Misoprostol
Description
Historical Context of Misoprostol Development and Initial Therapeutic Applications
This compound was first synthesized in 1973 by the pharmaceutical company G.D. Searle & Company. ids.ac.ukresearchgate.net Its development was aimed at preventing and treating gastric ulcers, particularly those induced by non-steroidal anti-inflammatory drugs (NSAIDs). researchgate.netdrugbank.com The U.S. Food and Drug Administration (FDA) approved it for this indication in 1988. ids.ac.uk Marketed under the brand name Cytotec, this compound entered the global market in the mid-1980s for the treatment of gastric ulcers. nih.gov Its mechanism of action involves reducing the secretion of gastric acid from parietal cells. drugbank.com
The initial therapeutic application of this compound was narrowly focused on its gastroprotective effects. However, a significant side effect noted during its use was its ability to induce uterine contractions, a property that would later redefine its research and clinical profile. ids.ac.uknih.gov
Evolution of this compound's Research Profile Beyond Original Indications
The recognition of this compound's uterotonic (uterine-contracting) properties sparked a wave of research into its off-label applications in obstetrics and gynecology. researchgate.netnih.gov This evolution began in the late 1980s when medical professionals in Brazil observed that women using this compound to terminate pregnancies experienced fewer severe complications from incomplete abortions. nih.gov This discovery led to extensive investigation into its use for various reproductive health purposes.
Decades of research have since established the safety and efficacy of this compound for a range of "off-label" uses, including:
Medical Abortion: Used alone or in combination with mifepristone, this compound has been shown to be effective for first-trimester pregnancy termination. nih.govgynuity.org
Postpartum Hemorrhage (PPH): It is used for both the prevention and treatment of PPH, a leading cause of maternal mortality worldwide. nih.govnih.gov
Incomplete Abortion and Miscarriage Management: this compound is utilized for the medical management of incomplete abortions and miscarriages. nih.govgynuity.org
Labor Induction and Cervical Ripening: The drug is employed to induce labor and to ripen the cervix before surgical procedures. researchgate.netwikipedia.org
This expansion of research has been so significant that the World Health Organization (WHO) has included this compound on its Model List of Essential Medicines for several obstetric and gynecological indications. nih.govajrh.info
Interactive Data Table: Evolution of WHO Recommendations for this compound
| Year of Inclusion/Recommendation | Indication |
| 2005 | Labor Induction |
| 2005 | Medical abortion (in combination with mifepristone) |
| 2010 | Post-abortion care (PAC) |
| 2011 | Prevention of postpartum hemorrhage (PPH) |
| 2015 | Treatment of postpartum hemorrhage (PPH) |
| 2018 | Medical abortion (as an alternative to the combination regimen) |
Significance of this compound in Contemporary Medical Science and Global Health Research
This compound holds a place of considerable importance in modern medical science and global health, primarily due to its potential to address significant health challenges in low-resource settings. nih.gov Its advantages over other uterotonics like oxytocin include its stability at room temperature, ease of administration (oral, sublingual, vaginal, or rectal), and relatively low cost. nih.govajrh.infoThis compound.org These characteristics make it a particularly valuable tool in regions where access to healthcare facilities and refrigerated storage is limited. nih.govnih.gov
Research has demonstrated that community-based distribution of this compound by trained health workers can be a safe and effective strategy for preventing PPH. nih.govnih.gov This is especially crucial in areas with high rates of home births. ids.ac.uknih.gov Furthermore, its role in providing a non-surgical option for abortion and post-abortion care has the potential to significantly reduce maternal morbidity and mortality from unsafe procedures. nih.govresearchgate.net
The ongoing research into this compound continues to explore its optimal use, including comparative studies of different administration routes and its effectiveness in various clinical scenarios. This compound.orgibisreproductivehealth.org Its "social life" as a drug that has transitioned from a niche gastrointestinal medication to a life-saving tool in global maternal health underscores its dynamic and evolving significance. nih.govresearchgate.net
Interactive Data Table: Key Research Findings on this compound's Off-Label Applications
| Application | Key Research Finding |
| Medical Abortion (First Trimester) | When used alone, this compound is about 75-85% successful in inducing abortion in the first trimester. gynuity.org When combined with mifepristone, the effectiveness is higher. wikipedia.orgoup.com |
| Postpartum Hemorrhage (PPH) Prevention | Studies have shown that this compound is an effective alternative to oxytocin for preventing PPH. nih.gov Community-based distribution has been found to be a feasible and effective strategy. ids.ac.uknih.gov |
| Incomplete Abortion Management | This compound is as effective as manual vacuum aspiration (MVA) for treating incomplete abortion. nih.gov |
| Labor Induction | Randomized trials have confirmed that this compound is more effective than a placebo and other prostaglandins for labor induction at term. |
Properties
IUPAC Name |
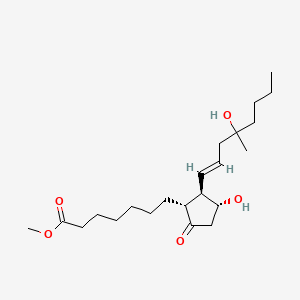
methyl 7-[(1R,2R,3R)-3-hydroxy-2-[(E)-4-hydroxy-4-methyloct-1-enyl]-5-oxocyclopentyl]heptanoate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H38O5/c1-4-5-14-22(2,26)15-10-12-18-17(19(23)16-20(18)24)11-8-6-7-9-13-21(25)27-3/h10,12,17-18,20,24,26H,4-9,11,13-16H2,1-3H3/b12-10+/t17-,18-,20-,22?/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OJLOPKGSLYJEMD-URPKTTJQSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCCC(C)(CC=CC1C(CC(=O)C1CCCCCCC(=O)OC)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCCCC(C)(C/C=C/[C@H]1[C@@H](CC(=O)[C@@H]1CCCCCCC(=O)OC)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H38O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7020897 | |
| Record name | Misoprostol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7020897 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
382.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Liquid | |
| Record name | Misoprostol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015064 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.6mg/mL, Water-soluble, 1.64e-02 g/L | |
| Record name | Misoprostol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00929 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MISOPROSTOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3573 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Misoprostol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015064 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Light yellow oil, Viscous liquid | |
CAS No. |
59122-46-2 | |
| Record name | Misoprostol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=59122-46-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Misoprostol [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0059122462 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Misoprostol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00929 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Misoprostol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7020897 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Misoprostol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MISOPROSTOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0E43V0BB57 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | MISOPROSTOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3573 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Misoprostol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015064 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
261-263 | |
| Record name | Misoprostol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00929 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Pharmacology and Molecular Mechanisms of Misoprostol
Classification as a Prostaglandin E1 Analog
Misoprostol is classified as a synthetic analog of prostaglandin E1 (PGE1). wikipedia.orgfebrasgo.org.brpatsnap.commdedge.com Prostaglandins are lipid compounds derived from fatty acids that act as autocrine and paracrine mediators in numerous physiological and pathophysiological processes. patsnap.comnih.gov PGE1, also known as alprostadil, is a naturally occurring prostaglandin. mdedge.com this compound is structurally similar to PGE1 but includes modifications, such as a methyl ester group at carbon-1 and a methyl and hydroxyl group at carbon-16, which enhance its oral activity and increase its duration of action compared to natural PGE1. mdedge.com Following absorption, this compound is rapidly de-esterified to its active metabolite, this compound acid. mdedge.comdrugbank.commims.comnih.gov
Receptor-Level Interactions and Agonist Activity
This compound, through its active metabolite, primarily acts as an agonist at specific E-prostanoid (EP) receptors, which are G protein-coupled receptors. wikipedia.orgnih.govdrugbank.comgenome.jp The four main subtypes of EP receptors are EP1, EP2, EP3, and EP4. This compound has been shown to bind to and stimulate EP2, EP3, and EP4 receptors, but not the EP1 receptor. wikipedia.org
Prostaglandin EP2 Receptors
This compound binds to and stimulates prostaglandin EP2 receptors. wikipedia.org EP2 receptors are typically coupled to the stimulatory G protein (Gs), and their activation leads to the activation of adenylyl cyclase and a subsequent increase in intracellular cyclic adenosine monophosphate (cAMP) levels. oncotarget.comyorku.ca Activation of EP2 receptors can have various effects depending on the tissue, including smooth muscle relaxation and modulation of immune responses. drugbank.comwikipedia.org
Prostaglandin EP3 Receptors
This compound binds to and stimulates prostaglandin EP3 receptors. wikipedia.org The EP3 receptor is a key target for this compound's actions, particularly in inducing uterine contractions and inhibiting gastric acid secretion. mdedge.comnih.govderangedphysiology.com EP3 receptors are unique among EP receptors as they can couple to multiple G proteins, including Gi, Gs, and Gq, leading to diverse downstream signaling effects depending on the splice variant and cellular context. oncotarget.comyorku.ca Activation of Gi-coupled EP3 receptors inhibits adenylyl cyclase, leading to a decrease in intracellular cAMP. oncotarget.comderangedphysiology.com EP3 receptors also play a role in calcium mobilization through pathways involving phosphoinositol turnover. mdedge.com
Prostaglandin EP4 Receptors
This compound also binds to and stimulates prostaglandin EP4 receptors. wikipedia.org Similar to EP2 receptors, EP4 receptors are primarily coupled to the stimulatory G protein (Gs), and their activation leads to increased intracellular cAMP levels via adenylyl cyclase activation. oncotarget.comyorku.ca EP4 receptor activation has been implicated in various physiological processes, including smooth muscle relaxation, immune modulation, and potential roles in cell proliferation and differentiation. drugbank.comresearchgate.net
Differentiation from Prostaglandin E2 Receptor Activation
While this compound activates EP2, EP3, and EP4 receptors, it is a synthetic analog of PGE1 and its receptor binding profile can differ from that of the naturally occurring prostaglandin E2 (PGE2). wikipedia.orgyorku.ca PGE2 acts on all four EP receptors (EP1, EP2, EP3, and EP4) with varying affinities, generally showing higher affinity for EP3 and EP4 compared to EP1 and EP2. oncotarget.com this compound, on the other hand, does not activate the EP1 receptor. wikipedia.org Although this compound can activate the same receptors as PGE2, its affinities for these receptors are generally lower than those of PGE2. oncotarget.comyorku.ca This differential receptor activation profile contributes to this compound's specific pharmacological effects and may result in a more restricted range of actions compared to PGE2 or other analogs that activate all four EP receptors. wikipedia.org
Intracellular Signaling Pathways Modulated by this compound
The binding of this compound to EP receptors triggers various intracellular signaling pathways mediated by their coupled G proteins.
cAMP Signaling Pathway: Activation of EP2 and EP4 receptors, which are Gs-coupled, leads to the stimulation of adenylyl cyclase and an increase in intracellular cAMP levels. oncotarget.comyorku.canih.gov Conversely, activation of Gi-coupled EP3 receptors inhibits adenylyl cyclase, resulting in decreased cAMP levels. oncotarget.comderangedphysiology.com The modulation of cAMP levels by this compound can influence the activity of protein kinase A (PKA), which in turn phosphorylates various downstream targets, including the transcription factor CREB. yorku.canih.gov Increased cAMP and PKA activity can modulate gene expression and cellular functions.
Phosphoinositol Turnover and Calcium Mobilization: Activation of EP3 receptors can also lead to an increase in intracellular calcium concentration, potentially mediated through Gq protein coupling and the activation of phospholipase C, which generates inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). mdedge.comyorku.ca IP3 can trigger the release of calcium from intracellular stores. yorku.ca This increase in intracellular calcium is particularly relevant to this compound's effects on smooth muscle contraction, such as in the uterus. mdedge.com
Other Pathways: this compound's interaction with EP receptors can also influence other signaling pathways, including those involving protein kinase A (PKA)-dependent mechanisms that affect intracellular calcium levels yorku.ca, and potentially pathways related to NFκB activity nih.gov and the WNT signaling pathway through EP4 receptor activation researchgate.net. The specific intracellular response depends on the EP receptor subtype activated and the cellular context.
