L-thyroxine
Description
L-thyroxine (L-T4), the synthetic form of the endogenous thyroid hormone thyroxine, is a cornerstone in the treatment of hypothyroidism and congenital hypothyroidism (CH). Chemically synthesized from L-tyrosine , it replaces deficient thyroid hormone levels, restoring metabolic homeostasis. Standard dosing ranges from 7–15 mcg/kg/day in neonates with CH to individualized regimens in adults, aiming for thyroid-stimulating hormone (TSH) normalization . L-T4’s efficacy extends to modulating lipid profiles, reducing atherosclerosis risk by lowering total cholesterol (-0.20 mmol/L) and low-density lipoprotein (LDL) cholesterol (-0.26 mmol/L) . Its pharmacokinetics, however, are influenced by formulation, administration timing, and interactions with binding proteins like human serum albumin (HSA) .
Properties
IUPAC Name |
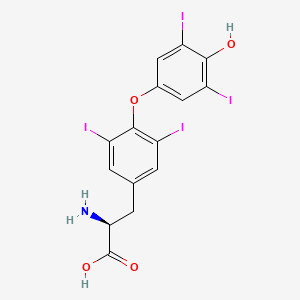
(2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C15H11I4NO4/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23/h1-2,4-5,12,21H,3,20H2,(H,22,23)/t12-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XUIIKFGFIJCVMT-LBPRGKRZSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=C(C=C(C(=C1I)OC2=CC(=C(C(=C2)I)O)I)I)CC(C(=O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=C(C=C(C(=C1I)OC2=CC(=C(C(=C2)I)O)I)I)C[C@@H](C(=O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C15H11I4NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8023214 | |
| Record name | Levothyroxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023214 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
776.87 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Thyroxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000248 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble in water, Insoluble in ethanol, benzene | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LEVOTHYROXINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3108 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals, Needles | |
CAS No. |
51-48-9 | |
| Record name | (-)-Thyroxine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=51-48-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Levothyroxine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000051489 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | levothyroxine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757434 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Levothyroxine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023214 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | LEVOTHYROXINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/Q51BO43MG4 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | LEVOTHYROXINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3108 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thyroxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000248 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
Decomposes at 235-236 °C, 235.5 °C | |
| Record name | Levothyroxine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00451 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | LEVOTHYROXINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3108 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thyroxine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0000248 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Preparation Methods
Classical Iodination of 3,5-Diiodothyronine
The foundational method involves iodinating 3,5-diiodothyronine using iodine in alkaline media. Patent US2579668A details a protocol where 3,5-diiodothyronine reacts with iodine in aqueous ammonia at 0–5°C for 24 hours, achieving 68–72% yields. Modern adaptations replace ammonia with methylamine-methanol systems, enhancing reaction control. For instance, dissolving 100 g of 3,5-diiodothyronine in methanolic methylamine (25–30°C) followed by iodine addition at −8°C produces L-thyroxine with 84.5–91.2% yield after sodium bisulfite quenching and potassium dihydrogen phosphate neutralization.
Critical Parameters
- Temperature: Maintaining −8 to 0°C during iodine addition prevents over-iodination.
- Stoichiometry: A 1:4 molar ratio of diiodothyronine to iodine ensures complete tetraiodination.
- Purification: Sequential washing with acetonitrile and vacuum drying at 55–60°C yields pharmaceutical-grade product.
Diiodination of N-tert-Butoxycarbonyl-L-Tyrosine Methyl Ester
Recent advancements prioritize modular synthesis from L-tyrosine. As demonstrated in Blucher Chemistry Proceedings, N-tert-butoxycarbonyl-L-tyrosine methyl ester undergoes regioselective diiodination using iodine (2.2 eq) and hydrogen peroxide (30%) in water at 25°C for 24 hours. This method achieves 80% yield of 3,5-diiodo-N-tert-butoxycarbonyl-L-tyrosine methyl ester, a key intermediate. Subsequent Boc deprotection with trifluoroacetic acid and coupling with 3,5-diiodothyronine derivatives completes the synthesis.
Advantages Over Classical Methods
- Selectivity: Hydrogen peroxide minimizes polyiodination byproducts.
- Scalability: Aqueous reaction media reduce organic solvent use.
