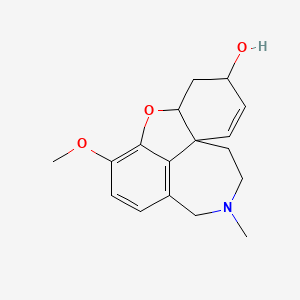
galanthamine
Description
Historical Context and Discovery of Galanthamine
The history of this compound is rooted in the traditional knowledge of plant-based remedies. The bioactive molecule was reportedly discovered accidentally in the early 1950s. jddtonline.infonih.gov Plant extracts containing this compound were initially utilized in Eastern Europe to address conditions such as nerve pain and poliomyelitis. jddtonline.infonih.govresearchgate.netresearchgate.net
Specifically, reports indicate that a Russian pharmacologist observed local villagers in the Caucasus Mountain region using wild Caucasian snowdrop (Galanthus woronowii) to treat poliomyelitis in children. jddtonline.inforesearchgate.net This observation spurred scientific investigation into the plant's constituents. This compound was first isolated in 1952 from Galanthus woronowii. jddtonline.inforesearchgate.nettandfonline.com Research published in 1951 had already demonstrated this compound's acetylcholinesterase (AChE) inhibiting properties and its ability to antagonize the effects of curare. jddtonline.inforesearchgate.net
Following these initial discoveries, preclinical pharmacological studies were conducted in the late 1950s, which further highlighted this compound's antagonistic effects against non-depolarizing neuromuscular blocking drugs. jddtonline.info The first industrial process for its production was developed in 1959. wikipedia.org this compound is an alkaloid found in various plants belonging to the Amaryllidaceae family, including species from the genera Galanthus (snowdrops), Narcissus (daffodils), and Leucojum (snowflakes). tandfonline.comwikipedia.org While chemical synthesis of this compound has been achieved, plants remain a primary source for its extraction. tandfonline.com
Significance of this compound in Neuropharmacology
This compound holds significant importance in neuropharmacology primarily due to its unique dual mechanism of action within the cholinergic system. It functions as a reversible, competitive inhibitor of the enzyme acetylcholinesterase (AChE). nih.govwikipedia.orgdrugbank.comnih.gov AChE is responsible for breaking down the neurotransmitter acetylcholine (ACh) in the synaptic cleft. By inhibiting AChE, this compound increases the concentration and prolongs the action of acetylcholine, thereby enhancing cholinergic neurotransmission. wikipedia.orgdrugbank.comnih.govijnrd.org
Beyond its AChE inhibitory activity, this compound also acts as a positive allosteric modulator of neuronal nicotinic acetylcholine receptors (nAChRs). wikipedia.orgdrugbank.comnih.govijnrd.orgnih.gov This modulation occurs at sites distinct from where acetylcholine binds, leading to a conformational change in the receptor that increases its response to acetylcholine. wikipedia.org This allosteric potentiation of nAChRs, particularly α4β2 and presynaptic α-7 receptors, facilitates the release of acetylcholine and other neurotransmitters like glutamate, GABA, dopamine, serotonin, and norepinephrine from presynaptic neurons. ijnrd.orgnih.govjst.go.jpresearchgate.net This dual mechanism is considered clinically significant. drugbank.comnih.govijnrd.org
The cholinergic system plays a crucial role in various cognitive functions, including memory, attention, and learning. drugbank.comnih.gov The degeneration of acetylcholine-producing neurons is a characteristic feature of neurodegenerative diseases like Alzheimer's disease (AD). drugbank.com By enhancing cholinergic signaling through its dual mechanism, this compound aims to compensate for the loss of cholinergic neurons and improve cognitive function. drugbank.comnih.govijnrd.org
Overview of Current Research Trajectories on this compound
Current research on this compound continues to explore its multifaceted pharmacological properties and potential therapeutic applications beyond its established use in Alzheimer's disease. While it is approved for the treatment of mild to moderate AD, investigations delve into its effects on other neurological and psychiatric conditions. wikipedia.orgdrugbank.comijnrd.orgresearchgate.net
Studies have explored this compound's potential in vascular dementia and Alzheimer's disease with cerebrovascular disease, showing therapeutic efficacy in some instances. drugbank.com The cognitive effects of this compound have also been studied in various psychiatric disorders, including mild cognitive impairment, cognitive impairment associated with schizophrenia and bipolar disorder, and autism. drugbank.comnih.govresearchgate.net Research in animal models of psychiatric disorders suggests that both its nicotinic receptor modulating properties and potential muscarinic receptor activation may contribute to effects on cognitive dysfunction. jst.go.jpresearchgate.net
Furthermore, research trajectories include investigating this compound's potential neuroprotective properties. Studies suggest it may protect against beta-amyloid toxicity and inhibit beta-amyloid aggregation and cytotoxicity in experimental systems. medlink.com It has also been shown to facilitate beta-amyloid clearance in rodent models of AD. medlink.com this compound has demonstrated antioxidant and anti-apoptotic actions in some studies. jddtonline.inforesearchgate.net
The biosynthesis of this compound is another area of active research. The recent elucidation of its biosynthetic pathway in plants provides insights into its production and opens avenues for sustainable and scalable production through synthetic biology approaches, potentially reducing reliance on natural plant sources. frontiersin.org
Clinical trials continue to evaluate this compound. As of recent reports, completed Phase 3 trials for Alzheimer's disease and dementia treatment are listed in clinical trial databases. drugbank.com Long-term studies, such as a 2-year randomized, placebo-controlled study, have investigated its effects on cognitive decline and mortality in patients with mild to moderate AD, reporting significant reductions in mortality and the decline in cognition and daily living activities. dovepress.comneurology.org A large observational study also indicated that this compound was associated with a lower risk of severe dementia and had a notable effect on cognitive decline compared to other cholinesterase inhibitors. neurology.org
Research also includes the exploration of related compounds, such as Benzgalantamine, a formulation closely related to this compound, which is being investigated for its effects on cognitive performance in conditions like Alzheimer's disease. patsnap.com
Data from clinical trials highlight the effects of this compound on cognitive function. For instance, in studies of 3 to 6 months duration, this compound at daily doses of 16-32 mg showed significant treatment effects on cognitive and global function. nih.govresearchgate.net A 2-year study reported a significant improvement in Mini-Mental State Examination (MMSE) scores in the this compound group compared to placebo. dovepress.com
Here is a representation of data points regarding cognitive effects from selected studies:
| Study Duration | This compound Dose Range | Observed Cognitive Benefit | Source |
| 3-6 months | 16-32 mg/day | Significant treatment effects on cognitive and global function. | nih.govresearchgate.net |
| 2 years | Not specified range | Significant improvement in MMSE scores; slower cognitive decline. | dovepress.com |
| Long-term (average 5 years) | Various (ChEI users vs nonusers) | Associated with higher MMSE score at each visit (0.13 points/year); Galantamine had strongest effect (0.18 points/year). | neurology.org |
This table summarizes some findings related to this compound's impact on cognitive measures in clinical research, illustrating the focus of current research trajectories on quantifying its effects over varying durations.
Properties
IUPAC Name |
(1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ASUTZQLVASHGKV-JDFRZJQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCC23C=CC(CC2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CC[C@@]23C=C[C@@H](C[C@@H]2OC4=C(C=CC(=C34)C1)OC)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H21NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2045606 | |
| Record name | Galanthamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2045606 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
287.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Crystals from water; decomposition 256-257 °C. Sparingly sol in cold; more sol in hot water. Very sparingly sol in alcohol, acetone. /Hydrochloride/, Fairly soluble in hot water; freely soluble in alcohol, acetone, chloroform. Less sol in benzene, ether., 1.70e+00 g/L | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Crystals from benzene | |
CAS No. |
357-70-0, 23173-12-8 | |
| Record name | (-)-Galantamine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=357-70-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Galantamine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000357700 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (+/-)-Galantamine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0023173128 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Galantamine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00674 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Galantamine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759861 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Galanthamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2045606 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | GALANTAMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0D3Q044KCA | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | GALANTAMINE, (±)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1T835Z585R | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
126-127 °C, 269 - 270 °C (hydrogen bromide salt) | |
| Record name | GALANTAMINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7361 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Galantamine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014812 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Bioproduction and Synthetic Methodologies of Galanthamine
Natural Sources and Biosynthesis of Galanthamine
This compound is a naturally occurring alkaloid found in specific plant species, where it is produced through a complex biosynthetic pathway.
Botanical Origins of this compound Alkaloids
This compound is predominantly found in plants belonging to the Amaryllidaceae family. Notable sources include various species of Galanthus (snowdrops), Narcissus (daffodils), and Leucojum aestivum (snowflake) wikipedia.orgnih.govrroij.comnih.gov. The alkaloid is extracted from the bulbs and flowers of these plants, although the concentration can be relatively low, typically less than 2% by dry weight wikipedia.orgchim.itwikipedia.org. Other genera within the Amaryllidaceae family, such as Lycoris and Hippeastrum, also contain this compound wikipedia.orgnih.govrroij.commdpi.com. The genus Lycoris, particularly Lycoris radiata, is used for commercial production of this compound in some regions rroij.comnih.gov. Wild Argentinian Amaryllidaceae species, including Zephyranthes filifolia, have also been identified as sources of this compound mdpi.com.
Table 1: Botanical Sources of this compound
| Botanical Genus | Common Name | Notes |
| Galanthus | Snowdrop | Early source, including G. nivalis, G. caucasicus, and G. woronowii wikipedia.orgnih.govnih.gov |
| Narcissus | Daffodil | Contains this compound, used for extraction wikipedia.orgrroij.comindena.com |
| Leucojum | Snowflake | L. aestivum is an industrial source wikipedia.orgnih.gov |
| Lycoris | Red Spider Lily | Used for commercial production in China wikipedia.orgrroij.comnih.gov |
| Hippeastrum | Amaryllis (certain species) | Found in Bolivian species nih.gov |
| Zephyranthes | Rain Lily (certain species) | Wild Argentinian species contain this compound mdpi.commdpi.com |
Enzymatic Pathways in this compound Biosynthesis
The biosynthesis of this compound in Amaryllidaceae plants is a fascinating example of natural product synthesis, starting from common amino acid precursors, phenylalanine and tyrosine chim.itmdpi.comfrontiersin.orgfrontiersin.orggoogle.com. The pathway involves a series of enzymatic steps. Tyrosine is decarboxylated by tyrosine decarboxylase to produce tyramine chim.itgoogle.com. Phenylalanine contributes to the catechol portion of norbelladine google.com. Tyramine then condenses with a phenylalanine-derived aldehyde to form a Schiff base, which is subsequently reduced to norbelladine chim.itgoogle.com.
A crucial step in the biosynthesis is the oxidative phenol coupling of 4'-O-methylnorbelladine, a precursor obtained after methylation of norbelladine chim.itfrontiersin.orgrsc.org. This oxidative coupling reaction proceeds in a para-ortho fashion and is likely catalyzed by cytochrome P-450 dependent enzymes, such as NtCYP96T6 chim.itfrontiersin.orgfrontiersin.org. This coupling forms a highly reactive dienone intermediate, which undergoes intramolecular 1,4-addition of a phenolic hydroxyl group onto the enone moiety, leading to the formation of the benzofuran ring system chim.it. Subsequent reduction of a carbonyl group and final N-methylation complete the biosynthesis of this compound chim.it.
Key intermediates and enzymatic steps in the proposed biosynthetic pathway include:
Tyrosine decarboxylase (TyDC) converting tyrosine to tyramine frontiersin.orggoogle.com.
Condensation of tyramine with a phenylalanine-derived unit to form norbelladine chim.itgoogle.com.
Methylation of norbelladine to 4'-O-methylnorbelladine, potentially catalyzed by norbelladine 4'-O-methyltransferase (N4OMT) chim.itgoogle.com.
Oxidative para-ortho phenol coupling of 4'-O-methylnorbelladine catalyzed by cytochrome P450 enzymes (e.g., NtCYP96T6) to form the this compound skeleton (specifically, N-demethylnarwedine) chim.itfrontiersin.orgfrontiersin.orgrsc.org.
Reduction of the keto group and N-methylation catalyzed by enzymes such as NtAKR1 and NtNMT1, respectively, to yield this compound frontiersin.orgfrontiersin.orgrsc.org.
Figure 1 illustrates a schematic representation of the proposed biosynthetic pathway for this compound. frontiersin.orgfrontiersin.org
Table 2: Key Enzymatic Steps in this compound Biosynthesis
| Enzyme | Catalyzed Reaction |
| Tyrosine decarboxylase (TyDC) | Decarboxylation of tyrosine to tyramine frontiersin.orggoogle.com |
| Norbelladine synthase/reductase | Condensation and reduction leading to norbelladine frontiersin.orggoogle.com |
| Norbelladine 4'-O-methyltransferase (N4OMT) | Methylation of norbelladine to 4'-O-methylnorbelladine chim.itgoogle.com |
| Cytochrome P450 (e.g., NtCYP96T6) | Oxidative para-ortho phenol coupling of 4'-O-methylnorbelladine chim.itfrontiersin.orgfrontiersin.org |
| NtAKR1 | Reduction step frontiersin.orgfrontiersin.org |
| NtNMT1 | N-methylation step frontiersin.orgfrontiersin.org |
Total Synthesis Strategies for this compound
Due to the limited supply from natural sources and the growing demand, significant efforts have been directed towards the total synthesis of this compound. These synthetic strategies aim to construct the complex tetracyclic framework of the alkaloid.
Retrosynthetic Approaches to this compound Core Structure
Various retrosynthetic strategies have been developed to access the this compound core structure. Many approaches converge on the formation of the key tetracyclic system, often involving the construction of the characteristic benzofuran and azepane rings.
A common retrosynthetic theme involves the oxidative phenol coupling as a key step to form the central C-C bond and the quaternary center, often from a norbelladine-type precursor chim.itwikipedia.orgrsc.orgrsc.org. This biomimetic approach, inspired by the natural biosynthesis, was first explored by Barton and co-workers chim.itrsc.org. The oxidative coupling of a diaryl ether precursor leads to a spirodienone intermediate (narwedine), which is then transformed into this compound wikipedia.orgrsc.org.
Other strategies have explored alternative ways to construct the tetracyclic core. Some approaches involve the formation of the azepane ring at a later stage bc.edu. Transition metal-catalyzed reactions, such as intramolecular Heck reactions, have been employed to build the quaternary center and the cyclic system rsc.orgsoton.ac.uk. Ring-closing metathesis (RCM) has also been utilized in the construction of the C ring rsc.org.
Electrochemical methods have emerged as a sustainable alternative for the key oxidative coupling step in some retrosynthetic plans rsc.orgrsc.orgcardiff.ac.uk. These methods aim to achieve the crucial aryl-phenol coupling electrochemically rsc.orgcardiff.ac.uk.
Table 3: Key Retrosynthetic Strategies
| Key Step in Retrosynthesis | Description | Example Approaches |
| Oxidative Phenol Coupling | Formation of central C-C bond and quaternary center from diaryl ether (e.g., norbelladine derivative). | Biomimetic approaches by Barton, industrial production based on this chim.itwikipedia.orgrsc.orgrsc.org |
| Intramolecular Heck Reaction | Palladium-catalyzed cyclization to form the quaternary center and cyclic system. | Trost synthesis rsc.orggoogle.com |
| Ring-Closing Metathesis (RCM) | Formation of a cyclic structure (e.g., the C ring) via alkene metathesis. | Brown synthesis rsc.orgsoton.ac.uk |
| Electrochemical Oxidative Coupling | Anodic oxidation to achieve aryl-phenol coupling. | Recent research by Wirth, Opatz, Waldvogel rsc.orgrsc.orgcardiff.ac.uk |
| [(3+2)+1] Cycloaddition | Rh(I)-catalyzed reaction to construct the cis-hydrobenzofuran skeleton and azepane ring simultaneously. | Yu and Feng synthesis bc.edu |
Stereoselective Synthesis of this compound Enantiomers
This compound is a chiral molecule, and its biological activity is associated with the (-)-enantiomer rsc.orgrsc.org. Achieving stereoselectivity in total synthesis is therefore crucial. Various methods have been employed to control the stereochemistry, particularly at the quaternary carbon center and the hydroxyl-bearing carbon.
One approach to obtaining enantiomerically pure this compound has been the resolution of racemic intermediates, such as narwedine wikipedia.orgrsc.orgrsc.orgx-mol.com. Racemic narwedine can be resolved through crystallization-induced dynamic chiral resolution using a chiral mediator wikipedia.orgrsc.org. This method has been utilized in industrial production wikipedia.orgrsc.org.
Asymmetric synthesis strategies have also been developed to directly construct the chiral centers with high enantioselectivity. These methods include:
Chiral pool synthesis, starting from enantiomerically pure precursors like L-tyrosine wikipedia.org.
Asymmetric induction using chiral reagents or catalysts in key steps, such as asymmetric ketone reduction wikipedia.orgrsc.org.
Asymmetric catalysis with chiral transition metal complexes or organocatalysts rsc.org.
Diastereoselective reactions where existing stereocenters in an intermediate influence the formation of new ones wikipedia.orgx-mol.com. For example, complementary reducing agents like Luche reduction and L-selectride reduction can lead to different diastereomers from a cyclic enone system x-mol.com.
Table 4: Methods for Achieving Stereoselectivity in this compound Synthesis
| Method | Description | Examples/Applications |
| Classical Resolution | Separation of enantiomers from a racemic mixture, often by crystallization with a chiral resolving agent. | Resolution of narwedine wikipedia.orgrsc.orgrsc.orgx-mol.com |
| Chiral Pool Synthesis | Utilizing readily available chiral starting materials to build the target molecule. | Synthesis starting from L-tyrosine wikipedia.org |
| Asymmetric Catalysis/Reagents | Using chiral catalysts or stoichiometric chiral reagents to induce asymmetry in a reaction. | Asymmetric ketone reduction, asymmetric allylic alkylation wikipedia.orgrsc.org |
| Diastereoselective Reactions | Control of stereochemistry based on existing chiral centers in the molecule. | Complementary reductions of cyclic enones wikipedia.orgx-mol.com |
| Crystallization-Induced Dynamic Resolution | A method where a racemic mixture is resolved while the enantiomers are in dynamic equilibrium. | Industrial production of (-)-galanthamine wikipedia.orgrsc.org |
Semi-synthetic Derivatives of this compound and Analogues
Beyond the total synthesis of this compound, research has also focused on the creation of semi-synthetic derivatives and analogues. This involves modifying the this compound structure or synthesizing compounds with similar structural features to explore their chemical and biological properties.
Semi-synthesis typically involves using naturally isolated this compound or a late-stage biosynthetic intermediate as a starting material for further chemical transformations. Modifications to the this compound structure, such as derivatization of the hydroxyl group at the C-6 position, have been explored mdpi.com.
The synthesis of this compound analogues involves creating compounds that share some structural resemblance to this compound but may have variations in the ring system, heteroatoms, or substituents google.comresearchgate.net. These analogues are synthesized through various organic chemistry methodologies, often employing strategies similar to those used in total synthesis but applied to modified scaffolds google.com. The aim of creating derivatives and analogues is often to investigate structure-activity relationships and potentially develop compounds with altered or improved properties mdpi.comresearchgate.net.
Examples of modifications explored include changes to the ring members, the nature and position of heteroatoms, and the synthesis of ring-opened structures researchgate.net. The availability of intermediates from total synthesis routes can also provide access to various analogues google.com.
Table 5: Approaches to Semi-synthetic Derivatives and Analogues
| Approach | Description | Focus |
| Semi-synthesis | Chemical modification of naturally derived this compound or intermediates. | Derivatization of functional groups (e.g., C-6 hydroxyl) mdpi.com |
| Analogue Synthesis | Creation of compounds with structural similarities but variations from this compound. | Modifications to the core skeleton, heteroatoms, substituents google.comresearchgate.net |
| Intermediate Diversion | Utilizing intermediates from total synthesis routes to synthesize related compounds. | Access to various structural analogues google.com |
Molecular and Cellular Pharmacology of Galanthamine
Mechanism of Action: Acetylcholinesterase Inhibition
Acetylcholinesterase (AChE) is an enzyme responsible for the hydrolysis of acetylcholine (ACh) in the synaptic cleft, thereby terminating cholinergic neurotransmission. drugbank.com Galanthamine acts as an inhibitor of AChE, leading to increased concentrations of ACh available for binding to acetylcholine receptors. wikipedia.orgdrugbank.com This inhibition is a primary mechanism by which this compound is thought to enhance cholinergic function. drugbank.com
Kinetics of Acetylcholinesterase Binding by this compound
This compound is described as a competitive and reversible inhibitor of AChE. drugbank.com Early analyses using conventional steady-state models suggested a competitive inhibition pattern. researchgate.net However, more recent studies employing pre-steady-state analysis of reaction progress curves have indicated that this compound exhibits time-dependent inhibition, suggesting a more complex interaction than simple competitive binding. windows.net This time-dependent nature implies a long drug-target residence time, which was previously overlooked by conventional kinetic analyses and led to an underestimation of this compound's potency against AChE. windows.net Studies on human AChE have reported varying Ki values, with some indicating a competitive nature and others suggesting a mixed-type inhibition depending on the source of the enzyme (e.g., recombinant human AChE vs. Torpedo californica AChE). windows.net
Structural Basis of this compound-Acetylcholinesterase Interaction
The structural interaction between this compound and AChE has been elucidated through X-ray crystallography, notably with Torpedo californica acetylcholinesterase (TcAChE). nih.govrcsb.org this compound binds within the active site gorge of AChE. nih.govrcsb.org This gorge is approximately 20 Å deep and contains the catalytic machinery and binding subsites. researchgate.net this compound interacts with both the choline-binding site, involving residues like Trp-84, and the acyl-binding pocket, which includes residues such as Phe-288 and Phe-290. nih.gov
Interestingly, the tertiary amine group of this compound does not directly interact closely with Trp-84. nih.govrcsb.org Instead, the double bond of its cyclohexene ring is involved in stacking interactions with the indole ring of Trp-84. nih.gov The tertiary amine appears to form a non-conventional hydrogen bond with Asp-72, located near the top of the gorge. nih.gov A strong hydrogen bond is also formed between the hydroxyl group of this compound and Glu-199. nih.gov The rigid structure of this compound contributes to a low entropy cost upon binding, which, coupled with these moderate to weak interactions, contributes to its relatively tight binding to AChE. nih.gov Molecular docking studies have supported the observed binding orientation within the active site. rcsb.orgresearchgate.net
Allosteric Potentiation of Nicotinic Acetylcholine Receptors (nAChRs)
In addition to inhibiting AChE, this compound acts as an allosterically potentiating ligand (APL) of neuronal nicotinic acetylcholine receptors (nAChRs). wikipedia.orgpnas.orgresearchgate.net Allosteric modulators bind to sites distinct from the orthosteric (agonist) binding site, inducing conformational changes that alter receptor function. pnas.orgmdpi.com This allosteric modulation by this compound enhances the response of nAChRs to acetylcholine and other nicotinic agonists. researchgate.net This mechanism is considered a crucial aspect of this compound's therapeutic profile, potentially contributing to its effects beyond mere AChE inhibition. researchgate.net
Subtype Specificity of nAChR Modulation by this compound
This compound has demonstrated allosteric potentiation on several neuronal nAChR subtypes. Studies using heterologous expression systems and patch-clamp recordings have shown this compound to be a potent APL for human α3β4, α4β2, and α6β4 nAChRs. wikipedia.orgresearchgate.net It also potentiates the activity of chicken/mouse chimeric α7/5-HT3 receptors. wikipedia.orgresearchgate.net The α4β2 and α7 subtypes are particularly abundant in the central nervous system and are implicated in cognitive function. mdpi.comnih.gov
While this compound potentiates agonist responses in a concentration-dependent manner, this effect is typically observed within a specific concentration window (e.g., 0.1-1 µM), with higher concentrations potentially leading to inhibition. researchgate.net This bell-shaped dose-response is characteristic of some positive allosteric modulators. researchgate.netmdpi.com Some studies, however, have reported no potentiation or even inhibitory effects of this compound on certain nAChR subtypes or under specific experimental conditions. scielo.br It is important to note that this compound selectively modulates nAChRs and does not significantly alter the activity of muscarinic acetylcholine receptors (mAChRs) at therapeutically relevant concentrations. researchgate.netfrontiersin.org
Impact on Receptor Function and Signaling Cascades
This compound's allosteric potentiation of nAChRs leads to several functional consequences. By binding to an allosteric site, this compound increases the probability of channel opening in response to acetylcholine and slows down receptor desensitization. This results in enhanced ion flux through the receptor channel.