Summary of this compound's Receptor Interactions and Signaling Pathways:
| Receptor Subtype | Primary G Protein Coupling | Key Intracellular Effect(s) |
| EP1 | Gq | Increased intracellular Ca²⁺ (this compound does not activate) wikipedia.orgyorku.ca |
| EP2 | Gs | Increased cAMP oncotarget.comyorku.canih.gov |
| EP3 | Gi, Gs, Gq | Decreased cAMP (Gi), Increased cAMP (Gs), Increased Ca²⁺ (Gq) mdedge.comoncotarget.comyorku.ca |
| EP4 | Gs | Increased cAMP oncotarget.comyorku.canih.gov |
Adenylate Cyclase Inhibition and Cyclic AMP Levels in Parietal Cells
In gastric parietal cells, this compound primarily exerts its inhibitory effect on acid secretion by interfering with the adenylate cyclase signaling pathway. nih.govnih.gov Histamine stimulates acid secretion by activating histamine H2 receptors, which are coupled to stimulatory G proteins (Gs) that activate adenylate cyclase, leading to an increase in intracellular cyclic AMP (cAMP) levels. researchgate.net this compound, acting as a prostaglandin analog, binds to prostaglandin receptors (likely EP-3 receptors) on parietal cells. These receptors are coupled to inhibitory G proteins (Gi), which, upon activation by this compound, inhibit adenylate cyclase activity. nih.govresearchgate.net This inhibition leads to a reduction in the formation of cAMP. nih.govnih.gov Studies have shown that this compound noncompetitively inhibits cAMP formation in response to histamine in isolated parietal cells, with an IC50 value similar to that for the inhibition of histamine-stimulated acid secretion. nih.gov This suggests a close relationship between the antisecretory action of this compound and its ability to reduce histamine-stimulated cAMP formation, likely by interfering with the coupling between histamine H2 receptors and adenylate cyclase. nih.gov
Phosphoinositol Turnover and Calcium Mobilization in Myometrial Cells
In myometrial cells, this compound binding to EP-3 receptors induces an increase in intracellular phosphoinositol turnover and calcium mobilization. mdedge.commdedge.comthe-hospitalist.org This process leads to an increase in intracellular free calcium, which is a critical step in triggering actin-myosin contractility in smooth muscle cells. mdedge.commdedge.comthe-hospitalist.org The increase in intracellular calcium is propagated between myometrial cells through gap junctions, facilitating coordinated contractions. mdedge.commdedge.comthe-hospitalist.org While EP1 receptor activation is also associated with increased phosphoinositol turnover and calcium mobilization leading to smooth muscle contraction, EP3 receptors are known to inhibit adenylate cyclase and cAMP production via Gi proteins. researchgate.net
VEGF Signaling Pathway Modulation
Network pharmacology studies suggest that this compound can modulate the VEGF signaling pathway. nih.govajrh.info The VEGF signaling pathway is crucial for angiogenesis, regulating endothelial cell proliferation, migration, and survival. mdpi.complos.org While some research indicates that the action of mifepristone plus this compound on blood vessels in human decidua may be mediated by factors other than VEGF, network analysis points to a potential interaction. nih.govajrh.infonih.gov The VEGF pathway involves the binding of VEGF to its receptors (VEGFRs), leading to the activation of downstream signaling cascades such as Raf-MEK-MAPK, PI3K/AKT, and ERK1/2/FAK, which influence endothelial cell function. mdpi.commdpi.com HSP90AA1, identified as a core target of this compound, has been shown to play a role in VEGF-mediated signaling by interacting with eNOS and promoting PI3K/AKT pathway activation, which is essential for endothelial cell survival. mdpi.com
Calcium Signaling Pathway Modulation
This compound has been shown to modulate calcium signaling pathways in various cell types. In myometrial cells, it increases intracellular calcium leading to contraction. mdedge.commdedge.comthe-hospitalist.org In neuronal-type cells, this compound can elevate intracellular calcium levels via a protein kinase A (PKA)-mediated pathway, with the EP4 receptor potentially playing an inhibitory role. yorku.canih.gov Furthermore, research in neonatal cardiomyocytes suggests that this compound can attenuate hypoxia-induced proliferation by influencing perinuclear calcium signaling and promoting nuclear calcium accumulation. researchgate.netahajournals.orgresearchgate.net This involves the modulation of Bnip3, a Bcl-2 family member, and its splice variants, which affect calcium transfer between the sarcoplasmic/endoplasmic reticulum and mitochondria. researchgate.netbiorxiv.org
NF-κB Signaling Pathway Modulation
Studies indicate that this compound can modulate the NF-κB signaling pathway. nih.govajrh.inforesearchgate.netnih.gov NF-κB is a key transcription factor involved in inflammatory responses and the regulation of various genes, including cytokines like TNF-α and IL-10. nih.govnih.gov this compound's anti-inflammatory actions have been linked to E2 prostanoid receptor-mediated increases in cAMP, activation of protein kinase A (PKA), and subsequent attenuation of NF-κB transcriptional activity. researchgate.net While this compound may not affect NF-κB nuclear translocation, it can significantly decrease transcriptional stimulation by NF-κB. nih.gov Chromatin immunoprecipitation studies have demonstrated that this compound treatment can alter transcription factor and RNA Polymerase II promoter binding, leading to changes in TNF-α and IL-10 mRNA levels. researchgate.netnih.govnih.gov
Multi-Target Pharmacological Network Analysis of this compound
Network pharmacology approaches have been employed to analyze the multi-target pharmacological network of this compound, particularly in the context of its use for pregnancy termination. nih.govajrh.inforesearchgate.net This involves identifying known and potential targets of this compound and constructing protein-protein interaction networks to understand the complex interplay of these targets and associated pathways. nih.govajrh.info
Identification of Core Therapeutic Targets (e.g., HSP90AA1, EGFR, MAPK1)
Network pharmacology studies have identified core therapeutic targets of this compound. nih.govajrh.inforesearchgate.net Among these core targets are Heat Shock Protein 90 Alpha A1 (HSP90AA1), Epidermal Growth Factor Receptor (EGFR), and Mitogen-Activated Protein Kinase 1 (MAPK1). nih.govajrh.inforesearchgate.net These targets are involved in various cellular processes, including protein phosphorylation, cell localization, and protein hydrolysis regulation processes. nih.govajrh.info Their modulation by this compound is suggested to contribute to its pharmacological effects, including its influence on pathways such as VEGF signaling, calcium signaling, and NF-κB signaling. nih.govajrh.info
Data Table: Core Therapeutic Targets of this compound
| Target Protein | Gene Name | Role in Cellular Processes | Relevant Pathways Modulated by this compound |
| HSP90AA1 | HSP90AA1 | Protein folding, stability, and activation of client proteins | VEGF signaling pathway |
| EGFR | EGFR | Cell growth, proliferation, differentiation, and survival | |
| MAPK1 | MAPK1 | Signal transduction, cell proliferation, differentiation | VEGF signaling pathway, Calcium signaling, NF-κB signaling |
Note: This table summarizes information from network pharmacology studies identifying core targets and their potential involvement in pathways modulated by this compound. nih.govajrh.inforesearchgate.netmdpi.com
Functional and Pathway Enrichment Analyses (GO and KEGG)
Network pharmacology and molecular docking studies have been employed to investigate the multi-target mechanisms of this compound, particularly in the context of pregnancy termination. ajrh.infonih.gov These analyses aim to identify the key genes and pathways involved in this compound's effects.
Gene Ontology (GO) functional and KEGG pathway enrichment analyses have shown that this compound can modulate several signaling pathways. ajrh.infonih.gov These include the VEGF signaling pathway, calcium signaling pathway, and NF-κB signaling pathway. ajrh.infonih.gov The analyses suggest that this compound primarily interferes with processes such as protein phosphorylation, cell localization, and protein hydrolysis regulation. ajrh.infonih.gov
Research utilizing transcriptomics and proteomics in animal models has also applied GO and pathway enrichment analyses to understand the effects of medical abortion regimens including this compound. nih.gov These studies can reveal changes in gene and protein expression in uterine tissues and highlight affected pathways, such as cholesterol metabolism, arginine biosynthesis, cell apoptosis, and the FoxO signaling pathway. researchgate.net
Uterotonic Mechanisms
This compound's uterotonic properties are central to its use in obstetrics and gynecology. drugbank.compatsnap.comnih.govogmagazine.org.au These effects are mediated through several mechanisms acting on uterine and cervical tissue. drugbank.com
Direct Stimulation of Uterine Smooth Muscle Contraction
This compound directly stimulates the contraction of smooth muscle cells in the uterine lining. drugbank.compatsnap.comuct.ac.za This is primarily achieved through the activation of prostaglandin EP receptors on myometrial cells. wikipedia.orgdrugbank.compatsnap.commdedge.comthieme-connect.de Activation of EP1 and EP3 receptors generally leads to smooth muscle contraction, while EP2 and EP4 receptors are typically associated with relaxation. conicet.gov.arthieme-connect.denih.govutoronto.ca this compound's binding to EP2, EP3, and EP4 receptors contributes to increased uterine contractility. wikipedia.orgthieme-connect.deutoronto.ca
The binding of this compound to myometrial EP-3 receptors, for example, induces an increase in intracellular phosphoinositol turnover and calcium mobilization. mdedge.com This rise in intracellular free calcium triggers the interaction of actin and myosin filaments, leading to muscle contraction. patsnap.comogmagazine.org.aumdedge.com
Collagen Degradation in Cervical Tissue
This compound contributes to cervical ripening, a process involving the softening and dilation of the cervix. wikipedia.orgdrugbank.com A key aspect of cervical ripening is the degradation of collagen in the connective tissue stroma of the cervix. drugbank.comresearchgate.netmdpi.com
Studies suggest that this compound softens the cervix, partly through an inflammatory response that leads to collagen degradation. researchgate.netmdpi.com This involves the release of matrix metalloproteinases (MMPs), such as MMP-8 and MMP-9, which break down collagen fibers. mdedge.comresearchgate.netmdpi.com Electron microscopy studies have shown that this compound treatment can result in pronounced splitting and disorganization of collagen fibers in cervical tissue. mdedge.com This process is similar to the changes observed during natural cervical ripening at term pregnancy and may involve the influx and activation of inflammatory cells. researchgate.netmdpi.com
Gap Junction Modulation in Myometrial Cells
The coordinated and efficient contraction of the myometrium during labor requires electrical synchrony between myometrial cells. ogmagazine.org.auuct.ac.zaiiarjournals.orgijabbr.com This synchrony is facilitated by gap junctions, which are low-resistance pathways allowing the flow of ions and small molecules between adjacent cells. ogmagazine.org.auuct.ac.zaiiarjournals.org
Preclinical Research on Misoprostol
In Vitro Studies of Cellular and Molecular Effects
In vitro research has explored the direct effects of misoprostol on various cell types, providing insights into its molecular mechanisms of action. This compound acts as an agonist for E-prostanoid (EP) receptors, particularly EP2, EP3, and EP4 receptors. researchgate.netwho.int Binding to these receptors can lead to an increase in intracellular cAMP levels, which in turn can modulate various cellular processes. researchgate.net
Studies on gastric parietal cells have shown that this compound acid, the active metabolite of this compound, binds to specific prostaglandin receptors, leading to the inhibition of gastric acid secretion. hres.cafda.gov Additionally, this compound has been shown to stimulate the secretion of mucus and bicarbonate in the gastrointestinal tract, contributing to mucosal protection. hres.cadiva-portal.org
Beyond its effects on the gastrointestinal system, in vitro studies have also investigated the immunomodulatory properties of this compound. In human and equine leukocytes, this compound has been demonstrated to modulate the production of pro-inflammatory cytokines. For instance, this compound reduced LPS-induced TNF-α and IL-6 protein production in equine leukocytes and inhibited their mRNA production. researchgate.net It also enhanced IL-1β protein synthesis under certain conditions while inhibiting it under others. researchgate.net In human subjects, this compound reduced LPS-inducible TNF and increased the production of the anti-inflammatory cytokine IL-10. researchgate.net These effects on cytokine expression are correlated with the activation of cAMP/PKA signaling and subsequent changes in CRE and NF-κB activity. researchgate.net
Furthermore, this compound has been shown in vitro to suppress macrophage TNF-α and chemokine generation following challenge with Clostridium sordellii or peptidoglycan. nih.gov It also impaired leukocyte phagocytosis of C. sordellii and inhibited uterine epithelial cell human β-defensin expression. nih.gov These immunosuppressive effects were linked to the activation of Gs protein-coupled EP2 and EP4 receptors in macrophages and EP4 receptors alone in uterine epithelial cells. nih.gov
In Vivo Animal Model Investigations
In vivo studies using various animal models have been conducted to assess the systemic effects and potential toxicities of this compound. These investigations provide crucial data on acute and chronic toxicity, mutagenicity, carcinogenicity, and reproductive and developmental effects.
Toxicity Assessments (Acute and Chronic)
Acute toxicity studies in rodents and non-rodents have shown that the predominant sign of toxicity following single oral administration is diarrhea. hres.catga.gov.au Threshold doses for diarrhea varied among species, with values of 1.6 mg/kg in mice, 0.3 mg/kg in rats and rabbits, and 0.03 mg/kg in dogs. tga.gov.au Other acute toxic effects observed in animals are similar to those reported for other prostaglandins, including relaxation of smooth muscle, respiratory difficulties, and depression of the central nervous system. who.inthres.ca Microscopic changes in the stomach, such as hypertrophy of mucous cells and deepening of gastric pits, were also observed in single and repeat dose studies. tga.gov.au
Chronic toxicity studies, with durations up to 52 weeks in dogs and 2 years in rats, have also been conducted. tga.gov.aunih.gov Daily oral doses of up to 300 µg/kg in dogs and 9000 µg/kg in rats were evaluated. nih.gov Increased rectal temperatures were noted in dogs at doses of 100 and 300 µg/kg, while increased serum iron was observed in rats at 9000 µg/kg. nih.gov Increased stomach weights were seen in both dogs and rats in a dose-correlated manner, partly attributed to an increase in the number of normal epithelial cells (gastric hyperplasia). who.intnih.gov These gastric effects were reversible upon cessation of drug treatment. nih.gov Hyperostosis, primarily in the medulla of sternebrae, was observed in female mice following prolonged high-dose treatment, but this finding was not noted in long-term studies in dogs or rats and has not been seen in humans. who.intfda.govnih.govpfizermedicalinformation.com
Table 1: Summary of Acute Toxicity Findings in Animal Models
| Species | Route of Administration | Predominant Sign of Toxicity | Threshold Dose for Diarrhea |
| Mouse | Oral | Diarrhea | 1.6 mg/kg |
| Rat | Oral | Diarrhea | 0.3 mg/kg |
| Rabbit | Oral | Diarrhea | 0.3 mg/kg |
| Dog | Oral | Diarrhea | 0.03 mg/kg |
Note: This table is intended to be interactive in a suitable rendering environment.
Table 2: Summary of Chronic Toxicity Findings in Animal Models
| Species | Duration | Dose Range (µg/kg/day) | Key Findings | Reversibility |
| Dog | Up to 52 weeks | 30 - 1000 | Increased rectal temperature, increased stomach weight (gastric hyperplasia) | Yes |
| Rat | Up to 2 years | 24 - 9000 | Increased serum iron, increased stomach weight (gastric hyperplasia) | Yes |
| Mouse | Up to 21 months | Up to 16000 | Hyperostosis (high doses, female mice) | Not specified |
Note: This table is intended to be interactive in a suitable rendering environment.
