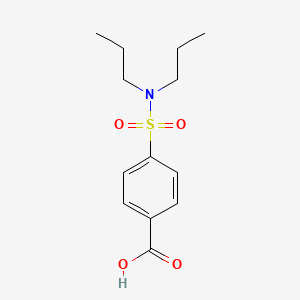
Probenecid
Description
This compound is a sulfonamide in which the nitrogen of 4-sulfamoylbenzoic acid is substituted with two propyl groups. It has a role as a uricosuric drug. It is a sulfonamide and a member of benzoic acids.
The prototypical uricosuric agent. It inhibits the renal excretion of organic anions and reduces tubular reabsorption of urate. This compound has also been used to treat patients with renal impairment, and, because it reduces the renal tubular excretion of other drugs, has been used as an adjunct to antibacterial therapy.
This compound is a uricosuric agent used for the treatment of gout usually in combination with other agents. This compound has been associated with minor serum aminotransferase elevations and very rarely with hypersensitivity reactions which, even more rarely, can be accompanied by acute liver injury.
This compound is a benzoic acid derivative with antihyperuricemic property. This compound competitively inhibits the active reabsorption of urate at the proximal tubule in the kidney thereby increasing urinary excretion of uric acid and lowering serum urate concentrations. This prevents urate deposition and promotes resolution of existing urate deposits. In addition, this compound modulates the transport of organic acids and acidic drugs at the proximal and distal renal tubule, thereby increasing the drug serum concentration.
See also: Colchicine; this compound (component of); Ampicillin/ampicillin trihydrate; this compound (component of).
Properties
IUPAC Name |
4-(dipropylsulfamoyl)benzoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C13H19NO4S/c1-3-9-14(10-4-2)19(17,18)12-7-5-11(6-8-12)13(15)16/h5-8H,3-4,9-10H2,1-2H3,(H,15,16) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
DBABZHXKTCFAPX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C13H19NO4S | |
| Record name | PROBENECID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20951 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9021188 | |
| Record name | Probenecid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9021188 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
285.36 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Probenecid appears as odorless white or almost white crystalline powder. Slightly bitter taste; pleasant aftertaste. (NTP, 1992), Solid | |
| Record name | PROBENECID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20951 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Probenecid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015166 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>42.8 [ug/mL] (The mean of the results at pH 7.4), less than 1 mg/mL at 68 °F (NTP, 1992), FREELY SOL IN WATER /SODIUM SALT/, SOL IN DIL ALKALI, ALCOHOL, CHLOROFORM & ACETONE; PRACTICALLY INSOL IN WATER & DIL ACIDS, 4.25e-01 g/L | |
| Record name | SID855953 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | PROBENECID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20951 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Probenecid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01032 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROBENECID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3387 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Probenecid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015166 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
CRYSTALS FROM DIL ALCOHOL, WHITE OR NEARLY WHITE, FINE, CRYSTALLINE POWDER | |
CAS No. |
57-66-9 | |
| Record name | PROBENECID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20951 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Probenecid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=57-66-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Probenecid [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000057669 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Probenecid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01032 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | probenecid | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757292 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | probenecid | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=18786 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Benzoic acid, 4-[(dipropylamino)sulfonyl]- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Probenecid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9021188 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Probenecid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.313 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PROBENECID | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/PO572Z7917 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PROBENECID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3387 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Probenecid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015166 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
381 to 385 °F (NTP, 1992), 194-196 °C, 195 °C | |
| Record name | PROBENECID | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20951 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Probenecid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01032 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PROBENECID | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3387 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Probenecid | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015166 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Historical Context and Evolution of Probenecid Research