The activation and potentiation of nAChRs by this compound can trigger downstream signaling cascades. For instance, nAChR activation, particularly of the α7 subtype, can lead to calcium influx into neurons and other cells. researchgate.netnih.gov This increase in intracellular calcium can then activate various signaling pathways. Research has shown that this compound-mediated potentiation of nAChRs can lead to the phosphorylation of Akt, a key effector of the phosphatidylinositol 3-kinase (PI3K) pathway. researchgate.net This α7 nAChR-PI3K-Akt pathway has been implicated in the neuroprotective effects of this compound. researchgate.net Furthermore, this compound-enhanced nAChR activity can modulate the release of neurotransmitters, including acetylcholine, glutamate, and serotonin, influencing synaptic plasticity and neuronal communication. drugbank.compnas.orgresearchgate.net In microglia, this compound-sensitized α7 nAChRs can induce calcium influx, leading to intracellular signaling cascades that stimulate processes like amyloid-β phagocytosis through actin reorganization. nih.gov
Neuroprotective Mechanisms of this compound
Beyond its effects on cholinergic neurotransmission, this compound has demonstrated neuroprotective properties through various molecular and cellular mechanisms. nih.govresearchgate.netiiarjournals.org These mechanisms are often linked to its ability to modulate nAChRs and downstream signaling pathways.
This compound has been shown to protect neurons against various insults, including β-amyloid-enhanced glutamate toxicity and NMDA-induced excitotoxicity. nih.govresearchgate.net This protection is mediated, at least in part, by the activation of α7 and α4β2 nAChRs. nih.govresearchgate.net The involvement of the α7 nAChR-PI3K-Akt pathway is crucial in this neuroprotection, as demonstrated by the blockage of protective effects by PI3K inhibitors or α7 nAChR antagonists. researchgate.net this compound's ability to induce Akt phosphorylation is linked to its allosteric potentiation of nAChRs. researchgate.net
Furthermore, this compound can influence microglial function, which plays a significant role in neuroinflammation and neurodegeneration. This compound can enhance microglial phagocytosis of amyloid-β, a key pathological feature of Alzheimer's disease. nih.gov This effect is mediated by the allosteric modulation of microglial α7 nAChRs, leading to calcium influx and activation of signaling cascades that promote phagocytosis. nih.gov This suggests a mechanism by which this compound may contribute to the clearance of toxic protein aggregates.
Studies also indicate that this compound can influence the expression of neuroprotective genes, such as nAChRα-7, Bcl-2 (an anti-apoptotic protein), and BDNF (Brain-Derived Neurotrophic Factor), potentially contributing to neuronal survival and plasticity. researchgate.netresearchgate.net While some research suggests potential involvement of muscarinic receptors in this compound's neuroprotective effects in specific contexts like glaucoma, the predominant evidence points towards nAChR-mediated mechanisms in the context of neurodegenerative conditions like Alzheimer's disease. arvojournals.org
Anti-inflammatory Effects in Neural Systems
Neuroinflammation is a significant factor in the pathogenesis of various neurological disorders. Research indicates that galantamine exhibits anti-inflammatory properties within neural systems. Studies have shown that galantamine can prevent the activation of microglia and astrocytes, key cellular mediators of neuroinflammation, in the hippocampus nih.govfrontiersin.orgresearchgate.net. This effect is associated with the inhibition of inflammatory signaling molecules, such as NF-κB p65, and a reduction in the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 nih.govfrontiersin.orgresearchgate.net. The cholinergic anti-inflammatory pathway, mediated by the binding of acetylcholine to α7 nicotinic receptors, is believed to play a role in this process, as it suppresses NF-κB activation and inhibits pro-inflammatory cytokine production jddtonline.infonih.gov. Galantamine, by increasing acetylcholine levels through AChE inhibition and allosterically modulating nAChRs, can activate this pathway jddtonline.infonih.gov. In vitro studies using microglia and hippocampal neuronal cell lines have further demonstrated galantamine's ability to reduce inflammatory responses nih.gov.
Antioxidant Properties and Oxidative Stress Mitigation
Oxidative stress, resulting from an imbalance between reactive oxygen/nitrogen species (ROS/RNS) and the body's antioxidant defenses, contributes to neuronal damage in neurodegenerative diseases arphahub.com. Galantamine has been shown to possess antioxidant properties and mitigate oxidative stress in neural systems eurekaselect.comnih.goveuropeanreview.org. It can act as a scavenger of reactive oxygen species, thereby lowering oxidative neuronal damage eurekaselect.comarphahub.com. Studies using in vitro models of hydrogen peroxide-induced oxidative stress in neuronal cells have shown that galantamine can reduce the release of ROS and prevent the loss of mitochondrial activity nih.gov. Furthermore, galantamine has been observed to prevent oxidative stress induced by amyloid-beta peptide in cortical neurons nih.gov. This includes preventing the increase in reactive oxygen species and lipoperoxidation caused by amyloid-beta and preventing alterations in the glutathione (GSH) antioxidant system nih.gov. The antioxidant effects of galantamine may be linked to its ability to prevent the activation of P2X7 receptors, protect mitochondrial membrane potential, and prevent membrane fluidity disturbances eurekaselect.com. Additionally, the increase in acetylcholine levels due to AChE inhibition and allosteric potentiation of α7 nAChRs by galantamine can lead to a decrease in the overproduction of reactive oxygen species eurekaselect.com.
Modulation of Apoptotic Pathways
Apoptosis, or programmed cell death, is another critical process involved in neurodegeneration. Galantamine has demonstrated the ability to modulate apoptotic pathways, offering neuroprotective effects researchgate.neteuropeanreview.org. Research indicates that galantamine can prevent apoptosis induced by various cytotoxic agents, including amyloid-beta peptide and thapsigargin eurekaselect.comresearchgate.net. This protective effect may involve the activation of nicotinic acetylcholine receptors and the upregulation of anti-apoptotic proteins such as Bcl-2 eurekaselect.comresearchgate.net. Studies have shown that galantamine treatment can increase the expression of Bcl-2 in neuronal cells researchgate.net. The allosteric potentiation of α7 nAChRs by galantamine is thought to contribute to this by inducing the phosphorylation of serine-threonine protein kinase, stimulating phosphoinositide 3-kinase, and elevating Bcl-2 expression eurekaselect.com. Furthermore, galantamine has been found to inhibit amyloid-beta-induced apoptosis by activating the JNK signaling pathway, enhancing α7 nAChR expression, and inhibiting the Akt pathway, which promotes autophagosome biogenesis and autophagy nih.gov.
Other Potential Pharmacological Targets of this compound
Beyond its well-established role as an AChE inhibitor and its effects on inflammation, oxidative stress, and apoptosis, galantamine interacts with other pharmacological targets, influencing various neurotransmitter systems.
Effects on Cholinergic Neurotransmission Beyond Acetylcholinesterase
While galantamine is known for inhibiting AChE, thereby increasing acetylcholine concentration in the synaptic cleft, it also has significant effects on cholinergic neurotransmission through its interaction with nicotinic acetylcholine receptors (nAChRs) nih.govdrugbank.compatsnap.com. Galantamine acts as a positive allosteric modulator of certain nAChR subtypes, including α4β2 and α7 receptors drugbank.comnih.govwikipedia.org. By binding to an allosteric site distinct from the acetylcholine binding site, galantamine enhances the sensitivity of these receptors to acetylcholine, leading to increased cholinergic signaling drugbank.compatsnap.comnih.gov. This allosteric modulation facilitates the release of acetylcholine from presynaptic neurons, further contributing to increased cholinergic tone nih.gov. This dual mechanism of action – AChE inhibition and nAChR modulation – is considered clinically significant nih.govnih.gov.
Interactions with Other Neurotransmitter Systems
Galantamine's allosteric modulation of nicotinic receptors can influence the release of neurotransmitters other than acetylcholine nih.govresearchgate.net. Nicotinic acetylcholine receptors are located on presynaptic neurons and regulate the release of various neurotransmitters, including dopamine, glutamate, norepinephrine, and serotonin nih.gov. By modulating these receptors, galantamine can indirectly affect the activity of these neurotransmitter systems nih.govresearchgate.net. Studies suggest that galantamine can enhance dopaminergic neurotransmission, possibly via the activation of nicotinic α7-receptors researchgate.net. The modulated release of other neurotransmitters by galantamine may contribute to its broader effects on cognitive function and potentially other neurological processes drugbank.comresearchgate.net.
Compound Names and PubChem CIDs
| Compound Name | PubChem CID |
| Galantamine | 9651 nih.gov |
| Acetylcholinesterase | 9000-81-1 (CAS) nih.gov |
| Acetylcholine | 187 nih.gov |
| Amyloid-beta peptide | |
| Hydrogen Peroxide | |
| TNF-α | |
| IL-1β | |
| IL-6 | |
| NF-κB | |
| Bcl-2 | |
| Caspase-3 | |
| Caspase-9 | |
| Glutathione (GSH) | |
| Reactive Oxygen Species (ROS) | |
| Nitric Oxide (NO) | |
| Dopamine | |
| Glutamate | |
| Serotonin | |
| Norepinephrine | |
| GABA |
Note: PubChem CIDs for some complex biological entities like proteins (TNF-α, IL-1β, IL-6, NF-κB, Bcl-2, Caspase-3, Caspase-9, Acetylcholinesterase) or general categories (Amyloid-beta peptide, Hydrogen Peroxide, GSH, ROS, NO, Dopamine, Glutamate, Serotonin, Norepinephrine, GABA) are not single, discrete chemical structures and therefore may not have a standard PubChem CID in the same way as small molecules like Galantamine or Acetylcholine. Where available, a relevant identifier like a CAS number for Acetylcholinesterase is provided.## The Molecular and Cellular Landscape of this compound's Actions
This compound, an alkaloid derived from Amaryllidaceae plants, is a compound with a well-established role in modulating cholinergic neurotransmission. Beyond its primary function as an acetylcholinesterase inhibitor, this compound exerts a range of molecular and cellular effects within the neural system, encompassing anti-inflammatory, antioxidant, and anti-apoptotic properties, as well as modulating nicotinic acetylcholine receptors and interacting with other neurotransmitter systems.
Anti-inflammatory Effects in Neural Systems
Neuroinflammation is increasingly recognized as a critical contributor to the progression of neurodegenerative diseases. This compound has demonstrated significant anti-inflammatory capabilities within the central nervous system. Studies have shown that this compound treatment can attenuate neuroinflammation by inhibiting the activation of glial cells, specifically microglia and astrocytes, in brain regions like the hippocampus nih.govfrontiersin.orgresearchgate.net. This inhibitory effect is associated with the suppression of key inflammatory signaling pathways. For instance, this compound has been shown to inhibit the activation and translocation of NF-κB p65, a crucial transcription factor involved in the expression of pro-inflammatory genes nih.govfrontiersin.orgresearchgate.net. Consequently, the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, is reduced following this compound administration nih.govfrontiersin.orgresearchgate.net.
A significant mechanism underlying this compound's anti-inflammatory effects is its interaction with the cholinergic anti-inflammatory pathway. This pathway is mediated by the binding of acetylcholine to α7 nicotinic acetylcholine receptors (α7 nAChRs), which leads to the suppression of NF-κB activation and the subsequent inhibition of cytokine release jddtonline.infonih.gov. As this compound inhibits acetylcholinesterase, it increases the availability of acetylcholine in the synaptic cleft. Furthermore, its allosteric modulation of nAChRs, including the α7 subtype, enhances the effects of acetylcholine on these receptors, thereby activating the cholinergic anti-inflammatory pathway jddtonline.infonih.gov. In vitro experiments using microglial and neuronal cell lines have provided further evidence of this compound's ability to mitigate inflammatory responses nih.gov.
Antioxidant Properties and Oxidative Stress Mitigation
Oxidative stress, characterized by an imbalance between the production of reactive oxygen/nitrogen species (ROS/RNS) and the capacity of antioxidant defense systems, is a major factor in neuronal damage observed in various neurological conditions arphahub.com. This compound exhibits antioxidant properties that contribute to its neuroprotective effects eurekaselect.comnih.goveuropeanreview.org. It functions as a scavenger of reactive oxygen species, thereby reducing oxidative damage to neurons eurekaselect.comarphahub.com.
Research utilizing in vitro models of oxidative stress, such as those induced by hydrogen peroxide, has shown that this compound can effectively decrease the generation of reactive oxygen species and preserve mitochondrial function in neuronal cells nih.gov. Furthermore, this compound has been shown to protect cortical neurons from oxidative stress induced by amyloid-beta peptide, a key pathological hallmark of Alzheimer's disease nih.gov. This protection involves preventing the increase in reactive oxygen species and lipid peroxidation, as well as mitigating alterations in the glutathione antioxidant system nih.gov. The mechanisms underlying this compound's antioxidant activity may include the prevention of P2X7 receptor activation, the protection of mitochondrial membrane potential, and the prevention of disturbances in membrane fluidity eurekaselect.com. Additionally, by increasing acetylcholine levels and potentiating α7 nAChRs, this compound can indirectly reduce the overproduction of reactive oxygen species eurekaselect.com.
Modulation of Apoptotic Pathways
Apoptosis, or programmed cell death, plays a crucial role in the neuronal loss observed in neurodegenerative disorders. This compound has demonstrated the capacity to modulate apoptotic pathways, thereby promoting neuronal survival researchgate.neteuropeanreview.org. Studies have indicated that this compound can protect neurons against apoptosis induced by various cytotoxic insults, including amyloid-beta peptide eurekaselect.comresearchgate.net.
This anti-apoptotic effect is closely linked to the modulation of nicotinic acetylcholine receptors and the subsequent activation of pro-survival signaling pathways. This compound has been shown to increase the expression of the anti-apoptotic protein Bcl-2 in neuronal cells researchgate.net. The allosteric potentiation of α7 nAChRs by this compound is thought to contribute to this effect by triggering intracellular signaling cascades, such as the phosphorylation of serine-threonine protein kinase and the stimulation of phosphoinositide 3-kinase, which ultimately lead to elevated Bcl-2 expression eurekaselect.com. Furthermore, this compound has been reported to inhibit amyloid-beta-induced apoptosis by activating the JNK signaling pathway, which enhances α7 nAChR expression, and by inhibiting the Akt pathway, which promotes autophagy – a cellular process involved in clearing damaged components and protein aggregates nih.gov.
Other Potential Pharmacological Targets of this compound
Beyond its direct effects on acetylcholinesterase and its influence on inflammation, oxidative stress, and apoptosis, this compound interacts with other pharmacological targets, impacting various neurotransmitter systems.
Effects on Cholinergic Neurotransmission Beyond Acetylcholinesterase
While this compound is widely recognized for its competitive and reversible inhibition of acetylcholinesterase, which increases acetylcholine concentrations in the synaptic cleft, its influence on cholinergic neurotransmission extends beyond this mechanism nih.govdrugbank.compatsnap.com. This compound also acts as a positive allosteric modulator of certain subtypes of neuronal nicotinic acetylcholine receptors (nAChRs), notably the α4β2 and α7 subtypes drugbank.comnih.govwikipedia.org. By binding to an allosteric site on these receptors, distinct from the orthosteric site where acetylcholine binds, this compound enhances the receptor's sensitivity to acetylcholine drugbank.compatsnap.comnih.gov. This allosteric modulation facilitates the release of acetylcholine from presynaptic terminals, further augmenting cholinergic signaling nih.gov. This dual mechanism of action, combining AChE inhibition with nAChR modulation, is considered a key aspect of this compound's pharmacological profile and contributes to its clinical significance nih.govnih.gov.
Interactions with Other Neurotransmitter Systems
The allosteric modulation of nicotinic receptors by this compound has implications for the activity of neurotransmitter systems beyond the cholinergic system nih.govresearchgate.net. Nicotinic acetylcholine receptors are strategically located on presynaptic neurons where they regulate the release of various neurotransmitters, including dopamine, glutamate, norepinephrine, and serotonin nih.gov. By modulating the function of these presynaptic nAChRs, this compound can indirectly influence the release and activity of these diverse neurotransmitters nih.govresearchgate.net. For example, studies suggest that this compound can enhance dopaminergic neurotransmission, potentially through the activation of α7 nAChRs researchgate.net. These interactions with other neurotransmitter systems may contribute to the broader cognitive and potentially behavioral effects observed with this compound drugbank.comresearchgate.net.