Mutagenicity and Carcinogenicity Studies
Evaluations of the mutagenic potential of this compound have been conducted using a battery of in vitro and in vivo assays. These studies, including tests for gene mutations and chromosomal damage, have consistently yielded negative results, indicating no evidence of genotoxicity. who.inttga.gov.aunih.govtga.gov.auhres.ca
Long-term carcinogenicity studies have been performed in rats and mice to assess the potential of this compound to induce tumors. In a 2-year study in rats, oral doses up to 2.4 mg/kg/day showed no evidence of an effect on tumor occurrence or incidence. tga.gov.aupfizermedicalinformation.comtga.gov.aunih.gov Similarly, a 21-month study in mice with oral doses up to 16 mg/kg/day did not reveal any tumorigenic effects. tga.gov.aupfizermedicalinformation.comtga.gov.au These doses represent significant multiples of typical human exposures. tga.gov.aupfizermedicalinformation.comtga.gov.au The most significant non-neoplastic finding in the rat carcinogenicity study was epithelial hyperplasia and hyperkeratosis of the gastric mucosa, which is considered characteristic of some prostaglandins. nih.gov
Reproductive Toxicity and Teratogenicity Mechanisms
Reproductive toxicity studies in animal models have demonstrated embryotoxicity and teratogenic potential for this compound, particularly at higher doses administered during organogenesis. tga.gov.autga.gov.aunih.gov Increased resorptions (embryotoxicity) were observed in rabbits at 1 mg/kg/day, in rats at 10 mg/kg/day, and in mice at 30 mg/kg/day when administered orally during the period of organogenesis. tga.gov.autga.gov.au
Teratogenic effects, such as an increased incidence of skeletal abnormalities, were noted in rabbits at 1 mg/kg/day, possibly due to maternal toxicity. tga.gov.autga.gov.au In mice, an increased incidence of skeletal abnormalities and cleft palate was observed at 30 mg/kg/day. tga.gov.au This dose in mice was found to be embryotoxic and teratogenic. tga.gov.au While some studies in rats and rabbits at certain dose levels did not find fetotoxic or teratogenic effects, other studies with prostaglandins have shown a dose-related increase in fetal malformations in rats. nih.govfda.gov
The mechanism underlying the observed reproductive and teratogenic effects in animals is thought to involve the pharmacological activity of this compound on uterine smooth muscle and the subsequent impact on fetal blood flow and development. nih.govnih.govwindows.netmedcraveonline.com
This compound is known to induce contractions of the smooth muscle fibers of the myometrium. who.intmims.com In pregnant animals, these intense uterine contractions can lead to compression of uterine and embryonic vessels. nih.govnih.govwindows.netresearchgate.netclinicalpub.comnih.gov This compression can disrupt blood flow to the placenta and the developing fetus, resulting in vascular disruption. researchgate.netpopcouncil.org
Animal studies indicate that temporary constriction of the uterine arteries, potentially caused by exposure to agents like this compound, can lead to hypoxic episodes in the embryo. clinicalpub.comresearchgate.net This vascular disruption and reduced blood flow are considered a plausible mechanism for the observed developmental abnormalities. medcraveonline.comresearchgate.netpopcouncil.org Experimental studies involving uterine manipulations in pregnant mice have demonstrated that such interventions during early gestation can result in hemorrhagic disruption of fetal structures, mediated by fetal hypoxia. clinicalpub.com
The disruption of fetal vascular supply due to this compound-induced uterine contractions can lead to embryonic hypoperfusion, characterized by decreased blood flow to fetal tissues. nih.govresearchgate.netresearchgate.net This reduced blood flow can result in tissue hypoxia, a state of oxygen deprivation. nih.govnih.govwindows.netresearchgate.netclinicalpub.comnih.gov
Hypoxia in the developing embryo can cause endothelial cell damage, hemorrhage, tissue loss, and subsequent abnormal development or structural abnormalities. researchgate.net The specific malformations observed can depend on the developmental stage at which the hypoxic event occurs. nih.govresearchgate.net Animal studies have shown that a single period of embryonic hypoxia can induce malformations similar to those associated with this compound exposure. nih.govwindows.netclinicalpub.com
In vitro studies using rat post-implantation embryo culture have shown that this compound can directly induce embryotoxic effects in a dose-dependent manner, leading to decreased embryo viability and function, including poor vascular development. researchgate.net Morphometric alterations were also observed in structures such as branchial arches, heart, and cephalic portions of the neural tube. researchgate.net These findings support the hypothesis that this compound can directly impact embryonic development, potentially through mechanisms related to vascular function and oxygenation.
Table 3: Summary of Reproductive Toxicity Findings in Animal Models
| Species | Route of Administration | Dosing Period | Key Findings | Relevant Dose (mg/kg/day) |
| Rabbit | Oral | Organogenesis | Increased resorptions, skeletal abnormalities (possibly maternal toxicity) | 1 |
| Rat | Oral | Organogenesis | Increased resorptions | 10 |
| Mouse | Oral | Organogenesis | Increased resorptions, skeletal abnormalities, cleft palate | 30 |
| Rat | Oral | Breeding | Dose-related pre- and post-implantation losses, decreased live pups (high dose) | 0.1 - 10 |
Note: This table is intended to be interactive in a suitable rendering environment.
Brainstem Ischemia and Limb Development Anomalies
Preclinical observations and studies in humans have suggested a link between in utero exposure to this compound and certain developmental anomalies, including those related to brainstem ischemia and limb development. The mechanism proposed involves uterine contractions induced by this compound, which can reduce blood flow to the uterine arteries, potentially leading to ischemic and hypoxic damage to the brainstem and contributing to limb development anomalies nih.gov.
Limb development anomalies associated with this compound exposure are often classified as "transverse," characterized by the absence of the distal part of a limb below the point where the aplasia begins nih.gov. These can include conditions such as arthrogryposis, clubfoot, distal reduction anomalies, constriction rings, syndactyly, or camptodactyly nih.gov. Studies have indicated that in utero exposure to this compound may increase the risk of developing transverse limb reduction anomalies nih.govnih.gov. Some research suggests this risk could be significantly higher compared to the general population nih.gov.
In a study involving infants exposed to this compound during the first trimester, deformities attributed to vascular disruption were observed researchgate.netcapes.gov.br. The uterine contractions induced by this compound are suggested to cause vascular disruption in the fetus, potentially leading to brainstem ischemia researchgate.netcapes.gov.br. The critical period for the pathogenesis of certain anomalies, such as Moebius sequence, is the first trimester, likely between gestational weeks four and eight scielo.br.
Moebius Sequence and Terminal Transverse Limb Defects
Moebius sequence is a rare congenital neurological disorder characterized by weakness or paralysis of multiple cranial nerves, most commonly the 6th (abducens) and 7th (facial) cranial nerves scielo.brpaojournal.com. This results in characteristic facial weakness and limited eye movement paojournal.com. Terminal transverse limb defects involve the absence of the end portion of a limb nih.gov.
Prenatal exposure to this compound has been associated with an increased risk of both Moebius sequence and terminal transverse limb defects nih.govresearchgate.net. Research, including systematic reviews and meta-analyses, has estimated a significantly increased odds ratio for these conditions following prenatal this compound exposure nih.govresearchgate.net.
The proposed mechanism linking this compound to Moebius sequence and terminal transverse limb defects involves vascular disruption during critical periods of embryonic development researchgate.netcapes.gov.brscielo.brpaojournal.com. Uterine contractions induced by this compound are thought to compromise blood flow, leading to ischemic damage in developing tissues, particularly the cranial nerve nuclei and limb buds nih.govresearchgate.netcapes.gov.brpaojournal.com. This vascular disruption sequence is considered a potential explanation for the observed pattern of anomalies scielo.brpaojournal.com.
Data from studies investigating congenital abnormalities in children exposed to this compound in utero have reported cases presenting with terminal transverse limb defects with or without Moebius sequence researchgate.netcapes.gov.br.
Neuroprotective Research in Animal Models (e.g., Alzheimer's Disease Models)
Preclinical research in animal models has explored the potential neuroprotective effects of this compound, particularly in the context of neurodegenerative conditions like Alzheimer's disease.
Studies using animal models, such as rats with chronic aluminum overload or APP/PS1 transgenic mice (models for Alzheimer's disease), have investigated the effects of this compound on neuronal health and cognitive function nih.govnih.govoncotarget.comresearchgate.netingentaconnect.com.
Modulation of Microsomal PGE2 Synthase (mPGES-1)
Microsomal prostaglandin E2 synthase-1 (mPGES-1) is a key enzyme involved in the synthesis of prostaglandin E2 (PGE2), a lipid mediator implicated in inflammation and various physiological processes nih.govresearchgate.net. PGE2 and its receptors (EP receptors) play a role in the central nervous system nih.govnih.govoncotarget.comresearchgate.netingentaconnect.com.
Research in APP/PS1 mice has shown that this compound administration can influence the expression of mPGES-1 and PGE2 receptors nih.govoncotarget.com. In these models, this compound was observed to reverse changes in the expression of mPGES-1, PGE2, and specific EP receptors (EP2, EP3, and EP4) nih.govoncotarget.com. Specifically, this compound administration decreased the expression of mPGES-1, PGE2, EP2, and EP4, while increasing EP3 expression in APP/PS1 mice nih.govoncotarget.com. These findings suggest that this compound's neuroprotective effects in this model may involve the modulation of the mPGES-1-PGE2-EP signaling pathway, particularly the activation of PGE2-EP3 signaling nih.govoncotarget.com.
Studies in aluminum-overload rats also indicated that this compound treatment could affect the PGES-PGE2-EP signal pathway, leading to a decrease in PGE2 levels and down-regulation of mPGES-1, EP2, and EP4 expression, alongside an up-regulation of EP3 expression nih.govresearchgate.netingentaconnect.com. This suggests a potential mechanism involving the rebuilding of the mPGES-1-PGE2-EP1-4 signal pathway balance nih.govingentaconnect.com.
Effects on Oxidative Stress Markers (e.g., SOD, MDA)
Oxidative stress is a significant factor in the pathogenesis of neurodegenerative diseases like Alzheimer's disease nih.govnih.gov. Markers such as superoxide dismutase (SOD) activity and malondialdehyde (MDA) content are commonly used to assess oxidative stress levels nih.govnih.govresearchgate.netmdpi.com. SOD is an important antioxidant enzyme, while MDA is an end-product of lipid peroxidation, indicating neuronal degeneration nih.govnih.gov.
Preclinical studies have investigated the effects of this compound on oxidative stress markers in animal models of neurodegeneration and other conditions. In APP/PS1 mice, this compound administration significantly reversed the decrease in SOD activity and the increase in MDA content observed in the hippocampus and cortex nih.govoncotarget.com. This suggests that this compound can reduce oxidative stress in the brain in this Alzheimer's disease model nih.govoncotarget.com.
Similarly, studies in rats with chronic aluminum overload showed that this compound treatment decreased MDA levels and increased SOD activity in hippocampal brain tissue, indicating a deceleration of oxidative stress nih.govresearchgate.netingentaconnect.com. This compound has also been shown to alleviate oxidative stress in the brain in models of systemic inflammation by decreasing cerebral MDA content and increasing antioxidant activity nih.govoncotarget.com.
Beyond neurodegeneration models, this compound has demonstrated effects on oxidative stress markers in other preclinical settings. For instance, studies on kidney injury in rats have shown that this compound treatment can lead to lower levels of MDA and higher activity of SOD and other antioxidant enzymes researchgate.netresearchgate.net. This suggests a broader antioxidative efficacy of this compound researchgate.net.
Clinical Research Applications and Efficacy of Misoprostol
Obstetric and Gynecological Research
Labor Induction Studies
Misoprostol has been widely studied as an agent for inducing labor, with research focusing on its comparison to established methods, its impact on the duration of labor, and its effectiveness in preparing the cervix for delivery.
Clinical trials have consistently compared the efficacy of this compound with that of oxytocin and dinoprostone, the standard agents for labor induction.
Research indicates that oral this compound is more effective than a placebo and shows equivalency to intravenous oxytocin, vaginal this compound, and vaginal dinoprostone for labor induction. utoronto.ca When compared directly with oxytocin, particularly in cases of prelabor rupture of membranes (PROM), oral this compound has been shown to significantly shorten the induction-to-delivery time. barpetaogs.co.in Multiple studies have reported a shorter duration of labor with this compound compared to oxytocin. barpetaogs.co.in However, one meta-analysis noted that while vaginal this compound was more effective than oxytocin in achieving vaginal delivery within 24 hours, it was also associated with a higher risk of uterine hyperstimulation. nih.gov
In comparisons with dinoprostone, a prostaglandin E2 analogue, this compound has demonstrated several advantages. A randomized study found that vaginal this compound resulted in a significantly shorter median induction-to-delivery interval compared to dinoprostone gel (14.6 hours vs. 19.0 hours). fetalmedicine.org This study also noted a higher rate of vaginal delivery within 24 hours and a reduced need for oxytocin augmentation in the this compound group. fetalmedicine.org Another meta-analysis of seven randomized trials concluded that this compound use led to an approximately 50% reduction in the need for oxytocin augmentation compared to dinoprostone. ufrgs.br While cesarean section rates were generally comparable between the two groups in several studies, some research suggests a lower requirement for oxytocin with this compound. fetalmedicine.orgufrgs.brfrontiersin.org
Table 1: Comparison of this compound with Oxytocin and Dinoprostone for Labor Induction
| Comparison Agent | Key Efficacy Findings | Citations |
|---|---|---|
| Oxytocin | Oral this compound is as effective as intravenous oxytocin. In cases of PROM, oral this compound leads to a shorter induction-to-delivery time. Vaginal this compound is more effective in achieving vaginal delivery within 24 hours. | utoronto.cabarpetaogs.co.innih.gov |
| Dinoprostone | Vaginal this compound results in a shorter induction-to-delivery interval and a higher rate of vaginal delivery within 24 hours. There is a significantly reduced need for oxytocin augmentation with this compound. | fetalmedicine.orgufrgs.brfrontiersin.org |
A significant focus of research on this compound for labor induction has been its effect on the time from the start of induction to delivery.
Numerous studies have demonstrated that this compound can significantly shorten the induction-to-delivery interval. barpetaogs.co.infetalmedicine.org In a study involving women with PROM, the induction-to-delivery time was significantly less in the this compound group (309.15 ± 57.74 minutes) compared to the oxytocin group (367.45 ± 78.88 minutes). barpetaogs.co.in Similarly, another trial reported a mean induction-to-delivery interval of 13.8 hours with vaginal this compound. jogcr.com When compared with dinoprostone, this compound was associated with a shorter median induction-to-delivery interval (14.6 hours versus 19.0 hours). fetalmedicine.org A study comparing different doses of intravaginal this compound with intracervical dinoprostone found the induction-to-vaginal-delivery interval to be significantly lower with a 50 µg dose of this compound (13.8±6.62 hours) compared to a 25 µg dose (16.4±7.34 hours) or dinoprostone (16.3±7.49 hours). europeanreview.org
Table 2: Research Findings on this compound's Effect on Induction-to-Delivery Interval
| Study Comparison | Induction-to-Delivery Interval with this compound | Citations |
|---|---|---|
| This compound vs. Oxytocin (PROM) | 309.15 ± 57.74 minutes | barpetaogs.co.in |
| Vaginal this compound Alone | 13.8 hours (mean) | jogcr.com |
| This compound vs. Dinoprostone | 14.6 hours (median) | fetalmedicine.org |
| 50 µg this compound vs. 25 µg this compound vs. Dinoprostone | 13.8 ± 6.62 hours | europeanreview.org |
This compound's utility in labor induction is closely linked to its ability to promote cervical ripening—the softening, dilating, and effacing of the cervix. clinicaltrials.govaafp.org
This compound acts on the intracellular matrix of the cervix, causing a breakdown of collagen fibrils, which leads to cervical softening. clinicaltrials.gov Research has shown a clear effect of this compound on cervical ripening, with one review noting a significant reduction in unchanged cervix at 12 to 24 hours compared to placebo. nih.gov Studies have demonstrated that both vaginal and oral this compound are effective for cervical ripening. utoronto.caclinicaltrials.gov A study comparing this compound with a combination of this compound and isosorbide mononitrate found that both were effective in achieving cervical ripening in terms of effacement, dilatation, and softening, though the combination was more effective. In a trial comparing different doses of this compound with dinoprostone, the 50 µg this compound group showed the maximum improvement in the Bishop's score, a measure of cervical favorability. europeanreview.org
Effects on Induction-to-Delivery Interval
Medical Abortion Research
This compound is a critical component in medical abortion regimens, both in combination with other drugs and as a standalone agent. Research has focused on establishing its efficacy and safety across different stages of pregnancy.