- Intermediate Stability: Boc protection prevents oxidative degradation during iodination.
Enzymatic Synthesis via Thyroglobulin Mimetics
Emerging biochemical approaches exploit thyroglobulin-like scaffolds for iodotyrosine coupling. A 2024 PMC study engineered a minimal protein precursor containing a conserved glutamate residue critical for thyroxine formation. In vitro iodination with thyroid peroxidase and potassium iodide (KI) generated this compound at acceptor tyrosine sites, mimicking natural biosynthesis. While yields remain suboptimal (≈45%), this method offers insights into evolutionary hormone-forming mechanisms.
Key Insights
- Glutamate Positioning: Carboxylate groups facilitate iodotyrosine deprotonation, enabling coupling.
- Proteolytic Release: Overlapping hormonogenic and proteolytic sites enable efficient thyroxine liberation.
Industrial-Scale Purification Protocols
Post-synthesis purification ensures compliance with pharmacopeial standards. The withdrawn OSHA method PV2117, though obsolete, provides a benchmark:
- Crude Product Dissolution : 2.0 mL methanol per 100 mg crude extract.
- Filtration : Glass fiber filters (0.45 µm) remove particulate contaminants.
- HPLC Analysis :
Modern facilities employ preparative HPLC with gradient elution (acetonitrile/0.1% formic acid) to isolate this compound at >99% purity.
Yield Optimization Strategies
Table 1: Comparative Analysis of Synthesis Methods
Optimization Levers
- Catalyst Addition : Potassium iodide (0.1 eq) accelerates iodine dissolution in methanol.
- pH Control : Maintaining pH 8.5–9.0 with potassium dihydrogen phosphate prevents thyroxine degradation.
- Reaction Quenching : Sodium bisulfite (1.0 eq) reduces residual iodine, curtailing over-reaction.
Challenges and Byproduct Mitigation
Major byproducts include 3,3',5-triiodothyronine (T3) and 3,5-diiodothyrosine, arising from incomplete iodination or premature coupling. Strategies to suppress these involve:
Chemical Reactions Analysis
Types of Reactions: Levothyroxine undergoes various chemical reactions, including:
Oxidation: Levothyroxine can be oxidized to form different derivatives.
Reduction: Reduction reactions can modify the iodine atoms in the molecule.
Substitution: Substitution reactions can occur at the phenolic hydroxyl group or the amino group.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and iodine.
Reduction: Reducing agents such as sodium borohydride can be used.
Substitution: Reagents like alkyl halides and acyl chlorides are commonly used for substitution reactions.
Major Products Formed:
Oxidation: Oxidized derivatives of levothyroxine.
Reduction: Reduced forms of levothyroxine with fewer iodine atoms.
Substitution: Substituted derivatives with different functional groups.
Scientific Research Applications
L-thyroxine, also known as levothyroxine or L-T4, is a synthetic form of thyroxine (T4), a thyroid hormone that plays a vital role in regulating the body's metabolism, growth, and development . It is primarily used to treat hypothyroidism, a condition characterized by the thyroid gland's inability to produce sufficient amounts of thyroid hormone .
Indications and Applications
Levothyroxine is indicated for a variety of conditions related to thyroid hormone deficiency :
- Hypothyroidism: this compound is a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism .
- Thyroid Cancer: It is used as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer .
- Subclinical Hypothyroidism: L-T4 can significantly increase free thyroxine (FT4) levels and decrease thyroid-stimulating hormone (TSH) levels. It may also decrease systolic blood pressure (SBP), T3, and total cholesterol (TC) while increasing FT3 levels .
- Prophylactic Treatment: this compound therapy can reduce the incidence and alleviate the symptoms of autoimmune thyroiditis in euthyroid patients .
Effects on Cardiovascular Risk Factors
Several studies suggest that this compound therapy can improve cardiovascular risk factors in patients with subclinical hypothyroidism :
- Lipid Profile Improvement: this compound treatment can lead to a significant reduction in total cholesterol and low-density lipoprotein (LDL) cholesterol levels .
- Endothelial Function: this compound treatment has been shown to improve endothelial function and reduce waist-to-hip ratio, both of which are markers of cardiovascular health .