Pharmacokinetics and Pharmacodynamics of Galanthamine
Absorption and Distribution Profiles
Galanthamine is characterized by rapid and nearly complete absorption following oral administration. fda.govfda.gov
Bioavailability Research and Factors Affecting Absorption
The absolute oral bioavailability of this compound is high, reported to be approximately 90% or ranging from 90% to 100%. nih.govwikipedia.orgfda.govnih.govdrugbank.comnih.govnih.govoup.com Pharmacokinetic studies indicate that galantamine exhibits a dose-linear profile over a range of 8 to 32 mg/day. nih.govfda.govnih.govdrugbank.comeurekaselect.com
Food can influence the rate, but generally not the extent, of galantamine absorption. fda.govhpra.iechula.ac.th While the area under the concentration-time curve (AUC) is typically unaffected by food, the peak plasma concentration (Cmax) may be reduced by approximately 25%, and the time to reach peak concentration (Tmax) can be delayed by about 1.5 hours when galantamine is administered with food. fda.govnih.govwikidoc.org For extended-release formulations, the rate of absorption may increase when administered with high-fat food, although Tmax may not be significantly different. chula.ac.th
Tissue Distribution and Blood-Brain Barrier Permeability Studies
This compound has a relatively large volume of distribution, with reported mean values around 175 L or 193 L. fda.govfda.govnih.govdrugbank.comnih.govnih.goveurekaselect.comwikidoc.org Plasma protein binding of galantamine is low, approximately 18% at therapeutic concentrations or ranging from 28.3% to 33.8%. nih.govfda.govnih.govdrugbank.comnih.govnih.goveurekaselect.comwikidoc.org In whole blood, galantamine is significantly distributed to red blood cells, accounting for about 52.7% of the distribution. fda.govnih.govdrugbank.comwikidoc.org The blood to plasma concentration ratio of galantamine is approximately 1.2. fda.govdrugbank.comwikidoc.org
This compound is known to cross the blood-brain barrier (BBB). nih.govnih.govdrugbank.com Studies, including those in rats, have shown distribution to various tissues, with notable levels observed in the liver, kidney, salivary glands, and adrenal glands. nih.govthieme-connect.com Distribution to the brain is also evident, although some research suggests that only a relatively small portion of the administered drug reaches the brain, as indicated by a brain-to-plasma ratio of approximately 1.3. google.comgoogle.com The distribution of unchanged galantamine to most tissues, particularly the brain, appears more pronounced than that of its metabolites. nih.gov Studies in rats have shown that tissue concentrations of unchanged drug and non-volatile radioactivity decline at a rate similar to plasma, indicating no significant tissue retention. nih.govthieme-connect.com Research also suggests that galantamine may decrease TBI-triggered blood-brain barrier permeability. nih.govnih.gov
| Parameter | Value(s) | Source(s) |
|---|---|---|
| Absolute Oral Bioavailability | ~90%, 90-100% | nih.govwikipedia.orgfda.govnih.govdrugbank.comnih.govnih.govoup.com |
| Tmax (Fasting) | ~1 hour | fda.govnih.govoup.comwikidoc.org |
| Effect of Food on AUC | Not affected | fda.govnih.govwikidoc.org |
| Effect of Food on Cmax | Reduced by ~25% | fda.govnih.govwikidoc.org |
| Effect of Food on Tmax | Delayed by ~1.5 hrs | fda.govnih.govwikidoc.org |
| Mean Volume of Distribution (Vd) | 175 L, 193 L | fda.govfda.govnih.govdrugbank.comnih.govnih.goveurekaselect.comwikidoc.org |
| Plasma Protein Binding | 18%, 28.3-33.8% | nih.govfda.govnih.govdrugbank.comnih.govnih.goveurekaselect.comwikidoc.org |
| Distribution to Red Blood Cells | 52.7% | fda.govnih.govdrugbank.comwikidoc.org |
| Blood to Plasma Ratio | 1.2 | fda.govdrugbank.comwikidoc.org |
| Crosses Blood-Brain Barrier | Yes | nih.govnih.govdrugbank.com |
Metabolism and Biotransformation of this compound
This compound undergoes significant metabolism, primarily in the liver, involving multiple metabolic pathways. nih.govfda.govnih.govhpra.ie Up to 75% of a galantamine dose is eliminated via metabolism. wikipedia.orghpra.ie
Cytochrome P450 Enzyme Involvement in this compound Metabolism
Hepatic cytochrome P450 (CYP) enzymes play a major role in the biotransformation of galantamine. nih.govfda.govnih.govhpra.ie In vitro studies indicate that CYP2D6 and CYP3A4 are the primary isoenzymes involved. nih.govwikipedia.orgphcogcommn.orgfda.govnih.govdrugbank.comnih.govnih.govoup.comhpra.ietg.org.auspringermedicine.com
CYP2D6 is primarily involved in the O-demethylation of galantamine, leading to the formation of O-desmethyl-galantamine. fda.govnih.govdrugbank.comhpra.iewikidoc.org CYP3A4 mediates the formation of galantamine-N-oxide. nih.govfda.govdrugbank.comhpra.iewikidoc.org O-demethylation mediated by CYP2D6 is more prominent in individuals classified as extensive metabolizers of CYP2D6 compared to poor metabolizers. fda.govnih.govwikidoc.org Despite this, unchanged galantamine and its glucuronide metabolite account for the majority of plasma radioactivity in both poor and extensive CYP2D6 metabolizers. fda.govnih.govhpra.iewikidoc.orgresearchgate.net
Inhibitors of CYP2D6 or CYP3A4 can affect galantamine metabolism. Potent inhibitors of these enzymes may increase the AUC of galantamine. fda.gov For instance, co-administration with paroxetine (a strong CYP2D6 inhibitor) or ketoconazole (a strong CYP3A4 inhibitor) has been shown to increase galantamine exposure. nih.govresearchgate.net Specifically, paroxetine increased galantamine bioavailability by 40%, while ketoconazole and erythromycin (another CYP3A4 inhibitor) increased it by 30% and 12%, respectively. wikipedia.orgjddtonline.info Conversely, drugs that induce CYP3A4 or CYP2D6, such as carbamazepine or rifampin, can accelerate galantamine metabolism. nih.govnih.gov
In vitro studies suggest that galantamine has low inhibitory potential towards major cytochrome P450 enzymes, including CYP1A2, CYP2A6, CYP3A4, CYP4A, CYP2C, CYP2D6, and CYP2E1. fda.govnih.gov
Identification and Activity of this compound Metabolites
Several metabolic pathways contribute to galantamine biotransformation, including O-demethylation, N-demethylation, N-oxidation, epimerization, and glucuronidation. nih.govwikipedia.orgfda.govnih.govdrugbank.comhpra.iewikidoc.orgresearchgate.net
Identified metabolites include O-desmethyl-galantamine, galantamine-N-oxide, norgalantamine (N-desmethyl-galantamine), O-desmethyl-norgalantamine, epigalantamine, and galantaminone. nih.govfda.govdrugbank.comwikidoc.org Glucuronidation results in the formation of galantamine glucuronide. nih.govwikipedia.orgfda.govnih.govdrugbank.comhpra.iewikidoc.orgresearchgate.net
While O-desmethyl-galantamine is considered a pharmacologically active metabolite, it has not been detected in unconjugated form in the plasma of either poor or extensive CYP2D6 metabolizers in some studies. researchgate.net Other metabolites, such as norgalantamine, O-desmethyl-norgalantamine, epigalantamine, and galantaminone, are reported not to retain clinically significant pharmacological activities. nih.govdrugbank.com Galantamine glucuronide is also a significant metabolite found in plasma and urine. fda.govnih.govhpra.iewikidoc.orgresearchgate.net
Elimination and Excretion Pathways of this compound
This compound is eliminated through multiple metabolic pathways and renal excretion. fda.govhpra.iewikidoc.orgresearchgate.net No single elimination pathway appears to be predominant. fda.govwikidoc.orgresearchgate.net
Excretion of galantamine and its metabolites occurs primarily via the urine. In a radiolabeled drug study, approximately 95% of the total radioactivity was detected in urine within 7 days, with about 5% recovered in feces. nih.govwikidoc.org
A significant portion of the administered dose is excreted unchanged in the urine. Following oral or intravenous administration, about 20% of the dose is excreted as unchanged galantamine in the urine within 24 hours. fda.govnih.govwikidoc.org Total urinary recovery of unchanged galantamine accounts for, on average, 32% of the dose, while galantamine glucuronide accounts for another 12% on average. wikidoc.org Approximately 20-25% of the total plasma clearance is attributed to renal clearance of unchanged galantamine, which is about 65 mL/min. nih.govfda.govnih.govwikidoc.org The total plasma clearance is approximately 300 mL/min. fda.govnih.govwikidoc.org
The terminal elimination half-life of galantamine is approximately 7 hours or ranges from 7 to 8 hours. wikipedia.orgfda.govnih.govnih.govoup.comeurekaselect.com
Renal function affects galantamine elimination. The elimination of galantamine is reduced in subjects with impaired kidney function. nih.gov Exposures to galantamine are higher in patients with moderate and severe renal impairment compared to healthy subjects. fda.goveurekaselect.comresearchgate.net Hepatic impairment also affects clearance; galantamine clearance was decreased by 60% in patients with moderate or severe hepatic impairment in one study. nih.gov
| Parameter | Value(s) | Source(s) |
|---|---|---|
| Primary Elimination Routes | Metabolism, Renal Excretion | fda.govhpra.iewikidoc.orgresearchgate.net |
| Urinary Excretion (Total Radioactivity) | ~95% (within 7 days) | nih.govwikidoc.org |
| Fecal Excretion (Total Radioactivity) | ~5% (within 7 days) | nih.govwikidoc.org |
| Urinary Excretion (Unchanged Drug) | ~20% (within 24 hrs), ~32% (total) | fda.govnih.govwikidoc.org |
| Urinary Excretion (Glucuronide) | ~12% (total) | wikidoc.org |
| Renal Clearance | ~65 mL/min | nih.govfda.govnih.govwikidoc.org |
| Total Plasma Clearance | ~300 mL/min | fda.govnih.govwikidoc.org |
| Terminal Elimination Half-life | ~7 hours, 7-8 hours | wikipedia.orgfda.govnih.govnih.govoup.comeurekaselect.com |
Pharmacodynamic Markers and Biomarker Research in this compound Studies
Research into the pharmacodynamics of this compound involves the investigation of various markers and biomarkers to understand its effects at a molecular and cellular level, particularly in the context of neurological conditions like Alzheimer's disease (AD). These studies aim to identify measurable indicators that reflect the drug's activity, its impact on disease processes, and potentially predict treatment response.
Acetylcholine Levels and Cholinesterase Activity:
A primary pharmacodynamic effect of this compound is the inhibition of acetylcholinesterase (AChE), the enzyme responsible for breaking down acetylcholine (ACh) in the synaptic cleft. This inhibition leads to increased levels of ACh, enhancing cholinergic neurotransmission. Studies have directly measured AChE levels to confirm this compound's inhibitory action. For instance, preclinical experiments in rabbits demonstrated that this compound treatment significantly reduced brain AChE levels. This reduction in AChE activity correlates with improved learning performance in these animal models.
While galantamine's primary mechanism involves AChE inhibition, its impact on acetylcholine levels in humans is often inferred from clinical outcomes rather than direct measurement, which can be challenging. However, the relationship between AChE inhibition and clinical response is a key area of pharmacodynamic investigation.
Nicotinic Acetylcholine Receptor Modulation:
Beyond AChE inhibition, this compound also acts as a positive allosteric modulator (PAM) of neuronal nicotinic acetylcholine receptors (nAChRs). This allosteric modulation enhances the response of these receptors to acetylcholine, further contributing to increased cholinergic signaling. Different subtypes of nAChRs, such as α4β2 and α7, are implicated in this compound's effects. Studies have investigated the binding of this compound to these receptors and their functional modulation as pharmacodynamic markers. Preclinical research has shown that this compound can increase nicotinic receptor binding, particularly the α4β2 subtype, in the brain. This increase in receptor binding was associated with facilitated learning in animal models.
However, research on this compound's PAM activity on human nAChRs has yielded some conflicting results, with some studies suggesting a lack of functional PAM activity on human α4β2 or α7 receptors. This highlights the complexity of fully characterizing this compound's pharmacodynamic profile across different species and receptor subtypes.
Neuroinflammation Markers:
Emerging research indicates that this compound may exert effects on neuroinflammatory pathways, suggesting potential biomarkers in this domain. Studies in animal models have shown that this compound can decrease markers of neuroinflammation. For example, in mice exposed to lipopolysaccharide (LPS), this compound treatment reduced the expression of microglia and astrocyte markers (CD11b and GFAP) and pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α in the hippocampus. This compound also inhibited the activation of NF-κB p65, a key signaling molecule in inflammatory responses. These findings suggest that modulation of neuroinflammatory markers could serve as pharmacodynamic indicators of this compound's effects, particularly in conditions where neuroinflammation plays a significant role.
Synaptic Plasticity Markers:
Synaptic dysfunction and loss are critical features of neurodegenerative diseases. Biomarkers related to synaptic plasticity and integrity are being investigated to understand this compound's impact on these processes. Studies in LPS-exposed mice showed that this compound treatment ameliorated the loss of synapse-associated proteins, specifically synaptophysin (SYN) and postsynaptic density protein 95 (PSD-95), in the hippocampus. SYN is a presynaptic vesicle protein, while PSD-95 is a major scaffolding protein in the postsynaptic density, and their levels are indicative of synaptic density and function. The preservation of these proteins by this compound suggests a potential beneficial effect on synaptic integrity.
Another potential biomarker in this category is neurogranin (Ng), a protein found in the postsynaptic density that is involved in synaptic plasticity. Changes in CSF neurogranin levels have been explored in the context of dementia and treatment effects. While one study investigating the effects of this compound on CSF biomarkers in patients with dementia did not find a significant impact on markers like neurogranin, research into synaptic biomarkers is ongoing.
Brain-Derived Neurotrophic Factor (BDNF) is a neurotrophic factor that plays a crucial role in neuronal survival, growth, and synaptic plasticity. Reduced levels of BDNF have been implicated in cognitive impairment and neurodegenerative diseases. Some studies in animal models have shown that this compound treatment can lead to increased levels of BDNF in the brain, suggesting a potential role for BDNF as a pharmacodynamic marker reflecting this compound's neurotrophic effects.
Other Potential Biomarkers:
Research also explores other potential biomarkers that might be influenced by this compound, reflecting broader effects on neuronal health and function. These can include markers of oxidative stress and apoptosis. For instance, a study in a rat model of doxorubicin-induced neurotoxicity showed that this compound positively impacted various biological markers, including those related to oxidative stress (malondialdehyde (MDA), superoxide dismutase (SOD)) and apoptosis (Bax, Bcl-2, caspase-3).
Furthermore, neuroimaging techniques such as Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) can provide insights into functional and structural changes in the brain that may serve as pharmacodynamic markers. PET studies have examined the effects of this compound treatment on regional cerebral blood flow (rCBF) and regional cerebral metabolic rate for glucose (rCMRglc) in patients with AD, finding correlations between changes in these measures and cognitive function, as well as AChE activity and nicotinic receptors. MRI has been used in preclinical studies to assess the prevention of structural brain damage by this compound.
Biomarker Research in Clinical Trials:
Biomarker research is increasingly integrated into clinical trials evaluating this compound. Studies aim to identify biomarkers that correlate with clinical outcomes, potentially allowing for the prediction of treatment response or monitoring disease progression. While some studies on AD biomarkers in cerebrospinal fluid (CSF), such as amyloid-β (Aβ), total tau (T-Tau), and phosphorylated tau (P-Tau), have not shown significant changes with this compound treatment, the investigation of various biomarkers in different biological fluids and using imaging techniques continues. The goal is to find reliable markers that can help personalize treatment strategies and better understand the in vivo effects of this compound in patient populations.
Data Tables:
While direct quantitative data from diverse human studies on specific pharmacodynamic markers can be highly variable due to differences in study design, patient populations, and measurement techniques, the following tables summarize the types of markers investigated and representative findings from the search results, primarily from preclinical studies where direct measurement of these markers is more common.
| Marker Category | Specific Markers Investigated | Representative Findings (Preclinical Studies) | Source(s) |
| Cholinesterase Activity | Acetylcholinesterase (AChE) | Reduced brain AChE levels. | |
| Nicotinic Receptors | Nicotinic acetylcholine receptors (nAChRs), α4β2 | Increased nicotinic receptor binding. | |
| Neuroinflammation | CD11b (microglia marker) | Decreased expression in hippocampus. | |
| GFAP (astrocyte marker) | Decreased expression in hippocampus. | ||
| IL-1β (pro-inflammatory cytokine) | Decreased expression in hippocampus. | ||
| IL-6 (pro-inflammatory cytokine) | Decreased expression in hippocampus. | ||
| TNF-α (pro-inflammatory cytokine) | Decreased expression in hippocampus. | ||
| NF-κB p65 (inflammatory signaling) | Inhibited activation/levels in hippocampus and cell lines. | ||
| COX-2 (inflammatory marker) | Positively impacted levels in a neurotoxicity model. | ||
| HMGB1 (neuroinflammatory indicator) | Reduced levels in an animal model. | ||
| Synaptic Plasticity | Synaptophysin (SYN) | Ameliorated loss in hippocampus. | |
| Postsynaptic density protein 95 (PSD-95) | Ameliorated loss in hippocampus. | ||
| Neurogranin (Ng) | Levels explored in CSF in clinical studies (no significant change observed with this compound in one study). | ||
| BDNF (Brain-Derived Neurotrophic Factor) | Increased levels in brain. | ||
| Oxidative Stress | Malondialdehyde (MDA) | Positively impacted levels in a neurotoxicity model. | |
| Superoxide dismutase (SOD) | Positively impacted levels in a neurotoxicity model. | ||
| Apoptosis | Bax | Positively impacted levels in a neurotoxicity model. | |
| Bcl-2 | Positively impacted levels in a neurotoxicity model. | ||
| Caspase-3 | Positively impacted levels in a neurotoxicity model. | ||
| Neuroimaging | Regional Cerebral Blood Flow (rCBF) | Increased in cortical areas, correlated with cognition and AChE activity. | |
| Regional Cerebral Metabolic Rate for Glucose (rCMRglc) | Stabilization in cortical areas, correlated with cognition. |
Detailed Research Findings:
Detailed research findings on this compound's pharmacodynamic markers provide insights into its multifaceted actions. Preclinical studies in rabbits using a classical eyeblink conditioning model, relevant to learning and memory deficits in AD, demonstrated that this compound treatment (3.0 mg/kg) for 15 days significantly improved learning, reduced brain AChE levels, and increased nicotinic receptor binding. Continuous treatment with 3.0 mg/kg this compound for 15 weeks ameliorated learning deficits during acquisition and retention. A statistically significant correlation was found between learning performance and brain AChE levels, with greater inhibition of brain AChE correlating with faster acquisition. Nicotinic receptor binding, specifically α4β2 nAChRs labeled with [³H]epibatidine, was significantly elevated in older rabbits treated with 3.0 mg/kg this compound for 15 days. However, after long-term therapy (15 weeks), tolerance to the nicotinic site up-regulation was observed.
In a study investigating this compound's effects on neuroinflammation and synaptic plasticity in LPS-exposed mice, this compound (4 mg/kg intraperitoneal injection for 14 days) prevented LPS-induced cognitive deficits. This was associated with a reduction in neuroinflammatory markers (CD11b, GFAP, IL-1β, IL-6, TNF-α, NF-κB p65) and an amelioration of the loss of synapse-associated proteins (SYN and PSD-95) in the hippocampus. The study also showed negative correlations between neuroinflammation markers and synaptic proteins, suggesting that this compound's anti-inflammatory effects may contribute to preserving synaptic integrity and improving cognition in this model.
Research in a rat model of doxorubicin-induced neurotoxicity explored the impact of this compound on markers of neuroinflammation, oxidative stress, and apoptosis. Doxorubicin treatment led to increased levels of NF-κB, COX-2, MDA, Bax, and caspase-3, and a decrease in SOD and Bcl-2. Co-administration of this compound was observed to positively impact these markers, suggesting a mitigating effect on doxorubicin-induced neurotoxicity. Specifically, this compound prevented the increase in Bax, Bcl-2, and caspase-3 concentrations induced by doxorubicin treatment alone.
Clinical studies investigating the effect of this compound on CSF biomarkers in patients with dementia have yielded varied results. One study comparing this compound and risperidone treatment found that this compound did not significantly affect the levels of AD biomarkers in CSF, including T-Tau, P-Tau, Aβ1–42, and Aβ40/42-ratio, after 12 weeks of treatment. However, this study also noted correlations between baseline levels of certain CSF biomarkers (low Aβ1–42, low Aβ42/40, and P-Tau) and the reduction of specific neuropsychiatric symptoms.
Neuroimaging studies using PET have provided evidence of this compound's impact on brain function. A study in patients with mild AD treated with this compound (16 to 24 mg/day) for up to 12 months showed significant increases in rCBF in different cortical areas throughout the study. These increases in rCBF positively correlated with AChE activity, nicotinic receptors, and cognitive function. After 12 months, an increase in rCMRglc in the frontal brain region and stabilization in other cortical areas were observed, which also correlated with a stabilization of cognition.
These detailed findings highlight the diverse range of pharmacodynamic markers and biomarkers being investigated to understand how this compound exerts its effects, from direct enzyme inhibition and receptor modulation to broader impacts on neuroinflammation, synaptic plasticity, and neuronal integrity.
| Study Type | Model/Population | Key Pharmacodynamic Markers Measured | Notable Findings | Source(s) |
| Preclinical | Rabbits (classical eyeblink conditioning model) | Brain AChE levels, Nicotinic receptor binding (α4β2) | Reduced brain AChE, increased nicotinic receptor binding, correlated with improved learning. | |
| Preclinical | LPS-exposed mice | CD11b, GFAP, IL-1β, IL-6, TNF-α, NF-κB p65 (neuroinflammation); Synaptophysin (SYN), PSD-95 (synaptic proteins) | Decreased neuroinflammation markers, ameliorated loss of synaptic proteins, correlated with cognitive improvement. | |
| Preclinical | Rat model of doxorubicin-induced neurotoxicity | NF-κB, COX-2 (neuroinflammation); MDA, SOD (oxidative stress); Bax, Bcl-2, caspase-3 (apoptosis) | Positively impacted markers of neuroinflammation, oxidative stress, and apoptosis. | |
| Preclinical | Animal models | BDNF | Increased BDNF levels in the brain. | |
| Clinical | Patients with dementia (CSF study) | T-Tau, P-Tau, Aβ1–42, Aβ40/42-ratio, Neurogranin | No significant effect on these CSF AD biomarkers observed with this compound treatment in one study; correlations between baseline biomarkers and neuropsychiatric symptoms noted. | |
| Clinical | Patients with mild AD (PET study) | Regional Cerebral Blood Flow (rCBF), Regional Cerebral Metabolic Rate for Glucose (rCMRglc), Brain AChE activity, Nicotinic receptors (inferred from correlation) | Increased rCBF and stabilized rCMRglc in cortical areas, correlated with cognition, AChE activity, and nicotinic receptors. |
Therapeutic Efficacy and Clinical Applications of Galanthamine
Galanthamine in Alzheimer's Disease Management
This compound is indicated for the symptomatic treatment of mild to moderate Alzheimer's disease (AD). drugbank.comcochrane.orgwikipedia.org Clinical trials have investigated its effects on various aspects of AD, including cognitive function, behavioral and psychological symptoms, and long-term disease progression.
Cognitive Enhancement in Mild-to-Moderate Alzheimer's Disease
Numerous randomized, double-blind, placebo-controlled trials have demonstrated the efficacy of this compound in improving cognitive function in patients with mild to moderate AD. cochrane.orge-century.usnih.govjacobimed.orgodprn.ca Measures such as the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-cog) have consistently shown statistically significant improvements or a slower rate of cognitive decline in this compound-treated patients compared to those receiving placebo. e-century.usnih.govjacobimed.orgnih.govnih.gov
For example, a meta-analysis of randomized controlled trials indicated that cognitive effects were significantly increased in the this compound group when compared with the placebo group, with a mean difference on the ADAS-cog subscale of -3.15 (95% CI: -3.70 to -2.60, P<0.00001) for a dosage of 24 mg daily. e-century.us Another study reported a mean treatment effect of 3.1 points (95% confidence interval 1.7 to 4.5) for a lower dose and 4.1 points (2.7 to 5.6) for a higher dose on the ADAS-cog/11 at six months, both statistically significant compared to placebo (P<0.001). nih.gov
Interactive Table 1: Change from Baseline in ADAS-cog Scores in this compound Trials
| Study Duration | This compound Group (Mean Change from Baseline ADAS-cog) | Placebo Group (Mean Change from Baseline ADAS-cog) | p-value |
| 6 months | -1.7 (SE 0.4) nih.gov | 1.0 (SE 0.5) nih.gov | <0.0001 nih.gov |
| 26 weeks | -1.8 nih.gov | -0.3 nih.gov | <0.001 nih.gov |
| 6 months | 2.9 points (lower dose) nih.gov | - | <0.001 nih.gov |
| 6 months | 3.1 points (higher dose) nih.gov | - | <0.001 nih.gov |
Note: Negative values typically indicate improvement or less decline on the ADAS-cog scale.
Studies utilizing computerized neuropsychological tests have also shown positive effects of this compound on specific cognitive measures, such as attention and episodic memory, resulting in statistically significant reductions in reaction times after 12 weeks of treatment in patients with mild to moderate AD. scielo.br
Impact on Behavioral and Psychological Symptoms of Dementia
Behavioral and psychological symptoms of dementia (BPSD) are common in AD and contribute significantly to caregiver distress and can influence the decision for institutionalization. doi.orgresearchgate.netnih.govnih.govkarger.com Research has explored the impact of this compound on these symptoms.
Pooled data from multiple studies involving patients with mild to moderate AD have indicated that this compound treatment is associated with a reduction in the emergence of behavioral disturbances and improvement in existing behavioral problems. doi.orgresearchgate.netnih.govpsychiatryonline.org Specific symptoms that showed improvement with this compound in clinical trials include aberrant motor behavior, agitation, anxiety, apathy, and disinhibition. doi.orgpsychiatryonline.org One analysis of pooled data from three trials showed a statistically significant benefit of this compound over placebo on total Neuropsychiatric Inventory (NPI) scores. doi.orgresearchgate.netnih.gov
Interactive Table 2: Effect of this compound on Neuropsychiatric Inventory (NPI) Scores
| Study Duration | Patient Population | This compound Group (Change in NPI Score) | Placebo Group (Change in NPI Score) | p-value |
| 5/6 months | Mild to Moderate AD | Improved (vs. baseline) doi.org | Worsened (vs. baseline) doi.org | 0.013 nih.gov |
| 3 months | Non-institutionalized AD | Significantly reduced disturbances nih.gov | - | <0.05 (caregiver burden) nih.gov |
The reduction in behavioral symptoms observed with this compound therapy has also been associated with a concomitant reduction in reported caregiver distress. researchgate.netnih.govpsychiatryonline.org
Long-term Efficacy and Potential for Disease Progression Modulation
Studies have investigated the long-term effects of this compound treatment in AD. While dementia is a progressive neurodegenerative disease and this compound is not known to alter the underlying disease process, long-term treatment has been associated with attenuated cognitive decline compared to predicted decline in untreated patients. drugbank.commedscape.comnih.govjnj.com
A 2-year randomized, placebo-controlled study in patients with mild to moderate AD reported that long-term treatment with this compound significantly reduced the decline in cognition and activities of daily living. dovepress.com Cognitive impairment, based on the mean change in Mini-Mental State Examination (MMSE) scores from baseline to month 24, significantly worsened in the placebo group (-2.14) compared with the this compound group (-1.41) (P<0.001). dovepress.com Functional impairment, assessed by the Disability Assessment in Dementia (DAD) score, also worsened significantly less in the this compound group (-8.16) versus the placebo group (-10.81) at month 24 (P=0.002). dovepress.com
Interactive Table 3: Long-term Outcomes in a 2-Year this compound Study in AD
| Measure | This compound Group (Mean Change from Baseline at Month 24) | Placebo Group (Mean Change from Baseline at Month 24) | p-value |
| MMSE Score (Cognition) | -1.41 (SD 4.05) dovepress.com | -2.14 (SD 4.34) dovepress.com | <0.001 dovepress.com |
| DAD Score (Activities of Daily Living) | -8.16 (SD 17.25) dovepress.com | -10.81 (SD 18.27) dovepress.com | 0.002 dovepress.com |
Note: For MMSE, a smaller negative change indicates less cognitive decline. For DAD, a smaller negative change indicates less functional decline.