In settings where mifepristone is unavailable, research has explored the efficacy of this compound-only regimens for medical abortion. nih.gov
First Trimester: In the first trimester, this compound alone is considered a reasonable option for abortion. nih.gov A systematic review found that for gestations up to 63 days, the efficacy of this compound-only regimens ranged from 84% to 96%. nih.gov Another review of 38 studies reported that 78% of women who used a this compound-only regimen had a complete abortion without the need for surgical intervention. societyfp.org Research suggests that success rates are higher with repeated doses of this compound. bohrium.com Studies on self-managed abortions using this compound-only regimens have reported high effectiveness, with 93-99% of participants having complete abortions without surgical intervention. ibisreproductivehealth.org However, it is noted that a combined regimen of mifepristone and this compound is significantly more effective than this compound alone. bohrium.com
Second Trimester: For second-trimester terminations, this compound-only regimens have also been shown to be effective, although the chances of complications increase as pregnancy advances. nih.govgynuity.org One study on second-trimester termination found that vaginal this compound was 100% successful in inducing abortion, with the induction-to-expulsion interval varying with gestational age. gynaecologyjournal.com For pregnancies between 12 and 16 weeks, the majority of terminations occurred within 11 to 15 hours, while for those between 17 and 21 weeks, the peak expulsion time was between 16 to 20 hours. gynaecologyjournal.com Another study comparing a mifepristone-misoprostol combination with a this compound-only regimen in the second trimester found that while the combination was more effective, this compound alone could also be used effectively, albeit requiring a higher total dose. nih.gov A study in Hong Kong found that the mifepristone-misoprostol group had a shorter time to fetal expulsion (7.3 hours vs. 11.3 hours) and a higher proportion of successful abortions within 24 hours (95.7% vs 79.2%) compared to the this compound-alone group. hkjgom.org
Table 3: Efficacy of this compound-Alone Regimens for Medical Abortion
| Gestational Age | Efficacy/Success Rate | Key Findings | Citations |
|---|---|---|---|
| First Trimester (≤63 days) | 84% - 96% | Efficacy varies with regimen; repeat dosing improves success. | nih.gov |
| First Trimester (Self-Managed) | 93% - 99% | High rate of complete abortion without surgical intervention. | ibisreproductivehealth.org |
| Second Trimester (12-21 weeks) | 100% successful induction | Induction-expulsion interval increases with gestational age. | gynaecologyjournal.com |
| Second Trimester | Effective, but less so than combination regimen | Requires higher total dose compared to mifepristone-misoprostol combination. | nih.govhkjgom.org |
Combination Regimens with Mifepristone or Methotrexate: Synergistic Effects
The combination of this compound with other compounds, such as mifepristone or methotrexate, has been shown to produce synergistic effects, enhancing the efficacy of medical abortion.
Mifepristone, a progesterone receptor antagonist, when used in conjunction with this compound, has demonstrated high success rates in terminating early pregnancies. researchgate.netglobalauthorid.com Clinical trials have shown that this combination is more effective than this compound alone. nihr.ac.uk One study reported that a mifepristone-pretreatment group had a complete expulsion rate of 83.8% after one dose of this compound, compared to 67.1% in the group that received this compound alone. medscape.com Another trial found that the combination of mifepristone and this compound resulted in an 83% resolution rate for missed miscarriages, compared to 76% for those who received a placebo with this compound. nihr.ac.uk This combined regimen has also been associated with a reduced need for subsequent surgical intervention. nihr.ac.uk
Cervical Priming for Surgical Abortion
This compound is frequently utilized for cervical priming, or ripening, prior to surgical abortion to facilitate cervical dilation and reduce the risk of complications. bioline.org.brnih.gov
Clinical studies have demonstrated the efficacy of this compound for this purpose. Research has shown that both sublingual and vaginal administration of this compound are effective for cervical ripening in first-trimester surgical abortions. impactfactor.org A randomized controlled trial involving 600 women found that cervical priming with this compound before manual vacuum aspiration (MVA) significantly reduced the need for further cervical dilation and decreased the operative time. nih.gov Another study showed that the success of cervical dilatation with this compound increases with the dosage. bioline.org.br
Comparative studies have also been conducted. One trial compared this compound to laminaria, an osmotic dilator, and found that vaginal this compound resulted in greater mean cervical dilatation than laminaria, although the difference was not statistically significant. societyfp.org The use of this compound for cervical priming has been associated with high patient satisfaction rates. societyfp.org
Miscarriage Management Research (Incomplete and Missed Abortion)
This compound has been investigated as a medical alternative to surgical evacuation for the management of incomplete and missed miscarriages.
Efficacy of Uterine Evacuation
Research has established this compound as an effective method for uterine evacuation in cases of incomplete and missed abortion. nih.gov Studies have reported high success rates, with some indicating that this compound is as effective as surgical management. ijrcog.orgspringermedizin.de A review of literature found sufficient evidence to support this compound as a safe and effective means of non-surgical uterine evacuation for incomplete abortion in the first trimester. nih.gov
Success rates for complete uterine evacuation with this compound in cases of incomplete abortion have been reported to be between 80% and 99%. ipas.org For missed abortions, success rates with this compound are also high, particularly when preceded by mifepristone. ipas.orgmiscarriageassociation.org.uk One study found that pretreatment with mifepristone before this compound administration significantly increased the success rate of medical management for missed miscarriage. nihr.ac.ukmiscarriageassociation.org.uk
Reduction in Need for Surgical Intervention
A significant advantage of using this compound for miscarriage management is the potential to reduce the need for surgical intervention, such as dilation and curettage (D&C). medscape.com
In a randomized controlled trial comparing medical therapy with this compound to D&C for incomplete or inevitable abortion, the success rate for the this compound group was 96.3%, compared to 91.5% for the surgical group. medscape.com Another study on missed miscarriage found that combining mifepristone with this compound reduced the need for surgery to 17%, compared to 25% in the group that received a placebo with this compound. nihr.ac.uk Similarly, a study comparing this compound with surgical management for incomplete and missed miscarriage reported a 97% success rate in the medical treatment group, with only 3% requiring subsequent surgical evacuation. ijrcog.org
Postpartum Hemorrhage (PPH) Prevention and Treatment Research
This compound has been studied for its role in both the prevention and treatment of postpartum hemorrhage (PPH), a leading cause of maternal mortality worldwide. nih.gov
Comparative Efficacy with Oxytocin
Oxytocin is the standard uterotonic agent used for the prevention of PPH. However, research has explored this compound as an alternative, particularly in settings where oxytocin may not be readily available. nih.govclinicaltrials.gov
Several studies have compared the efficacy of this compound with oxytocin for PPH prevention. A double-blind randomized controlled trial found that the quantity of blood loss was lower in the group receiving this compound compared to the oxytocin group. nih.gov Another study concluded that oral this compound is as effective as intramuscular oxytocin in preventing PPH. scivisionpub.com However, some research has indicated no significant difference between the two in terms of the decrease in hemoglobin and hematocrit levels. nih.gov
The combination of this compound and oxytocin has also been investigated. A randomized clinical trial showed that the combined use of this compound and oxytocin significantly reduced postpartum blood loss and the incidence of PPH compared to oxytocin alone. medznat.ru
Community-Level Distribution and Impact on Maternal Mortality
This compound's stability at room temperature and ease of administration in tablet form make it a suitable intervention for preventing postpartum hemorrhage (PPH) in low-resource settings where a high proportion of births occur at home without skilled attendants. gynuity.org Community-level distribution programs have been implemented to increase access to this life-saving medication. gynuity.orgscispace.com
Research indicates that community-based distribution of this compound is a feasible, safe, and acceptable strategy. scispace.comdovepress.com Studies in several countries, including Nigeria, Uganda, and in rural areas of other nations like Ethiopia and Pakistan, have demonstrated that trained community health workers (CHWs) or traditional birth attendants (TBAs) can successfully distribute and administer this compound. gynuity.orgscispace.com In some programs, this compound is provided to pregnant women during their third trimester for self-administration after home births. gynuity.org
A significant concern regarding community distribution has been the potential for misuse or a reduction in facility-based deliveries. nih.gov However, evidence does not support these concerns. nih.govnih.gov A scoping review of 14 studies found no significant difference in the rates of facility delivery between groups that received community-based this compound and control groups. nih.govnih.gov Furthermore, the review, which included data from over 11,000 mothers, reported that self-administration of this compound before delivery was very low, occurring in less than 2% of cases, with no adverse outcomes from such misuse noted. nih.govnih.gov
The primary impact of these programs is the increased access to a uterotonic for PPH prevention for women who would otherwise not have it. scispace.com For instance, a study in northern Nigeria showed that the availability of this compound protected 83% of women who delivered at home against PPH. scispace.com Pooled analysis of randomized controlled trials has shown that this compound use in home or primary care settings can reduce the incidence of PPH by 24% and severe PPH by 41% compared to a placebo. nih.gov An integrative review of 18 programs reported that out of more than 86,000 women who took this compound, there were 51 maternal deaths, none of which were directly attributed to the use of the drug. healthynewbornnetwork.org
Table 1: Impact of Community-Based this compound Distribution Programs
| Study/Region | Key Findings | Citation |
| Northern Nigeria | Protected 83% of women delivering at home from PPH. | scispace.com |
| Scoping Review (Multiple Countries) | No significant impact on facility delivery rates; misuse was rare (<2%). | nih.govnih.gov |
| Pooled RCT Analysis | Reduced PPH incidence by 24% and severe PPH by 41% compared to placebo. | nih.gov |
| Integrative Review (18 programs) | No maternal deaths directly attributed to this compound use among 86,732 women. | healthynewbornnetwork.org |
| Uganda Pilot Study | Demonstrated safe and feasible self-administration by women delivering at home. | gynuity.org |
Cervical Ripening Prior to Gynecological Procedures (e.g., Hysteroscopy, Dilation and Curettage)
This compound is widely researched for its efficacy in cervical ripening, or softening and dilating the cervix, before gynecological procedures such as hysteroscopy and dilation and curettage (D&C). nih.govnih.gov This pre-operative ripening can make the procedure easier to perform and may reduce the risk of complications like cervical injury and uterine perforation. nih.govnih.gov
For hysteroscopy , a systematic review and meta-analysis of 25 randomized controlled trials involving over 2,200 women found that using this compound beforehand significantly reduced the need for mechanical cervical dilatation. nih.gov The analysis also showed a greater initial cervical width and fewer complications, such as cervical lacerations and the creation of false passages, compared to placebo or no treatment. nih.gov The effectiveness of this compound can be influenced by factors like menopausal status, with some research suggesting it is more beneficial in premenopausal women. mdpi.com One study found that in postmenopausal women, pretreatment with estradiol enhanced the cervical dilating effect of this compound. mdpi.com
In the context of dilation and curettage (D&C) , research also supports the use of this compound for cervical priming. nih.gov A clinical trial in Iran demonstrated that this compound was effective for cervical ripening in premenopausal women undergoing diagnostic D&C for abnormal uterine bleeding. nih.gov The study highlighted that a ripened cervix simplifies the procedure and lowers the risk of trauma associated with mechanical dilation. nih.gov However, the efficacy may decrease with age, potentially due to lower estrogen levels. nih.gov
Table 2: Efficacy of this compound for Cervical Ripening in Gynecological Procedures
| Procedure | Key Research Finding | Patient Population | Citation |
| Hysteroscopy | Significantly reduced need for cervical dilatation and lowered complication rates (cervical laceration, false passage). | 2,203 females across 25 RCTs | nih.gov |
| Hysteroscopy | More beneficial for cervical dilation in premenopausal women compared to postmenopausal women. | Premenopausal and Postmenopausal women | mdpi.com |
| Hysteroscopy | Vaginal administration found to be more effective than oral route for cervical ripening. | Premenopausal women | |
| Dilation and Curettage | Effective for cervical priming, making the procedure easier and reducing risks of cervical injury. | Premenopausal women | nih.gov |
| Dilation and Curettage | Efficacy may decline with increasing age. | Premenopausal women | nih.gov |
Gastrointestinal Research
Prevention of NSAID-Induced Gastric Ulcers
This compound, a synthetic prostaglandin E1 analog, is approved for the prevention of gastric ulcers induced by nonsteroidal anti-inflammatory drugs (NSAIDs). nih.gov NSAIDs can deplete endogenous prostaglandins in the gastric mucosa, which play a protective role, making the stomach more susceptible to injury. nih.govacpjournals.org this compound works by replacing these protective prostaglandins. hres.ca
Numerous clinical trials have demonstrated its efficacy. A double-blind, placebo-controlled study found that this compound significantly reduced the incidence of NSAID-induced gastric ulcers. nih.gov In that study, ulcers developed in 21.7% of patients in the placebo group, compared to only 1.4% in the group receiving 200 micrograms of this compound. nih.gov Another 12-month, placebo-controlled trial confirmed that this compound decreases the cumulative development of these ulcers over the long term. tandfonline.com
Comparison with Proton Pump Inhibitors (PPIs)
Proton pump inhibitors (PPIs) are another class of drugs used to prevent NSAID-induced ulcers. has-sante.fr Comparative studies have been conducted to evaluate the relative efficacy of this compound and PPIs.