Initial Development and Early Therapeutic Rationale for Probenecid
This compound, a sulfonamide derivative, was synthesized in 1949 by Miller, Ziegler, and Sprague with the primary objective of decreasing the renal clearance of antibiotics, particularly penicillin. nih.govnih.gov It was introduced to the market in 1950. nih.gov The rationale behind this development was to increase and prolong the plasma concentrations of co-administered antibiotics, thereby enhancing their effectiveness and allowing for lower dosing, which was especially relevant during periods of limited antibiotic supply, such as World War II. nih.govnewdrugapprovals.org
Role in Penicillin Co-administration and Renal Clearance Modulation
This compound's mechanism in this context involves the competitive inhibition of organic anion transporters (OATs) in the renal tubules. nih.govresearchgate.netmedicinesinformation.co.nz These transporters are responsible for actively secreting organic acids, including penicillin and other beta-lactam antibiotics, from the bloodstream into the urine for excretion. newdrugapprovals.orgmedicinesinformation.co.nzdrugbank.com By blocking these transporters, this compound reduces the rate at which these antibiotics are removed from the body, leading to higher and more sustained plasma levels. nih.govresearchgate.netmedicinesinformation.co.nzpatsnap.com This interaction was therapeutically exploited to extend the plasma half-life of penicillins and cephalosporins, improving pharmacokinetic/pharmacodynamic target attainment. nih.govdrugbank.com Studies have shown that this compound can significantly increase the area under the curve (AUC), peak concentration (Cmax), and serum half-life (t½) of co-administered beta-lactam antibiotics. nih.gov
An example of pharmacokinetic data illustrating the effect of this compound on antibiotic levels is shown below, based on findings from studies on beta-lactam co-administration:
| Antibiotic | Effect of this compound on AUC | Effect of this compound on Cmax | Effect of this compound on t½ |
| Flucloxacillin | Increased (approx. 53-55%) | Not specified | Not specified |
| Cefalexin | Increased | Increased | Increased |
| Penicillin V | Increased | Not specified | Increased |
Introduction for Gout Treatment
Subsequent to its initial use with antibiotics, it was fortuitously discovered that this compound also possessed uricosuric properties. nih.govnih.gov This led to its introduction and approval for the treatment of gout and hyperuricemia. newdrugapprovals.orgpatsnap.comnih.gov Gout is a condition characterized by the accumulation of uric acid crystals in the joints, resulting from elevated serum uric acid levels (hyperuricemia). patsnap.comarthritisaustralia.com.au this compound addresses this by inhibiting the tubular reabsorption of uric acid in the kidneys, primarily by blocking the urate transporter 1 (URAT1) and other organic anion transporters (OAT1, OAT3, and GLUT9) in the renal tubules. drugbank.comexplorationpub.com This action increases the excretion of uric acid in the urine, thereby lowering serum uric acid concentrations and helping to prevent the formation and deposition of urate crystals. drugbank.compatsnap.com this compound was the first urate-lowering therapy to be widely commercialized. explorationpub.com
Shifting Clinical Relevance and Research Focus of this compound
Despite its initial importance in antibiotic therapy and its approval for gout treatment, the clinical relevance of this compound has significantly declined over the years. nih.govresearchgate.netnih.gov The expanded production of more diverse, cheaper, and safer antibiotics reduced the necessity of using this compound to boost antibiotic levels for many common infections in the post-World War II era. nih.govresearchgate.net In the treatment of gout, the introduction of alternative therapies, such as allopurinol in the 1960s, which inhibits uric acid production, and the use of colchicine and steroids for acute attacks, diminished this compound's role as a primary treatment. researchgate.netexplorationpub.com While still approved for gout and hyperuricemia, it is rarely used today for either indication compared to its past prevalence. researchgate.netexplorationpub.com Its efficacy in lowering serum uric acid is considered moderate, and it may not be effective in patients with significant renal impairment. explorationpub.commedsafe.govt.nz
However, the decline in its traditional clinical use has coincided with a shifting research focus, exploring other pharmacological properties and potential applications. nih.govresearchgate.netnih.gov
Emergence of New Therapeutic Potentials and Research Avenues for this compound
In recent years, research into this compound has revealed its ability to interact with various membrane proteins beyond the well-known OATs and URAT1, suggesting new potential therapeutic utilities. nih.govmdpi.comresearchgate.net These interactions include the blockade of pannexin-1 (Panx1) hemichannels and the activation of Transient Receptor Potential Vanilloid 2 (TRPV2) channels. nih.govresearchgate.netmdpi.com