Furthermore, one long-term study suggested a significantly lower mortality rate in patients treated with this compound compared to placebo over two years. jnj.comdovepress.com The mortality rate was 3.2% in the this compound group and 5.5% in the placebo group (P=0.011). jnj.com While this compound is considered a symptomatic treatment, these long-term findings suggest potential benefits beyond just cognitive effects. drugbank.comjnj.com
Investigational Applications of this compound
Beyond its approved use in AD, this compound has been explored in other types of dementia and cognitive impairment.
Vascular Dementia Research
Vascular dementia (VaD) is the second most common type of dementia. nih.govcochrane.orgbmj.com Given the cholinergic deficit observed in VaD, similar to AD, cholinesterase inhibitors like this compound have been investigated for their potential therapeutic effects. nih.govcochrane.orgneurology.org
Clinical trials have evaluated the efficacy of this compound in patients with probable VaD or AD with concomitant cerebrovascular disease. nih.govnih.govcochrane.orgresearchgate.netjwatch.org Some studies have indicated that this compound may be effective in improving cognition, including executive function, in patients with vascular dementia. nih.govresearchgate.net
In a randomized trial involving patients with probable VaD, this compound treatment resulted in a greater improvement in ADAS-cog/11 scores after 26 weeks compared with placebo (-1.8 vs -0.3; p < 0.001). nih.govresearchgate.net A difference favoring this compound was also detected on the EXIT-25, a measure of executive functioning (p = 0.041). nih.govresearchgate.net However, this study did not find a significant difference between this compound and placebo on measures of activities of daily living. nih.govresearchgate.net
A review of two trials in vascular cognitive impairment or VaD found evidence of benefit for this compound over placebo in measures of cognition and global clinical state. cochrane.org Another randomized trial in patients with probable vascular dementia or AD combined with cerebrovascular disease showed that this compound had greater efficacy than placebo on ADAS-cog and Clinician's Interview-Based Impression of Change Plus Caregiver Input (CIBIC-plus). nih.gov Activities of daily living and behavioral symptoms were also significantly improved compared with placebo in this study. nih.gov
Interactive Table 4: Efficacy of this compound in Vascular Dementia/AD with CVD
| Patient Population | Study Duration | Outcome Measure | This compound vs. Placebo Result | p-value |
| Probable VaD | 26 weeks | ADAS-cog/11 | Greater improvement (-1.8 vs -0.3) nih.gov | <0.001 nih.gov |
| Probable VaD | 26 weeks | EXIT-25 | Difference favoring this compound nih.gov | 0.041 nih.gov |
| Probable VaD or AD with CVD | 6 months | ADAS-cog | Greater efficacy (treatment effect 2.7 points) nih.gov | <0.0001 nih.gov |
| Probable VaD or AD with CVD | 6 months | CIBIC-plus | 74% stable or improved vs 59% nih.gov | 0.0001 nih.gov |
| Vascular Cognitive Impairment or VaD | - | Cognition, Global State | Some advantage over placebo cochrane.org | - |
Lewy Body Dementia Research
Dementia with Lewy bodies (DLB) is another common form of dementia characterized by a significant cholinergic deficit. tandfonline.comnih.govnih.gov Cholinesterase inhibitors are often considered for the management of cognitive and neuropsychiatric symptoms in DLB. tandfonline.comnih.gov
Research on this compound specifically in DLB is less extensive compared to AD. A small, open-label study evaluated the efficacy of this compound in 50 patients with DLB over 24 weeks. tandfonline.comnih.govresearchgate.net This study reported beneficial effects on neuropsychiatric symptoms, particularly visual hallucinations and nighttime behaviors, as measured by the Neuropsychiatric Inventory (NPI-12). nih.govresearchgate.net The NPI-12 total score improved by 8.24 points from baseline (p = 0.01). nih.govresearchgate.net Improvements were also observed in the Clinician's Global Impression of Change scores. nih.govresearchgate.net However, this study did not find a significant change in cognitive function as assessed by a computerized cognitive assessment system. nih.govresearchgate.net
While donepezil and rivastigmine have more robust evidence and are recommended as first-line treatments for DLB in some guidelines, open-label trial data for this compound provides preliminary evidence of potential benefits on cognitive fluctuation, sleep disturbances, and psychiatric symptoms in DLB. tandfonline.comnih.gov Further large-scale, controlled studies are needed to definitively establish the role of this compound in the treatment of DLB. tandfonline.comnih.gov
Potential in Other Neurodegenerative Disorders (e.g., Parkinson's Disease Dementia)
This compound, an acetylcholinesterase inhibitor (AChEI) and a positive allosteric modulator of nicotinic acetylcholine receptors (nAChRs), has been investigated for its potential therapeutic effects in neurodegenerative disorders beyond Alzheimer's disease, particularly in Parkinson's disease dementia (PDD) nih.govmdpi.com. PDD is a progressive neurodegenerative condition characterized by cognitive impairment in individuals with Parkinson's disease nih.govresearchgate.net. The rationale for exploring this compound in PDD stems from the significant cholinergic deficit observed in this population, similar to Alzheimer's disease nih.govnih.gov.
Studies have explored this compound's impact on cognitive and neuropsychiatric symptoms in PDD patients. An open-label trial involving participants with PDD demonstrated statistically significant improvements in several cognitive assessments, including the Mini-Mental State Examination (MMSE), the Cognitive Alzheimer's Disease Assessment Scale (ADAS-cog), the clock drawing test, and the Frontal Assessment Battery (FAB) nih.gov. Additionally, this trial indicated benefits in neuropsychiatric symptoms such as hallucinations, anxiety, sleep disturbance, and apathy, as measured by the Neuropsychiatric Inventory (NPI) nih.gov. Another open-label trial in PDD patients also suggested that this compound may be useful, with observed improvements in clock drawing and trends towards improvement in MMSE and verbal fluency capes.gov.brresearchgate.net.
While randomized controlled trials (RCTs) specifically for this compound alone in PD have not consistently shown success, the combination of this compound and memantine has demonstrated synergistic effects on cognitive enhancement in animal models and is considered to have therapeutic potential that warrants further assessment in RCTs nih.gov. This potential combination therapy is thought to target multiple pathways involved in PD pathophysiology, including the kynurenine pathway nih.gov.
The dual mechanism of action of this compound – AChE inhibition and positive allosteric modulation of nAChRs, particularly the α7 and α4β2 subtypes – is considered advantageous nih.govmdpi.com. Stimulation of nicotinic receptors may help counteract the downregulation that can occur with cholinesterase inhibition and facilitate the release of neurotransmitters like dopamine, which is relevant in PD capes.gov.brresearchgate.net.
The International Parkinson and Movement Disorder Society Evidence-Based Medicine Review considers this compound potentially useful for treating PDD nih.gov.
Exploratory Studies in Neurological and Psychiatric Conditions
Beyond its primary indication in Alzheimer's disease and its potential in PDD, this compound has been the subject of exploratory research in a range of other neurological and psychiatric conditions. These studies investigate its potential based on its cholinergic effects and its modulation of nicotinic receptors, which are involved in various neural circuits.
Research suggests potential benefits in cognitive dysfunction associated with bipolar disorder nih.govjst.go.jp. Proof-of-concept studies and case reports have explored this compound's effects on cognitive impairment in this population jst.go.jp.
Exploratory studies have also examined this compound in conditions like chronic post-stroke aphasia, where it has shown beneficial effects nih.gov. Its potential in managing dementia associated with Down syndrome has also been noted nih.gov. Furthermore, this compound has been explored as a potential treatment for cognitive impairment associated with electroconvulsive therapy, both alone and in combination with memantine nih.gov.
The drug's influence on nicotinic receptors and its impact on various neurotransmitter systems like monoamines, glutamate, and GABA are thought to contribute to its potential effects in these diverse neurological and psychiatric conditions jst.go.jp.
Anti-Inflammatory and Immunomodulatory Potentials Beyond Neurodegeneration
Emerging research highlights this compound's anti-inflammatory and immunomodulatory properties, effects that extend beyond its established role in neurodegenerative conditions. These actions are significantly linked to the cholinergic anti-inflammatory pathway (CAP), a physiological mechanism by which the nervous system regulates immune responses plos.orgfrontiersin.orgnih.govnih.gov.
This compound is understood to activate the CAP, primarily through its interaction with α7 nicotinic acetylcholine receptors (α7nAChR) plos.orgfrontiersin.orgnih.govnih.govjddtonline.info. Activation of α7nAChRs on immune cells, such as macrophages, can suppress the release of pro-inflammatory cytokines nih.govnih.gov. Studies have shown that this compound can reduce levels of key inflammatory mediators like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) plos.orgnih.govmedrxiv.orgprobiologists.comnih.gov.
Preclinical studies in various inflammatory models have demonstrated this compound's anti-inflammatory effects. These include murine models of endotoxemia, inflammatory bowel disease (IBD), hemorrhagic shock, lupus, sepsis, and colitis frontiersin.orgmedrxiv.orgprobiologists.comnih.gov. In a mouse model of familial Mediterranean fever (FMF), an autoinflammatory disease, long-term this compound treatment attenuated markers of inflammation and chronic disease complications, potentially by inhibiting macrophage overactivation nih.gov. Research in a tularemia-infected mouse model showed that this compound influenced the immune response by down-regulating IL-6 and up-regulating interferon-gamma (IFN-gamma), leading to mitigation of nephropathy and reduced mortality nih.gov.
Clinical research is also exploring these anti-inflammatory effects. A randomized, double-blind, placebo-controlled trial in patients with metabolic syndrome (MetS) demonstrated that this compound treatment significantly alleviated systemic inflammation and insulin resistance medrxiv.orgnorthwell.edu. The study reported a reduction in pro-inflammatory molecules like TNF and leptin and an increase in the anti-inflammatory molecule adiponectin medrxiv.org. This effect was associated with beneficial alterations in autonomic regulation, as indicated by changes in heart rate variability medrxiv.org.
Furthermore, studies suggest this compound's potential in conditions like acute pancreatitis, where it has shown to attenuate pancreatic histologic injury and modulate inflammatory cytokines in experimental models, although the mechanism may not solely involve previously established CAP components researchgate.net. In collagen-induced arthritis models, this compound has shown a positive impact on joint collagen degradation, suggesting an immunomodulatory activity in this context probiologists.comprobiologists.com.
The anti-inflammatory and immunomodulatory potentials of this compound, mediated significantly through the CAP, indicate a broader therapeutic scope beyond its primary neurological applications.
| Study Type | Condition | Key Finding | Relevant Outcome Measures | Citation |
| Open-label Trial | Parkinson's Disease Dementia | Improved cognitive function and neuropsychiatric symptoms | MMSE, ADAS-cog, clock drawing test, FAB, NPI (hallucinations, anxiety, sleep disturbance, apathy) | nih.gov |
| Open-label Trial | Parkinson's Disease Dementia | Improvement in clock drawing, trends in MMSE and verbal fluency | MMSE, clock drawing test, verbal fluency, NPI | capes.gov.brresearchgate.net |
| Preclinical Study | Animal Models (various) | Alleviated inflammation and metabolic derangements | Levels of inflammatory mediators (e.g., TNF-α, IL-6), insulin resistance, body weight, food intake, adipose depots | frontiersin.orgmedrxiv.org |
| Clinical Trial | Metabolic Syndrome | Alleviated systemic inflammation and insulin resistance | Levels of pro-inflammatory (TNF, leptin) and anti-inflammatory (adiponectin) molecules, insulin levels, insulin resistance (HOMA-IR), heart rate variability | medrxiv.orgnorthwell.edu |
| Preclinical Study | Familial Mediterranean Fever | Attenuated markers of inflammation and chronic disease complications | Inflammatory markers, disease complications | nih.gov |
| Preclinical Study | Tularemia (mouse model) | Modulated immune response, mitigated nephropathy, reduced mortality | IL-6, IFN-gamma, biochemical markers (inorganic phosphate, uric acid), mortality | nih.gov |
| Preclinical Study | Acute Pancreatitis (mice) | Attenuated pancreatic histologic injury, modulated inflammatory cytokines | Pancreatic histology, serum amylase, pancreatic inflammatory cytokines, IL-10 | researchgate.net |
| Preclinical Study | Collagen-Induced Arthritis | Positive impact on joint collagen degradation | Joint collagen content, thickness of collagen fibers | probiologists.comprobiologists.com |
| Clinical Study | Schizophrenia (Adjunctive) | Potential benefit on negative symptoms (alogia, attention) | PANSS total score, alogia, attention subscales | nih.govbrieflands.com |
| Exploratory Study | Chronic Post-Stroke Aphasia | Beneficial effects | Not specified in snippets | nih.gov |
| Exploratory Study | Dementia with Down Syndrome | Potential in management | Not specified in snippets | nih.gov |
| Exploratory Study | Cognitive Impairment (ECT) | Helpful for treatment (alone or with memantine) | Not specified in snippets | nih.gov |
Adverse Event Profile and Safety Considerations of Galanthamine
Characterization of Adverse Drug Reactions (ADRs)
Adverse drug reactions associated with galanthamine primarily affect the gastrointestinal, central nervous system, and cardiovascular systems due to its mechanism of action which increases acetylcholine levels. drugs.comwikipedia.orgdrugbank.comnih.gov
Gastrointestinal System Manifestations
Gastrointestinal adverse events are among the most common reactions reported with this compound. These are largely attributed to the increased cholinergic activity in the digestive tract.
| Adverse Event | Incidence (Very Common: ≥10%, Common: 1% to 10%) | Notes |
| Nausea | Very Common (≥10%) drugs.com | Most frequent, often transient (median duration 5-7 days) medicinenet.comdrugs.com |
| Vomiting | Very Common (≥10%) drugs.com | Common during titration cbg-meb.nl |
| Diarrhea | Common (1% to 10%) drugs.com | |
| Abdominal Pain | Common (1% to 10%) drugs.com | Includes upper abdominal pain drugs.com |
| Dyspepsia | Common (1% to 10%) drugs.com | Indigestion rxlist.com |
| Stomach Discomfort | Common (1% to 10%) drugs.com | Includes abdominal discomfort drugs.com |
| Decreased Appetite | Common (1% to 10%) drugs.com | Can lead to weight loss patsnap.comhres.ca |
| Weight Loss | Common (1% to 10%) nih.gov | Occurs early in treatment, dose-related hres.ca |
| Retching | Uncommon (0.1% to 1%) drugs.com | |
| GI Bleeding | Postmarketing reports drugs.com | Includes upper and lower GI bleeding drugs.com |
| Stomach Ulcers | Increased risk in susceptible individuals healthline.commayoclinic.org | Especially with history of ulcer disease or NSAID use cbg-meb.nlhealthline.commayoclinic.orghres.ca |
Nausea and vomiting are particularly frequent, especially during the dose escalation phase, and often resolve within about a week. medicinenet.comdrugs.comhres.ca While clinical trials have not shown an increased incidence of peptic ulcer disease or gastrointestinal bleeding compared to placebo, patients with a history of these conditions or those taking NSAIDs should be monitored due to the potential for increased gastric acid secretion with increased cholinergic activity. nih.govcbg-meb.nlhealthline.commayoclinic.orghres.cagov.bc.ca
Central Nervous System Effects
This compound's effects on the central nervous system are also related to its cholinergic activity.
| Adverse Event | Incidence (Common: 1% to 10%) | Notes |
| Headache | Common (1% to 10%) drugs.com | One of the most common side effects nih.gov |
| Dizziness | Common (1% to 10%) drugs.com | Particularly noticeable when starting or increasing dose patsnap.com |
| Tremor | Common (1% to 10%) drugs.com | Shaking of a body part medlineplus.gov |
| Somnolence | Common (1% to 10%) drugs.com | Sleepiness rxlist.com |
| Lethargy | Common (1% to 10%) drugs.com | Extreme tiredness medlineplus.gov |
| Insomnia | Common rxlist.com | Difficulty falling asleep or staying asleep medlineplus.gov |
| Fatigue | Common (1% to 10%) drugs.com | Unusual tiredness or weakness drugs.commedlineplus.gov |
| Confusion | Incidence not known drugs.com | Can be a symptom of overdose drugs.commedlineplus.gov |
| Seizures | Postmarketing reports drugs.com | Rare, but reported; patients with a history of seizures may be at increased risk healthline.commayoclinic.orghres.camedlineplus.gov |
| Hallucinations | Postmarketing reports rxlist.com | Reported, including in a patient with a history of hallucinations drugbank.com |
| Depression | Common medlineplus.gov | Feeling sad or empty, loss of interest or pleasure drugs.com |
| Paresthesia | Frequency not reported drugs.com | Abnormal skin sensations medicinenet.com |
| Dysgeusia | Frequency not reported drugs.com | Unusual or unpleasant taste medicinenet.comrxlist.com |
| Hypersomnia | Frequency not reported drugs.com | Excessive sleepiness medicinenet.com |
| Ataxia | Potential overdose symptom wikipedia.org | Loss of coordination wikipedia.org |
| Restlessness | Potential overdose symptom wikipedia.org | wikipedia.org |
| Cardiorespiratory Paralysis | Potential overdose symptom wikipedia.org | wikipedia.org |
| Coma | Potential overdose symptom wikipedia.org | wikipedia.org |
Headache and dizziness are frequently reported. nih.gov While seizures are uncommon, they have been reported, and individuals with a history of seizures may be more susceptible. healthline.commayoclinic.orghres.camedlineplus.gov Hallucinations have also been observed. drugbank.comrxlist.com
Cardiovascular System Effects
This compound can exert vagotonic effects on the cardiac conduction system, potentially leading to cardiovascular adverse events. nih.gov
| Adverse Event | Incidence (Common: 1% to 10%, Uncommon: 0.1% to 1%) | Notes |
| Bradycardia | Common (1% to 10%) drugs.com | Slow heart rate; more common in patients with heart problems healthline.commayoclinic.orgmedlineplus.govnih.gov |
| Syncope | Common (1% to 10%) drugs.com | Fainting; increased risk with higher doses and in patients with heart problems nih.govhres.cahealthline.commayoclinic.orgnih.gov |
| Hypertension | Frequent hres.ca | High blood pressure; reported in postmarketing experience rxlist.com |
| Hypotension | Frequency not reported drugs.com | Low blood pressure; postural hypotension also reported hres.ca |
| First Degree AV Block | Frequency not reported drugs.com | Atrioventricular block medicinenet.comrxlist.com |
| Complete AV Block | Postmarketing reports drugs.com | Complete atrioventricular block rxlist.com |
| Palpitations | Frequency not reported drugs.com | Awareness of heartbeat medicinenet.com |
| Sinus Bradycardia | Frequency not reported drugs.com | Slow heart rhythm originating in the sinus node medicinenet.comdrugs.com |
| Supraventricular Extrasystoles | Frequency not reported drugs.com | Extra heartbeats originating above the ventricles medicinenet.comdrugs.com |
| Flushing | Frequency not reported drugs.com | Redness of the face, neck, arms, and upper chest drugs.com |
| Chest Pain | Common unboundmedicine.com | Can be a serious adverse effect wikipedia.org |
| QT Prolongation | Potential risk nih.gov | May occasionally be associated with QT prolongation and torsades de pointes ventricular tachycardia nih.gov |
Bradycardia (slow heart rate) and syncope (fainting) are commonly reported cardiovascular effects, with an increased risk in individuals with pre-existing cardiac issues. healthline.commayoclinic.orgnih.gov While less common than gastrointestinal effects, these cardiovascular events are important considerations, particularly in susceptible patients. nih.gov Heart block and pauses in heart rate have also been observed, especially with faster dose escalation. hres.cahres.ca
Risk Factors for Adverse Events
Several factors can influence an individual's risk of experiencing adverse events while taking this compound, including genetic makeup, metabolic status, existing medical conditions, and concurrent medication use.
Genetic Polymorphisms and Metabolic Status
This compound is primarily metabolized in the liver by cytochrome P450 enzymes, specifically CYP2D6 and CYP3A4. nih.govwikipedia.orgdrugbank.comunboundmedicine.comdrugsporphyria.net Genetic variations (polymorphisms) in the genes encoding these enzymes can affect how efficiently this compound is metabolized, leading to variations in drug concentrations and potentially influencing the risk of adverse effects.
Approximately 75% of this compound is metabolized by CYP2D6 and CYP3A4. nih.govdrugbank.com Individuals who are poor metabolizers due to CYP2D6 genetic polymorphisms may have significantly higher this compound concentrations, increasing the risk of adverse effects. medlink.comunboundmedicine.com Studies have indicated that carrying certain detrimental allelic variants of CYP2D6, CYP3A5, and UGT1A1 may be associated with higher this compound plasma concentrations. nih.gov
Comorbidities and Concomitant Medications
The presence of certain comorbidities and the use of concomitant medications can also increase the risk or severity of this compound-associated adverse events.