Generally, PPIs are often considered first-line treatment due to a better tolerability profile, as this compound is associated with gastrointestinal side effects like diarrhea and abdominal pain. has-sante.frnih.gov this compound is often recommended as a second-line treatment for patients who have contraindications or intolerance to PPIs. has-sante.fr
Table 3: Comparison of this compound and PPIs for NSAID-Induced Ulcer Prevention
| Agent | Efficacy | Key Considerations | Citations |
| This compound | Effective in preventing NSAID-induced gastric and duodenal ulcers. | Associated with gastrointestinal side effects (e.g., diarrhea, abdominal pain). Often considered second-line therapy. | nih.govhas-sante.frnih.govcochranelibrary.com |
| Proton Pump Inhibitors (e.g., Omeprazole) | Effective in preventing NSAID-induced gastric and duodenal ulcers. Lower relapse rate in maintenance therapy compared to this compound. | Generally better tolerated than this compound. Often considered first-line therapy. | has-sante.frnih.govcochranelibrary.comdovepress.com |
Gastric Mucosal Cytoprotection Mechanisms
Beyond its role in inhibiting acid secretion, this compound exhibits a direct protective effect on the gastric mucosa, a mechanism known as cytoprotection. nih.govijpp.com This means it helps protect the stomach lining from damage by various irritants, including NSAIDs and alcohol, through mechanisms independent of acid reduction. nih.govhres.ca This cytoprotective action is thought to involve several key processes that enhance the mucosal defense system. hres.cancats.io These mechanisms include increasing mucosal blood flow, stabilizing vascular endothelium, and improving the capacity for mucosal regeneration. hres.cancats.io
Increased Mucus and Bicarbonate Secretion
A primary component of this compound's cytoprotective effect is its ability to stimulate the secretion of mucus and bicarbonate from the gastric and duodenal mucosa. nih.govnih.gov
Mucus Secretion: The mucus layer acts as a physical barrier, preventing damaging agents from reaching the epithelial cells. this compound has been shown to increase both the secretion and the thickness of this protective mucus layer. ncats.ionih.gov One study in healthy volunteers demonstrated that this compound significantly increased gastric mucus secretion in a dose-dependent manner. nih.gov Following administration, mucus secretion increased by 37%, 82%, and 95% during the basal period. nih.gov
Bicarbonate Secretion: Bicarbonate is secreted into the mucus layer, where it neutralizes acid that diffuses back from the stomach lumen, thus maintaining a near-neutral pH at the cell surface. this compound enhances the secretion of bicarbonate, further strengthening this chemical barrier. hres.cancats.io
These actions collectively fortify the gastroduodenal mucosal barrier, making it more resilient to injury from substances like NSAIDs. hres.ca
Thickening of Mucosal Bilayer
This compound, a synthetic prostaglandin E1 analog, demonstrates significant mucosal protective effects, a mechanism that is understood to involve the thickening of the mucosal bilayer. medicaldialogues.indrugbank.comnih.gov This cytoprotective action is attributed to several integrated processes. nih.gov Research indicates that this compound enhances the secretion of both mucus and bicarbonate. jove.com This leads to a thickening of the adherent mucus gel layer that protects the gastric and duodenal mucosa from aggressors like acid and pepsin. nih.gov
Studies in healthy human volunteers have quantified this effect. The administration of this compound resulted in a dose-dependent increase in gastric mucus secretion. hres.cahres.ca Similarly, this compound has been shown to stimulate duodenal bicarbonate secretion in a dose-dependent manner. hres.cahres.ca This enhanced secretion of mucus and bicarbonate contributes directly to the thickening and integrity of the mucosal barrier. medicaldialogues.indrugbank.comnih.gov The mechanism is thought to involve the replacement of depleted prostaglandins and an increase in mucosal blood flow, which aids in the preservation and regeneration of mucosal cells. hres.cahres.ca
Table 1: Effect of this compound on Gastric Mucus Secretion in Healthy Volunteers
| Parameter | Placebo | This compound 200 mcg | This compound 400 mcg | This compound 800 mcg |
|---|---|---|---|---|
| Increase in Basal Mucus Secretion | - | 37% | 82% | 95% |
| Increase in Mucus Secretion (during maximal acid inhibition) | - | 27% | 31% | 38% |
Data sourced from a comparative study in eight healthy volunteers. hres.cahres.ca
Exploratory and Emerging Clinical Applications
This compound is being explored for its hemostatic effects to reduce blood loss during myomectomy, a common gynecological surgery. binasss.sa.cr Uterine myomas are benign tumors, and their surgical removal can be associated with significant hemorrhage. nih.gov this compound's uterotonic properties, which cause uterine contractions, are believed to constrict the blood vessels supplying the fibroids, thereby reducing operative blood loss. ekb.egnih.gov
Multiple systematic reviews and meta-analyses of randomized controlled trials have established that preoperative administration of this compound is associated with significantly lower intraoperative blood loss compared to placebo. binasss.sa.crnih.govnih.govmdpi.com These analyses also report a corresponding lower drop in hemoglobin levels and a reduced need for blood transfusions in patients receiving this compound. binasss.sa.crnih.govnih.govmdpi.com Furthermore, a reduction in the mean operative time has been observed in the this compound groups. binasss.sa.crnih.govmdpi.com These findings suggest that this compound is an effective intervention for minimizing blood loss during open myomectomy. nih.gov
Table 2: Meta-Analysis of this compound Efficacy in Open Myomectomy
| Outcome Measure | Reduction Associated with this compound | Confidence Interval (95% CI) |
|---|---|---|
| Intraoperative Blood Loss | 180.2 mL | -224.04 to -136.35 |
| Hemoglobin Drop | 0.58 g/dL | -0.82 to -0.35 |
| Operative Time | 12.95 minutes | -19.89 to -6.01 |
| Perioperative Blood Transfusion (Risk Ratio) | 0.43 | 0.29 to 0.63 |
Data synthesized from a meta-analysis of 16 randomized controlled trials involving 975 women. mdpi.com
Emerging research has investigated the use of this compound in combination with other agents, such as the nitric oxide donor isosorbide mononitrate (ISMN), to enhance clinical efficacy in specific applications. springermedizin.de Nitric oxide donors like ISMN are known to promote cervical ripening by facilitating the breakdown of collagen and dilating blood vessels in the cervix. springermedizin.deinnovareacademics.in The rationale for combination therapy is that the different mechanisms of this compound (a prostaglandin analog) and ISMN could have a synergistic effect. barpetaogs.co.in
Studies evaluating this combination have yielded mixed results depending on the clinical context. In second-trimester abortions, the addition of ISMN to vaginal this compound was found to significantly shorten the induction-to-abortion interval and increase the rate of complete abortion compared to this compound alone. springermedizin.de Conversely, for pre-operative cervical ripening in the first trimester, some randomized controlled trials have not demonstrated a significant advantage of combining this compound with ISMN over using this compound by itself. springermedizin.denih.gov However, other studies in a similar setting found the combination to be more effective in achieving cervical ripening and shortening the operative duration.
Table 3: Comparative Efficacy of this compound vs. Combination Therapy (this compound + ISMN)
| Clinical Application | Key Finding | Study Conclusion |
|---|---|---|
| Second-Trimester Abortion | Combination therapy significantly reduced the induction-abortion interval by 4.21 hours. springermedizin.de | The addition of ISMN to this compound is more effective than this compound alone. springermedizin.de |
| First-Trimester Cervical Ripening | No significant difference in cervical resistance between combination therapy and this compound alone. nih.gov | No advantage of combining this compound with ISMN was shown. nih.gov |
| First-Trimester Missed Abortion | The combination group was more effective in achieving cervical ripening and had a shorter operative duration. | The combination of this compound and a nitric oxide donor offers a synergistic action. |
Findings are based on separate randomized clinical trials. springermedizin.denih.gov
The topical application of this compound as an oral rinse has been investigated for its potential to reduce the severity of oral mucositis, a common and debilitating side effect of high-dose chemotherapy. nih.govresearchgate.net The anti-inflammatory and mucosa-protecting properties of this compound provide the rationale for this exploratory application. nih.govfrontiersin.org
Pharmacokinetic and Pharmacodynamic Research Across Administration Routes
Bioavailability and Absorption Kinetics
The method by which misoprostol is introduced into the body fundamentally alters its absorption and subsequent availability. Research has explored several routes, each presenting a unique pharmacokinetic profile. The primary pharmacokinetic parameters considered are the peak plasma concentration (Cmax), the time to reach peak concentration (Tmax), and the total drug exposure over time, represented by the area under the serum concentration-time curve (AUC). This compound.org
Oral Administration
Following oral administration, this compound is absorbed rapidly and almost entirely from the gastrointestinal tract. This compound.org It undergoes extensive and swift first-pass metabolism, where it is de-esterified into its active metabolite, this compound acid. This compound.orgnih.gov Studies have shown that after an oral dose, the plasma concentration of this compound acid rises quickly, reaching its peak in approximately 12 to 30 minutes. This compound.orgnih.govjogi.co.in However, this peak is followed by a rapid decline, with levels decreasing significantly within 120 minutes and remaining low thereafter. This compound.orgjogi.co.in The quick onset of action is a key characteristic of this route. oup.com
Sublingual Administration
Sublingual administration involves placing the tablet under the tongue, where it dissolves and is absorbed through the sublingual mucosa. This compound.org This route allows for rapid absorption, bypassing the first-pass metabolism in the liver. This compound.orgoup.com Consequently, sublingual administration typically results in a higher peak plasma concentration (Cmax) compared to oral and vaginal routes. This compound.org The time to peak concentration (Tmax) for the sublingual route is comparable to the oral route, occurring at around 30 minutes. This compound.org Studies have indicated that the systemic bioavailability, as measured by the AUC, is greatest with sublingual administration, suggesting it is a highly potent route. oup.comoup.com
Buccal Administration
Buccal administration, where the tablet is placed between the cheek and gum, offers another alternative. This method is characterized by slower absorption over a more extended period, leading to sustained serum levels but lower peak concentrations compared to sublingual administration. gynuity.org Research comparing buccal and sublingual routes has shown that sublingual administration results in a significantly higher Cmax and AUC. The rate of buccal absorption is reported to be faster than vaginal dosing. nih.gov
Rectal Administration
Interactive Table: Pharmacokinetic Parameters of this compound by Administration Route
| Administration Route | Peak Concentration (Cmax) | Time to Peak (Tmax) | Bioavailability (AUC) |
| Oral | High, but declines rapidly This compound.orgnih.gov | ~30 minutes This compound.org | Lower than vaginal and sublingual oup.comoup.com |
| Sublingual | Highest among routes This compound.org | ~30 minutes This compound.org | Highest among routes oup.comoup.com |
| Vaginal | Lower than oral and sublingual This compound.org | 70-80 minutes This compound.orgoup.com | Higher than oral This compound.orgoup.comnih.gov |
| Buccal | Lower than sublingual | Slower than sublingual gynuity.org | Lower than sublingual |
| Rectal | Lower than oral nih.gov | 40-65 minutes This compound.org | Lower than vaginal nih.gov |
Metabolite Analysis: this compound Acid (SC-30695) and Further Metabolites
This compound itself is a prodrug, a methyl ester that is rapidly and extensively metabolized into its pharmacologically active free acid form, known as this compound acid or SC-30695. nih.govnih.gov This de-esterification process occurs quickly, to the extent that intact this compound is often undetectable in plasma. nih.govjogi.co.in The serum protein binding of this active metabolite is approximately 81-89%.
Following its formation, this compound acid undergoes further metabolic changes. These subsequent metabolic pathways include the β-oxidation of the α-side chain and the ω-oxidation of the β-side chain. This leads to the formation of dinor and tetranor metabolites. drugbank.com Additionally, reduction of the ketone group on the cyclopentane ring results in the formation of prostaglandin F analogues. These further metabolites are generally considered inactive. The primary route for the elimination of these metabolites is through the urine.
Time-to-Peak Concentration (Tmax) and Duration of Action by Route
The time to reach maximum plasma concentration (Tmax) and the duration of the compound's action are critical pharmacokinetic parameters that vary substantially with the route of administration. These differences are primarily due to the unique physiological characteristics of the absorption sites, such as blood supply and pH, and whether the route is subject to first-pass metabolism in the liver. This compound.org
Oral Administration: Following oral administration, this compound is absorbed very quickly. fda.gov Studies report that the peak plasma concentration of its active metabolite, this compound acid, is reached in approximately 12 to 30 minutes. fda.govThis compound.org This rapid absorption leads to a fast onset of action, observed in as little as 8 minutes. This compound.orgdrugbank.com However, the concentration declines swiftly after reaching its peak, leading to a relatively short duration of action of about 2 hours. This compound.orgdrugbank.com
Sublingual Administration: The sublingual route, where a tablet is placed under the tongue, also provides rapid absorption, bypassing the first-pass effect in the liver. This compound.org The Tmax for sublingual administration is comparable to the oral route, occurring at approximately 26 to 30 minutes. This compound.orgoup.comnih.gov This results in a similarly quick onset of action (around 11 minutes), but the duration of clinical effect is sustained for longer than the oral route, at approximately 3 hours. This compound.orgdrugbank.com
Vaginal Administration: In contrast to the oral and sublingual routes, vaginal administration results in a much slower and more gradual absorption of the compound. This compound.org This leads to a significantly delayed Tmax, typically observed between 70 and 80 minutes. This compound.org While the onset of action is slower (around 20 minutes), the slower absorption profile results in sustained plasma concentrations and a prolonged duration of action, lasting approximately 4 hours, with detectable serum levels still present after 6 hours. This compound.orgdrugbank.com
Buccal Administration: Buccal administration, where the tablet is placed between the cheek and gum, is characterized by slower absorption over a longer period compared to the sublingual route. gynuity.org This results in a Tmax of about 78 minutes. drugbank.com The absorption pattern is similar to the vaginal route, leading to sustained serum levels. nih.govgynuity.org
The following table summarizes the time-to-peak concentration and duration of action for this compound acid by different administration routes.
| Administration Route | Time to Peak (Tmax) (minutes) | Onset of Action (minutes) | Duration of Action (hours) |
| Oral | 12 - 30 fda.govThis compound.org | ~ 8 This compound.orgdrugbank.com | ~ 2 This compound.orgdrugbank.com |
| Sublingual | 26 - 30 oup.comnih.gov | ~ 11 This compound.orgdrugbank.com | ~ 3 This compound.orgdrugbank.com |
| Vaginal | 70 - 80 This compound.org | ~ 20 This compound.orgdrugbank.com | ~ 4 This compound.orgdrugbank.com |
| Buccal | ~ 78 drugbank.com | Slower than oral/sublingual gynuity.org | Sustained, similar to vaginal gynuity.org |
| Rectal | 40 - 65 This compound.org | Slower than oral/sublingual drugbank.com | ~ 4 drugbank.com |
Relationship Between Administration Route, Systemic Exposure (AUC), and Clinical Effects
Sublingual Route: Research consistently shows that sublingual administration results in the highest systemic bioavailability. This compound.orgoup.com In one comparative study, the AUC for the sublingual route was significantly greater than for the oral and vaginal (without water) routes. oup.comnih.gov This high bioavailability is attributed to the rapid absorption through the sublingual mucosa, which is rich in blood vessels, and the avoidance of extensive first-pass metabolism in the liver. This compound.org This leads to a more pronounced and rapid increase in uterine tonus compared to vaginal administration. This compound.org
Vaginal Route: Vaginal administration provides a higher AUC compared to the oral route, suggesting greater bioavailability. This compound.org This may explain why vaginal administration is often found to be more clinically effective than oral administration for certain applications. oup.com The sustained plasma concentrations achieved with the vaginal route lead to long-lasting uterine contractility. oup.com However, there is wide individual variability in absorption from the vaginal mucosa. nih.govoup.com Adding water to the tablets before vaginal insertion can improve absorption and increase the AUC, making it more comparable to the sublingual route. oup.comnih.gov
Oral Route: The oral route undergoes extensive and rapid first-pass metabolism, where the compound is quickly de-esterified to this compound acid. This compound.org This "first-pass effect" significantly reduces the total systemic exposure. oup.com Studies have shown the AUC after oral administration to be substantially lower than that after sublingual or vaginal administration. This compound.orgoup.com For instance, one study found the AUC after oral administration was only 54% of that after sublingual administration. oup.com
The table below presents comparative data on the Area Under the Curve (AUC) for this compound acid following administration of a 400 μg dose.
| Administration Route | Area Under the Curve (AUC₀₋₃₆₀) (pg·h/mL) | Relative Bioavailability Compared to Sublingual |
| Sublingual | 743.7 ± 291.2 oup.comnih.gov | 100% |
| Oral | 402.8 ± 151.6 oup.comnih.gov | ~54% oup.com |
| Vaginal (dry) | 433.7 ± 182.6 oup.comnih.gov | ~58% oup.com |
| Vaginal (with water) | 649.3 ± 333.8 oup.comnih.gov | Not significantly different from sublingual oup.com |
| Buccal | Lower than sublingual, similar to oral tga.gov.au | - |
| Rectal | Lower than oral and vaginal nih.gov | - |
The interplay between the route of administration, the resulting pharmacokinetic profile (Tmax, AUC), and the pharmacodynamic response is crucial. Routes that bypass the first-pass effect and allow for rapid, high absorption (sublingual) or sustained absorption (vaginal) tend to provide greater systemic exposure and, consequently, a more pronounced clinical effect on the uterus compared to the oral route. This compound.orgoup.com
Drug Interaction Research
Interactions with Antacids (Magnesium, Aluminum, Calcium-containing)
The concurrent administration of antacids with misoprostol can influence its absorption and side effect profile. Research has specifically highlighted the differential effects of magnesium-containing antacids compared to those containing aluminum or calcium.
