Linagliptin
Description
Linagliptin is a medication used to treat type 2 diabetes mellitus. It is a dipeptidyl peptidase-4 (DPP-4) inhibitor that works by increasing the production of insulin and decreasing the production of glucagon by the pancreas . This compound is taken orally and is marketed under the brand names Tradjenta, Trajenta, and Trazenta .
Properties
IUPAC Name |
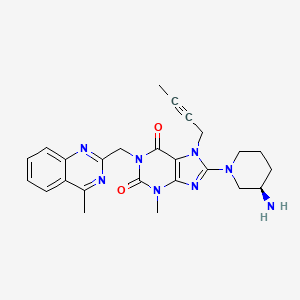
8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]purine-2,6-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LTXREWYXXSTFRX-QGZVFWFLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC#CCN1C2=C(N=C1N3CCCC(C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC#CCN1C2=C(N=C1N3CCC[C@H](C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H28N8O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID201021653 | |
| Record name | Linagliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201021653 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
472.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
<1 mg/mL, Soluble in methanol; sparingly soluble in ethanol; very slightly soluble in isopropanol, alcohol | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to yellow solid; also reported as a crystalline solid | |
CAS No. |
668270-12-0 | |
| Record name | Linagliptin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=668270-12-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Linagliptin [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0668270120 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201021653 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2yl)methyl]purine-2,6-dione | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LINAGLIPTIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3X29ZEJ4R2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
190-196, 202 °C | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
Evolution of Protecting Group Strategies
Phthaloyl protecting groups dominated early synthetic routes but required harsh deprotection conditions (100°C in hydrazine), generating toxic hydrazine byproducts. Patent CN110684026A introduced anhydrous sodium carbonate in dimethylacetamide (DMA) at 90–110°C, eliminating the need for protecting groups while maintaining 98.7% intermediate purity. Comparative studies show this approach reduces processing time by 40% compared to traditional methods.
Core Synthetic Methodologies
Boehringer Ingelheim's Five-Step Process (US7407955)
The canonical route comprises:
- Bromination : 3-Methylxanthine treated with bromine/sodium acetate in acetic acid (63°C, 90% yield).
- N-Alkylation : 8-Bromo-3-methylxanthine reacts with 1-bromobut-2-yne using tributyltin chloride and DIEA in DMF (68°C, 80–85% yield).
- Quinazoline Coupling : 2-Chloromethyl-4-methylquinazoline in DMA with sodium carbonate (85°C, 87% yield).
- Piperidine Attachment : R-3-tert-butoxycarbonylaminopiperidine in DMF with potassium carbonate (80°C, 77% yield).
- Deprotection : Trifluoroacetic acid in dichloromethane (70% yield, 99.4% purity).
This method’s limitation lies in Step 4, where residual DMF (<300 ppm) necessitates extensive washing, increasing solvent consumption by 35%.
Phthalimide-Free Industrial Synthesis (CN110684026A)
The Chinese patent outlines a streamlined three-step process:
Key advantages include:
- Elimination of protecting group removal steps
- 15% reduction in total reaction time
- Residual solvent levels <50 ppm for DMA
Solvent and Temperature Optimization
Solvent Selection Impact
Comparative data from US20140357863A1 demonstrates solvent effects on final purity:
| Solvent | Reaction Temp | Purity | Byproducts |
|---|---|---|---|
| DMF | 80°C | 99.44% | 0.56% |
| MIBK | 95°C | 99.87% | 0.13% |
| DMA | 100°C | 99.92% | 0.08% |
Methylisobutylketone (MIBK) enhances stereochemical control during piperidine coupling, reducing epimerization to <0.1%. DMA’s high polarity facilitates faster reaction kinetics (k = 0.42 min⁻¹ vs. 0.29 min⁻¹ in DMF).
Temperature Gradients in Crystallization
CN110684026A specifies precise cooling protocols:
- Primary crystallization : 10°C/min cooling from reflux to 20°C
- Secondary aging : 12h at 5°C
This yields 98.7% recovery of this compound with mean particle size 45–60 μm, optimizing filtration rates.