- Cardiac Function: Thyroxine therapy may improve left ventricular diastolic function in patients with subclinical hypothyroidism, suggesting a positive impact on cardiac function .
One study showed that this compound treatment reduced total cholesterol from 231.6 to 220 mg/dl (P < 0.001), improved flow-mediated dilation (FMD) from 4.2 to 5.9% (P < 0.001), and reduced waist to hip ratio from 0.83 to 0.81 (P < 0.006) .
This compound Monotherapy
Initially, there were concerns about using this compound monotherapy due to the possibility of T3 deficiency . However, two major discoveries in the 1970s led to changes in clinical practice that justified this compound monotherapy :
- Peripheral deiodinase-mediated T4 to T3 conversion: It was discovered that T3 is generated from L-T4 in the body .
- Development of serum thyroid hormone and thyroid-stimulating hormone radioimmunoassays: These assays allowed for accurate measurement of thyroid hormone levels in the blood .
It has been shown that T3 is predominantly produced by peripheral conversion through the 5′-deiodination of T4, with only 20% of T3 in the circulation secreted directly by the thyroid . Studies have confirmed the restoration of the prohormone pool and endogenous generation of T3 in those treated with this compound monotherapy, providing a solid mechanism to explain the documented ability of this compound monotherapy clinically to normalize thyroid function .
Case Studies
- One study treated twenty women with subclinical hypothyroidism with this compound and placebo in a double-blind cross-over design during 2 x 6 months. The study found that approximately one woman in four with this 'subclinical' condition will benefit from this compound treatment .
- Another study showed that physiological this compound replacement in patients with subclinical hypothyroidism has a beneficial effect on low-density lipoprotein cholesterol levels and clinical symptoms of hypothyroidism. An important risk reduction of cardiovascular mortality of 9–31% can be estimated from the observed improvement in low-density lipoprotein cholesterol .
Impact on Autoimmune Thyroiditis
Mechanism of Action
Levothyroxine exerts its effects by replacing the thyroid hormone that is normally produced by the thyroid gland. It is converted to triiodothyronine (T3) in peripheral tissues, which then binds to thyroid hormone receptors in the nucleus of cells. This binding activates gene transcription and protein synthesis, leading to increased metabolism, growth, and development . The primary molecular targets are thyroid hormone receptors, and the pathways involved include the regulation of metabolic processes and energy expenditure .
Comparison with Similar Compounds
Desiccated Thyroid Extract (DTE)
DTE, derived from porcine thyroid glands, contains both thyroxine (T4) and triiodothyronine (T3). Clinical studies comparing DTE and L-T4 show comparable effectiveness in symptom relief, but DTE may result in transiently elevated T3 levels, raising concerns about cardiac risks in susceptible patients. L-T4 remains preferred due to its predictable pharmacokinetics and standardized dosing .
Liothyronine (L-T3)
Liothyronine, the synthetic T3 hormone, has a shorter half-life (1–2 days) than L-T4 (7 days), necessitating multiple daily doses. A randomized trial found L-T3 monotherapy inferior to L-T4 in maintaining stable TSH and free T4 levels, with higher rates of iatrogenic hyperthyroidism.
D-Thyroxine (D-T4)
The D-enantiomer of thyroxine exhibits distinct binding properties. While its affinity for cyclodextrin-based supramolecular systems is ~7,000-fold higher than L-T4 due to stereoselective hydrophobic interactions , binding to HSA mutants (e.g., R218H and R218P) shows similar dissociation constants (Kd: ~2.6 μM for L-T4 vs. ~2.2 μM for D-T4) . Despite comparable protein binding, D-T4 lacks therapeutic relevance due to negligible hormonal activity in humans.
Liquid and Softgel Formulations
Compared to tablets, liquid L-T4 formulations (e.g., Tirosint® and Tifactor®) demonstrate enhanced bioavailability, particularly when taken 15–30 minutes before meals . In neonates with CH, liquid L-T4 (Tifactor®) required lower starting doses (7–12 mcg/kg/day) to avoid overtreatment (TSH: 0.08 ± 0.02 mcUI/ml vs. 36.7 ± 14.7 mcUI/ml with Tirosint®) . Softgel capsules similarly improve adherence and reduce food/drug interactions, offering a 10–15% increase in bioavailability over conventional tablets .
