Piracetam
Description
Molecular Formula and IUPAC Nomenclature
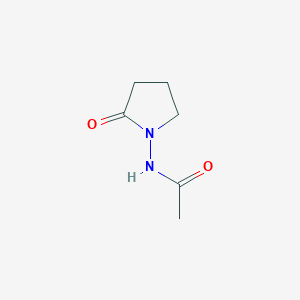
Piracetam is a synthetic nootropic compound with the molecular formula C₆H₁₀N₂O₂ and a molar mass of 142.16 g/mol. Its systematic IUPAC name is 2-(2-oxopyrrolidin-1-yl)acetamide , which reflects its structural components: a 2-oxopyrrolidine ring linked to an acetamide group via a methylene bridge. The numbering of the pyrrolidine ring begins at the nitrogen atom, with the carbonyl oxygen at position 2 (Figure 1).
Table 1: Key chemical identifiers of this compound
| Property | Value |
|---|---|
| Molecular formula | C₆H₁₀N₂O₂ |
| IUPAC name | 2-(2-oxopyrrolidin-1-yl)acetamide |
| CAS Registry Number | 7491-74-9 |
| SMILES | NC(=O)CN1CCCC1=O |
| InChI Key | GMZVRMREEHBGGF-UHFFFAOYSA-N |
Structural Relationship to 2-Oxo-Pyrrolidine Derivatives
This compound belongs to the racetam family, characterized by a 2-oxo-pyrrolidine core structure. This five-membered lactam ring differentiates racetams from related compounds like γ-aminobutyric acid (GABA), despite this compound being a cyclic derivative of GABA. The 2-oxo group (a ketone at position 2) and the acetamide side chain at position 1 are critical for its biological activity (Figure 2).
Structurally, this compound shares similarities with pyroglutamic acid (5-oxoproline), another 2-oxo-pyrrolidine derivative, but lacks the carboxylic acid moiety. Comparative analysis reveals that the acetamide group in this compound enhances its ability to interact with lipid membranes and proteins, unlike the acidic side chain of pyroglutamic acid.
Table 2: Structural comparison of this compound with related 2-oxo-pyrrolidine derivatives
| Compound | Core Structure | Functional Groups | Biological Role |
|---|---|---|---|
| This compound | 2-oxo-pyrrolidine | Acetamide | Nootropic activity |
| Pyroglutamic acid | 5-oxoproline | Carboxylic acid | Metabolic intermediate |
| Aniracetam | 2-oxo-pyrrolidine | p-Anisoyl group | Cognitive enhancer |
Conformational Analysis via NMR and Computational Modeling
This compound exhibits conformational flexibility due to rotation around the C–N bond connecting the pyrrolidine ring and acetamide group. Nuclear Magnetic Resonance (NMR) and computational modeling have elucidated its dominant conformers and polymorphic behavior:
NMR Studies
- Solid-state ³¹P NMR revealed that this compound interacts with phospholipid headgroups, inducing isotropic motion in model membranes. This interaction stabilizes membrane structure by preventing negative curvature formation.
- ²H NMR of deuterated lipid bicelles demonstrated that this compound reduces peptide-induced membrane destabilization, likely by coating phospholipid headgroups.
Computational Modeling
- Density Functional Theory (DFT) calculations identified two stable gas-phase conformers: an exo conformation (N–H bond oriented away from the ring) and an endo conformation (N–H bond oriented toward the ring). The exo form is marginally more stable due to an intramolecular N–H···O=C hydrogen bond.
- Molecular dynamics simulations predicted that this compound’s amphipathic nature (hydrophobic pyrrolidine ring and polar acetamide) facilitates its insertion into lipid bilayers, aligning with experimental NMR data.
Table 3: Key findings from conformational studies
Polymorphism and Crystal Structure
This compound exists in multiple polymorphic forms (e.g., Forms I–IV), with Forms II and III being polytypes differing in layer stacking. X-ray diffraction and DFT-based crystal structure prediction revealed that Form III has a herringbone arrangement, while Form II adopts face-to-face molecular packing. These structural differences influence mechanical properties, such as Form III’s higher elastic modulus.