Patients with a history of stomach problems, ulcers, or bleeding are at increased risk of gastrointestinal complications, including stomach ulcers and bleeding, as this compound can increase stomach acid. healthline.commayoclinic.org Concurrent use of non-steroidal anti-inflammatory drugs (NSAIDs) further elevates this risk. cbg-meb.nlhealthline.commayoclinic.orghres.ca
Individuals with heart problems, particularly those with a history of slow or irregular heart rate, are at a higher risk of experiencing bradycardia and syncope. healthline.commayoclinic.org this compound should be used with caution in patients with supraventricular cardiac conduction defects or those taking medications that slow heart rate, such as digoxin and beta-blockers. cbg-meb.nlunboundmedicine.com
Patients with a history of asthma or other lung diseases may experience a worsening of their condition with this compound use. healthline.commayoclinic.org
Renal and hepatic impairment can affect the clearance of this compound, potentially leading to increased drug levels and a higher risk of adverse effects. mayoclinic.orgunboundmedicine.comgmmmg.nhs.uk Severe hepatic or renal impairment is a contraindication for this compound use. mayoclinic.orgunboundmedicine.com
Concomitant use of medications that inhibit CYP2D6 or CYP3A4 can decrease this compound metabolism and increase its concentration, potentially leading to increased cholinergic adverse effects, predominantly nausea and vomiting. wikipedia.orggov.bc.cagmmmg.nhs.uk Examples of such inhibitors include paroxetine (CYP2D6) and ketoconazole and erythromycin (CYP3A4). wikipedia.org Conversely, inducers of CYP2D6 and CYP3A4 may decrease this compound levels. gov.bc.ca
This compound can also interact with other medications affecting the cholinergic system, potentially increasing cholinergic effects when combined with other cholinergic agonists or decreasing the effectiveness of anticholinergic medications. medlink.comdrugbank.comunboundmedicine.commedscape.commedindia.net Caution is also advised when this compound is used with certain anesthetic agents, as it may prolong the effects of succinylcholine-type neuromuscular blocking agents and potentially antagonize the effects of non-depolarizing neuromuscular blocking drugs. nih.govunboundmedicine.comgmmmg.nhs.uk
| Comorbidity/Factor | Associated Risk | Notes |
| History of Ulcer Disease/GI Bleeding | Increased risk of stomach ulcers and bleeding cbg-meb.nlhealthline.commayoclinic.orghres.ca | Monitor closely, especially with concurrent NSAID use cbg-meb.nlhealthline.commayoclinic.orghres.ca |
| Concurrent NSAID Use | Increased risk of stomach ulcers and bleeding cbg-meb.nlhealthline.commayoclinic.orghres.ca | |
| Heart Problems (slow/irregular heart rate, conduction defects) | Increased risk of bradycardia and syncope cbg-meb.nlhealthline.commayoclinic.orgunboundmedicine.com | Caution with concurrent heart rate-slowing medications cbg-meb.nlunboundmedicine.com |
| Asthma or other Lung Diseases | Potential worsening of condition healthline.commayoclinic.org | Use with caution healthline.commayoclinic.org |
| Severe Renal Impairment | Increased this compound levels, increased risk of adverse effects mayoclinic.orgunboundmedicine.comgmmmg.nhs.uk | Contraindicated mayoclinic.orgunboundmedicine.com |
| Severe Hepatic Impairment | Increased this compound levels, increased risk of adverse effects mayoclinic.orgunboundmedicine.comgmmmg.nhs.uk | Contraindicated mayoclinic.orgunboundmedicine.com |
| Mild to Moderate Renal/Hepatic Impairment | Potential for increased this compound levels mayoclinic.orgunboundmedicine.comgmmmg.nhs.uk | Cautious dose titration recommended unboundmedicine.comgmmmg.nhs.uk |
| History of Seizures | May be at increased risk of seizures healthline.commayoclinic.orghres.ca | Use with caution healthline.commayoclinic.org |
| Concurrent CYP2D6 or CYP3A4 Inhibitors | Increased this compound levels, increased cholinergic adverse effects wikipedia.orggov.bc.cagmmmg.nhs.uk | Consider dose reduction based on tolerability gmmmg.nhs.uk |
| Concurrent Cholinergic Agonists | Increased cholinergic effects medlink.comdrugbank.comunboundmedicine.commedscape.commedindia.net | Use with caution medscape.com |
| Concurrent Anticholinergic Medications | Decreased effectiveness of anticholinergic medications medlink.comunboundmedicine.com | Effect of interaction may be unclear, use caution medscape.com |
| Concurrent Neuromuscular Blocking Agents (Succinylcholine-type) | Prolonged neuromuscular blockade nih.govunboundmedicine.comgmmmg.nhs.uk | Inform healthcare provider before surgery/procedures healthline.com |
| Concurrent Neuromuscular Blocking Agents (Non-depolarizing) | Potential antagonism of effects unboundmedicine.comgmmmg.nhs.uk | Higher doses may be required for paralysis; neostigmine reversal may be ineffective gmmmg.nhs.uk |
| Genetic Polymorphisms (CYP2D6, CYP3A4, CYP3A5, UGT1A1) | Can affect this compound metabolism and plasma concentrations medlink.comunboundmedicine.comnih.gov | May influence therapeutic outcome and risk of adverse effects medlink.comnih.gov |
| Female Sex | May be more sensitive to cholinergic adverse effects (nausea, vomiting) hres.ca | |
| Elderly Patients (>85 years old) with Low Body Weight | Dose escalation should be undertaken with particular caution hres.ca | Especially females hres.ca |
Management Strategies for Adverse Events
Management strategies for adverse events associated with this compound primarily focus on mitigating cholinergic effects, particularly those affecting the gastrointestinal system. Common adverse effects reported in clinical trials and post-marketing surveillance include nausea, vomiting, diarrhea, decreased appetite, and weight decrease. nih.govfda.gov These often occur during the upward titration of dosages. nih.gov
Several strategies can be employed to manage these gastrointestinal issues:
Administration with Food: Taking this compound with food, preferably a full meal, can help reduce gastrointestinal upset. goodrx.commedlineplus.govnih.gov
Adequate Fluid Intake: Ensuring adequate fluid intake is important for all patients taking this compound, and it can help manage diarrhea and prevent dehydration. nih.govgoodrx.comnih.gov Dehydration, including rare severe cases leading to renal insufficiency and failure, has been reported in postmarketing experience. nih.gov
Dietary Modifications: Avoiding spicy and high-fat foods may make this compound easier on the stomach. goodrx.com Eating a bland diet for short periods may also help manage diarrhea. goodrx.com
Smaller, More Frequent Meals: This approach can be beneficial while the body is adjusting to the medication. goodrx.com
Antiemetic Agents: The use of antiemetic agents may help reduce the impact of nausea and vomiting. nih.gov
Over-the-Counter Diarrhea Medications: Medications such as loperamide may be used for short-term management of diarrhea, but it is advisable to consult a pharmacist. goodrx.com
If gastrointestinal issues persist or are severe despite these measures, healthcare providers may consider a lower dose or a slower dose increase. goodrx.com In some cases, switching to a different medication might be recommended. goodrx.com
Other potential adverse effects, such as dizziness, may become less noticeable as the patient adjusts to the medication. goodrx.com Caution is advised when first starting this compound or after a dose increase, particularly when getting up from a seated position, to reduce the risk of falls, which is already higher in people living with dementia. goodrx.com Discussing fall prevention measures with a healthcare provider is recommended. goodrx.com
Bradycardia and heart block have been reported, and all patients should be considered at risk for adverse effects on cardiac conduction. nih.gov While bradycardia was more frequent in this compound-treated patients in randomized controlled trials, it was rarely severe or led to discontinuation. nih.gov
In cases of suspected overdose, which can present as a cholinergic crisis with symptoms like salivation, severe nausea, and muscle weakness, consulting a poison control center for the latest recommendations is crucial as management strategies are continually advancing. nih.govnih.gov
Long-term Safety Surveillance and Pharmacovigilance Data for this compound
Long-term safety surveillance and pharmacovigilance are essential for monitoring the safety profile of this compound once it is available for wider use in the general population. Post-marketing surveillance (Phase 4 clinical trials) focuses on collecting real-world data that may reveal long-term effects or rare adverse drug reactions not apparent in pre-approval studies. lindushealth.comfda.gov
Pharmacovigilance activities for this compound involve monitoring its safety and effectiveness in diverse patient populations under varying clinical conditions. This includes identifying any long-term effects, detecting rare adverse drug reactions, and ensuring that the benefits of the product continue to outweigh the risks. lindushealth.comfgk-cro.com Regulatory agencies like the FDA and EMA require post-marketing surveillance as part of the approval process, mandating regular reports on the product's safety and efficacy. fda.govfgk-cro.com
Data from long-term studies and post-marketing surveillance contribute to a comprehensive understanding of this compound's safety profile over extended treatment periods. For example, a 72-week post-marketing surveillance study aimed at evaluating the long-term efficacy and safety of this compound in routine clinical practice reported adverse events in 28.5% of patients, with 8.41% being serious. researchgate.net These reported events generally aligned with the safety profile observed in previous studies. researchgate.net
Another long-term, randomized, placebo-controlled study over two years assessed patient survival and drug efficacy. dovepress.com While patients treated with this compound reported numerically more treatment-emergent adverse events (TEAEs), primarily gastrointestinal disorders, the incidence and types of serious adverse events were similar between the this compound and placebo groups, with the exception of mortality. jnj.com Interestingly, this study indicated a statistically significantly lower mortality rate in patients treated with this compound compared to the placebo group. dovepress.comjnj.com
Postmarketing surveillance of marketed anticholinesterase inhibitors, including this compound, have shown reports of bradycardia and various types of heart block in patients with and without known underlying cardiac conduction abnormalities. nih.gov
Pharmacovigilance relies on various methods, including spontaneous reporting by healthcare professionals and consumers, registry studies, observational studies, and analysis of electronic health records. fgk-cro.com Systems like the FDA Adverse Event Reporting System (FAERS) are crucial databases for supporting post-marketing safety surveillance by collecting and analyzing safety reports to detect signals and monitor drug safety. fda.gov This ongoing monitoring can lead to regulatory actions such as updating product labeling or re-evaluating approval decisions if necessary. fda.gov
Data from clinical trials indicate that discontinuations due to adverse events are more likely with this compound compared to placebo, particularly at higher doses. fda.govnih.gov The most common adverse reactions leading to discontinuation in double-blind clinical trials included nausea, vomiting, decreased appetite, and dizziness. fda.gov
Long-term studies and pharmacovigilance data are vital for confirming the safety profile established in initial clinical trials and identifying any potential long-term or rare risks associated with this compound use in a broader patient population.
Data Table: Incidence of Common Adverse Events Leading to Discontinuation in this compound Clinical Trials
| Adverse Event | This compound-Treated Patients (N=2932) | Placebo Patients (N=1525) |
| Nausea | 7.7% | 0.9% |
| Vomiting | 4.1% | 1.6% |
| Decreased Appetite | 1.9% | 0.9% |
| Dizziness | 1.6% | 0.5% |
| Diarrhea | 0.9% | <0.5% |
| Headache | 0.9% | <0.5% |
| Decreased Weight | 0.8% | <0.5% |
| Abdominal Pain | 0.5% | <0.5% |
Source: Pooled data from 7 placebo-controlled studies. fda.gov
Data Table: Mortality in a 2-Year Randomized, Placebo-Controlled Study
| Group | Number of Deaths | Percentage of Deaths | P-value |
| This compound | 33 | 3.2% | 0.011 |
| Placebo | 56 | 5.5% |
Source: Final analysis of a 2-year study. jnj.com
Drug-drug Interactions Involving Galanthamine
Pharmacokinetic Interactions
Pharmacokinetic interactions involve alterations in the absorption, distribution, metabolism, or excretion of galanthamine due to the presence of other medications.
Interactions with CYP450 Enzyme Inhibitors and Inducers
This compound is primarily metabolized in the liver, with hepatic cytochrome P450 (CYP) enzymes, specifically CYP2D6 and CYP3A4, playing significant roles in its metabolic pathways. CYP2D6 is involved in the O-demethylation of this compound to form O-desmethyl-galanthamine, while CYP3A4 mediates the formation of this compound-N-oxide. drugbank.comnih.govfda.gov Approximately 75% of the drug is metabolized by these two enzymes. drugbank.com
Inhibitors of CYP2D6 and CYP3A4 can decrease the metabolism of this compound, leading to increased plasma concentrations and potentially enhanced cholinergic effects. nih.govfda.govwikipedia.orgjddtonline.infotaylorandfrancis.comresearchgate.net Studies have shown that potent inhibitors of these enzymes can increase the bioavailability of this compound. For instance, paroxetine, a strong CYP2D6 inhibitor, has been shown to increase this compound bioavailability by 40%. wikipedia.orgjddtonline.infocbg-meb.nlgmmmg.nhs.uk Similarly, the CYP3A4 inhibitors ketoconazole and erythromycin have been reported to increase this compound bioavailability by 30% and 12%, respectively. fda.govwikipedia.orgjddtonline.infocbg-meb.nl
Conversely, drugs that induce CYP3A4 or CYP2D6 enzymes, such as carbamazepine, phenytoin, phenobarbital, rifampin, or dexamethasone, can accelerate the metabolism of this compound, potentially leading to decreased plasma concentrations and reduced efficacy. nih.govnih.gov
The following table summarizes the observed effects of some CYP enzyme inhibitors on this compound bioavailability:
| Interacting Drug | CYP Enzyme Inhibition | Effect on this compound Bioavailability | Source |
|---|---|---|---|
| Paroxetine | Potent CYP2D6 | Increased by ~40% | wikipedia.orgjddtonline.infocbg-meb.nlgmmmg.nhs.uk |
| Ketoconazole | Strong CYP3A4, also inhibits CYP2D6 | Increased by ~30% | fda.govwikipedia.orgjddtonline.infocbg-meb.nl |
| Erythromycin | CYP3A4 | Increased by ~10-12% | fda.govwikipedia.orgjddtonline.infocbg-meb.nl |
| Cimetidine | CYP3A4 | Increased by ~16% | hres.ca |
Note: This table presents data from specific studies and the magnitude of interaction may vary depending on individual patient factors and study design.
Genetic variations in CYP2D6 activity can also influence this compound pharmacokinetics. Individuals classified as poor metabolizers of CYP2D6 may exhibit higher plasma concentrations of this compound compared to extensive metabolizers. nih.govfda.govhres.ca
Protein Binding Displacement Interactions
This compound exhibits relatively low plasma protein binding, reported to be around 18% at therapeutically relevant concentrations or between 28.3% and 33.8%. drugbank.comwikipedia.orgjddtonline.infonih.gov Due to this low protein binding, displacement interactions with other highly protein-bound drugs are generally not considered clinically significant. nice.org.uk While displacement from protein binding can theoretically increase the free fraction of a drug, this typically only leads to a detectable increase in effect for extensively bound drugs (>90%) that are not widely distributed in the body. nice.org.uk
Pharmacodynamic Interactions
Pharmacodynamic interactions occur when drugs with similar or opposing pharmacological effects are co-administered, leading to additive or antagonistic outcomes on the body's systems.
Interactions with Cholinergic Agents
As a cholinesterase inhibitor, this compound increases the availability of acetylcholine in the synaptic cleft. wikipedia.orgnih.govmedlink.com Therefore, co-administration with other cholinergic agents or cholinesterase inhibitors can result in synergistic effects, potentially increasing the risk of cholinergic overstimulation. nih.govnih.govhres.cadrugs.com This includes other acetylcholinesterase inhibitors (such as donepezil, rivastigmine, neostigmine, pyridostigmine, ambenonium) and cholinergic agonists (such as bethanechol or systemically administered pilocarpine). nih.govcbg-meb.nlnih.govhres.cadrugs.com
A notable interaction exists with succinylcholine and similar neuromuscular blocking agents. This compound is likely to exaggerate the muscle relaxation effects of succinylcholine-type agents during anesthesia due to its inhibition of cholinesterase, which is responsible for the breakdown of succinylcholine. nih.govjddtonline.inforesearchgate.netcbg-meb.nlgmmmg.nhs.ukdrugs.com
| Interacting Drug Class/Agent | Mechanism of Interaction | Potential Outcome | Source |
|---|---|---|---|
| Other Cholinesterase Inhibitors | Additive increase in acetylcholine | Increased cholinergic effects, potential overstimulation | nih.govcbg-meb.nlnih.govhres.cadrugs.com |
| Cholinergic Agonists | Additive cholinergic effects | Increased cholinergic effects, potential overstimulation | nih.govcbg-meb.nlnih.govhres.cadrugs.com |
| Succinylcholine | Inhibition of breakdown by cholinesterase | Exaggerated and prolonged muscle relaxation | nih.govjddtonline.inforesearchgate.netcbg-meb.nlgmmmg.nhs.ukdrugs.com |
Interactions with Anticholinergic Agents
Anticholinergic medications have effects opposite to those of cholinergic agents by blocking the action of acetylcholine. drugs.com Therefore, co-administration of this compound with anticholinergic drugs can lead to a pharmacodynamic antagonism, where the effects of both medications may be reduced. nih.govcbg-meb.nlhres.cadrugs.commedscape.com this compound has the potential to interfere with the activity of anticholinergic medications. hres.cadrugs.com If anticholinergic medication is abruptly stopped, there is a potential risk that this compound's effect could be exacerbated. cbg-meb.nl This interaction is particularly relevant as some medications, including certain antipsychotics (e.g., clozapine, olanzapine), possess anticholinergic properties that could counteract this compound's beneficial effects. droracle.ai
Interactions with Cardiovascular Medications
This compound can have vagotonic effects on the sinoatrial and atrioventricular nodes, potentially leading to bradycardia or heart block. nih.govdrugs.comdrugs.com Consequently, a pharmacodynamic interaction is possible when this compound is administered concurrently with other medications that significantly reduce heart rate. jddtonline.inforesearchgate.netcbg-meb.nlmedlink.com This includes agents such as digoxin, beta-blockers (e.g., acebutolol, carvedilol, labetalol, metoprolol, nadolol, nebivolol, pindolol, propranolol, timolol), and certain calcium-channel blocking agents (e.g., diltiazem, verapamil), and amiodarone. nih.govcbg-meb.nlnice.org.ukmedlink.com While a study showed that multiple doses of this compound did not affect the pharmacokinetics of digoxin or warfarin, pharmacodynamic interactions, such as heart block, have been reported with digoxin co-administration. nih.govjddtonline.infocbg-meb.nl Caution should also be exercised with medicinal products that have the potential to cause torsades de pointes. cbg-meb.nl
| Interacting Drug Class/Agent | Potential Pharmacodynamic Effect with this compound | Potential Outcome | Source |
|---|---|---|---|
| Digoxin | Bradycardia, vagotonic effects | Increased risk of bradycardia, heart block | nih.govjddtonline.inforesearchgate.netcbg-meb.nlmedlink.com |
| Beta-blockers | Bradycardia | Increased risk of bradycardia, AV block | nih.govcbg-meb.nlnice.org.ukmedlink.com |
| Calcium Channel Blockers (certain) | Reduced heart rate | Increased risk of bradycardia, AV block | cbg-meb.nlnice.org.uk |
| Amiodarone | Reduced heart rate, potential for torsades de pointes | Increased risk of bradycardia, potential for arrhythmias | cbg-meb.nl |
Interactions with Centrally Acting Medications
This compound, a centrally acting acetylcholinesterase inhibitor and positive allosteric modulator of neuronal nicotinic acetylcholine receptors, can interact with various centrally acting medications. These interactions can be pharmacodynamic, affecting the drugs' actions, or pharmacokinetic, influencing their metabolism and concentration in the body.