Impurity Profiling and Control
Major Process-Related Impurities
Purification Techniques
US20140357863A1 details a four-step purification:
- Aqueous wash : 3x with dichloromethane (removes 89% polar impurities).
- Solvent swap : MIBK to ethanol via vacuum distillation (ΔT = 50°C).
- Activated carbon treatment : 0.5% w/v, 60min contact time.
- Anti-solvent crystallization : Methyl tert-butyl ether (MTBE) added at 10°C.
This sequence reduces total impurities from 1.2% to 0.08% while maintaining yield at 86%.
Comparative Analysis of Industrial Methods
| Parameter | Boehringer (US7407955) | CN110684026A | Glenmark (WO2014033746) |
|---|---|---|---|
| Total Steps | 5 | 3 | 4 |
| Total Yield | 68% | 85% | 72% |
| Purity | 99.4% | 99.8% | 99.6% |
| Solvent Usage | 12 L/kg | 8 L/kg | 10 L/kg |
| Energy Cost | 1,200 kWh/batch | 850 kWh/batch | 950 kWh/batch |
The Chinese method achieves superior metrics through DMA-enabled kinetic enhancement and in-situ crystallization. Glenmark’s approach using dibenzoyl-D-tartarate salt adds 15% material cost but improves enantiomeric excess to 99.9%.
Chemical Reactions Analysis
Linagliptin undergoes various chemical reactions, including oxidation, reduction, and substitution. Common reagents used in these reactions include oxidizing agents, reducing agents, and catalysts . For example, this compound is particularly susceptible to degradation when exposed to acid and peroxide, leading to the formation of degradation products . Understanding these reactions is crucial for optimizing its formulation and ensuring its therapeutic effectiveness .
Scientific Research Applications
Management of Type 2 Diabetes Mellitus
Linagliptin is primarily indicated for the treatment of type 2 diabetes. It can be used as monotherapy or in combination with other antidiabetic agents. Clinical trials have demonstrated that this compound effectively lowers hemoglobin A1c levels with a low risk of hypoglycemia.
- Efficacy Comparison : In a study comparing this compound to glimepiride, both medications showed similar reductions in hemoglobin A1c, but this compound had a significantly lower incidence of hypoglycemia and cardiovascular events .
| Study | Treatment | HbA1c Reduction | Hypoglycemia Incidence |
|---|---|---|---|
| This compound vs Glimepiride | -0.16% | 1% | |
| This compound vs Glimepiride | -0.36% | 2% |
Cardiovascular Benefits
Recent studies suggest that this compound may offer cardiovascular protection. It has been associated with improved endothelial function and reduced cardiovascular events in patients with type 2 diabetes.
- Endothelial Function Improvement : A randomized study showed that after 16 weeks of treatment with this compound, patients exhibited significant improvements in flow-mediated dilation, indicating enhanced endothelial function .
Renal Protection
This compound has shown nephroprotective effects in preclinical models and clinical settings. It mitigates kidney fibrosis and improves albuminuria without altering glucose levels.
- Mechanisms : The protective effects are attributed to the suppression of pro-inflammatory cytokines and oxidative stress pathways .
| Study | Outcome | Findings |
|---|---|---|
| Kidney Health | Reduced kidney fibrosis and albuminuria in animal models | |
| In Vitro Studies | Inhibition of TGF-β activation |
Potential Use in COVID-19 Management
A clinical trial investigated the efficacy of this compound in hospitalized patients with type 2 diabetes and COVID-19. Although no significant difference was found compared to standard care, the study highlighted the need for further research into its immunomodulatory effects .
Case Study 1: Cardiovascular Outcomes
A long-term follow-up study involving over 3000 participants assessed the impact of this compound on major adverse cardiovascular events. The results indicated a lower incidence of cardiovascular complications compared to traditional therapies .
Case Study 2: Renal Health Impact
In a cohort study focusing on diabetic patients with renal impairment, this compound treatment was associated with significant reductions in markers of kidney damage, suggesting its role as a protective agent against diabetic nephropathy .
Mechanism of Action
Linagliptin exerts its effects by inhibiting the DPP-4 enzyme, which is responsible for the degradation of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) . By inhibiting DPP-4, this compound increases the levels of active incretin hormones, leading to increased insulin production and decreased glucagon production . This helps to regulate blood glucose levels in patients with type 2 diabetes .