Properties
IUPAC Name |
2-(2-oxopyrrolidin-1-yl)acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GMZVRMREEHBGGF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(=O)N(C1)CC(=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H10N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5044491 | |
| Record name | 2-Oxo-1-Pyrrolidineacetamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5044491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
142.16 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Boiling Point |
Decomposes | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Color/Form |
Crystals from isopropanol | |
CAS No. |
7491-74-9 | |
| Record name | Piracetam | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=7491-74-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Piracetam [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007491749 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | piracetam | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758191 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 2-Oxo-1-Pyrrolidineacetamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5044491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Piracetam | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.028.466 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PIRACETAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ZH516LNZ10 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
151.5 - 152.5 °C | |
| Record name | Piracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09210 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PIRACETAM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7529 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
Reaction Mechanism and Stepwise Procedure
The synthesis begins with the deprotonation of α-pyrrolidone using sodium methoxide in methanol, forming a reactive sodium enolate. Subsequent alkylation with methyl chloroacetate in toluene at 20–110°C produces methyl 2-(2-oxopyrrolidin-1-yl)acetate. The final amination step employs ammonia-saturated methanol at 50–70°C, yielding crude this compound, which is purified via recrystallization in isopropanol.
Key operational parameters include:
Solvent and Catalyst Optimization
Methanol serves dual roles as both solvent and proton donor in the amination step, while toluene facilitates azeotropic distillation during methanol removal. The patent specifies that substituting methanol with ethanol or isopropanol reduces yields by 12–15% due to decreased ammonia solubility.
Glycine-Derived Synthetic Pathways
Alternative routes utilizing glycine derivatives, though less prevalent, offer insights into precursor flexibility. A 1979 British patent describes condensing trimethylsilyl glycine with γ-chlorobutyryl chloride, followed by ammonolysis and cyclization.
Challenges in Industrial Implementation
While theoretically feasible, this method faces practical limitations:
- Hygroscopic intermediates : Glycinamide hydrochloride’s moisture sensitivity necessitates stringent humidity control (<5% RH)
- Multi-step purification : Column chromatography required after cyclization increases production costs
- Yield : Reported at 58–62%, significantly lower than α-pyrrolidone routes
Succinic Anhydride Approach: A Cost-Prohibitive Alternative
The succinic anhydride method, involving glycine ammonolysis and sodium tetrafluoroborate reduction, demonstrates academic interest but limited commercial applicability:
Reaction Economics and Scalability Issues
- Catalyst cost : Sodium tetrafluoroborate prices exceed $450/kg, rendering large-scale production economically unfeasible
- Electrolytic reduction requirements : Energy-intensive steps (15–20 kWh/kg product) inflate operational costs
Single-Step Synthesis: Theoretical Promise vs. Practical Limitations
Direct condensation of 4-chlorobutyric acid ethyl ester with glycinamide hydrochloride in ethanol represents the most atom-economical route. However, competing side reactions—particularly N-alkylation at multiple sites—result in complex mixtures requiring HPLC purification. Industrial batches using this method rarely exceed 35% yield.
Crystallization Dynamics and Polymorphic Control
Recent advancements in this compound crystallization have identified six polymorphic forms, with Form II (thermodynamically stable) and Form VI (metastable) being most pharmacologically relevant.
Solvent-Mediated Polymorphic Transitions
Induction time experiments (1,200 trials) reveal:
| Solvent | Preferred Polymorph | Induction Time (288 K) | Interfacial Energy (mJ/m²) |
|---|---|---|---|
| Ethanol | Form II | 15 min | 4.2 ± 0.3 |
| Isopropanol | Form VI | >6 hours | 5.1 ± 0.4 |
Isopropanol’s higher viscosity (2.4 cP vs. ethanol’s 1.2 cP) and lower dielectric constant (19.9 vs. 24.3) stabilize Form VI nucleation by reducing molecular mobility. This kinetic trapping enables isolation of the metastable form for specialized formulations requiring rapid dissolution profiles.
Recrystallization Protocols for Pharmaceutical-Grade Material
Industrial purification typically employs:
- Primary crystallization : Methanol/water (70:30 v/v) at 278 K
- Annealing : 323 K for 2 hours under nitrogen
- Final polishing : Anti-solvent addition (n-heptane) with controlled cooling (1 K/min)
These steps yield 99.9% pure this compound with mean particle size 50–70 μm, suitable for direct compression tableting.
Comparative Analysis of Synthetic Methodologies
A comprehensive evaluation of this compound synthesis routes reveals critical trade-offs:
α-Pyrrolidone method :
- Advantages : High yield (82%), minimal purification, commodity chemicals
- Disadvantages : Requires anhydrous conditions, generates sodium chloride waste
Glycine route :
- Advantages : Novel intellectual property opportunities
- Disadvantages : Low yield (58%), expensive silylation reagents
Single-step synthesis :
- Advantages : Atom economy (92%)
- Disadvantages : Poor regioselectivity, complex workup
Chemical Reactions Analysis
Types of Reactions
Piracetam undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized to form 2-pyrrolidone-5-carboxylic acid.
Reduction: Reduction of this compound can yield 2-pyrrolidone.