Antacids can reduce the bioavailability of this compound's active metabolite, this compound acid. medscape.compfizermedicalinformation.com However, the primary clinical concern is the exacerbation of diarrhea, a known side effect of this compound. Magnesium-containing antacids, which themselves can have a laxative effect, may potentiate this compound-induced diarrhea. medscape.compfizermedicalinformation.comdrugs.comnih.gov For this reason, the concomitant use of magnesium-containing antacids is generally not recommended. drugs.comscbdd.com
If antacid therapy is required during treatment with this compound, preparations containing aluminum or calcium are considered preferable alternatives. medscape.comdrugs.comrxhealthmed.ca Studies suggest that while antacids may delay the absorption of the diclofenac component in combination products like Arthrotec, this effect is not typically considered clinically important. pfizermedicalinformation.comscbdd.com
| Antacid Type | Nature of Interaction with this compound | Research Findings |
|---|---|---|
| Magnesium-containing (e.g., Magnesium Hydroxide, Magaldrate) | Pharmacodynamic / Side Effect Potentiation | Exacerbates or potentiates this compound-associated diarrhea. medscape.compfizermedicalinformation.comdrugs.comdrugbank.com Concomitant use is generally not recommended. scbdd.com |
| General Antacids | Pharmacokinetic | Can reduce the bioavailability of this compound acid. medscape.compfizermedicalinformation.com This effect is not always considered clinically significant. scbdd.com |
| Aluminum or Calcium-containing (e.g., Aluminum Hydroxide, Calcium Carbonate) | Neutral / Alternative Option | Recommended as alternatives if an antacid is necessary during this compound therapy. medscape.comdrugs.comrxhealthmed.ca |
Interactions with Oxytocic Agents (e.g., Oxytocin, Carboprost Tromethamine)
This compound exhibits pharmacodynamic synergism with other oxytocic agents, which are substances that stimulate uterine contractions. This interaction is of significant clinical importance.
Prostaglandins like this compound may potentiate the uterine response to oxytocin. drugs.com The combined use can augment the effects of oxytocic agents, potentially leading to uterine hyperstimulation. medscape.comdrugs.com This heightened activity increases risks such as uterine rupture or cervical laceration. drugs.comrxlist.com
Due to this synergistic effect, the concurrent use of this compound and other oxytocic agents is approached with caution. Specific time intervals between the administration of each drug are recommended to mitigate the risk of adverse uterine events. For instance, it is suggested that oxytocin infusion should not commence for at least 4 hours after the last oral dose of this compound. drugs.commedscape.com
| Oxytocic Agent | Nature of Interaction with this compound | Research Findings and Management |
|---|---|---|
| Oxytocin | Pharmacodynamic Synergism | This compound increases the effects of oxytocin, potentially augmenting uterine response. medscape.comdrugbank.commedscape.com It is recommended to delay the start of oxytocin infusion for at least 4 hours after the last this compound dose. drugs.com |
| Carboprost Tromethamine | Pharmacodynamic Synergism | Carboprost tromethamine may increase the uterotonic activities of this compound. drugbank.com Additive pharmacodynamic effects may occur. drugs.com Concomitant use is generally not recommended. drugs.com |
Interactions with Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
The interaction between this compound and NSAIDs is unique, as this compound is primarily indicated to reduce the risk of gastric ulcers induced by NSAID therapy. nih.govrxlist.com Therefore, they are frequently used concomitantly.
Research has focused on two main aspects of this interaction: the pharmacokinetic effects and the potential for NSAIDs to interfere with the prostaglandin-mediated actions of this compound.
Pharmacokinetic studies have shown no clinically significant interactions. Drug interaction studies demonstrated that this compound had no effect on the kinetics of ibuprofen or diclofenac. rxlist.com A minor decrease (around 20%) in the area under the curve (AUC) of aspirin was observed, but this was not considered to be clinically significant. rxlist.com
Furthermore, concerns that NSAIDs, which inhibit prostaglandin synthesis, might counteract the effects of exogenous prostaglandins like this compound have been investigated. Studies have found that the co-administration of NSAIDs does not interfere with the action of this compound to induce uterine contractions in the context of medical abortion. nih.govnih.gov
| NSAID | Nature of Interaction with this compound | Research Findings |
|---|---|---|
| Ibuprofen, Diclofenac | Pharmacokinetic | No clinically significant effect on the kinetics of these NSAIDs was observed in drug interaction studies. rxlist.com |
| Aspirin | Pharmacokinetic | A 20% decrease in aspirin AUC was noted, which was not deemed clinically significant. rxlist.com this compound does not interfere with the antiplatelet effects of therapeutic aspirin doses. |
| General NSAIDs | Pharmacodynamic | Studies show that NSAIDs do not appear to interfere with or attenuate the prostaglandin-mediated effects of this compound, such as the induction of uterine contractions. nih.govnih.gov |
Interactions with Methotrexate
The direct interaction between this compound and methotrexate has not been extensively documented in dedicated interaction studies. However, an interaction is relevant when considering combination products that contain both this compound and an NSAID, such as diclofenac/misoprostol.
The established interaction exists between the NSAID component and methotrexate. Concomitant use of NSAIDs and methotrexate may elevate the risk of methotrexate toxicity, which can manifest as neutropenia, thrombocytopenia, and renal dysfunction. pfizermedicalinformation.com
In the context of medical abortion regimens that use both methotrexate and this compound, one review of analgesic use found that women who took an NSAID after this compound administration did not have a reduced rate of abortion, suggesting no negative interference by the NSAID on the this compound-induced process. nih.gov A case report noted methotrexate toxicity in a patient taking multiple medications, including this compound, but a direct causal link was not established. researchgate.net
| Interacting Drug | Nature of Interaction | Clinical Impact |
|---|---|---|
| Methotrexate (with Diclofenac/Misoprostol) | Pharmacokinetic / Pharmacodynamic (via NSAID component) | Concomitant use of NSAIDs (like the diclofenac in the combination product) and methotrexate may increase the risk for methotrexate toxicity. pfizermedicalinformation.comwebmd.com |
Cytochrome P450 Enzyme System Considerations (CYP3A4)
The cytochrome P450 (CYP) system is a critical pathway for the metabolism of many drugs. CYP3A4 is a particularly important enzyme in this system, responsible for the metabolism of approximately half of all drugs on the market. pharmacytimes.com
Predictive models based on molecular structure provide some insight into this compound's relationship with the CYP450 system. According to these models, this compound is predicted to be a substrate for the CYP3A4 enzyme. drugbank.com This means that its metabolism may be influenced by other drugs that are strong inhibitors or inducers of CYP3A4.
However, the same models predict that this compound itself is not a significant inhibitor of major CYP enzymes, including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. drugbank.com This suggests that this compound is unlikely to affect the metabolism of other drugs that are substrates for these enzymes. It is important to note that mifepristone, a drug often used in combination with this compound, is metabolized by CYP3A4. oatext.com
| CYP450 Enzyme Interaction | Predicted Role of this compound | Source of Finding |
|---|---|---|
| CYP3A4 Substrate | Substrate | drugbank.com |
| CYP1A2 Inhibitor | Non-Inhibitor | drugbank.com |
| CYP2C9 Inhibitor | Non-Inhibitor | drugbank.com |
| CYP2C19 Inhibitor | Non-Inhibitor | drugbank.com |
| CYP2D6 Inhibitor | Non-Inhibitor | drugbank.com |
| CYP3A4 Inhibitor | Non-Inhibitor | drugbank.com |
Adverse Event and Safety Profile Research
Gastrointestinal Adverse Effects: Mechanisms and Incidence
Misoprostol's action as a prostaglandin E1 analog contributes to its gastrointestinal side effects. It can increase intestinal motility and fluid secretion, as well as inhibit gastric acid secretion and promote mucus and bicarbonate secretion nih.govdroracle.aisimsrc.edu.in. These mechanisms underlie the common gastrointestinal adverse events associated with its use nih.govdroracle.ai.
Diarrhea, Abdominal Pain, Nausea, Vomiting, Flatulence, Dyspepsia, Constipation
Diarrhea is frequently reported and is often the most common gastrointestinal side effect nih.govwikipedia.orgrxlist.comdrugs.comdroracle.ai. In clinical trials for the prevention of NSAID-induced gastric ulcers, the incidence of diarrhea at a dose of 800 mcg daily averaged 13%, ranging from 14-40% in controlled trials rxlist.compfizermedicalinformation.com. Diarrhea is dose-related and typically develops early in the course of therapy, often resolving within about 8 days wikipedia.orgrxlist.comdrugs.compfizermedicalinformation.com. In some cases (around 2% in clinical trials), it may necessitate discontinuation of the medication wikipedia.orgrxlist.comdrugs.compfizermedicalinformation.com. Rare instances of severe diarrhea leading to dehydration have been reported rxlist.compfizermedicalinformation.commedicalnewstoday.com.
Abdominal pain is another common gastrointestinal adverse effect nih.govrxlist.comdrugs.comdroracle.ai. In NSAID trials, abdominal pain occurred in 13-20% of patients, and in all studies, it was reported in about 7% of patients rxlist.compfizermedicalinformation.com. However, there was no consistent difference in incidence compared to placebo in these trials wikipedia.orgrxlist.compfizermedicalinformation.com.
Other reported gastrointestinal effects with incidences greater than 1% in clinical trials include nausea (3.2%), flatulence (2.9%), dyspepsia (2.0%), vomiting (1.3%), and constipation (1.1%) rxlist.comdroracle.aipfizermedicalinformation.com. Similar to abdominal pain, the incidence of these effects was not significantly different from placebo in clinical trials wikipedia.orgrxlist.compfizermedicalinformation.com.
Taking this compound with food can help minimize the incidence of diarrhea rxlist.comdrugs.compfizermedicalinformation.com. Coadministration with magnesium-containing antacids may increase the incidence of diarrhea and should be avoided nih.govrxlist.compfizermedicalinformation.com.
| Gastrointestinal Adverse Effect | Incidence (Clinical Trials, Prevention of NSAID-induced Ulcers) | Notes |
| Diarrhea | 13% (average), 14-40% (range) at 800 mcg/day | Dose-related, often self-limiting wikipedia.orgrxlist.com |
| Abdominal Pain | 7% (all studies), 13-20% (NSAID trials) | Incidence similar to placebo wikipedia.orgrxlist.com |
| Nausea | 3.2% | Incidence similar to placebo rxlist.compfizermedicalinformation.com |
| Flatulence | 2.9% | Incidence similar to placebo rxlist.compfizermedicalinformation.com |
| Dyspepsia | 2.0% | Incidence similar to placebo rxlist.compfizermedicalinformation.com |
| Vomiting | 1.3% | Incidence similar to placebo rxlist.compfizermedicalinformation.com |
| Constipation | 1.1% | Incidence similar to placebo rxlist.compfizermedicalinformation.com |
Note: Incidence rates are based on clinical trials for the prevention of NSAID-induced gastric ulcers unless otherwise specified. rxlist.compfizermedicalinformation.com
Uterine-Related Adverse Effects: Mechanisms and Incidence
This compound's uterotonic properties, stemming from its prostaglandin E1 analog activity, lead to increased uterine tone and contractions nih.govnih.govhres.cawikipedia.org. This mechanism is central to its use in obstetrics and gynecology but is also the basis for several uterine-related adverse effects nih.govhres.cawikipedia.org.
Uterine Hyperstimulation
Uterine hyperstimulation, characterized by excessive frequency or duration of uterine contractions, is a known risk associated with the use of uterotonic agents, including this compound wikipedia.orgpfizermedicalinformation.com. This can potentially compromise uteroplacental blood flow wikipedia.orgpfizermedicalinformation.com. The risk of uterine hyperstimulation is a concern when this compound is used for cervical ripening or labor induction wikipedia.orgpfizermedicalinformation.com. Some research suggests that this compound-induced hyperstimulation may be more challenging to manage compared to hyperstimulation caused by other agents wikipedia.org.
Post-Administration Bleeding and Cramping
Bleeding and cramping are commonly experienced following the administration of this compound, particularly when used for gynecological indications such as medical abortion or management of early pregnancy loss wikipedia.orgdroracle.aiaafp.org. This is a direct result of this compound's ability to induce uterine contractions hres.cawikipedia.orgdroracle.aiaafp.org. The bleeding can be heavier than typical menstrual bleeding wikipedia.orgaafp.org. While generally expected effects, excessive bleeding requiring medical attention is possible though uncommon droracle.ai. Cramping and bleeding typically commence within a few hours of administration and may persist for several days, with lighter bleeding potentially lasting longer aafp.org. In clinical trials for the prevention of NSAID-induced ulcers, gynecological disorders reported with incidences less than 1% included spotting (0.7%), cramps (0.6%), hypermenorrhea (0.5%), menstrual disorder (0.3%), and dysmenorrhea (0.1%) rxlist.compfizermedicalinformation.com. Postmenopausal vaginal bleeding has also been related to this compound administration rxlist.compfizermedicalinformation.com.
Systemic Adverse Effects: Mechanisms and Incidence
Beyond the gastrointestinal and uterine systems, this compound can also cause systemic adverse effects. These effects are generally less common than the gastrointestinal issues but can include a range of symptoms nih.govdrugs.comghsupplychain.org. This compound's systemic activity, even at low concentrations, can elicit prostaglandin-like effects in various tissues hres.ca.