Comparison with Similar Compounds
Linagliptin is one of several DPP-4 inhibitors used to treat type 2 diabetes. Other similar compounds include sitagliptin, saxagliptin, and alogliptin . Compared to these compounds, this compound has a unique pharmacokinetic profile, with a long terminal half-life and predominantly non-renal elimination . This allows for once-daily dosing without the need for dose adjustment in patients with renal impairment . Additionally, this compound has shown a sustained and maximal inhibition of DPP-4 activity, which is not observed with some other DPP-4 inhibitors .
Biological Activity
Linagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor primarily used in the management of type 2 diabetes mellitus. It has gained attention not only for its efficacy in glycemic control but also for its potential biological activities beyond glucose regulation. This article explores the biological activity of this compound, including its pharmacodynamics, pharmacokinetics, and emerging therapeutic roles.
Pharmacodynamics
Mechanism of Action
this compound selectively inhibits DPP-4, an enzyme that degrades incretin hormones, which are critical for insulin secretion and glucose homeostasis. The compound exhibits a potent inhibition profile with an IC50 value of 1 nM, outperforming other DPP-4 inhibitors like sitagliptin and saxagliptin . this compound's high selectivity for DPP-4 (over 10,000-fold compared to other dipeptidyl peptidases) enhances its therapeutic profile by minimizing off-target effects .
Biological Effects
Research indicates that this compound may exert antioxidant effects due to its xanthine-based structure, which is significant in reducing oxidative stress in various tissues . In experimental models of autoimmune myocarditis, this compound treatment resulted in a marked reduction in inflammatory cell infiltration and myocardial damage .
Table 1: Comparison of DPP-4 Inhibitors
| Compound | IC50 (nM) | Selectivity for DPP-4 |
|---|---|---|
| This compound | 1 | >10,000-fold |
| Sitagliptin | 19 | Not specified |
| Alogliptin | 24 | Not specified |
| Saxagliptin | 50 | Not specified |
| Vildagliptin | 62 | Not specified |
Pharmacokinetics
This compound demonstrates unique pharmacokinetic properties characterized by extensive tissue distribution and a nonlinear increase in tissue concentrations with rising doses. Following administration, this compound binds to DPP-4 primarily in tissues such as the kidney, liver, and lung . Its absorption is influenced by intestinal P-glycoprotein, indicating a complex interaction with gastrointestinal physiology .
Table 2: Pharmacokinetic Parameters of this compound
| Parameter | Value |
|---|---|
| Bioavailability | High |
| Half-life | ~12 hours |
| Volume of distribution | High |
| Tissue binding | Significant in DPP-4 rich tissues |
Case Study: this compound in COVID-19 Patients
A randomized clinical trial assessed the efficacy of this compound compared to standard care in hospitalized patients with diabetes and COVID-19. The study involved 64 participants who received either this compound (5 mg daily) or standard therapy. Although there was no significant difference in the time to clinical improvement between groups (7 days for this compound vs. 8 days for standard care), this compound was associated with lower in-hospital mortality rates .
Table 3: Clinical Outcomes from the COVID-19 Study
| Outcome | This compound Group (n=32) | Standard Care Group (n=32) |
|---|---|---|
| Median Time to Improvement | 7 days (IQR: 3.5–15) | 8 days (IQR: 3.5–28) |
| In-hospital Mortality | 15.6% | 25.0% |
Emerging Therapeutic Roles
Recent studies suggest potential applications of this compound beyond diabetes management. Its anti-inflammatory properties may be beneficial in conditions like autoimmune diseases and cardiovascular disorders due to its ability to modulate immune responses and reduce oxidative stress .
Q & A
Q. How can preclinical studies on this compound adhere to NIH guidelines for rigor and reproducibility?
- Methodological Answer : Follow ARRIVE 2.0 guidelines for animal studies, including randomization, blinding, and sample size justification. For in vitro work, document cell line authentication (e.g., STR profiling) and mycoplasma testing. Pre-register protocols on platforms like Open Science Framework to mitigate bias .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