Substitution: Substitution reactions can occur at the acetamide group, leading to the formation of various derivatives
Common Reagents and Conditions
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Reduction: Reducing agents such as lithium aluminum hydride can be used.
Substitution: Reagents like alkyl halides and acyl chlorides are commonly used for substitution reactions
Major Products Formed
Oxidation: 2-pyrrolidone-5-carboxylic acid
Reduction: 2-pyrrolidone
Substitution: Various acetamide derivatives
Scientific Research Applications
Piracetam has a wide range of scientific research applications:
Chemistry: Used as a model compound for studying the properties of cyclic amides and their derivatives.
Biology: Investigated for its effects on cell membrane fluidity and neuroprotection.
Medicine: Used in the treatment of cognitive disorders, myoclonus, and sickle cell anemia. It has also been studied for its potential benefits in conditions like dementia, vertigo, and dyslexia.
Industry: Utilized in the development of nootropic supplements and cognitive enhancers .
Mechanism of Action
Piracetam’s mechanism of action is not fully understood, but it is believed to involve several pathways:
Neurotransmitter Modulation: this compound modulates neurotransmission in cholinergic and glutamatergic systems.
Neuroprotection: It has neuroprotective properties, enhancing neural plasticity and protecting against hypoxia.
Vascular Effects: This compound reduces erythrocyte adhesion to vascular endothelium, hindering vasospasms and facilitating microcirculation .
Comparison with Similar Compounds
Comparison with Structural Analogs
Chemical Classification and Potency
Piracetam analogs are categorized into three subgroups based on structure and clinical use :
| Compound | Structural Features | Relative Potency vs. This compound | Key Indications |
|---|---|---|---|
| This compound | 2-oxopyrrolidine ring | 1× (reference) | Myoclonus, stroke, cognitive decline |
| Phenylthis compound | This compound + phenyl group | 10–100× | Cognitive enhancement, physical fatigue |
| Aniracetam | Fat-soluble with additional aryl group | 5–20× | Anxiety, memory enhancement |
| Levetiracetam | Pyrrolidone + ethyl group | N/A (distinct mechanism) | Epilepsy |
| DM235 (Sunifiram) | Modified pyrrolidine core | 1,000–10,000× | Experimental cognitive enhancer |
Key Insights :
Mechanistic Differences
Controversies : While this compound and analogs like oxiracetam were initially thought to act via cholinergic pathways, species-specific responses (e.g., dose discrepancies in mice vs. rats) suggest multifactorial mechanisms .
Efficacy in Specific Conditions
Stroke and Neuroprotection
- Oxiracetam: Limited data, but one trial noted improved aphasia recovery vs. placebo .
Cognitive Decline
Epilepsy
- Levetiracetam : Superior to this compound due to targeted SV2A binding, reducing seizure frequency by >50% in refractory cases .
Biological Activity
Piracetam, a nootropic compound first synthesized in the 1960s, is widely recognized for its cognitive-enhancing properties. It belongs to the racetam family of drugs and has been extensively studied for its effects on brain function, neuroprotection, and various neurological disorders. This article delves into the biological activity of this compound, highlighting its mechanisms of action, therapeutic applications, and relevant research findings.
This compound exhibits several mechanisms that contribute to its biological activity:
- Neurotransmitter Modulation : this compound modulates neurotransmission by enhancing cholinergic, serotonergic, noradrenergic, and glutamatergic pathways. It increases the density of postsynaptic receptors without binding strongly to them (Ki > 10μM) . This modulation facilitates improved cognitive processes such as learning and memory.
- Membrane Fluidity : The compound interacts with phospholipid membranes, promoting membrane fluidity and stability. This action is crucial for maintaining the structure and function of transmembrane proteins involved in neurotransmission .
- Neuroprotection : this compound has demonstrated neuroprotective effects against various forms of neuronal injury, including oxidative stress induced by lipopolysaccharides (LPS). It reduces intracellular reactive oxygen species (ROS) and nitric oxide (NO), which are implicated in neuroinflammation and cell death .
Therapeutic Applications
This compound has been investigated for a range of clinical applications:
- Cognitive Enhancement : Numerous studies have reported improvements in cognitive functions such as memory, attention, and consciousness in both healthy individuals and those with cognitive impairments .
- Neurodegenerative Disorders : Research suggests that this compound may have potential benefits in conditions like Alzheimer's disease and vascular dementia by enhancing synaptic plasticity and protecting neurons from damage .
- Anticonvulsant Properties : this compound has been shown to possess anticonvulsant properties, making it a candidate for treating epilepsy .