Common systemic adverse effects reported in clinical trials with incidences greater than 1% include headache (2.4%) rxlist.comdroracle.aipfizermedicalinformation.com. Other systemic effects reported with lower incidences include shivering, chills, and fever nih.govdrugs.comghsupplychain.orgnih.govzu.edu.pk. Shivering and fever are particularly noted when this compound is used for labor induction or prevention of postpartum hemorrhage and are thought to be related to prostaglandin effects on thermoregulation centers ghsupplychain.orgnih.govzu.edu.pkelsevier.esscirp.org. These effects are often transient ghsupplychain.org.
Less frequently reported mild systemic adverse effects include syncope, lethargy, weakness, and vertigo nih.gov. Moderate-to-severe systemic reactions are less common but can include hypotension, sinus tachycardia, edema, and, rarely, myocardial infarction nih.gov. Allergic reactions to this compound can occur, although their frequency is not clearly established in studies medicalnewstoday.com.
| Systemic Adverse Effect | Incidence (Clinical Trials, Prevention of NSAID-induced Ulcers) | Notes |
| Headache | 2.4% | Incidence similar to placebo rxlist.compfizermedicalinformation.com |
| Shivering | Not specified (>1%) in this context | Common in obstetrical use (e.g., PPH prevention), incidence varies ghsupplychain.orgzu.edu.pkscirp.org |
| Fever/Hyperthermia | Not specified (>1%) in this context | Common in obstetrical use (e.g., PPH prevention), incidence varies, often transient ghsupplychain.orgzu.edu.pkelsevier.esscirp.org |
| Nausea | 3.2% | Also listed under GI effects rxlist.comdroracle.aipfizermedicalinformation.com |
| Vomiting | 1.3% | Also listed under GI effects rxlist.comdroracle.aipfizermedicalinformation.com |
| Dizziness | Not specified (>1%) in this context | Less common hres.cadroracle.ai |
| Chills | Not specified (>1%) in this context | Common in obstetrical use, incidence varies ghsupplychain.orgnih.govelsevier.es |
Note: Incidence rates for headache, nausea, and vomiting are based on clinical trials for the prevention of NSAID-induced gastric ulcers. Incidence for shivering, fever, and chills can vary significantly based on the indication and route of administration, particularly in obstetrical settings. rxlist.compfizermedicalinformation.comghsupplychain.orgzu.edu.pkscirp.org
Headache
Headache is another adverse effect that has been reported in individuals using this compound. medscape.compfizermedicalinformation.comnih.govdroracle.ai In clinical trials, headache has been reported with varying frequencies. For example, in some trials, headache was reported by more than 1% of subjects receiving this compound, with an incidence of around 2.4%. pfizermedicalinformation.comdroracle.ai However, some studies have indicated that the incidence of headache in this compound groups was not significantly different compared to placebo groups. pfizermedicalinformation.com
In studies comparing this compound to other medications, such as oxytocin for postpartum hemorrhage prevention, headache was more commonly associated with the oxytocin group than the this compound group. scirp.org
Reported incidence rates of headache in clinical trials have included:
| Study Context | This compound Incidence (%) | Comparator Incidence (%) |
| Clinical Trials (general adverse reactions) pfizermedicalinformation.comdroracle.ai | 2.4 | Not significantly different from placebo pfizermedicalinformation.com |
| Postpartum Hemorrhage Prevention scirp.org | 0.1 | 1.9 (Oxytocin) scirp.org |
| Medical Abortion (after mifepristone) | 33.0 - 34.0 | 31.0 (Oral this compound) |
Note: Incidence rates can vary based on the specific study and patient population.
Safety in Specific Patient Populations (e.g., Age, Renal Impairment, Smoking Status)
The safety profile of this compound has been investigated in various patient populations, including different age groups and individuals with specific medical conditions.
Age:
Clinical studies have generally indicated that there are no significant differences in the safety profile of this compound in patients aged 65 years and older compared to younger patients when used for indications like the prevention of NSAID-induced ulcers. pfizer.commedicalnewstoday.com While the AUC (area under the curve) for this compound acid may be increased in subjects over 64 years of age, routine dosage adjustment is typically not recommended unless the usual dose is not tolerated. hres.cae-lactancia.org For pediatric populations, the safety and efficacy of this compound have not been established for conditions like peptic ulceration or NSAID-induced peptic ulcer disease. nih.govpfizer.commayoclinic.org
Renal Impairment:
Caution is advised when using this compound in patients with renal impairment. medscape.com Pharmacokinetic studies in individuals with varying degrees of renal impairment have shown an approximate doubling of the elimination half-life, peak plasma concentration (Cmax), and AUC of this compound acid compared to individuals with normal renal function. hres.cae-lactancia.org However, a clear correlation between the degree of impairment and the AUC has not always been observed. hres.cae-lactancia.org While routine dosage adjustment is generally not recommended, a dose reduction may be necessary if the standard dose is not tolerated in patients with renal impairment. nih.govhres.ca Careful monitoring is advised for patients in whom dehydration would be dangerous, including those with renal impairment. pfizer.com
Smoking Status:
Smoking status has been considered in the context of this compound use, particularly in clinical trials. Some studies have noted that heavy smokers were not included in certain clinical trials for NSAID-induced ulcer prevention, suggesting a potential consideration for this population. medscape.com Smoking is known to increase irritation to the stomach and can make it more susceptible to damage from medications like NSAIDs, which this compound is used to prevent ulcers from. clevelandclinic.org While some research protocols for studies involving this compound, particularly in the context of medical abortion, have excluded heavy smokers, specific detailed research findings solely on the safety profile of this compound directly related to smoking status across various indications are less extensively documented in the provided search results compared to age and renal impairment. researchgate.net
Risk-Benefit Analyses in Clinical Applications
The risk-benefit analysis for this compound varies significantly depending on the specific clinical application.
For the prevention of NSAID-induced gastric ulcers, this compound has demonstrated efficacy in reducing the incidence of these ulcers, particularly in patients at high risk for complications. fda.gov The benefits in this context include the prevention of potentially serious gastrointestinal events such as bleeding, ulceration, and perforation that can be associated with chronic NSAID use. fda.gov The risks include common adverse effects like diarrhea, abdominal pain, and nausea, as well as the significant risk of teratogenicity and adverse pregnancy outcomes if used in pregnant women. medscape.comnih.gov Therefore, in women of childbearing potential, the risks associated with this compound use for ulcer prevention generally outweigh the benefits unless the patient is at high risk for complications from gastric ulcers and can comply with effective contraceptive measures. medscape.comnih.govpfizer.com
In obstetric and gynecological applications, where this compound is widely used off-label for indications such as cervical ripening, labor induction, and management of postpartum hemorrhage, the risk-benefit analysis involves different considerations. medscape.comnih.gov this compound's uterotonic properties are beneficial in these settings. nih.govmims.com However, risks such as uterine hyperstimulation, uterine rupture (particularly in women with prior uterine surgery), and potential adverse effects on the fetus or neonate need to be carefully weighed against the clinical need. medscape.comnih.govmayoclinic.org For instance, while this compound is effective for labor induction, the risk of uterine rupture increases with advancing gestational age and in patients with a history of cesarean delivery or major uterine surgery. medscape.commayoclinic.org
In the context of medical abortion, the combination of mifepristone and this compound is FDA-approved and considered a highly effective and safe option. nih.gov The benefit is the successful termination of pregnancy, while the risks include expected effects like cramping and bleeding, as well as less common but serious complications. nih.govmedicalnewstoday.com
Risk-benefit analyses are ongoing in various clinical scenarios, including surgical procedures like abdominal myomectomy, where this compound is explored for its hemostatic effects. mdpi.com Studies in this area analyze benefits such as reduced intraoperative blood loss and decreased need for blood transfusion against the safety profile in this specific surgical context. mdpi.com
Ultimately, the decision to use this compound requires a careful consideration of the potential benefits in treating or preventing a specific condition against the potential risks and adverse effects for the individual patient, taking into account their medical history, physiological status, and the specific clinical indication.
Formulation and Drug Delivery Research
Tablet Formulations (e.g., Angusta vs. Cytotec)
Tablet formulations are the most common presentation of misoprostol. Early formulations, such as Cytotec, typically contain 100 mcg or 200 mcg of this compound. rxlist.comfda.gov These tablets utilize excipients like hydrogenated castor oil, hypromellose, microcrystalline cellulose, and sodium starch glycolate. rxlist.comhres.capfizermedicalinformation.com The formulation of Cytotec was designed to optimize the rapid release of this compound in the stomach. hres.ca
More recent developments include lower-dose formulations specifically designed for certain indications, such as Angusta tablets containing 25 micrograms of this compound. medicines.org.uk The development of lower-dose tablets like Angusta addresses the need for precise dosing in applications requiring smaller quantities of the active pharmaceutical ingredient. frontiersin.orgtandfonline.com
Comparative studies evaluating the bioavailability of different this compound tablet formulations have been conducted. One study compared the relative bioavailability of Angusta 25 µg and Cytotec 200 µg tablets administered orally or sublingually. frontiersin.orgnih.gov While the ratios for AUC (Area Under the Curve) and Cmax (maximum concentration) were similar between the two formulations, the confidence intervals were outside the standard bioequivalence range, suggesting that their bioavailability might not be equivalent at the 25 µg or 50 µg dose levels under the study conditions. frontiersin.orgnih.gov The study noted that the need to cut or dissolve 200 µg Cytotec tablets to achieve lower doses could contribute to dosage inaccuracy and exposure to atmospheric conditions, potentially affecting stability. frontiersin.org
Excipients commonly found in this compound tablets include microcrystalline cellulose, hydrogenated castor oil, hypromellose, and sodium starch glycolate. rxlist.comhres.capfizermedicalinformation.comrjptonline.org These excipients play roles as fillers, binders, disintegrants, and stabilizers. For instance, microcrystalline cellulose is used as a direct compression vehicle and its low moisture content is important for this compound stability. rjptonline.org Hypromellose is included to protect the active component from ambient humidity and slow down water penetration. nih.gov
Enteric-Coated Tablet Development for Gastric Protection
The development of enteric-coated this compound tablets is explored, particularly in combination formulations with NSAIDs, to protect the stomach from the potential irritant effects of the NSAID component while ensuring this compound release in the small intestine. In such formulations, the NSAID is typically in the core tablet, which is then enteric-coated, and this compound is applied as an overcoat or in the outer layer. researchgate.netbiorxiv.orggoogle.com
Research has focused on developing compression-coated tablets where an enteric-coated core containing an NSAID (like diclofenac sodium) is surrounded by an outer layer containing this compound. researchgate.netbiorxiv.org Enteric coating polymers such as Eudragit L 100-55 are utilized to prevent drug release in the acidic environment of the stomach. researchgate.netbiorxiv.org Dissolution testing is a key evaluation method for enteric-coated tablets, confirming minimal drug release in simulated gastric fluid (pH 1.2) and subsequent release in phosphate buffer (pH 6.8), simulating intestinal fluid. researchgate.netbiorxiv.orgsymbiosisonlinepublishing.com Studies have shown that enteric-coated this compound formulations can achieve less than 10% drug dissolution in acidic conditions and high dissolution rates in basic conditions. biorxiv.org
Suppository Formulations for Rectal Administration
Suppository formulations of this compound have been developed as an alternative route of administration, particularly for indications where oral administration is not feasible or less effective. bayviewrx.comoup.com Rectal administration of this compound tablets has been practiced, but a dedicated suppository formulation offers potential advantages in terms of ease of administration and potentially more consistent absorption. bayviewrx.comoup.comscirp.orgresearchgate.netunige.ch
Research into this compound suppositories involves evaluating different suppository bases and the inclusion of surfactants to optimize drug release. Lipophilic bases such as Hard fat (Adeps solidus), Witepsol, and Suppocire have been investigated. scirp.orgresearchgate.netunige.chunige.ch Surfactants like Tween 20, Tween 80, and sodium lauryl sulfate (SLS) have been tested to facilitate this compound release from the lipophilic base. scirp.orgresearchgate.netunige.chunige.ch Studies have shown that adding surfactants to the base can enhance in vitro this compound release. For example, formulations using Adeps solidus with 5% Tween 20 or 1% SLS demonstrated significant this compound release within 30 minutes. scirp.orgresearchgate.netunige.chunige.ch The stability of this compound in suppository formulations has also been assessed, with studies indicating stability for several months when stored at refrigerated temperatures. scirp.orgresearchgate.netunige.chunige.ch
Excipient Research and Stability Considerations (e.g., Moisture Sensitivity)
This compound is known to be sensitive to moisture, which significantly impacts its stability in pharmaceutical formulations. hres.carjptonline.orgnih.govghsupplychain.orgnih.govwho.int Degradation of this compound is accelerated by high temperature, moisture, and high humidity. hres.ca The presence of water catalyzes degradation processes, leading to the formation of inactive degradation products such as type A, type B, and 8-epimer this compound. nih.govghsupplychain.org
Excipient selection plays a crucial role in mitigating moisture-induced degradation. The use of a solid dispersion of this compound with hydroxypropyl methylcellulose (HPMC) is a common strategy to enhance stability. nih.govghsupplychain.orgnih.gov HPMC is designed to protect this compound from ambient humidity and slow down water penetration into the tablet matrix. nih.gov Studies have shown that the rate of this compound degradation increases sharply when the water content of the dispersion exceeds a certain threshold, such as 2%. nih.govnih.gov Therefore, excipients with low moisture content, such as certain grades of microcrystalline cellulose and sodium starch glycolate, are preferred in this compound tablet formulations. rjptonline.orgghsupplychain.org
Packaging is also critical for maintaining this compound stability. Aluminum-aluminum blister packs are recommended to protect tablets from moisture, particularly in environments with high humidity and temperature. ghsupplychain.orgnih.govwho.intresearchgate.net Studies have demonstrated that this compound tablets in PVC-aluminum blisters degrade more rapidly than those in aluminum-aluminum blisters under challenging storage conditions. ghsupplychain.orgnih.govresearchgate.net Maintaining intact primary packaging is essential for ensuring the quality and stability of this compound tablets. nih.govresearchgate.net
Ethical and Societal Considerations in Misoprostol Research
Off-Label Use and Regulatory Landscape
Misoprostol's journey from a gastric ulcer medication to a crucial tool in reproductive health highlights the complexities of off-label use and the varied regulatory landscapes it navigates globally nih.govresearchgate.net. While approved by the FDA for gastric ulcer prevention and treatment, its widespread and often critical applications in obstetrics and gynecology are frequently considered off-label in the United States nih.gov. This means that while clinicians may legally prescribe it for these uses based on established medical evidence, these specific applications are not included in the drug's official labeling approved by the regulatory authority researchgate.net.
The legal status of this compound for reproductive health varies significantly across different countries, reflecting diverse cultural, religious, and political factors numberanalytics.com. Despite its recognition by the World Health Organization (WHO) as an essential medicine for several obstetric and gynecological indications, including postpartum hemorrhage (PPH) prevention and treatment, incomplete abortion management, and labor induction, its registration and availability for these uses are not uniform globally nih.govnumberanalytics.com. Some countries have placed this compound on their national Essential Medicines Lists for these purposes, integrating it into their procurement systems healthynewbornnetwork.org. However, concerns about its potential misuse for unsafe abortion have historically contributed to its non-registration for obstetric and gynecological indications in some regions, even where extensive study and off-label use have demonstrated its effectiveness oup.com.