The blockade of Panx1 hemichannels has garnered attention for its potential role in reducing neuroinflammation, a key factor in various central nervous system (CNS) disorders. nih.govmdpi.comresearchgate.net Panx1 hemichannels are involved in the release of ATP and other molecules that contribute to inflammatory responses and affect neuronal communication. mdpi.com By inhibiting these channels, this compound may exert neuroprotective, antiepileptic, and anti-inflammatory effects, which are being investigated in the context of conditions like ischemia, sepsis, epilepsy, Parkinson's, and Alzheimer's diseases. nih.govmdpi.comresearchgate.net Furthermore, this compound's ability to inhibit organic anion and cation transporters in the brain (OATs and OCTs) can increase the bioavailability of certain drugs in the CNS, suggesting its potential as an adjuvant therapy to improve the effectiveness of conventional treatments for psychiatric and neurological disorders where drug delivery to the brain is a challenge. nih.govmdpi.comresearchgate.net
The identification of this compound as an agonist of TRPV2 channels has opened up research avenues in cardiology and neurology. researchgate.netnih.govnih.gov TRPV2 channels are involved in various physiological processes, including calcium signaling. nih.govmdpi.com Studies have shown that this compound can exert a positive inotropic effect on cardiomyocytes, mediated by TRPV2 channels, suggesting a potential role in treating heart conditions. nih.gov
Beyond these specific targets, this compound's interaction with OATs and its effect on drug excretion continue to be relevant in research for optimizing the pharmacokinetics of other medications, including some antivirals like oseltamivir, aciclovir, ganciclovir, and zidovudine, as well as certain nonsteroidal anti-inflammatory drugs (NSAIDs) and methotrexate. medicinesinformation.co.nzmedsafe.govt.nzebi.ac.ukwikipedia.orgspandidos-publications.com This property is being explored to enhance the systemic exposure and efficacy of these drugs or to reduce their renal toxicity. newdrugapprovals.orgmedicinesinformation.co.nzmedsafe.govt.nz
Pharmacological Mechanisms of Action of Probenecid
Modulation of Organic Anion Transporters (OATs) by Probenecid
This compound is a well-established inhibitor of organic anion transporters (OATs), a family of proteins crucial for the transport of a wide range of endogenous and exogenous organic anions across cell membranes. nih.govpatsnap.com OATs are predominantly located in the kidneys and liver, playing a significant role in the body's elimination of toxins and drugs. patsnap.com this compound's interaction with OATs is primarily characterized by competitive inhibition, where it competes with other organic anions for binding sites on the transporter proteins. patsnap.compatsnap.com
Competitive Inhibition of Urate Transporter 1 (URAT1) by this compound
A key target of this compound is Urate Transporter 1 (URAT1), also known as SLC22A12. nih.gov URAT1 is primarily expressed on the apical membrane of renal proximal tubule cells and is responsible for the reabsorption of uric acid from the tubular fluid back into the bloodstream. patsnap.comnih.gov this compound acts as a competitive inhibitor of URAT1, binding to the transporter and reducing the reabsorption of uric acid. wikipedia.orgpatsnap.com This inhibition leads to increased excretion of uric acid in the urine, consequently lowering serum urate levels. wikipedia.orgpatsnap.com Research has shown that this compound binds to a similar region as other URAT1 inhibitors and stabilizes the transporter in an inward-facing state. acs.org Studies comparing the inhibitory potency of this compound on human and rat URAT1 have shown a significantly higher affinity for the human transporter. nih.gov
| Inhibitor | Human URAT1 IC50 (µM) | Rat URAT1 IC50 (µM) |
| This compound | 22 | 786 |
| Benzbromarone | 0.22 | 26 |
| Sulfinpyrazone | 32 | 680 |
| Lesinurad | 3.5 | 81 |
Table 1: Inhibitory Potency (IC50) of Uricosuric Agents on Human and Rat URAT1. nih.gov
Inhibition of OAT1 and OAT3 by this compound
This compound also inhibits Organic Anion Transporter 1 (OAT1, SLC22A6) and Organic Anion Transporter 3 (OAT3, SLC22A8). taylorandfrancis.comnih.govnih.gov These transporters are located on the basolateral membrane of renal proximal tubule cells and are involved in the uptake of organic anions from the blood into the cells for secretion into the tubular fluid. mdpi.comoup.com By inhibiting OAT1 and OAT3, this compound reduces the active tubular secretion of various organic anions, including numerous drugs. medicinesinformation.co.nzsolvobiotech.com Studies using HEK293 cells expressing OAT1 and OAT3 have demonstrated that this compound inhibits the uptake of model substrates like 6-carboxyfluorescein (6-CF) in a dose-dependent manner. researchgate.net
| Transporter | This compound IC50 (µM) |
| OAT1 | 29.42 |
| OAT3 | 8.662 |
Table 2: Estimated IC50 Values of this compound for OAT1 and OAT3 Inhibition in HEK293 Cells. researchgate.net
This inhibition by this compound can lead to increased plasma concentrations and prolonged half-lives of co-administered drugs that are substrates for OAT1 and OAT3, such as certain antibiotics. medicinesinformation.co.nzpatsnap.com
Impact on Renal Tubular Secretion of Organic Anions by this compound
