges arise in quantifying HCTZ in biological fluids, and how are they addressed?
- Methodological Answer : Overcome matrix effects (e.g., plasma proteins) via liquid-liquid extraction (LLE) or solid-phase extraction (SPE). Validated HPTLC methods achieve LODs of 0.5 ng/mL for HCTZ in plasma using methanol-ammonia (9:1) mobile phases . For capillary electrophoresis, amperometric detection enhances sensitivity in urine samples .
Methodological Tables
Table 1 : Key Factors in HCTZ Dissolution Optimization via Box-Behnken Design
| Factor | Optimal Range | Impact on Dissolution |
|---|---|---|
| Outlet Temperature | 50–60°C | ↑ Solubility |
| Atomization Pressure | 1.5–2.0 bar | ↓ Particle Size |
| Drug Load | 10–20% | Balanced Flowability |
Table 2 : Genetic Variants Associated with HCTZ Response
| Gene | SNP ID | Allele | Effect Size (∆SBP, mmHg) |
|---|---|---|---|
| PRKAG2 | rs2727563 | C | −3.2 |
| DCC | rs12604940 | C | −2.8 |
| EPHX2 | rs13262930 | T | −2.5 |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