Key Clinical and Pharmacological Data
Critical Research Findings and Controversies
- Formulation Variability : Liquid L-T4 poses overtreatment risks in neonates if initial doses exceed 12 mcg/kg/day , while softgel capsules improve consistency in adults .
- Bone Health: Long-term L-T4 therapy, particularly at suppressive doses (TSH <0.1 mIU/L), correlates with cortical bone mineral density (BMD) reduction in pre- and postmenopausal women .
- Protein Binding : Conflicting HSA binding data exist, with one study reporting an 80-fold difference in Kd between wild-type and R218P HSA , while others show minimal variation .
- Timing of Administration : Bedtime dosing increases L-T4 absorption, yielding higher free T4 (+1.2 ng/dL) and lower TSH (-0.8 mIU/L) versus morning dosing .
Biological Activity
L-thyroxine (T4) is a synthetic form of the thyroid hormone thyroxine, primarily used in the treatment of hypothyroidism. Its biological activity extends beyond its role as a prohormone, influencing various physiological processes through both genomic and nongenomic mechanisms. This article delves into the biological activity of this compound, supported by data tables, case studies, and detailed research findings.
Genomic Actions : T4 exerts its effects primarily through binding to thyroid hormone receptors (TRs), which regulate gene expression. Although traditionally viewed as a prohormone that requires conversion to triiodothyronine (T3) for biological activity, recent studies indicate that T4 possesses intrinsic activity that can influence gene expression independently of T3. For instance, research involving triple knockout mice demonstrated that T4 administration could regulate gene expression in the liver, impacting pathways related to cell proliferation and cholesterol metabolism .
Nongenomic Actions : T4 also exhibits rapid nongenomic effects that do not involve direct interaction with DNA. It can bind to integrin αvβ3 on cell membranes, activating signaling pathways that influence angiogenesis and cell proliferation. This mechanism has implications for cancer biology and neurodevelopment .
Clinical Efficacy
This compound is widely recognized for its effectiveness in managing hypothyroidism. A meta-analysis of randomized controlled trials involving 1,735 patients revealed that this compound significantly decreased thyroid-stimulating hormone (TSH) levels and improved free T4 levels compared to placebo. In patients with subclinical hypothyroidism, this compound treatment also resulted in notable improvements in cardiovascular risk factors .
Table 1: Clinical Outcomes of this compound Treatment
| Parameter | Baseline Levels | Post-Treatment Levels | P-Value |
|---|---|---|---|
| Total Cholesterol (mg/dl) | 231.6 | 220 | <0.001 |
| LDL Cholesterol (mg/dl) | 142.9 | 131.3 | <0.05 |
| Waist-to-Hip Ratio | 0.83 | 0.81 | <0.006 |
| Endothelial Function (FMD %) | 4.2 | 5.9 | <0.001 |
Case Studies
- Long-term Use and Colorectal Cancer Risk : A case-control study indicated that prolonged use of levothyroxine (≥5 years) was associated with a statistically significant reduction in colorectal cancer risk (OR = 0.60, 95% CI = 0.44 to 0.81) among a cohort of patients . This suggests potential protective effects of this compound beyond thyroid function.
- Impact on Cardiovascular Health : In a double-blind study focusing on subclinical hypothyroidism, patients treated with this compound showed significant reductions in total cholesterol and LDL levels over 48 weeks, highlighting its role in cardiovascular risk management .
Research Findings
Recent investigations have explored the multifaceted roles of this compound:
- Intrinsic Activity : Studies have demonstrated that T4 can induce growth hormone responses in vitro without converting to T3, suggesting a direct hormonal action .
- Antimicrobial Properties : Emerging research has identified antibacterial properties associated with this compound, indicating potential applications beyond endocrine therapy .
- Effects on Symptoms : Patients receiving this compound reported improvements in symptoms of fatigue and overall well-being, correlating with biochemical changes in thyroid hormone levels .
Q & A
Q. What documentation standards are essential for replicating this compound studies?
- Methodological Answer : Publish full experimental sections with batch numbers for reagents (e.g., Sigma-Aldryl this compound, ≥98% HPLC) . Supplementary files should include raw lipid profiles, TSH assay protocols, and randomization sequences .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