The practice of off-label prescribing of this compound for reproductive health indications is often justified by a strong evidence base from published clinical trials and reviews demonstrating its efficacy and safety in these contexts researchgate.net. However, using this compound off-label can raise issues of inequity in patient management, particularly concerning access and potential costs if the medication is not subsidized for these uses researchgate.net. Clinicians prescribing this compound off-label are also advised to use evidence-based protocols to mitigate potential medicolegal challenges researchgate.net.
Access to this compound in Resource-Limited Settings
Access to this compound is a critical factor in improving reproductive health outcomes, particularly in resource-limited settings where access to comprehensive medical care may be limited nih.gov. This compound possesses several characteristics that make it particularly well-suited for these environments: it is relatively inexpensive, heat-stable (not requiring refrigeration), and can be administered via multiple routes (oral, sublingual, vaginal, rectal), potentially by non-physician health workers or through self-administration nih.govisrctn.comnih.gov.
Despite its potential, access to this compound in resource-limited settings faces several barriers. These include inconsistencies in supply and distribution, inadequate staffing, lack of knowledge among both providers and end-users, lack of drug registration for reproductive health indications, fear and apprehension regarding perceived misuse at policy and provider levels, and insufficient integration of this compound into basic healthcare services aku.edu. Stigma surrounding abortion also significantly hinders the integration of this compound into routine obstetric care and its availability to the public .
Studies highlight the feasibility and effectiveness of community-based distribution of this compound for PPH prevention in settings where access to skilled birth attendants and oxytocin is limited nih.gov. Research in countries like Ethiopia and Nigeria has explored the implementation of this compound use for PPH prevention and post-abortion care (PAC), identifying challenges and potential strategies for improving access healthynewbornnetwork.orgbioline.org.br. For instance, a study in Senegal indicated that pharmacies are one of the few places women can access this compound where it is not widely available, and research in this area has led to commitments from the Ministry of Health to train pharmacists on essential medicines, including this compound service.gov.uk.
Impact on Maternal Health Outcomes in Developing Countries
This compound has the potential to significantly impact maternal health outcomes in developing countries, where maternal mortality rates are disproportionately high aku.edujsr.org. Postpartum hemorrhage and complications from unsafe abortions are leading causes of maternal death in these regions nih.govaku.edu. This compound's ability to prevent and treat PPH and manage incomplete abortions offers a vital intervention to reduce these preventable deaths numberanalytics.comnih.gov.
Research indicates that the availability of this compound for safe abortion, PAC, and PPH could avert more maternal deaths than other large-scale interventions in developing countries . Studies have shown that transitioning from no this compound coverage to high coverage could lead to a significant reduction in maternal mortality jsr.org. For example, research in Sub-Saharan Africa, a region with a high burden of abortion-related mortality and maternal death from hemorrhage, underscores this compound's promise for improving reproductive health outcomes .
However, the full impact of this compound on maternal health in developing countries is yet to be realized due to the implementation barriers mentioned earlier aku.edu. While this compound is recognized as an essential obstetric medication, its application remains contested, partly because its use for abortion disrupts traditional medical and legal authority over pregnancy and abortion . Despite this, evidence supports the safety and effectiveness of this compound when used correctly, and healthcare workers in settings like Malawi have expressed satisfaction with its outcomes for managing incomplete abortions mdpi.com.
Research on this compound's Role in Self-Administered Regimens
Research has increasingly explored the role of this compound in self-administered regimens, particularly for medication abortion and PPH prevention in contexts where clinical access is limited clacaidigital.infoallaboveall.orgibisreproductivehealth.orgtandfonline.comnih.gov. Self-administration of this compound, defined as a person taking the medication without direct clinical supervision, has become more common globally and is credited with contributing to declines in maternal morbidity and mortality, especially in settings with restrictive abortion laws allaboveall.orgibisreproductivehealth.org.
Studies on self-managed this compound-alone regimens for abortion have reported high levels of effectiveness, with a significant percentage of participants achieving complete abortion without requiring surgical intervention allaboveall.orgibisreproductivehealth.orgtandfonline.com. For instance, a prospective cohort study involving callers to safe abortion hotlines and accompaniment groups in Nigeria, Southeast Asia, and Argentina found that a very high percentage of participants who used this compound alone reported a complete abortion without procedural intervention at follow-up nih.gov. Effectiveness rates in self-managed contexts have sometimes appeared higher than in some clinical studies, potentially due to longer follow-up periods and the allowance for additional doses in self-managed protocols allaboveall.orgibisreproductivehealth.org.
Research also supports the feasibility and safety of community-based distribution of this compound for PPH prevention, enabling women to self-administer the medication at home births or in settings without skilled attendance nih.gov. Studies have found no evidence to support fears of misuse or increased adverse pregnancy outcomes when this compound is distributed at the community level nih.gov.
The increasing evidence on the safety and effectiveness of self-administered this compound regimens is informing counseling and support models outside of traditional clinical settings, and influencing clinical policy and practice to expand the range of providers offering medication abortion ibisreproductivehealth.org.
Summary of Research Findings on Self-Administered this compound Regimens
| Study Location(s) | Context | Regimen Type | Reported Effectiveness (Complete Abortion without Surgical Intervention) | Notes | Source |
| United States | Self-administered | This compound alone | 97% (up to 56 days gestation) | Based on two studies of 1000 women; 98% preferred home administration. | clacaidigital.info |
| Various (Meta-analysis of clinical studies) | Clinically managed | This compound alone | 78% (across 13 studies, <13 weeks gestation) | Wide variation in regimens and observation periods. | allaboveall.orgtandfonline.com |
| Argentina, Burma/Thai border, Nigeria, Pakistan, United States | Self-managed (Prospective cohort studies) | This compound alone | 88% - 100% | Effectiveness range observed across multiple studies. Longer evaluation periods (3-4 weeks) may contribute to higher rates. | ibisreproductivehealth.org |
| Thai-Burma border | Self-managed with support | This compound alone | 96.4% | Assessed after one month. | tandfonline.com |
| Nigeria | Self-managed (purchased from drug sellers) | This compound alone | 94% | Assessed about a month after taking medication. Varied regimens used. | tandfonline.com |
| Argentina, Nigeria, Southeast Asia | Self-managed (Hotlines/accompaniment groups) | This compound alone | 98.1% | At last follow-up (median 22 days). High effectiveness observed. | nih.gov |
Note: Effectiveness rates and study parameters vary across research. This table provides a summary of reported findings in the context of self-administration.
Future Directions and Unanswered Research Questions
Elucidating Novel Pharmacological Targets and Pathways
While Misoprostol is known to act primarily by stimulating prostaglandin E1 receptors, research is ongoing to fully understand all its pharmacological targets and the downstream pathways they influence. Studies suggest that this compound may prevent the triggering of inflammatory processes and the release of tissue-damaging cytokines and mediators involved in various conditions. nih.gov This indicates potential mechanisms of action beyond its direct effects on smooth muscle contraction and gastric acid secretion. Further research is needed to identify these novel targets and pathways, which could unlock potential new therapeutic uses for this compound. nih.govscirp.org Understanding these mechanisms at a molecular level could lead to the development of modified this compound analogs with enhanced selectivity or efficacy for specific conditions.
Optimization of Dosing Regimens and Routes for Specific Clinical Indications
Optimizing the dosing regimens and routes of administration for this compound across its various indications remains a key area of research. Different routes, including oral, sublingual, buccal, vaginal, and rectal, exhibit varying pharmacokinetic profiles, which can influence efficacy and the occurrence of side effects. ekb.eggynuity.orggynuity.orgnih.gov While studies have compared different routes and doses for indications like medical abortion and labor induction, the optimal regimens for specific patient populations and clinical scenarios are still being refined. ekb.eggynuity.orgnih.govekb.egscirp.orgijrcog.orgsocietyfp.orgijrcog.org For instance, research continues to explore the ideal candidate for this compound use before hysteroscopy and the optimal dosing regimen for cervical ripening in this context. nih.gov Further comparative studies are necessary to determine if there are significant differences between sublingual and buccal routes for obstetric and gynecological indications. gynuity.org The influence of factors like vaginal pH on the effectiveness of vaginal this compound also requires further investigation.
Comparative Effectiveness Research with Newer Agents and Combination Therapies
Comparative effectiveness research is crucial to evaluate this compound's performance against newer pharmaceutical agents and in combination with other therapies. For example, studies compare the effectiveness of this compound alone versus in combination with mifepristone for medical abortion, investigating different doses, routes, and intervals between the medications. researchgate.netnih.gov Research also compares this compound to other uterotonics like oxytocin for the prevention and treatment of postpartum hemorrhage, particularly in low-resource settings. scirp.orggynuity.orgnih.gov While this compound offers advantages in terms of cost, storage, and administration in such settings, ongoing research is needed to confirm its comparative effectiveness and explore optimal combination strategies. scirp.orggynuity.orgnih.gov Evaluating this compound in combination with advanced drug delivery systems is also a promising area.
Long-Term Safety and Efficacy Studies Across Diverse Populations
While this compound has been used by millions globally, more long-term safety and efficacy studies are needed, especially in diverse populations. This includes evaluating its effects in specific age groups, different ethnicities, and individuals with comorbidities. The long-term implications of repeated this compound exposure for certain indications also warrant further investigation. Although some studies suggest this compound is not associated with long-term effects on women's health, comprehensive long-term data across varied demographics and health statuses would strengthen the evidence base. gynuity.org Research should also focus on understanding the potential for rare adverse outcomes, which may not be apparent in smaller or shorter-term studies. nih.gov
Development of Advanced Drug Delivery Systems
The development of advanced drug delivery systems for this compound is an active area of research aimed at improving its pharmacokinetic profile, reducing systemic side effects, and enhancing targeted delivery. kcl.ac.uknih.govresearchgate.net This includes exploring novel formulations such as gastric-directed microcapsules for the treatment of gastric ulcers, designed to improve chemical stability and reduce systemic exposure. kcl.ac.uknih.gov Research is also being conducted on innovative vaginal drug delivery systems, including inserts, hydrogel systems, and nanoparticles, to optimize dispersion, retention, and controlled release for gynecological applications. researchgate.net These advancements could lead to more effective and patient-friendly this compound products.
Research into this compound's Potential in Other Therapeutic Areas
Based on its pharmacological properties, there is potential for this compound to be explored for therapeutic uses beyond its current established indications. Its effects on inflammatory pathways suggest potential applications in conditions characterized by inflammation. nih.gov While currently licensed primarily for gastric ulcer prevention and treatment, further work indicates its potential in preventing inflammatory processes and the release of damaging mediators involved in conditions like asthma and osteoarthritis. nih.gov Research into these and other potential therapeutic areas could uncover new applications for this versatile compound.
Global Health Policy Research and Implementation Strategies
Research into global health policy and implementation strategies is critical to ensure equitable access to and appropriate use of this compound, particularly in low-resource settings where it offers significant advantages due to its low cost, stability, and ease of administration. gynuity.orgnih.govpopcouncil.orgnih.govids.ac.ukmhtf.org Studies are needed to identify and address barriers to its widespread and appropriate use, including reluctance within some healthcare systems, political controversies, and the lack of clear guidelines based on local contexts. popcouncil.orgmhtf.org Research focusing on community-based distribution models and the training of lower-level health providers, such as community health workers and traditional birth attendants, is essential to improve access for indications like postpartum hemorrhage prevention and treatment. gynuity.orgids.ac.ukglobaldoctorsforchoice.orgnih.gov Policy research should also focus on facilitating the registration of this compound for its women's health indications in more countries. popcouncil.orgmhtf.org
Q & A
Basic Research Questions
Q. What experimental designs are optimal for evaluating Misoprostol’s pharmacokinetics in human serum?
- Methodological Answer : Utilize ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) for high sensitivity and specificity. Optimize chromatographic conditions (e.g., retention time of 2.2 minutes for this compound acid) and validate the method using spiked serum samples across a dynamic range (e.g., 3.1–18.4 ppb). Include longitudinal study designs to track metabolite clearance rates .
Q. How can clinical trials standardize outcome measures for this compound’s efficacy in postpartum hemorrhage (PPH) prevention?
- Methodological Answer : Adopt primary endpoints such as blood loss volume (mL), hemoglobin levels post-delivery, and incidence of adverse effects (e.g., pyrexia). Use randomized controlled trials (RCTs) with control groups receiving placebo or alternative uterotonics (e.g., oxytocin). Statistical significance should be assessed via χ² tests or t-tests for categorical and continuous variables, respectively, with P<0.05 .
Q. What are best practices for formulating research questions on this compound’s role in maternal health?
- Methodological Answer : Align questions with gaps identified in systematic reviews (e.g., dose optimization, mortality reduction). Ensure specificity (e.g., “Does 400 µg oral this compound reduce PPH incidence compared to 600 µg?”) and relevance to clinical guidelines. Use frameworks like PICOT (Population, Intervention, Comparison, Outcome, Time) for rigor .
Advanced Research Questions
Q. How can researchers resolve contradictions in maternal mortality data from this compound trials?
- Methodological Answer : Conduct meta-analyses with sensitivity analyses to address heterogeneity. For example, exclude hyperpyrexia from composite outcomes (e.g., “maternal death or severe morbidity”) to isolate blood loss-related effects. Use random-effects models to account for variability across studies (I² >50% indicates high heterogeneity) .
Q. What statistical methods are robust for analyzing adverse effects in this compound trials?
- Methodological Answer : Apply multivariate logistic regression to adjust for confounders (e.g., gestational age, parity). For rare outcomes like maternal death, use Monte Carlo simulations to assess bias-corrected effectiveness. Report risk ratios (RR) with 95% confidence intervals (CI) for outcomes such as meconium-stained liquor or pyrexia .
Q. How can mixed-methods approaches enhance understanding of this compound implementation barriers?
- Methodological Answer : Combine quantitative surveys (e.g., 21-item questionnaires on prescribing practices) with qualitative interviews to explore contextual factors (e.g., healthcare provider attitudes). Use thematic analysis for qualitative data and χ² tests for quantitative trends. Ensure data saturation in interviews for reliability .
Q. What methodologies optimize dose-response studies for this compound in labor induction?
- Methodological Answer : Compare lower doses (e.g., 25–50 µg) to standard regimens using RCTs stratified by gestational age. Measure outcomes such as time to vaginal delivery, cesarean rates, and uterine hyperstimulation. Employ survival analysis (e.g., Kaplan-Meier curves) to evaluate induction timelines .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