The inhibition of OAT1 and OAT3 by this compound significantly impacts the renal tubular secretion of organic anions. nih.govpatsnap.comdrugbank.comnih.gov Renal tubular secretion is a crucial process for the elimination of many drugs, toxins, and endogenous compounds from the body. patsnap.comnih.gov By blocking the transporters responsible for the uptake of these substances from the blood into the renal tubule cells, this compound reduces their secretion into the urine. medicinesinformation.co.nzdrugbank.com This mechanism is the basis for this compound's historical use in increasing the plasma levels of antibiotics like penicillin, thereby enhancing their therapeutic efficacy. medicinesinformation.co.nzwikipedia.org The competitive nature of this inhibition means that this compound competes with other organic anions for excretion pathways in the kidney. patsnap.compatsnap.com
Modulation of Transient Receptor Potential Vanilloid 2 (TRPV2) Channels by this compound
Beyond its effects on organic anion transporters, this compound has also been identified as a modulator of Transient Receptor Potential Vanilloid 2 (TRPV2) channels. taylorandfrancis.comnih.govcellphysiolbiochem.commendeley.comguidetopharmacology.orgnih.gov TRPV2 is a non-selective cation channel expressed in various tissues, including the heart, neurons, and immune cells. mdpi.comnih.govbiorxiv.org
Activation of TRPV2 Channels by this compound
This compound has been shown to act as an agonist of TRPV2 channels, leading to their activation. nih.govcellphysiolbiochem.comguidetopharmacology.orgselleckchem.com Studies using TRPV2-transfected cells have demonstrated that this compound can induce increases in intracellular calcium levels, consistent with TRPV2 channel activation. selleckchem.combiorxiv.orgbiorxiv.org While the exact mechanism of TRPV2 activation by this compound is not fully understood, research suggests it may involve direct interaction with an intracellular binding pocket on the channel protein. nih.gov This interaction appears to potentiate the channel's response to other stimuli and may prevent it from entering an inactivated state. nih.gov It is noted that higher micromolar concentrations of this compound are typically required to activate TRPV2 channels. cellphysiolbiochem.comresearchgate.net
Consequences for Intracellular Calcium Signaling and Cellular Processes
Activation of TRPV2 channels by this compound leads to an influx of calcium ions into the cell, impacting intracellular calcium signaling. mdpi.comnih.govbiorxiv.orgbiorxiv.org This rise in intracellular calcium can trigger a cascade of downstream cellular processes. In cardiomyocytes, TRPV2 activation by this compound has been linked to positive inotropic effects, suggesting a role in modulating cardiac contractility. taylorandfrancis.comnih.gov In neuronal cells, TRPV2 activation by this compound has been shown to cause rapid changes in the actin cytoskeleton and influence neurite initiation and branching, potentially involving alterations in cellular cAMP levels. biorxiv.orgbiorxiv.org The transient nature of the this compound-induced increase in intracellular calcium has been observed in some cell types. biorxiv.orgbiorxiv.org These findings suggest that this compound's modulation of TRPV2 channels can influence diverse cellular functions depending on the cell type and its physiological context.
Modulation of Pannexin-1 (Panx1) Hemichannels by this compound
Pannexin-1 (Panx1) channels are ATP-permeable channels expressed in various cell types, including neurons and glial cells mdpi.comroyalsocietypublishing.org. They play a role in paracrine cell communication through the release of ATP into the extracellular space mdpi.com. This extracellular ATP can activate purinergic receptors, such as P2X7 receptors (P2X7R), which can further influence cellular processes, including calcium influx and inflammasome activation mdpi.comresearchgate.netdovepress.comnih.gov.
This compound has been identified as an inhibitor of Panx1 channels nih.govnih.gov. Studies have shown that this compound can inhibit voltage-induced Panx1 currents in experimental settings researchgate.net. The inhibition of Panx1 by this compound is concentration-dependent nih.gov. While this compound is considered a Panx1 inhibitor, it is important to note that it may require millimolar concentrations for this effect and has been reported to block other ion channels as well biorxiv.org. Some studies suggest that this compound's effect on calcium release may be independent of its Panx1 blocking activity rupress.org.
Table 1: this compound Inhibition of Pannexin-1 Channels
| Target Channel | Inhibitor | IC50 (µM) | Reference |
| Pannexin 1 | This compound | ~150 | nih.gov |
| Pannexin 1 | Carbenoxolone | ~5 | nih.gov |
| Connexins | This compound | Insensitive | nih.gov |
Panx1 hemichannels are involved in the release of ATP from cells mdpi.comroyalsocietypublishing.org. This released ATP can contribute to inflammatory mechanisms, including the activation of the inflammasome researchgate.netdovepress.comelifesciences.org. The inflammasome is a multiprotein complex that plays a key role in innate immunity and leads to the processing and release of inflammatory cytokines like IL-1β and IL-18 researchgate.netdovepress.comnih.gov.
Research suggests that this compound, by blocking Panx1 channels, can affect ATP release and subsequently modulate inflammasome activation researchgate.netdovepress.comnih.govfrontiersin.org. Studies have shown that this compound can inhibit inflammasome activation in various cell types, including neurons and astrocytes nih.govfrontiersin.org. This inhibition of inflammation through Panx1 blockade may contribute to potential therapeutic effects of this compound in conditions involving neuroinflammation mdpi.comresearchgate.net.