Anticholinergic Medications: this compound has the potential to interfere with the activity of anticholinergic medications. drugs.comnih.gov This is due to its mechanism of increasing cholinergic tone, which is functionally opposite to the effects of anticholinergic drugs. drugs.comnih.gov
Cholinomimetics and Other Cholinesterase Inhibitors: A synergistic effect is anticipated when this compound is administered concurrently with other cholinesterase inhibitors or cholinergic agonists, such as bethanechol or succinylcholine. drugs.comnih.gov This synergy arises from their shared mechanism of enhancing cholinergic neurotransmission. drugs.comnih.gov Galantamine is likely to exaggerate the muscle relaxation effects of succinylcholine-type and similar neuromuscular blocking agents, particularly in individuals with pseudocholinesterase deficiency. nih.govcbg-meb.nl
Antipsychotics: Caution is advised when combining this compound with antipsychotic medications, and such combinations should be closely medically supervised. droracle.ai The interaction can potentially increase the risk of extrapyramidal symptoms (movement disorders) with certain antipsychotics. droracle.aifrontiersin.org Some antipsychotics possessing strong anticholinergic properties, such as clozapine or olanzapine, might counteract the beneficial effects of this compound, potentially reducing its effectiveness. droracle.ai Research in animal models suggests that cholinesterase inhibitors like this compound can potentiate antipsychotic-induced extrapyramidal symptoms in a synergistic manner. frontiersin.orgresearchgate.net Specifically, studies in mice have shown that while this compound alone rarely induces extrapyramidal signs, it markedly potentiated bradykinesia induced by low-dose haloperidol in a dose-dependent and synergistic fashion. frontiersin.orgresearchgate.net Despite these concerns, some findings suggest that the combination of this compound and antipsychotics may be effective for cognitive impairments associated with schizophrenia. droracle.ai For example, a study in a mouse model of schizophrenia found that co-administration of this compound and risperidone showed synergistic effects on impairments of social interaction and dopamine release in the medial prefrontal cortex. nih.gov
Opioids: Preliminary research suggests that this compound may hold promise as a treatment for opioid addiction. medscape.comnews-medical.net A secondary analysis of a randomized controlled trial in methadone-maintained individuals with cocaine use disorder found that this compound treatment was associated with fewer opioid-positive urine samples compared to placebo. medscape.comnih.govpharmaceutical-journal.comnih.gov This effect was observed both during the treatment period and the subsequent 6-month follow-up. medscape.compharmaceutical-journal.comnih.gov Participants receiving this compound also reported significantly more days of abstinence from illicit opioids and had a longer time to first opioid use. pharmaceutical-journal.comnih.gov These findings suggest a potential "anti-addictive" effect of this compound, possibly mediated through its dual mechanism of increasing acetylcholine levels and binding to nicotinic receptors, which are involved in addiction. medscape.comnews-medical.net
Benzodiazepines: No interactions were found between this compound and alprazolam (a benzodiazepine) in one drug interaction checker. drugs.com However, this does not definitively rule out all potential interactions with other benzodiazepines. Benzodiazepines primarily exert their effects on the central nervous system by binding to GABA type A receptors. jiaci.org Some benzodiazepines are metabolized by cytochrome P450 enzymes, particularly CYP3A4 and CYP2C19. jiaci.org this compound is metabolized by CYP2D6 and CYP3A4, and inhibitors of these enzymes can increase this compound's bioavailability and potentially enhance cholinergic effects. nih.govnih.govcbg-meb.nljddtonline.infowikipedia.orgdrugs.com While some studies have examined the use of psychotropic drugs, including benzodiazepines, in Alzheimer's patients treated with cholinesterase inhibitors, they have primarily focused on the association with cognitive performance and mortality risk rather than specific pharmacokinetic or pharmacodynamic interactions with this compound itself. nih.govresearchgate.net
Data Table: Summary of Potential Interactions with Centrally Acting Medications
| Medication Class | Potential Interaction | Mechanism | Research Findings |
| Anticholinergics | Interference with activity | Opposing effects on cholinergic tone. drugs.comnih.gov | This compound can interfere with anticholinergic medication activity. drugs.comnih.gov |
| Cholinomimetics/Other ChEIs | Synergistic effects | Shared mechanism of enhancing cholinergic neurotransmission. drugs.comnih.gov Exaggeration of muscle relaxation with neuromuscular blocking agents. | Expected synergy when given concurrently. drugs.comnih.gov Likely to exaggerate succinylcholine-type muscle relaxation. nih.govcbg-meb.nl |
| Antidepressants | Mixed findings on cognitive/mood effects when used adjunctively; potential improvement in depressive symptoms in AD. | Potential influence on cholinergic and other neurotransmitter systems. researchgate.netfrontiersin.org | Pilot study in older adults with depression showed no significant benefit on mood or cognition with this compound augmentation of venlafaxine XR or citalopram over 24 weeks. nih.gov Some studies in AD patients suggest improvement in depressive symptoms with chronic ChEI use. frontiersin.org |
| Antipsychotics | Increased risk of extrapyramidal symptoms; potential reduced this compound effectiveness (with anticholinergic types). | Potentiation of antipsychotic-induced EPS; anticholinergic properties of some antipsychotics. droracle.aifrontiersin.orgresearchgate.net | Caution advised due to potential for increased EPS risk, particularly with certain antipsychotics. droracle.aifrontiersin.org Animal studies show synergistic potentiation of haloperidol-induced bradykinesia. frontiersin.orgresearchgate.net Some findings suggest potential efficacy for cognitive impairments in schizophrenia. droracle.ai Synergistic effects with risperidone on social interaction and dopamine release observed in mouse model. nih.gov |
| Opioids | Potential reduction in illicit opioid use. | Dual mechanism: increased acetylcholine and binding to nicotinic receptors involved in addiction. medscape.comnews-medical.net | Secondary analysis of a trial in methadone-maintained individuals showed fewer opioid-positive urine samples and increased abstinence days with this compound compared to placebo. medscape.comnih.govpharmaceutical-journal.comnih.gov Longer time to first opioid use observed. pharmaceutical-journal.comnih.gov |
| Benzodiazepines | No interaction found with alprazolam in one checker; potential for pharmacokinetic interactions with some benzodiazepines. | Metabolism via CYP enzymes (this compound by CYP2D6/3A4; some benzodiazepines by CYP3A4/2C19). nih.govnih.govcbg-meb.nldrugs.comjiaci.orgjddtonline.infowikipedia.orgdrugs.com | No interaction found between this compound and alprazolam in one checker. drugs.com this compound metabolism can be affected by CYP inhibitors. nih.govnih.govcbg-meb.nljddtonline.infowikipedia.orgdrugs.com Research on co-use in AD patients has focused on cognitive outcomes and mortality rather than specific interaction mechanisms with this compound. nih.govresearchgate.net |
Detailed Research Findings:
Research into the interactions between this compound and centrally acting medications highlights both pharmacokinetic and pharmacodynamic considerations. Pharmacokinetically, this compound is metabolized by cytochrome P450 enzymes, primarily CYP2D6 and CYP3A4. nih.govnih.govcbg-meb.nljddtonline.infowikipedia.orgdrugs.comresearchgate.net Inhibitors of these enzymes can increase the systemic exposure and bioavailability of this compound. For instance, paroxetine, a strong CYP2D6 inhibitor, has been shown to increase this compound's oral bioavailability by approximately 40%. drugs.comnih.govcbg-meb.nljddtonline.infowikipedia.org Similarly, CYP3A4 inhibitors like ketoconazole and erythromycin can increase this compound bioavailability by about 30% and 12%, respectively. drugs.comnih.govcbg-meb.nljddtonline.infowikipedia.org Concurrent administration with potent inhibitors of CYP2D6 (e.g., quinidine, fluoxetine) or CYP3A4 (e.g., ketoconazole, ritonavir) may lead to an increased incidence of cholinergic adverse reactions. cbg-meb.nl
Pharmacodynamically, this compound's enhancement of cholinergic activity can lead to additive or synergistic effects when combined with other cholinergic agents. drugs.comnih.gov This is particularly relevant for medications affecting neuromuscular transmission, such as succinylcholine. drugs.comnih.govcbg-meb.nl
Pre-clinical Research and in Vitro/in Vivo Models of Galanthamine
In Vitro Studies on Cellular Mechanisms
In vitro research provides controlled environments to investigate the direct effects of galanthamine at the cellular and molecular levels. These studies are crucial for identifying primary targets and understanding the initial interactions of the compound.
Receptor Binding Assays
Receptor binding assays are used to determine the affinity and selectivity of this compound for specific receptors. This compound is known to interact with nicotinic acetylcholine receptors (nAChRs). Studies have shown that this compound can bind to distinct sites on nAChRs, including at the α-γ and δ-β subunit interfaces in the extracellular domain, particularly in the presence of an agonist jneurosci.orgnih.gov. This binding is separate from the primary acetylcholine binding site jneurosci.org. This compound acts as an allosteric potentiating ligand (APL) for several human nAChR subtypes, including α3β4, α4β2, and α6β4, as well as the chicken/mouse chimeric α7/5-hydroxytryptamine3 receptor . The concentrations at which this compound potentiates these receptors in vitro correlate with levels found in cerebrospinal fluid at therapeutic doses in humans . Unlike its effects on nAChRs, this compound does not appear to alter the activity of human muscarinic acetylcholine receptors (mAChRs) at various concentrations, indicating a selective modulation of nAChRs .
Enzyme Inhibition Assays
This compound is a well-established inhibitor of acetylcholinesterase (AChE), the enzyme responsible for breaking down acetylcholine in the synaptic cleft. This inhibition leads to increased levels of acetylcholine, enhancing cholinergic neurotransmission mdpi.comnih.gov. Enzyme inhibition assays, often utilizing methods like the Ellman method, are employed to quantify the potency of this compound in inhibiting AChE and butyrylcholinesterase (BChE). This compound has been shown to be a reversible, competitive, and selective inhibitor of AChE mdpi.comnih.gov. Studies have determined IC50 values for this compound against AChE from various sources, including human and electric eel guidetopharmacology.orgresearchgate.net. While primarily known for AChE inhibition, this compound also exhibits inhibitory effects on BChE, although typically with lower potency compared to AChE d-nb.infod-nb.info.
Data from enzyme inhibition assays:
| Enzyme | Source | IC50 (µM) | Reference |
| Acetylcholinesterase (AChE) | Human | 1.18 - 1.29 | guidetopharmacology.org |
| Acetylcholinesterase (AChE) | Electric eel | ~1.44 - 1.48 | nih.gov |
| Acetylcholinesterase (AChE) | Unknown origin | 1.0 - 1.27 | guidetopharmacology.org |
| Butyrylcholinesterase (BChE) | Human | 6.86 | d-nb.info |
Note: IC50 values can vary depending on the specific assay conditions and enzyme source.
Cell Culture Models for Neuroprotection and Toxicity Assessment
Cell culture models, particularly neuronal cell lines like SH-SY5Y, are widely used to investigate the neuroprotective effects of this compound against various insults mimicking neurodegenerative conditions. These models allow for the assessment of cell viability, apoptosis, and the modulation of specific signaling pathways. This compound has demonstrated the ability to protect SH-SY5Y cells against amyloid-β (Aβ)-induced toxicity mdpi.comresearchgate.net. This neuroprotective effect is associated with increased expression of α7 nAChRs and the activation of signaling pathways such as MAPK/JNK, while inhibiting pathways like PI3K/Akt/mTOR, which are involved in apoptosis and autophagy regulation researchgate.netnih.gov. Studies have shown that this compound can enhance α7 nAChR expression in a dose-dependent manner in SH-SY5Y cells nih.gov. Furthermore, this compound has been investigated for its potential neuroprotective effects in models of Parkinson's disease, such as SH-SY5Y cells treated with 6-hydroxydopamine (6-OHDA) zenodo.org.
Data from cell culture studies on neuroprotection against Aβ toxicity:
| Cell Line | Insult | This compound Concentration | Effect on Cell Viability | Reference |
| SH-SY5Y | Aβ-induced toxicity | 0.3 µM | Maximum protection against apoptosis researchgate.net | researchgate.net |
| SH-SY5Y | Aβ-induced toxicity | 250 µM | Reduced cell viability compared to this compound alone mdpi.com | mdpi.com |
| SH-SY5Y | Aβ-induced toxicity | 500 µM | Increased cell survival compared to Aβ alone mdpi.com | mdpi.com |
| SH-SY5Y | Aβ₁₋₄₂-induced neurotoxicity | 0.1 - 10 µM | No neurotoxicity from this compound alone nih.gov | nih.gov |
Note: The effects of this compound on cell viability in the presence of Aβ can be concentration-dependent and exhibit a U-shaped curve in some models researchgate.net.
This compound has also been shown to potentiate the neuroprotective effect of memantine against NMDA-induced excitotoxicity in cultured rat cortical neurons nih.gov.
Animal Models of Neurodegenerative Diseases
Animal models are essential for studying the effects of this compound in a more complex biological system, allowing for the investigation of behavioral outcomes, neuropathological changes, and pharmacokinetic properties in vivo.
Rodent Models of Alzheimer's Disease and Other Dementias
Rodent models, particularly mice and rats, are widely used to study neurodegenerative diseases like AD and evaluate potential therapeutic agents such as this compound. Various rodent models exist, including those induced by neurotoxins or genetically modified to express mutations associated with familial AD frontiersin.orgmdpi.com.
In scopolamine-induced models of dementia in mice, this compound and its derivatives have been evaluated for their effects on memory and cognitive function nih.gov. Scopolamine is a muscarinic receptor antagonist that induces memory deficits, mimicking some aspects of cognitive impairment seen in dementia frontiersin.orgmdpi.com. Studies in this model have shown that this compound derivatives can improve both short- and long-term memory and affect exploratory activity nih.gov.
Transgenic mouse models of AD, such as the 5XFAD model which overexpresses mutated human amyloid precursor protein (APP) and presenilin 1 (PS1), are used to study the impact of this compound on amyloid pathology and behavioral decline plos.orgnih.gov. Chronic treatment with this compound in the 5XFAD mouse model has been shown to slow down amyloid plaque deposition and improve performance in behavioral tests like the open field and light-dark avoidance plos.orgnih.gov. This compound treatment in this model also reduced astrogliosis, a marker of neuroinflammation plos.org.
Another rodent model used is the anti-nerve growth factor (anti-NGF) transgenic mouse (AD11 mice), which exhibits a progressive neurodegenerative phenotype resembling AD nih.gov. This compound has been investigated in this model for its ability to ameliorate early signs of neurodegeneration nih.gov.
In rat models, this compound has been studied for its effects on scopolamine-induced amnesia, with assessments including maze tests to evaluate memory function mdpi.com.
Data from rodent studies:
| Animal Model | Disease Model | Key Findings | Reference |
| Mice (Scopolamine-induced) | Dementia | Improved short- and long-term memory; affected exploratory activity. nih.gov | nih.gov |
| 5XFAD Mouse | Alzheimer's Disease | Slowed amyloid plaque deposition; improved behavioral performance; reduced astrogliosis. plos.orgnih.gov | plos.orgnih.gov |
| Anti-NGF Mouse (AD11) | Neurodegeneration (AD-like) | Ameliorated early signs of neurodegeneration. nih.gov | nih.gov |
| Wistar Rats (Scopolamine-induced) | Amnesia | Evaluated effects on memory and behavior using maze tests. mdpi.com | mdpi.com |
Behavioral and Cognitive Assessments in Animal Models
Pre-clinical research utilizing various animal models has investigated the effects of galantamine on behavior and cognitive function. These studies often employ standardized behavioral tests designed to assess different aspects of learning and memory.
One common approach involves the use of rodent models, such as rats and mice, where cognitive impairment is induced through various methods to mimic neurodegenerative conditions or other factors affecting cognitive function researchgate.netnih.govxiahepublishing.com. Galantamine's impact is then evaluated using tests like the Morris water maze, novel object recognition, and passive avoidance tasks researchgate.netnih.govnih.govnih.gov.
Studies have demonstrated that galantamine can lead to significant improvements in cognitive behavioral outcomes in these models. For example, in rats, galantamine administration resulted in improved performance in both Atlantis watermaze training and novel object recognition tests compared to vehicle-treated controls researchgate.netnih.gov. Similarly, in mouse models of Alzheimer's disease (AD), galantamine administration has been shown to ameliorate memory decline in tests such as the Morris water maze and novel object recognition nih.gov.
Furthermore, research in mice suggests that galantamine can prevent cognitive deficits induced by substances like lipopolysaccharide (LPS), which is used to model neuroinflammation. In these studies, galantamine treatment prevented LPS-induced deficits in spatial learning and memory, as well as memory acquisition in passive avoidance tests nih.gov. The ameliorating effects of galantamine on cognitive impairment induced by amyloid-β peptide fragments or phencyclidine in mice have also been linked to the augmentation of dopaminergic neurotransmission following the activation of nicotinic acetylcholine receptors (nAChRs) oup.com.
Beyond cognitive improvements, galantamine has also been investigated for its effects on other behaviors in animal models. For instance, chronic treatment with galantamine in a mouse model of AD resulted in improved performance in behavioral tests such as open field and light-dark avoidance plos.org.
The behavioral and cognitive improvements observed in these pre-clinical studies are often correlated with neurobiological changes. For instance, in the study using LPS-exposed mice, galantamine inhibited gliosis and the activation of microglia and astrocytes in the hippocampus, suggesting that its cognitive benefits may be linked to anti-neuroinflammatory effects nih.gov. In the 5XFAD mouse model of AD, galantamine treatment not only improved behavioral performance but also significantly lowered amyloid-β plaque density in brain regions like the hippocampus and entorhinal cortex, along with reduced gliosis plos.org.
Data from behavioral and cognitive assessments in animal models highlight galantamine's potential to improve various aspects of cognitive function, particularly in models of cognitive impairment.
| Animal Model | Cognitive/Behavioral Test | Key Finding | Citation |
| Rats | Atlantis Watermaze, Novel Object Recognition | Improved cognitive behavioral outcomes | researchgate.netnih.gov |
| APPswe/PS1dE9 Mouse Model of AD | Morris Water Maze, Novel Object Recognition | Ameliorated memory decline | nih.gov |
| LPS-exposed Mice | Spatial learning/memory, Passive Avoidance | Prevented cognitive deficits, inhibited neuroinflammation | nih.gov |
| Amyloid-β peptide/Phencyclidine-treated Mice | Novel Object Recognition, Conditioned Fear Learning | Ameliorated recognition memory impairment, augmented dopaminergic neurotransmission | oup.com |
| 5XFAD Mouse Model of AD | Open Field, Light-Dark Avoidance | Improved behavioral performance, slowed plaque formation, reduced gliosis | plos.org |
Toxicological Studies and Safety Pharmacology in Pre-clinical Settings
Pre-clinical toxicological studies and safety pharmacology assessments are fundamental in evaluating the potential risks associated with galantamine administration. These studies in animal models provide crucial data on acute and chronic toxicity, as well as effects on various physiological systems.
Single-dose toxicity studies have been conducted in rats, mice, and dogs via oral and intravenous routes fda.gov. Chronic toxicity has been evaluated in longer-term studies, including 1-, 6-, and 12-month studies in rats and dogs, and 3-month studies in mice fda.gov. Many of the observed side effects in these studies were consistent with the expected pharmacological effects of galantamine as a reversible acetylcholinesterase inhibitor, primarily involving the central nervous system and gastrointestinal systems due to increased cholinergic stimulation fda.gov.
Repeated-dose toxicity studies have further characterized the effects of galantamine. For instance, in 6- to 12-month studies in rats and dogs, a dose of 1.6 mg/kg was considered non-toxic hres.ca. Higher doses in these studies often led to clinical signs related to exaggerated cholinergic stimulation hres.ca.
Specific toxicological endpoints investigated in pre-clinical settings include carcinogenicity and reproductive toxicity. In a 2-year oral carcinogenicity study in rats, an increased incidence of endometrial adenocarcinomas was observed at higher doses nih.gov. However, galantamine was not found to be carcinogenic in 6-month or 24-month oral carcinogenicity studies in mice at specified dose levels nih.gov.
Reproductive toxicity studies in animals have indicated potential risks. In studies with pregnant rats, galantamine demonstrated teratogenic effects on fetuses nih.gov. A slightly increased incidence of skeletal variations was observed in rats dosed before mating and during organogenesis hres.ca. Decreased pup weights were noted in rats dosed from the beginning of organogenesis through the post-partum period hres.ca. However, no drug-related teratogenic effects were observed in rabbits given galantamine during organogenesis hres.ca.
Safety pharmacology studies in animals have also assessed the effects of galantamine on key physiological systems, such as the cardiovascular system. As a cholinesterase inhibitor, galantamine can have vagotonic effects on the heart, potentially leading to bradycardia and heart block hres.ca. These effects are a predictable consequence of its pharmacological action hres.ca.
Pre-clinical studies have also explored specific motor effects, such as the induction of tremulous jaw movements in rats, which are considered a rodent model of parkinsonian tremor researchgate.net. These movements were dose-related and appeared to be mediated through actions on muscarinic acetylcholine receptors researchgate.net.
| Study Type | Animal Species | Duration | Key Findings | Citation |
| Single-dose Toxicity | Rats, Mice, Dogs | Single dose | Side effects related to cholinergic stimulation (CNS, GI) | fda.gov |
| Chronic Toxicity | Rats, Dogs | 1, 6, 12 months | Clinical signs related to exaggerated cholinergic stimulation; 1.6 mg/kg considered non-toxic in longer studies | fda.govhres.ca |
| Chronic Toxicity | Mice | 3 months | Side effects related to cholinergic stimulation | fda.gov |
| Carcinogenicity | Rats | 2 years | Increased incidence of endometrial adenocarcinomas at higher doses | nih.gov |
| Carcinogenicity | Mice | 6, 24 months | Not carcinogenic at tested doses | nih.gov |
| Reproductive Toxicity | Rats | Gestation/Post-partum | Teratogenic effects, decreased pup weights, skeletal variations | hres.canih.gov |
| Reproductive Toxicity | Rabbits | Organogenesis | No drug-related teratogenic effects | hres.ca |
| Safety Pharmacology (Cardiac) | Animals | - | Vagotonic effects, potential for bradycardia/heart block | hres.ca |
| Motor Effects | Rats | - | Induced tremulous jaw movements (model of parkinsonian tremor) | researchgate.net |
Clinical Trial Design and Outcomes for Galanthamine
Phase I Clinical Trials: Safety and Pharmacokinetics in Healthy Volunteers
Phase I clinical trials are typically the initial step in testing a new medical treatment in humans after preclinical studies. slideshare.netclinicaltrial.be The primary aims of these trials are to assess the safety, tolerability, and pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted by the body) of the compound. clinicaltrial.bequanticate.com These studies are often conducted in a small group of healthy volunteers to establish a safe dosage range and understand how the body processes the drug without the confounding factors of an underlying disease. slideshare.netclinicaltrial.beecrin.org
Studies have investigated the pharmacokinetics of galantamine in healthy volunteers. For instance, an open-label study administered a single oral 4-mg dose of galantamine to healthy control subjects to assess its pharmacokinetic parameters over 6 days. nih.gov The findings from such studies contribute to determining appropriate dosing for later-phase trials. clinicaltrial.beecrin.org
Phase II Clinical Trials: Efficacy and Dose-Finding in Patient Populations
Following Phase I, Phase II trials enroll a larger group of patients to further evaluate safety, begin assessing efficacy, and determine the optimal dosage range. slideshare.net These studies aim to gather preliminary data on whether the drug has a beneficial effect on the target condition and to identify the most effective doses with acceptable tolerability. slideshare.net
While specific details on dedicated Phase II trials for galantamine focusing solely on dose-finding in patient populations were not extensively detailed in the search results, the dose-finding aspect is often integrated into early efficacy studies. For example, some Phase III trials included multiple dose arms to compare the effects of different galantamine doses against placebo. jacobimed.orgnih.govnih.govresearchgate.netneurology.org These trials, while primarily designed as Phase III, also served to confirm dose-response relationships observed in earlier stages.
Phase III Clinical Trials: Definitive Efficacy and Safety Studies
Phase III clinical trials are large-scale studies designed to definitively assess the efficacy of a new drug in a larger and more diverse patient population. slideshare.net These trials compare the investigational treatment to a placebo or a standard treatment to confirm its effectiveness and monitor for adverse events in a larger group. slideshare.net Galantamine has been evaluated in multiple Phase III trials for the treatment of mild to moderate Alzheimer's disease. nih.gov
These trials have demonstrated that galantamine can significantly improve cognitive function compared to placebo. jacobimed.orgnih.govnih.govresearchgate.netneurology.org Efficacy has been measured using standardized scales such as the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-cog) and the Clinician's Interview-Based Impression of Change plus Caregiver Input (CIBIC-plus). jacobimed.orgnih.govnih.govresearchgate.netneurology.org
Randomized Controlled Trials (RCTs)
Randomized controlled trials (RCTs) are considered the gold standard for evaluating the efficacy of interventions. frontiersin.orgfda.gov In RCTs of galantamine, patients are randomly assigned to receive either galantamine or a placebo, minimizing bias in treatment allocation. jacobimed.orgnih.govnih.govcmaj.ca Multiple multicenter, double-blind, parallel-group, placebo-controlled RCTs have investigated galantamine in patients with mild to moderate Alzheimer's disease. nih.govnih.govresearchgate.netneurology.org
These trials have consistently shown a significant benefit of galantamine on cognitive function. For example, in a 5-month trial, galantamine-treated patients showed improvement on the ADAS-cog scale compared to placebo, with treatment effects favoring galantamine. jacobimed.org Another 6-month trial reported that patients receiving galantamine had significantly better cognitive function than the placebo group, with observed treatment effects on the ADAS-cog subscale. nih.govnih.gov The effects on the ADAS-cog scale have been reported as statistically significant, with mean differences in change from baseline favoring galantamine. nih.govnih.gov Galantamine has also shown a better outcome on global clinical impression scales like the CIBIC-plus compared to placebo. jacobimed.orgnih.govnih.govresearchgate.netneurology.org
Interactive Table 1: Efficacy Outcomes in Selected Galantamine RCTs (vs. Placebo)
| Study Duration | Patient Population | Outcome Measure | Galantamine Effect (vs. Placebo) | Statistical Significance | Source |
| 5 months | Mild to moderate AD | ADAS-cog | Improvement (points) | p < 0.05 to p < 0.001 | jacobimed.org |
| 6 months | Mild to moderate AD | ADAS-cog subscale | Mean change favoring galantamine | P < 0.001 | nih.govnih.gov |
| 6 months | Mild to moderate AD | CIBIC-plus | Better outcome | P < 0.05 | nih.govnih.gov |
| 6 months | Mild to moderate AD | ADAS-cog/11 | Treatment effects (points) | p < 0.001 | researchgate.netneurology.org |
| 6 months | Mild to moderate AD | CIBIC-plus | Better outcome | p < 0.05 | researchgate.netneurology.org |
*Note: Specific numerical values for improvement/change vary across studies and doses.