Case Studies and Clinical Trials
A summary of key studies investigating the biological activity of this compound is presented in Table 1:
| Study | Findings | Dosage | Population |
|---|---|---|---|
| Ahmed & Oswal (2010) | Continuous positive allosteric modulation on AMPA receptors; enhances neuronal excitation | 200 mg/kg | Rats |
| Colucci et al. (2012) | Improves acetylcholine functions via muscarinic receptors | 400 mg/kg | Rats |
| Braz. J. Pharm. Sci. (2022) | Reduces LPS-induced oxidative stress; improves SOD levels | 200-400 mg/kg | Mice |
Neuroprotective Effects
A notable study highlighted this compound's capacity to protect against LPS-induced neuronal toxicity. The treatment significantly reduced levels of inflammatory cytokines like IL-6 and amyloid-beta, indicating its potential role in mitigating neuroinflammation . Additionally, this compound was found to enhance antioxidant enzyme activities such as catalase and superoxide dismutase (SOD), further supporting its neuroprotective profile.
Q & A
Q. What are the primary neuropharmacological mechanisms of action for Piracetam, and how are they experimentally validated?
this compound enhances cell membrane fluidity, modulates neurotransmission (particularly cholinergic and glutamatergic systems), and increases oxygen consumption via adenylate kinase activity in the brain . These mechanisms are validated through in vivo models assessing acetylcholine release, membrane permeability assays, and behavioral tests in rodents. For example, studies measuring synaptic plasticity changes or oxygen utilization in ischemic brains provide direct evidence .
Q. What experimental evidence supports this compound’s neuroprotective efficacy in preclinical stroke models?
Meta-analyses of animal stroke models show this compound and derivatives improve outcomes by 30.2% (95% CI: 16.1–44.4), primarily via infarct size reduction and neurological score improvements. However, only two studies directly tested this compound in stroke models, with median quality scores of 4/10, highlighting methodological limitations . Key assays include histopathological analysis of infarct regions and Morris water maze tests for functional recovery .
Q. How is this compound’s dosage optimized in experimental settings, and what factors influence dose-response relationships?
Preclinical studies use dose-ranging protocols (e.g., 80–100 mg/kg/day in rodents) to establish efficacy and safety thresholds. For instance, LD50 values in mice (455.5 mg/kg) inform toxicity limits . Dose-response linearity is observed in epilepsy trials, where 24 g/day in humans showed superior efficacy compared to lower doses . Optimization requires pharmacokinetic profiling (e.g., half-life, blood-brain barrier penetration) and endpoint-specific titration .
Advanced Research Questions
Q. How do contradictory findings in this compound’s clinical efficacy for cognitive impairment arise, and what methodological strategies resolve them?
Meta-analyses report odds ratios of 3.43–3.55 for global improvement in dementia but note heterogeneity (I² > 50%) and publication bias . Contradictions stem from:
- Study design : Crossover trials with unanalyzed first-period data inflate effect sizes .
- Population diversity : Mixed cohorts (Alzheimer’s, vascular dementia) obscure subtype-specific responses . Solutions include stratified randomization, intention-to-treat analysis, and adherence to PRISMA guidelines for systematic reviews .
Q. What critiques apply to the translational gap between this compound’s preclinical promise and clinical trial outcomes?
Preclinical data for stroke were published 10 years after clinical trials began, suggesting animal models were not foundational to trial design . Discrepancies arise from:
- Timing : Animal studies administer this compound ≤6 hours post-stroke, whereas trials allowed 12-hour delays .
- Outcome measures : Rodent infarct size vs. human functional independence scales (e.g., modified Rankin) lack alignment. Bridging this gap requires co-development of preclinical-clinical protocols and biomarker validation (e.g., neuroimaging correlates) .
Q. Why does this compound fail to enhance cognition in Down syndrome despite efficacy in other neurodevelopmental models?
A randomized crossover trial (N=25) found no cognitive improvement in Down syndrome, with adverse effects (e.g., agitation) . Potential explanations:
- Pathophysiological divergence : Down syndrome’s trisomy 21 may alter drug targets (e.g., GABAergic pathways).
- Dosing mismatch : 80–100 mg/kg/day may inadequately penetrate the blood-brain barrier in this population. Mechanistic studies comparing this compound’s effects on Shank3 vs. DYRK1A pathways could clarify etiology-specific responses .
Methodological Recommendations
- For preclinical studies : Use the STAIR criteria for stroke models, including blinded outcome assessment and sample size calculations .
- For clinical trials : Prioritize adaptive designs to refine dosing (e.g., Bayesian response-adaptive randomization) .
- For meta-analyses : Apply GRADE criteria to evaluate evidence certainty and adjust for unpublished data via trim-and-fill analyses .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