Table 2: this compound's Impact on ATP Release and Inflammasome Activation
| Mechanism Affected | This compound Effect | Outcome | Reference |
| Panx1-mediated ATP release | Inhibition | Decreased extracellular ATP | nih.govelifesciences.orgresearchgate.net |
| Inflammasome activation (e.g., AIM2, NLRP3) | Inhibition | Reduced processing/release of inflammatory cytokines (IL-1β, IL-18) | researchgate.netdovepress.comnih.govfrontiersin.org |
Blockade of Panx1 Hemichannels by this compound
Inhibition of Multidrug Resistance-Associated Proteins (MRPs) by this compound
Multidrug Resistance-Associated Proteins (MRPs), members of the ATP-binding cassette (ABC) transporter family, are involved in the efflux of a wide range of substrates, including organic anions, conjugated drugs, and xenobiotics oup.comresearchgate.net. They play a significant role in drug resistance in various contexts, including cancer and the transport of endogenous metabolites oup.comresearchgate.net.
This compound has been identified as an inhibitor of MRPs oup.comtocris.com. Specifically, it has been shown to inhibit organic acid transport mediated by MRPs, such as MRP1 and OAT3 researchgate.nettocris.comnih.govnih.gov. This inhibition can affect the cellular accumulation and excretion of various compounds, including certain antibiotics and endogenous substances drugbank.comwikipedia.orgpatsnap.comnih.gov. While this compound can inhibit several MRPs, its inhibitory profile can vary among different MRP subtypes oup.com.
Potential Interaction with Other Molecular Targets of this compound
Beyond its established interactions with organic anion transporters and pannexin-1 channels, research using techniques like systems pharmacology and molecular docking has suggested potential interactions of this compound with other molecular targets nih.govfrontiersin.org. These findings offer insights into additional possible mechanisms underlying this compound's effects.
AKT1 (Protein Kinase B alpha) is a serine/threonine kinase involved in various cellular processes, including cell survival, proliferation, and metabolism spandidos-publications.com. It is a key component in signal transduction pathways.
Studies utilizing molecular docking and simulations have indicated that this compound may exhibit binding affinity with AKT1 nih.govfrontiersin.orgresearchgate.net. These computational analyses suggest a potential interaction, although further experimental validation is typically required to confirm the functional implications of such binding. AKT1 has been identified as one of the hub targets in network analysis exploring the pharmacological mechanisms of this compound in specific conditions nih.govfrontiersin.org.
Albumin (ALB) is the most abundant protein in plasma and plays crucial roles in transporting various molecules, maintaining oncotic pressure, and modulating the activity of other substances drugbank.comnih.gov.
Research, including systems pharmacology analyses, has identified Albumin as a potential interaction partner for this compound nih.govfrontiersin.orgresearchgate.net. This compound is known to have a high percentage of binding to plasma proteins, specifically binding significantly to albumin drugbank.comnih.gov. This binding can influence the pharmacokinetics of this compound and other drugs that also bind to albumin drugbank.comnih.gov.
Table 3: Potential Molecular Targets of this compound Identified Through Research
| Target Protein | Type of Interaction Suggested | Research Method | Reference |
| AKT1 | Binding Affinity | Molecular Docking, Systems Pharmacology | nih.govfrontiersin.orgresearchgate.net |
| ALB (Albumin) | Binding | Plasma Protein Binding Studies, Systems Pharmacology | drugbank.comnih.govfrontiersin.orgresearchgate.netnih.gov |
EGFR (Epidermal Growth Factor Receptor)
Research suggests that this compound may interact with the Epidermal Growth Factor Receptor (EGFR). Molecular docking and dynamics simulations indicate that this compound exhibits binding affinity with EGFR. nih.govresearchgate.net These studies suggest that this compound can form conventional hydrogen bonds with EGFR. nih.gov The interaction between this compound and EGFR has been explored in the context of SARS-CoV-2 and RSV co-infection, where EGFR is activated in lung epithelial cells and promotes pro-inflammatory responses. frontiersin.org Molecular dynamics simulations have shown stable interactions between this compound and EGFR, suggesting EGFR as a potential target mediating this compound's effects in this context. nih.gov
CASP3 (Caspase 3)
This compound has been identified as potentially affecting Caspase 3 (CASP3). nih.govresearchgate.net CASP3 is a protease critical for mediating apoptosis and plays a role in maintaining cellular homeostasis. frontiersin.org Activation of CASP3 can serve as a defense mechanism against viral-induced damage. frontiersin.org Studies investigating this compound in the context of SARS-CoV-2 and RSV co-infection have identified CASP3 as one of the top hub targets of this compound. nih.govresearchgate.net Additionally, research on breast cancer cells has shown that co-treatment with this compound enhanced the effects of bisphosphonates on caspase 3/7 activity, suggesting an influence on apoptotic pathways. nih.gov
CTNNB1 (Beta-Catenin)
Beta-Catenin (CTNNB1) is another protein identified as a potential target of this compound. nih.govresearchgate.net CTNNB1 is a key transcription factor in the Wnt signaling pathway, playing essential roles in cell proliferation, differentiation, apoptosis, migration, invasion, and tissue homeostasis. mdpi.cominnovareacademics.in Research indicates a direct interaction between Pannexin1 (PANX1) and beta-catenin, and this compound, as a PANX1 blocker, was shown to decrease beta-catenin levels at the protein level in melanoma cells. nih.gov This suggests that this compound's interaction with PANX1 may indirectly influence beta-catenin signaling. CTNNB1 was also identified as a hub target of this compound in the context of SARS-CoV-2/RSV co-infection. nih.govresearchgate.net
SRC Kinase
This compound has been shown to interact with SRC Kinase (SRC). nih.govresearchgate.net Molecular docking and molecular dynamics simulations indicate strong binding affinities and stable interactions between this compound and SRC. nih.govresearchgate.net this compound has been shown to form conventional hydrogen bonds with SRC. nih.gov Structure-activity relationship analysis further supports SRC as a potential binding target of this compound. researchgate.net The interaction with SRC Kinase suggests a potential influence on various cellular processes regulated by this non-receptor tyrosine kinase, including cell growth, differentiation, and survival.