Some RCTs also investigated the effect of galantamine on activities of daily living and behavioral symptoms, with some studies showing benefits in these domains as well. jacobimed.orgnih.govnih.govresearchgate.netneurology.org For instance, a 5-month study indicated that galantamine significantly benefited activities of daily living and behavioral symptoms compared to placebo. jacobimed.org Another trial found that the higher dose of galantamine produced a significantly better outcome on the disability assessment for dementia scale than placebo at six months. nih.govnih.gov
RCTs have also been conducted to evaluate galantamine in other conditions, such as vascular dementia, where it showed effectiveness in improving cognition, including executive function. researchgate.net However, studies in mild cognitive impairment (MCI) did not consistently show a significant effect of galantamine on delaying conversion to dementia. researchgate.net
Open-label Extension Studies
Open-label extension studies typically follow the completion of a double-blind, placebo-controlled trial. In these extensions, all participants, regardless of their original treatment assignment, receive the active drug. The primary purpose is often to gather longer-term data on the safety and tolerability of the treatment, but they can also provide insights into sustained efficacy. researchgate.netneurology.orgnih.govsemanticscholar.orgingentaconnect.com
Studies involving open-label extensions of galantamine trials have provided evidence regarding the longer-term effects of the treatment. researchgate.netneurology.orgnih.govsemanticscholar.orgingentaconnect.com For example, a 12-month open-label extension of an earlier trial evaluated the long-term outcomes of galantamine treatment. nih.gov Patients who continued to receive galantamine throughout the double-blind and open-label phases showed sustained cognitive benefits. nih.gov Another study with a 6-month open-label extension reported that cognitive and daily function were maintained for 12 months in patients who received galantamine continuously. researchgate.netneurology.org Pooled analyses of open-label extensions have also suggested attenuated cognitive decline over time in galantamine-treated patients compared to predicted decline in untreated individuals. semanticscholar.org
Meta-analyses and Systematic Reviews of Galanthamine Clinical Efficacy
Meta-analyses and systematic reviews synthesize data from multiple clinical trials to provide a more robust estimate of a treatment's effect. These analyses pool results from various studies, increasing the statistical power to detect treatment effects and evaluate consistency across trials. e-century.usresearchgate.netnih.govumw.edu.plnih.gov
Several meta-analyses and systematic reviews have assessed the efficacy of galantamine in the treatment of Alzheimer's disease. e-century.usresearchgate.netnih.govumw.edu.plnih.gov These analyses have generally concluded that galantamine is effective in improving cognitive function in patients with mild to moderate AD compared to placebo. e-century.usresearchgate.netnih.govumw.edu.plnih.gov
A meta-analysis of eight randomized clinical trials found that cognitive effects were significantly increased for galantamine compared to placebo, as measured by the ADAS-cog subscale. e-century.usresearchgate.net This analysis reported a significant mean difference favoring galantamine. e-century.usresearchgate.net The beneficial effect was also observed on the Clinicians' Global Impression of Change scale. e-century.usresearchgate.net
Another systematic review and meta-analysis concluded that cholinesterase inhibitors, including galantamine, were able to stabilize or slow decline in cognition, function, behavior, and global change when compared with placebo. nih.gov This review found a significant improvement in cognitive function based on the ADAS-cog and in functional outcomes based on the Disability Assessment for Dementia (DAD) scale for galantamine compared with placebo. nih.gov
Interactive Table 2: Summary of Efficacy Findings from Meta-analyses
| Analysis Type | Outcome Domain | Galantamine Effect (vs. Placebo) | Statistical Significance | Source |
| Meta-analysis (8 RCTs) | Cognition (ADAS-cog) | Significant increase in cognitive effects (MD = -3.15) | P < 0.00001 | e-century.usresearchgate.net |
| Meta-analysis (8 RCTs) | Global (CIBIC) | Significant effect (OR = 1.30) | P = 0.01 | e-century.usresearchgate.net |
| Systematic Review & Meta-analysis | Cognition (ADAS-cog) | Stabilize or slow decline | Significant | nih.gov |
| Systematic Review & Meta-analysis | Function (DAD) | Significant improvement | Significant | nih.gov |
| Systematic Review & Meta-analysis | Behavior (NPI) | Significant improvement (WMD = -1.44) | Significant | nih.gov |
| Meta-analysis | Cognition | Significantly improved cognitive abilities | Significant | umw.edu.pl |
*MD: Mean Difference, OR: Odds Ratio, WMD: Weighted Mean Difference
These reviews generally support the efficacy of galantamine in improving cognitive and global function in patients with mild to moderate Alzheimer's disease. nih.govumw.edu.pl
Real-World Evidence and Observational Studies on Galantamine Use
Real-world evidence (RWE) is data collected outside the controlled environment of traditional clinical trials, often reflecting routine clinical practice. frontiersin.org Observational studies are a common source of RWE, where researchers observe outcomes in patients receiving a treatment as part of their usual care, without random assignment. frontiersin.orgfda.gov RWE can complement findings from RCTs by providing insights into the effectiveness and use of a drug in broader patient populations and over longer periods. frontiersin.org
While RCTs are the primary source of evidence for regulatory approval, RWE and observational studies can provide supplementary information, particularly regarding long-term efficacy and outcomes in diverse clinical settings. frontiersin.orgfda.govnih.gov
An observational study evaluated the long-term efficacy of galantamine in routine clinical practice over 72 weeks. nih.gov This study compared the actual cognitive function of patients receiving galantamine to a simulated disease trajectory without treatment. nih.gov The results indicated that the cognitive decline observed in patients treated with galantamine was significantly smaller than the simulated decline. nih.gov Individual analysis in this study demonstrated that a majority of patients had better actual cognitive scores than their predicted scores without treatment. nih.gov
Real-world data has been utilized in post-marketing surveillance to monitor the long-term efficacy of drugs. frontiersin.org While some manufacturers have submitted observational data to support the effectiveness of other treatments beyond the duration of RCTs, the availability and use of extensive observational data specifically for galantamine's long-term effectiveness in regulatory assessments can vary. frontiersin.org
Research Methodologies and Analytical Techniques for Galanthamine Studies
Analytical Chemistry Techniques for Galanthamine Quantification
Analytical chemistry plays a crucial role in the precise identification and quantification of this compound in various matrices, including plant extracts, pharmaceutical formulations, and biological fluids. Diverse methods have been developed for this purpose, with key techniques including High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS), and Nuclear Magnetic Resonance (NMR) spectroscopy. researchgate.net
High-Performance Liquid Chromatography (HPLC)
HPLC is a widely used technique for the separation and quantification of this compound. It offers advantages in terms of sensitivity, accuracy, and the ability to analyze complex samples. Numerous HPLC methods have been developed for the determination of this compound in sources such as Amaryllidaceae plants and pharmaceutical dosage forms. jocpr.comresearchgate.netjrespharm.comturkjps.orgresearchgate.netjournaljpri.com These methods often involve reversed-phase columns and specific mobile phases optimized for this compound separation. Detection is commonly achieved using UV photodiode-array, fluorescence, or mass spectrometric detectors. researchgate.netjrespharm.comnih.gov For instance, an isocratic HPLC system utilizing a mobile phase of trifluoroacetic acid-water-acetonitrile has been successfully applied for the simultaneous quantification of this compound and lycorine in Galanthus fosteri. jrespharm.com Another developed and validated RP-HPLC method uses a C18 column and a mobile phase of phosphate buffer and acetonitrile for this compound hydrobromide estimation in extended-release formulations. journaljpri.com Highly sensitive HPLC methods involving derivatization with agents like dansyl chloride and fluorescence detection have also been established for quantifying this compound in biological fluids such as human plasma and urine. nih.gov
Table 1: Examples of HPLC Methods for this compound Analysis
| Matrix Analyzed | Column Type | Mobile Phase | Detection Method | Limit of Quantification (LOQ) | Reference |
| Galanthus fosteri | C18 | TFA-water-acetonitrile (0.01:90:10, v/v/v) | DAD | 0.03675 µg mL⁻¹ | jrespharm.com |
| Sternbergia species | Not specified | Not specified | HPLC | 25 µg | turkjps.org |
| Pharmaceutical formulation | Thermo Scientific C18 | 0.1M phosphate buffer: Acetonitrile (40:60V/V) pH 4.5 | UV (203 nm) | 1-10 µg/ml (Linear Range) | journaljpri.com |
| Human plasma and urine | Inertsil C18 | 40% acetonitrile and 60% 10 mM o-phosphoric acid | Fluorescence (Ex 375/Em 537 nm) | 18.81-212.97 ng/ml | nih.gov |
Mass Spectrometry (MS) Applications
Mass spectrometry, often coupled with chromatographic techniques like HPLC or Gas Chromatography (GC-MS), is a powerful tool for the identification, structural elucidation, and quantification of this compound and its metabolites. researchgate.netresearchgate.netjrmds.inscielo.br LC-MS and LC-MS/MS methods offer high sensitivity and selectivity, making them suitable for analyzing complex biological samples. researchgate.netscirp.orghep.com.cn GC-MS is particularly valuable for the reliable and fast identification of Amaryllidaceae alkaloids, including this compound, and for comparative studies of their percentage contribution in alkaloid mixtures. researchgate.netscielo.br MS imaging (MSI) techniques, such as those utilizing probe electrospray ionization (PESI) or MALDI coupled with quadrupole time-of-flight (Q-TOF) MS, can visualize the distribution of this compound within plant tissues, aiding in identifying regions with high alkaloid content for efficient extraction. shimadzu.comjst.go.jp LC-MS/MS methods have also been developed for the simultaneous determination of this compound and other alkaloids in human serum, crucial for assessing exposure or poisoning cases. scirp.org
Nuclear Magnetic Resonance (NMR) Spectroscopy for Structural Research
NMR spectroscopy is a fundamental technique for the structural elucidation of organic compounds, including this compound. researchgate.netjrmds.in Both 1D and 2D NMR experiments (such as COSY, HMQC, and NOESY) are traditionally used to determine the relative configuration of natural products like this compound. acs.org While NMR alone may be limited in establishing the absolute configuration, its combination with other techniques like vibrational circular dichroism (VCD) can provide a comprehensive analysis of both relative and absolute configurations. acs.orgcore.ac.uk NMR can also be employed in conjunction with theoretical calculations, such as Density Functional Theory (DFT), to gain insights into the molecular structure and conformational behavior of this compound and its derivatives. researchgate.net
Neuroimaging Techniques in this compound Research
Neuroimaging techniques are employed to investigate the effects of this compound on brain function and metabolism in vivo. These techniques provide valuable insights into the neurobiological mechanisms underlying the effects of this compound, particularly in the context of neurological conditions.
Positron Emission Tomography (PET) for Receptor Occupancy and Metabolic Studies
Positron Emission Tomography (PET) is a nuclear medicine technique that uses radioactive tracers to visualize and measure metabolic processes or receptor binding in the body, including the brain. PET is a valuable tool in this compound research for assessing receptor occupancy and studying changes in brain metabolism. PET studies using specific radiotracers can measure the in vivo brain acetylcholinesterase (AChE) activity and nicotinic receptor binding following this compound administration. nih.govfrontiersin.org For example, studies using tracers like [¹¹C]-PMP have demonstrated that this compound can cause sustained inhibition of cortical AChE activity. nih.gov PET with [¹⁸F]-fluorodeoxyglucose (FDG-PET), which measures glucose metabolism, has been used to differentiate brain metabolic patterns associated with treatment responses to this compound. ucla.edumdpi.commdpi.com These metabolic studies can help identify brain regions where glucose uptake changes in response to this compound treatment, potentially serving as biomarkers for treatment effects. ucla.edumdpi.com
Table 2: Examples of Neuroimaging Techniques in this compound Research
| Technique | Application in this compound Research | Key Findings/Applications | Reference |
| fMRI | Studying brain activation and functional connectivity changes | Enhancement of dorsal visual system processing; investigation of default mode network connectivity. | medlink.comnih.govtajdearobpharma.com |
| PET (AChE) | Measuring acetylcholinesterase activity and receptor binding | Assessment of cortical AChE inhibition; investigation of nicotinic receptor binding. | nih.govfrontiersin.org |
| PET (FDG) | Studying brain glucose metabolism | Differentiation of metabolic patterns associated with treatment response; identification of regions with metabolic changes. | ucla.edumdpi.commdpi.com |
Biomarker Discovery and Validation Methodologies in this compound Research
Biomarker discovery and validation are critical steps in understanding disease progression, identifying patient populations likely to respond to treatment, and monitoring therapeutic effects. The process typically involves hypothesis generation, sample collection, data collection, data analysis, assay development, assay validation, and ultimately, regulatory approval bioanalysis-zone.comverisimlife.com. For this compound research, biomarkers could potentially indicate the severity of cholinergic deficits, predict response to this compound treatment, or track the impact of the drug on specific biological pathways.
Biomarker discovery often begins by defining a target biological process, such as those affected by this compound's action on cholinergic systems bioanalysis-zone.com. Multiple candidate biomarkers may be identified through high-throughput technologies, including mass spectrometry, proteomic, and epigenetic methods bioanalysis-zone.com. Once candidates are identified, they require rigorous validation before being used in clinical studies bioanalysis-zone.comverisimlife.com.
Validation involves establishing the performance characteristics of a biomarker, such as sensitivity, specificity, accuracy, and precision nih.gov. Analytical validation ensures the biomarker provides consistent measurements, while clinical validation demonstrates its usefulness in a clinical context nih.gov. This can involve retrospective analysis of clinical trial data or prospective clinical trials nih.gov. A key challenge in biomarker discovery is the lack of well-established validation methods for candidates bioanalysis-zone.com. Affinity proteomics, utilizing pre-qualified antibody pools, has been suggested as a method to aid in both screening and validation stages bioanalysis-zone.com.
Biomarkers can serve different purposes: they can indicate a biological response to a treatment, predict the likelihood of a clinical event, or identify individuals more likely to benefit from a specific therapy verisimlife.com. In the context of this compound, research might focus on identifying biomarkers related to cholinergic function or neuronal integrity that change with treatment.
Electrophysiological Techniques for Neuronal Activity Assessment
One common technique is electroencephalography (EEG), which records electrical activity from the cortex mdpi.com. EEG can capture neural oscillations, rhythmic electrical activity in neural assemblies, which are thought to play a role in coordinating brain regions nih.gov. Studies using magnetoencephalography (MEG), a non-invasive electrophysiological imaging technique, have been employed to assess the effect of acetylcholinesterase inhibitors like this compound on neural oscillations, such as beta amplitude in the sensorimotor region nih.gov. Changes in these oscillations can be indicative of altered cognitive control mechanisms nih.gov.
In vitro electrophysiological methods, such as patch-clamp recordings, are used to study the effects of this compound on ion channels and receptor function at the cellular level ceon.rsresearchgate.net. These techniques can demonstrate how this compound potentiates the response of nicotinic acetylcholine receptors to acetylcholine, supporting its proposed role as an allosteric potentiating ligand researchgate.net. Studies have shown that this compound can enhance excitatory postsynaptic potentials (EPSPs) measured by stimulating specific synaptic pathways, such as CA3-CA1 synapses in the hippocampus nih.gov. Preliminary data also suggest this compound can reduce the afterhyperpolarization (AHP) and spike accommodation in hippocampal pyramidal neurons, indicating increased neuronal excitability nih.gov.
Electrophysiological recordings, sometimes combined with EEG, can also assess the impact of this compound on the activity of specific brain regions, such as the basal forebrain, which provides cholinergic projections to the cortex and limbic structures mdpi.com. Age-related declines in neuronal activity and theta-gamma coupling in the brain, which are linked to cognitive impairment, have been shown to be potentially reversed by cholinesterase inhibitors, highlighting the utility of electrophysiological measures in evaluating the effects of this compound mdpi.com.
Proteomics and Genomics Approaches in this compound Research
Proteomics and genomics provide powerful tools for investigating the molecular pathways affected by this compound and identifying potential genetic factors influencing treatment response or disease progression. Genomics focuses on the study of genes and heredity, while proteomics is the comprehensive study of proteins, including their structure, function, and interactions ebsco.comcdc.govopenstax.org.
Genomic approaches in this compound research can involve identifying genetic variations, such as single nucleotide polymorphisms (SNPs), that may serve as biomarkers for prognosis or treatment response openstax.orgtataa.com. Genome-wide association studies (GWAS) can be used to identify genetic differences associated with diseases like Alzheimer's, which this compound is used to treat openstax.org. These identified genes can become targets for further research into disease mechanisms and potential therapies openstax.org. Transcriptome analysis, which studies the complete set of RNA transcripts, can also be used to identify genes associated with the biosynthesis of compounds like this compound in plants frontiersin.org.
Proteomics complements genomics by studying the entire set of proteins produced by a cell or organism cdc.govopenstax.org. Since genes code for proteins, studying proteomes provides insights into how genetic information is expressed and how protein function is altered in disease states or in response to treatment ebsco.comopenstax.org. Proteomic approaches can be used to identify proteins whose expression levels are affected by a disease process, potentially serving as biomarkers openstax.org. Affinity-based proteomic panels and mass spectrometry-based techniques are employed for protein biomarker discovery and analysis tataa.com.
In the context of this compound, proteomics could help researchers understand how the drug influences protein expression profiles in the brain or other relevant tissues. This could reveal downstream effects of cholinergic modulation and identify protein signatures associated with treatment efficacy or lack thereof openstax.org. Integrating genomic and proteomic data (proteogenomics) can provide a more comprehensive understanding of the complex interplay between genes and proteins in disease and in response to interventions like this compound treatment cdc.gov.
Advanced Statistical Methods for Clinical Trial Data Analysis
Advanced statistical methods are fundamental to the design, analysis, and interpretation of clinical trials evaluating the efficacy and safety of this compound. These methods ensure that trial results are robust, reliable, and can inform healthcare decisions spssanalysis.com.
Biostatisticians play a crucial role in clinical trials, involved in aspects such as trial design, randomization, sample size determination, data analysis, and reporting andywillan.com. Choosing the appropriate statistical method depends on the study design, the type of data collected, and the specific research questions being addressed spssanalysis.com.
Commonly used statistical methods in clinical trials include t-tests, ANOVA (Analysis of Variance), and methods for analyzing survival data, such as Kaplan-Meier curves and Cox proportional hazards models spssanalysis.com. For analyzing repeated measurements from clinical trials, mixed-effects models are often employed spssanalysis.com. Bayesian methods provide an alternative probabilistic framework for making inferences based on trial data spssanalysis.com.
In this compound clinical trials, statistical analyses are used to compare outcomes between treatment groups (e.g., this compound vs. placebo) and assess changes from baseline nih.govnih.govneurology.org. For instance, studies have used mixed model analyses to evaluate the impact of this compound on behavioral measures and caregiver time nih.gov. Statistical comparisons are often performed using methods like one-way ANOVA for continuous variables and the Cochran-Mantel-Haenszel test for categorical variables nih.gov.
When evaluating multiple biomarkers or outcomes, controlling for multiple comparisons is important to minimize the risk of false discoveries nih.gov. Measures of false discovery rate (FDR) are particularly useful when dealing with high-dimensional data, such as from genomic or proteomic studies nih.gov. Statistical models are also used to identify predictive biomarkers by testing for interactions between treatment and the potential biomarker nih.gov.
Detailed statistical analysis plans are typically developed prior to receiving trial data to avoid bias nih.gov. These plans define outcomes, hypotheses, and criteria for success nih.gov. Statistical analysis of clinical trial data on this compound has demonstrated significant improvements in cognitive function and activities of daily living compared to placebo in patients with mild to moderate Alzheimer's disease nih.govneurology.org.
| Compound Name | PubChem CID |
| This compound | 9651 nih.gov |
| This compound HBr | 121587 mybiosource.com |
Note: While this compound has CID 9651, this compound Hydrobromide, a common salt form used in research and pharmaceuticals, has CID 121587. Both are relevant in the context of this compound research.this compound is a chemical compound primarily known for its role as an acetylcholinesterase inhibitor and an allosteric potentiating ligand of nicotinic acetylcholine receptors wikipedia.orgnih.gov. Research into this compound involves a variety of sophisticated methodologies aimed at understanding its mechanisms of action, identifying potential biomarkers for treatment response, assessing its effects on neuronal activity, and analyzing clinical trial data. This article focuses exclusively on the research methodologies and analytical techniques employed in this compound studies, adhering strictly to the specified outline.
Biomarker Discovery and Validation Methodologies in this compound Research
Biomarker discovery and validation are critical steps in understanding disease progression, identifying patient populations likely to respond to treatment, and monitoring therapeutic effects bioanalysis-zone.comverisimlife.com. For this compound research, biomarkers could potentially indicate the severity of cholinergic deficits, predict response to this compound treatment, or track the impact of the drug on specific biological pathways.
The process of biomarker discovery and development is extensive, involving hypothesis generation, sample collection, data collection, data analysis, assay development, assay validation, and ultimately, regulatory approval bioanalysis-zone.comverisimlife.com. Biomarker discovery often begins by defining a target biological process, such as those affected by this compound's action on cholinergic systems bioanalysis-zone.com. High-throughput technologies, including mass spectrometry, proteomic, and epigenetic methods, can be used to identify multiple candidate biomarkers bioanalysis-zone.com.
Once candidate biomarkers are identified, they require rigorous validation to establish their performance characteristics, such as sensitivity, specificity, accuracy, and precision verisimlife.comnih.gov. Analytical validation focuses on ensuring consistent measurements, while clinical validation demonstrates the biomarker's usefulness in a clinical setting nih.gov. This validation can be performed through retrospective analysis of existing clinical trial data or by conducting prospective clinical trials nih.gov. A significant challenge in this field is the lack of standardized validation methods for candidate biomarkers bioanalysis-zone.com. Affinity proteomics, which utilizes pre-qualified antibody pools, has been suggested as a method that can assist in both the screening and validation phases of biomarker discovery bioanalysis-zone.com.
Biomarkers can serve various functions, including indicating a biological response to treatment, predicting the likelihood of a clinical event, or identifying individuals who are more likely to benefit from a specific therapy verisimlife.com. In the context of this compound, research may focus on identifying biomarkers related to cholinergic function or neuronal health that change in response to treatment.