HSP90AA1 (Heat Shock Protein 90 Alpha Family Class A Member 1)
Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) is another protein identified as a potential target for this compound. nih.govresearchgate.net Molecular docking and molecular dynamics simulations have indicated strong binding affinities and stable interactions between this compound and HSP90AA1. nih.govresearchgate.net this compound can form conventional hydrogen bonds and Pi-Donor hydrogen bonds with HSP90AA1, as well as a Pi-Sulfur bond. nih.gov Structure-activity relationship analysis also suggests HSP90AA1 as a potential binding target of this compound. researchgate.net HSP90AA1 is a molecular chaperone involved in the folding and stability of many client proteins, including various kinases and transcription factors, suggesting that this compound's interaction could impact multiple signaling pathways.
PPARG (Peroxisome Proliferator-Activated Receptor Gamma)
This compound's interaction with Peroxisome Proliferator-Activated Receptor Gamma (PPARG) has also been explored. nih.govresearchgate.net PPARG is a ligand-activated transcription factor belonging to the nuclear receptor superfamily, involved in regulating genes related to metabolic processes, inflammation, and neuronal protection. nih.govmdpi.com Molecular docking analysis suggests that this compound can bind to PPARG, forming conventional hydrogen bonds and a Pi-Sulfur bond. nih.gov While molecular dynamics simulations showed more fluctuating RMSD values for the PPARG-probenecid system compared to other targets, molecular docking indicated effective binding. nih.govresearchgate.net
Alpha-Adrenergic Receptor
This compound has been shown to inhibit the alpha-adrenergic receptor. biomolther.orgfrontiersin.orgnih.govif-pan.krakow.pl Studies in spontaneously hypertensive rats demonstrated that this compound administration led to a significant decrease in systolic blood pressure, and this effect was associated with the inhibition of phenylephrine-induced blood vessel contraction. nih.gov This inhibition was observed even in endothelium-removed rat aorta, suggesting a direct interaction with the alpha-adrenergic receptor. nih.govif-pan.krakow.pl Furthermore, this compound inhibited the alpha-adrenergic-receptor-mediated activation of ERK I/II in MC3TC-E1 cells. nih.gov These findings indicate that this compound may exert anti-hypertensive effects partly through the inhibition of the alpha-adrenergic receptor, particularly the alpha1 subtype which is prevalent in the aorta.
COX-2 and JNK Pathways
Research indicates that this compound can influence cellular processes by interacting with the Cyclooxygenase-2 (COX-2) and c-Jun N-terminal kinase (JNK) pathways. Studies have shown that this compound inhibits osteoclast formation, and this effect may involve the inhibition of JNK phosphorylation and the subsequent blocking of COX-2 expression. nih.govbiomolther.orgsemanticscholar.org This suggests a potential antiosteoclastogenic activity mediated through these pathways.
Specifically, investigations using lipopolysaccharide (LPS)-induced RAW264.7 cells have demonstrated that this compound dose-dependently reduces reactive oxygen species (ROS) levels. nih.govbiomolther.orgsemanticscholar.orgnih.gov Western blot analysis in these studies revealed that this compound impacts COX-2 and JNK, which are downstream signaling molecules of ROS. nih.govbiomolther.orgsemanticscholar.orgnih.gov The inhibition of ROS generation by this compound appears to contribute to its antiosteoclastogenic activity by inhibiting the COX-2 and JNK pathways. nih.govbiomolther.orgsemanticscholar.orgnih.gov
The JNK signaling pathway is known to regulate various cellular processes and is involved in osteoclastogenesis. biomolther.orgsemanticscholar.org this compound has been shown to inhibit JNK phosphorylation. nih.govbiomolther.orgsemanticscholar.org This inhibition of JNK phosphorylation, alongside the reduction in ROS production and blocking of COX-2 expression, is presented as a mechanism by which this compound inhibits osteoclastogenesis. biomolther.orgsemanticscholar.org
This compound's influence on JNK and COX-2 pathways has also been investigated in the context of antiviral responses. Studies on respiratory syncytial virus (RSV) replication in human respiratory epithelial cells (A549) indicate that this compound inhibits RSV-induced phosphorylation of JNKs and ERKs, and the downstream phosphorylation of c-jun. mdpi.comnih.gov This suggests that this compound's inhibition of JNK and ERK phosphorylation involves the MAPK pathway, which can preclude virus replication. mdpi.comnih.gov The inhibition of JNKs by this compound has also been shown to reverse the repression of the transcription factor HNF-4. mdpi.comnih.gov
Uridine Diphosphate Glucuronosyltransferase (UGT) Inhibition
This compound is recognized as an inhibitor of Uridine Diphosphate Glucuronosyltransferase (UGT) enzymes, which are crucial for the glucuronidation of various compounds, including drugs and endogenous substances. nih.govnih.govscienceopen.comresearchgate.netdrugbank.com Glucuronidation is a major phase II metabolic pathway that increases the water solubility of substrates, facilitating their excretion. drugbank.comtandfonline.com
Studies have utilized in vitro systems, such as those integrating capillary electrophoresis with immobilized rat liver microsomes, to investigate UGT inhibition. nih.gov These studies have shown that this compound can inhibit the glucuronidation of acetaminophen, a widely used analgesic. nih.gov The extent of inhibition observed with encapsulated UGT was comparable to that seen with free UGT. nih.gov
This compound is considered a general inhibitor of a variety of UGT isozymes. researchgate.net Research has indicated that this compound inhibits UGT1A1, UGT1A6, UGT1A7, UGT1A9, UGT1A10, and UGT2B7. scienceopen.com It is also noted as inhibiting almost all types of glucuronidation-related enzymes, which can interfere with the clinical pharmacokinetics of drugs undergoing glucuronidation. nih.govresearchgate.net
The inhibitory effect of this compound on UGT enzymes has been demonstrated in drug-drug interaction studies. For instance, coadministration of this compound with quetiapine, a drug metabolized significantly by UGT enzymes, resulted in a significant increase in the maximum plasma concentration (Cmax) of quetiapine, likely due to UGT enzyme inhibition. nih.govresearchgate.net Similarly, this compound has been found to increase the levels of olanzapine, another atypical antipsychotic metabolized by UGT1A4, through the inhibition of UGT glucuronidation enzymes. nih.gov
In vitro studies evaluating the inhibitory effects of various compounds on human UGT isoforms have included this compound as a known inhibitor. mdpi.comresearchgate.net These studies have shown that this compound inhibits the glucuronidation activity of several UGT isoforms in a concentration-dependent manner. researchgate.net For example, inhibition by this compound ranged from 5% to 82% at 0.5 mM and 20% to 95% at 2 mM across different isoforms. researchgate.net
Pharmacokinetic and Pharmacodynamic Research of Probenecid
Absorption and Distribution Studies of Probenecid
Following oral administration, this compound is reported to be completely absorbed by the intestinal tract. mdpi.commedsafe.govt.nz Peak plasma levels are typically reached within two to four hours after oral administration. medsafe.govt.nz this compound exhibits a high degree of plasma protein binding, primarily to albumin, with reported binding percentages ranging from 85% to 95%. mdpi.commedsafe.govt.nz The apparent volume of distribution has been reported as approximately 11 liters or 0.003–0.014 L/kg. mdpi.commedsafe.govt.nznih.gov Tissue concentrations of this compound are generally lower than plasma concentrations, based on animal studies. nih.gov
Blood-Brain Barrier Transport and Central Nervous System Exposure of this compound
This compound is described as diffusing freely across the blood-brain barrier (BBB) due to its high lipid solubility. mdpi.com However, it is also subject to active transport mechanisms that facilitate its efflux from the brain. mdpi.com Research indicates that this compound can block active transport across the BBB, leading to decreased excretion of acidic metabolites of neurotransmitters like dopamine (homovanillic acid) and serotonin (5-hydroxy indoleacetic acid). mdpi.com Studies involving coadministration with other compounds, such as morphine and fluorescein, have shown that this compound can increase their concentrations in the brain and cerebrospinal fluid, suggesting an impact on their transport across the BBB. mdpi.com This effect is attributed, in part, to this compound's ability to inhibit organic anion transporters (OATs) and organic cation transporters (OCTs) present in the choroid plexus and the BBB, which can increase the levels of endogenous organic anions and cations in the blood, CSF, and brain. mdpi.comresearchgate.net OAT3, expressed on the basolateral side of endothelial cells at the BBB and epithelial cells of the choroid plexus, is one such transporter that this compound inhibits, potentially enhancing brain penetration of its substrates. nih.gov For instance, this compound coadministration increased N-acetylcysteine (NAC) brain exposure by nearly 2.5-fold in a study, suggesting a transporter effect at the BBB or blood-CSF barrier. nih.gov