Electrophysiological Techniques for Neuronal Activity Assessment
Electroencephalography (EEG) is a common non-invasive technique used to record electrical activity from the cortex mdpi.com. EEG can detect neural oscillations, which are rhythmic electrical activities in neuronal populations believed to be involved in coordinating brain regions nih.gov. Studies utilizing magnetoencephalography (MEG), another non-invasive electrophysiological imaging technique, have investigated the effects of acetylcholinesterase inhibitors like this compound on neural oscillations, such as beta amplitude in the sensorimotor cortex nih.gov. Changes in these oscillations can provide insights into altered cognitive control mechanisms nih.gov.
In vitro electrophysiological methods, such as patch-clamp recordings, are employed to study the effects of this compound on ion channels and receptor function at the cellular level ceon.rsresearchgate.net. These techniques can demonstrate how this compound potentiates the response of nicotinic acetylcholine receptors to acetylcholine, supporting its role as an allosteric potentiating ligand researchgate.net. Research has shown that this compound can enhance excitatory postsynaptic potentials (EPSPs) in specific synaptic pathways, such as CA3-CA1 synapses in the hippocampus nih.gov. Preliminary data also suggests that this compound can reduce the afterhyperpolarization (AHP) and spike accommodation in hippocampal pyramidal neurons, indicating an increase in neuronal excitability nih.gov.
Electrophysiological recordings, sometimes combined with EEG, can also be used to assess the impact of this compound on the activity of specific brain regions, such as the basal forebrain, which provides significant cholinergic projections to the cortex and limbic structures mdpi.com. Age-related declines in neuronal activity and theta-gamma coupling, linked to cognitive impairment, have been shown to be potentially reversible by cholinesterase inhibitors, highlighting the utility of electrophysiological measures in evaluating this compound's effects mdpi.com.
Proteomics and Genomics Approaches in this compound Research
Proteomics and genomics offer powerful tools for investigating the molecular pathways influenced by this compound and identifying potential genetic factors that may affect treatment response or disease progression. Genomics is the study of genes and heredity, while proteomics involves the comprehensive study of proteins, including their structure, function, and interactions ebsco.comcdc.govopenstax.org.
Genomic approaches in this compound research may involve identifying genetic variations, such as single nucleotide polymorphisms (SNPs), that could serve as biomarkers for prognosis or treatment response openstax.orgtataa.com. Genome-wide association studies (GWAS) can be utilized to identify genetic differences associated with conditions such as Alzheimer's disease, for which this compound is prescribed openstax.org. The genes identified through these studies can then become targets for further investigation into disease mechanisms and potential therapeutic strategies openstax.org. Transcriptome analysis, which examines the complete set of RNA molecules, can also be used to identify genes involved in the biosynthesis of compounds like this compound in plants frontiersin.org.
Proteomics complements genomic studies by examining the entire set of proteins produced by a cell or organism cdc.govopenstax.org. Since proteins are encoded by genes, studying proteomes provides insights into how genetic information is expressed and how protein function is altered in disease states or in response to therapeutic interventions ebsco.comopenstax.org. Proteomic methods can be used to identify proteins with altered expression levels in disease, which may serve as biomarkers openstax.org. Techniques such as affinity-based proteomic panels and mass spectrometry are employed for protein biomarker discovery and analysis tataa.com.
In the context of this compound, proteomics could help researchers understand how the drug influences protein expression profiles in relevant tissues, such as the brain. This could reveal the downstream effects of cholinergic modulation and identify protein signatures associated with treatment efficacy or lack of response openstax.org. Integrating genomic and proteomic data through proteogenomics can provide a more holistic understanding of the complex interactions between genes and proteins in disease and in response to treatments like this compound cdc.gov.
Advanced Statistical Methods for Clinical Trial Data Analysis
Advanced statistical methods are essential for the design, analysis, and interpretation of clinical trials evaluating the efficacy and safety of this compound. These methods are critical for ensuring that trial results are reliable, robust, and can effectively inform clinical practice spssanalysis.com.
Biostatisticians play a vital role throughout the clinical trial process, contributing to trial design, randomization strategies, sample size determination, data analysis, and reporting andywillan.com. The selection of appropriate statistical methods is guided by the specific study design, the type of data collected, and the research questions being investigated spssanalysis.com.
Commonly used statistical methods in clinical trials include t-tests, Analysis of Variance (ANOVA), and techniques for analyzing time-to-event data, such as Kaplan-Meier survival analysis and Cox proportional hazards models spssanalysis.com. Mixed-effects models are frequently used for the analysis of repeated measurements collected over time in clinical trials spssanalysis.com. Bayesian methods offer an alternative statistical framework for drawing inferences from trial data spssanalysis.com.
In clinical trials evaluating this compound, statistical analyses are used to compare outcomes between the this compound group and control groups (e.g., placebo) and to assess changes from baseline measurements nih.govnih.govneurology.org. For example, mixed model analyses have been used to evaluate the impact of this compound on behavioral measures and the amount of caregiver time required nih.gov. Statistical comparisons between groups often utilize methods such as one-way ANOVA for continuous variables and the Cochran-Mantel-Haenszel test for categorical variables nih.gov.
When multiple biomarkers or outcomes are assessed, it is important to control for multiple comparisons to reduce the likelihood of false positive findings nih.gov. Measures such as the false discovery rate (FDR) are particularly useful when analyzing high-dimensional data from genomic or proteomic studies nih.gov. Statistical models are also employed to identify predictive biomarkers by examining the interaction between the treatment effect and the potential biomarker nih.gov.
Formulation Science and Drug Delivery Research for Galanthamine
Development of Sustained-Release and Extended-Release Formulations
The development of sustained-release (SR) and extended-release (ER) formulations for galanthamine aims to reduce the frequency of administration and maintain more consistent drug levels in the body. Various techniques and materials have been investigated for this purpose.
One approach involves the preparation of modified release pellets using techniques like extrusion-spheronization. Studies have explored the use of release retardants such as Compritol 888 ATO and ethyl cellulose (EC) in developing this compound hydrobromide modified release pellets. rjptonline.org The concentration of these polymers has been identified as a critical factor influencing the drug release profile. rjptonline.org Pregelatinized starch has also been explored as a dry binder in the post-wet extrusion step to improve pellet properties. rjptonline.org
Another method for developing extended-release formulations is pelletization. Extended-release capsules of this compound hydrobromide (8mg) have been prepared using this technique, with in vitro dissolution studies conducted to compare their release profiles to reference listed drugs. researchgate.netijsra.netresearchgate.net Formulations have been evaluated for preformulation characteristics, as well as physical and chemical parameters. researchgate.netijsra.net Dissolution studies using the USP type II (paddle apparatus) in pH 6.5 phosphate buffer have shown that certain formulations can achieve a drug release profile pharmaceutically equivalent to reference products, based on similarity factors. researchgate.netijsra.netresearchgate.net Stability studies on optimized formulations have indicated their stability. researchgate.netijsra.netresearchgate.net
Matrix tablets incorporating hydrophobic polymers like Eudragit® RL and Eudragit® RS have also been investigated for developing sustained-release systems of this compound hydrobromide. researchgate.net Design of experiments has been utilized to study the influence of factors affecting release characteristics. researchgate.net Compatibility studies between galantamine hydrobromide and excipients, as well as swelling and erosion rates, have been performed. researchgate.net Data suggests that the drug release mechanism from these matrix systems is diffusion-controlled. researchgate.net
Novel sustained-release capsules of galantamine hydrobromide have been prepared using the extrusion-spheronization method. banglajol.infonih.gov In vitro release studies comparing these novel capsules to marketed extended-release capsules have shown that the developed formulations can exhibit superior sustained-release properties, with total release proportions reaching over 90% within 12 hours. banglajol.infonih.gov The release profile has been found to fit the Higuchi model. banglajol.info
Research on Novel Drug Delivery Systems (e.g., Nanoparticles, Liposomes, Microencapsulation)
Research into novel drug delivery systems for this compound aims to enhance its delivery to target sites, improve bioavailability, and potentially reduce side effects. Nanoparticles, liposomes, and microspheres are among the systems being explored. mdpi.comwalshmedicalmedia.comacs.org
Solid lipid nanoparticles (SLNs) have been investigated as carriers for this compound hydrobromide to enhance brain delivery. mdpi.comtandfonline.comtandfonline.com SLNs prepared using biocompatible components have shown potential in improving the bioavailability of this compound. mdpi.comtandfonline.com Studies have demonstrated that this compound-loaded SLNs can provide a controlled release profile over a period, with a significant percentage of the drug released over 24 hours. tandfonline.com The encapsulation of this compound in a lipidic layer has been observed to modulate its release profile compared to the fast release of the free drug. tandfonline.com
Liposomes have also been explored as carriers for this compound. Galantamine-loaded liposomes have been reported to offer increased drug bioavailability and superior pharmacokinetic behavior in studies involving rivastigmine, another acetylcholinesterase inhibitor, suggesting potential for this compound as well. mdpi.com Liposomes can potentially enhance drug permeation and lead to higher drug concentrations at the target site. mdpi.com
Polymeric nanoparticles, such as those prepared with PLGA (poly(lactide-co-glycolide)), have been developed for this compound delivery. mdpi.com These nanoparticles are being investigated for their potential to improve drug bioavailability and therapeutic activity. mdpi.com
Microencapsulation techniques are also being applied to develop sustained-release formulations. Galantamine pamoate loaded poly (lactide-co-glycolide) (PLGA) microspheres have been developed using an oil/water emulsion solvent evaporation method. frontiersin.org These microspheres have shown sustained release in vitro for an extended period and maintained stable plasma drug concentrations in in vivo studies. frontiersin.org The morphology of optimized microspheres has been characterized as spherical with smooth surfaces and a core-shell interior structure. frontiersin.org
Transdermal Patch Technology Development and Evaluation
The development of this compound transdermal patches is an attractive alternative route of administration, particularly for patients with conditions like Alzheimer's disease, as it can potentially eliminate gastrointestinal side effects and improve compliance by reducing dosing frequency. iajps.comupm.edu.mydoi.orgresearchgate.netsci-hub.se
Matrix-type transdermal patches have been developed using various polymers, including Eudragit-L100, HPMCk4M, and HPMCk15M, prepared by the solvent casting method. iajps.com Permeation enhancers like propylene glycol and Tween80 have been selected to improve drug delivery through the skin. iajps.com Formulations with different polymer concentrations have been evaluated for physical parameters and in vitro drug release using dialysis membranes. iajps.com Studies have identified formulations showing significant drug release over a period, with the release kinetics potentially following models like the peppas mechanism. iajps.com Drug-excipient compatibility studies using techniques like FTIR have been conducted to assess potential interactions. iajps.com
Pressure sensitive adhesive (PSA) patches for the transdermal delivery of this compound free base have also been developed and evaluated. doi.org Different PSAs, penetration enhancers, and drug loadings have been tested to optimize patch formulations. doi.org The use of penetration enhancers such as limonene, alone or in combination with crystallization inhibitors like oleic acid, has been shown to increase the flux of this compound across human cadaver skin. doi.org Optimized patches have exhibited diffusion release kinetics, fitting well to models like Higuchi's model. doi.org
Bilayered matrix-type this compound hydrobromide patches have been prepared using polymers like HPMC 4000 in the drug layer and Eudragit RS in the backing layer, also utilizing the solvent casting technique. sci-hub.se These patches have been evaluated for properties such as plasticity and in vitro drug release over an extended period. sci-hub.se
The development process involves evaluating prepared patches for various physical parameters, including appearance, thickness, weight variation, flatness, folding endurance, moisture uptake, moisture content, drug content uniformity, and surface pH. iajps.comupm.edu.my In vitro permeation studies, often using Franz diffusion cells and membranes like dialysis membrane or human cadaver skin, are crucial for evaluating the drug release and permeation profiles of the patches. iajps.comdoi.orgsci-hub.se
Studies on this compound-loaded gels as drug reservoirs within transdermal patches have also been conducted. upm.edu.myresearchgate.net The composition of the gel, including the amount of polymer (e.g., carbopol), neutralizer (e.g., triethanolamine), drug, and permeation enhancer (e.g., propylene glycol), has been shown to affect drug release. upm.edu.myresearchgate.net Optimized gel formulations have been developed and evaluated for properties like homogeneity, pH, and drug content uniformity. upm.edu.myresearchgate.net Histological examinations have been performed to assess skin irritation potential. upm.edu.my
Challenges, Future Directions, and Unresolved Questions in Galanthamine Research
Identifying Predictors of Treatment Response and Non-Response
A significant challenge in galanthamine treatment is the variability in patient response ijnrd.org. Not all patients with mild to moderate AD benefit equally, and identifying those most likely to respond remains an active area of research. Studies have explored whether baseline characteristics, such as demographic features, disease severity, and neuropsychological profiles, can predict long-term outcomes, but these have shown limited predictive value nih.govresearchgate.net.
Research suggests that the initial response to this compound administration in the early weeks of treatment may serve as a more reliable predictor of subsequent response nih.govresearchgate.netchdr.nl. Specifically, improvements in episodic memory function during the first four weeks of treatment have been associated with a greater likelihood of benefiting from continued this compound therapy nih.govresearchgate.net. For instance, changes in scores on the ADAS-J cog subscales, particularly word recognition, at week 4 have shown approximately 75% predictive performance for subsequent response nih.govresearchgate.net.
Further research is needed to identify robust biomarkers or clinical indicators that can accurately predict individual patient response to this compound, allowing for more personalized treatment strategies.
Exploring Combination Therapies with this compound
Given the complex multifactorial nature of AD, combination therapies targeting multiple pathological pathways are a rational approach nih.govnih.gov. Research is exploring the potential benefits of combining this compound with other therapeutic agents. One prominent area of investigation is the combination of this compound with memantine, an N-methyl-D-aspartate (NMDA) receptor antagonist nih.govnih.govmdpi.com. Cholinergic and glutamatergic dysfunction are believed to underlie AD symptomatology, and combining agents that address both systems may offer enhanced therapeutic effects nih.gov.
Beyond AD, combination therapies involving this compound are also being explored for other neurological and psychiatric conditions, such as Parkinson's disease dementia and cognitive impairment in schizophrenia mdpi.comnih.gov.
Investigating this compound's Role in Early Disease Stages and Prevention
Current approvals for this compound are primarily for mild to moderate AD drugbank.comwikipedia.org. However, there is interest in understanding its potential role in earlier stages of the disease, such as mild cognitive impairment (MCI), and its possible preventative effects. While some studies in people with MCI showed little to no difference in improving memory or self-care activities compared to placebo, participants receiving this compound in one study likely had a lower risk of progression to dementia after two years cochrane.org.
The potential for this compound to influence the trajectory of cognitive decline in the prodromal or very early phases of AD remains an important area for future research ijnrd.orgcochrane.org. Understanding if early intervention with this compound can delay the onset or slow the progression of dementia is a key unresolved question.
Expanding Therapeutic Indications for this compound Beyond Current Approvals
This compound's primary indication is AD, but its pharmacological properties, particularly its modulation of the cholinergic system, suggest potential therapeutic applications in other conditions nih.gov. Research is exploring its use in vascular dementia, Alzheimer's disease with cerebrovascular disease (mixed dementia), dementia associated with Parkinson's disease, and cognitive impairment in Lewy body disease drugbank.comnih.gov.
Additionally, this compound is being investigated for its potential in treating cognitive impairment due to traumatic brain injury and in managing symptoms in schizophrenia nih.gov. Its peripheral actions, including its ability to antagonize residual non-depolarizing neuromuscular blockade, have also led to investigations in anesthetic settings . The potential for repurposing this compound for conditions beyond its current approvals is an active area of clinical investigation nih.gov.
Mechanistic Elucidation of Peripheral Effects and Systemic Impact
While this compound is primarily known for its central nervous system effects in AD, it also acts peripherally by inhibiting acetylcholinesterase in muscle and other tissues, thereby increasing cholinergic tone drugbank.comwikipedia.org. The full extent and clinical significance of these peripheral effects and their systemic impact are not yet completely understood.
Studies have indicated potential neuroprotective effects of this compound in the peripheral nervous system, observed in experimental models of sciatic nerve compression injury nih.gov. This neuroprotective effect may be related to anti-inflammatory mechanisms nih.gov. Furthermore, research in animal models suggests potential antidiabetic effects of this compound, possibly mediated through stimulation of the cholinergic pathway and its anti-oxidant, anti-apoptotic, and anti-inflammatory properties plos.org. These findings highlight the need for further mechanistic studies to fully elucidate this compound's actions beyond the central nervous system and their potential therapeutic implications.
Addressing Inter-individual Variability in Response and Metabolism
Inter-individual variability in response to this compound treatment is a significant clinical issue unil.chelsevier.es. This variability may be partly attributed to differences in drug metabolism and elimination unil.ch. This compound is primarily metabolized by cytochrome P450 enzymes, particularly CYP2D6 and, to a lesser extent, CYP3A4 unil.chtandfonline.com. Genetic variations in these enzymes can influence the rate at which this compound is metabolized, leading to variations in plasma concentrations and potentially affecting efficacy and tolerability unil.chtandfonline.com.
Pharmacogenetic studies aim to understand how genetic factors contribute to this variability and to identify genetic biomarkers that could predict drug response unil.chelsevier.es. While some studies have explored the influence of genetic variants, such as those in CYP2D6 and APOE, on this compound response, the findings have not always been consistent elsevier.estandfonline.com. Further research is needed to fully understand the genetic and non-genetic factors contributing to inter-individual variability and to develop strategies for optimizing this compound therapy based on individual patient profiles.
Development of Improved Analogues and Derivatives with Enhanced Profiles
Despite the therapeutic benefits of this compound, there is ongoing research into developing improved analogues and derivatives with potentially enhanced efficacy, reduced side effects, or novel mechanisms of action chim.itmdpi.comresearchgate.net. The natural origin and complex structure of this compound have made it an appealing template for the synthesis of new compounds chim.itresearchgate.net.
Research groups are exploring structural modifications to the this compound molecule to improve its biological profile chim.itresearchgate.net. This includes the synthesis of derivatives with peptide fragments, aiming to lower toxicity and potentially confer new properties, such as inhibition of β-secretase activity researchgate.netnih.gov. Other modifications are being investigated to enhance acetylcholinesterase inhibitory activity or explore activity against other targets relevant to neurodegenerative diseases mdpi.comresearchgate.net. For instance, some this compound derivatives have shown promising antimycobacterial activity, indicating the potential for expanding the therapeutic scope of this compound class mdpi.com. The development and evaluation of these novel compounds represent a key future direction in this compound research.
Q & A
Q. What methodological approaches are standard for quantifying galanthamine in plant extracts?
High-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS) or nuclear magnetic resonance (¹H NMR) is widely used for precise quantification. For instance, ¹H NMR metabolic profiling enables simultaneous identification of this compound and related metabolites in plant matrices, as demonstrated in fertilizer studies on Narcissus bulbs . Calibration curves using this compound standards (10–100 µg/mL) with internal controls (e.g., codeine) ensure accuracy, achieving R² > 0.99 .
Q. How do agricultural practices influence this compound accumulation in Narcissus bulbs?
Standard nitrogen and potassium fertilization significantly enhances this compound yield compared to unfertilized controls. However, doubling nitrogen levels increases amino acids and citric acid intermediates without boosting this compound, suggesting metabolic trade-offs. Multivariate analysis of ¹H NMR data is critical to disentangle nutrient effects on secondary metabolism .
Q. What in vitro models validate this compound’s cholinesterase inhibitory activity?
Competitive inhibition assays using acetylcholinesterase (AChE) from Torpedo californica are standard. This compound binds reversibly to the active site gorge, interacting with Trp84 and other residues. Butyrylcholinesterase (BChE) assays are less common due to this compound’s higher AChE selectivity (IC₅₀ ~0.5 µM vs. ~10 µM for BChE) .
Advanced Research Questions
Q. How can artificial neural networks (ANNs) optimize enzymatic synthesis of this compound derivatives?
ANNs with backpropagation algorithms (e.g., batch back propagation) model reaction parameters (temperature, enzyme concentration, time) to predict esterification yields (>60%). The optimal ANN architecture (4 inputs, 7 hidden nodes, 1 output) reduces experimental iterations, validated by <0.2% deviation between predicted and empirical yields .
Q. What strategies resolve contradictions in fertilizer studies on this compound production?
Conflicting results (e.g., nitrogen effects) require metabolic flux analysis. While ¹H NMR reveals amino acid accumulation under high nitrogen, targeted gene expression profiling (e.g., TYDC, NCS) can clarify whether precursor availability limits this compound biosynthesis. Controlled hydroponic systems minimize soil variability .
Q. How does this compound’s conformation change upon binding to AChE?
Density functional theory (DFT) and docking studies show that this compound’s outer core (methoxy group, cyclohexene ring) undergoes conformational shifts to fit the AChE active site. The dipole moment increases from 2.09 D (isolated) to 2.67 D (bound), enhancing electrostatic interactions with Trp84 and Phe330 .
Q. What multi-target effects does this compound exhibit in Alzheimer’s models?
Beyond AChE inhibition, this compound prevents Aβ₁–₄₂ oligomerization by stabilizing unfolded conformations via electrostatic interactions (e.g., with Asp23, Lys28). Circular dichroism (CD) and fluorescence quenching assays validate this anti-aggregation activity .
Q. How to design experiments for this compound derivatives with non-AChE activities?
Biomimetic diversity-oriented synthesis (DOS) generates libraries based on this compound’s rigid core. For secretory pathway modulation (unrelated to AChE), high-throughput screening in mammalian cells combined with structure-activity relationship (SAR) analysis identifies critical functional groups (e.g., tertiary amine, aromatic rings) .
Q. What in silico methods predict this compound’s pharmacokinetics and hydrogen-bonding properties?
Molecular dynamics (MD) simulations and quantum chemical calculations (MP2/6-311++G**) map hydrogen-bond donor/acceptor strengths. Natural bond orbital (NBO) analysis identifies hyperconjugation between lone pairs of furanyl oxygen and σ*(C-H) orbitals, explaining solvent-dependent NOE patterns .
Q. How can metabolic profiling elucidate this compound biosynthesis pathways?
Full-factor experimental designs (e.g., 2⁴ factorial) with modified Murashige-Skoog (MS) media optimize Leucojum aestivum shoot cultures. Regression models (R² > 0.88) link ammonium, nitrate, and sucrose levels to this compound yields, while ¹H NMR tracks downstream metabolites (e.g., tyramine, norbelladine) .
Methodological Notes
- Contradiction Management : Use multivariate statistical tools (e.g., PCA, PLS-DA) to differentiate environmental vs. genetic factors in this compound production .
- Structural Insights : Combine X-ray crystallography (e.g., AChE-galanthamine complexes) with in silico docking (AUTODOCK) to validate binding modes .
- Dosage Optimization : Pilot studies in animal models (e.g., Morris water maze) recommend 2.5 mg/kg this compound for balancing AChE inhibition (50–70%) and minimal side effects .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