Metabolism and Excretion Pathways of this compound
This compound undergoes biotransformation primarily in the liver. mdpi.com Metabolism involves both phase 1 oxidation of the alkyl side chains (approximately 70%) and phase 2 conjugation with glucuronic acid (approximately 20%). mdpi.com The major metabolites, including the acyl glucuronide, retain the ability to block the tubular secretion of organic acids but exhibit lower binding affinity to plasma proteins compared to the parent drug. mdpi.commedsafe.govt.nz The acyl glucuronide metabolite accounts for a significant portion of the dose, close to 50%. medsafe.govt.nz Other metabolites, such as mono-n-propyl, secondary alcohol, and carboxylic acid derivatives, are also formed, totaling about 30% of the dose. medsafe.govt.nznih.gov
Elimination of this compound and its metabolites occurs mainly via the kidneys. mdpi.comnih.gov Renal excretion is the primary route for metabolites, while excretion of the unchanged parent drug is minimal and dependent on urinary pH. nih.gov Approximately 75-88% of the administered dose is recovered in the urine, predominantly as metabolites. mdpi.commedsafe.govt.nz Studies have indicated that the elimination process involves parallel pathways, with the excretion of the major metabolite following Michaelis-Menten kinetics and oxidized metabolites adhering to pseudo-first-order kinetics influenced by product inhibition. nih.gov Renal clearance accounts for a notable portion of the total plasma clearance of this compound. nih.gov
Pharmacokinetic and Pharmacodynamic Interactions of this compound with Other Agents
This compound is well-known for its ability to interact with the disposition of other drugs, primarily by inhibiting renal tubular transport of organic acids. drugbank.comnih.govmedicinesinformation.co.nz This competitive inhibition, particularly of organic anion transporters (OATs) in the kidney, leads to increased plasma concentrations and prolonged half-lives of coadministered drugs that are substrates for these transporters. drugbank.commedicinesinformation.co.nz this compound also has been shown to inhibit glucuronidation, which can further influence the concentrations of drugs metabolized by this pathway. medicinesinformation.co.nznih.gov
Impact on Antibiotic Pharmacokinetics and Pharmacodynamics (e.g., Penicillins, Cephalosporins, Flucloxacillin, Carbapenems)
This compound competitively inhibits the renal tubular secretion of many weak organic acids, including various beta-lactam antibiotics such as penicillins and most cephalosporins. drugbank.com This inhibition results in elevated and prolonged plasma concentrations of these antibiotics, which can be therapeutically advantageous in certain infections. drugbank.commedicinesinformation.co.nztaylorandfrancis.com Studies have shown that coadministration with this compound leads to decreased renal excretion and increased AUC (Area Under the Curve) for antibiotics like amoxicillin, ampicillin, cefotaxime, and meropenem, with AUC increases ranging from 50% to 100%. seq.es For penicillins, this compound can also potentially decrease their volume of distribution. mdpi.com While this interaction is generally not harmful and can be used therapeutically, the clinical significance in specific patient populations, such as those in the ICU, may vary depending on underlying conditions like renal clearance and fluid balance. nih.gov Flucloxacillin, an isoxazolyl penicillin, has also been noted to have increased exposure when coadministered with this compound. nih.gov
Interactions with Antivirals (e.g., Acyclovir, Zidovudine, Cidofovir)
This compound interacts with the pharmacokinetics of several antiviral agents, primarily through inhibition of renal tubular secretion. nih.govmedicinesinformation.co.nzeuropa.eu Coadministration of this compound with acyclovir has been shown to decrease acyclovir clearance by 33%, resulting in a 50% increase in its concentrations. medicinesinformation.co.nz While no change to usual management is typically required, dose reduction may be considered for high-dose acyclovir therapy. medicinesinformation.co.nz
This compound also increases the AUC of zidovudine by reducing its metabolic clearance. hiv-druginteractions.orggilead.com Patients receiving both drugs require close monitoring for potential zidovudine-induced hematological toxicity. hiv-druginteractions.orggilead.com It is recommended that patients temporarily discontinue or decrease their zidovudine dose by 50% on days when cidofovir is administered with this compound. europa.eugilead.com
For cidofovir, concomitant oral this compound decreases its renal clearance by blocking active tubular secretion. nih.gov This interaction is the basis for the clinical use of this compound as a nephroprotective agent during cidofovir therapy. seq.esnih.gov Studies have shown that approximately 70% to 85% of the cidofovir dose administered with this compound is excreted unchanged in the urine within 24 hours. gilead.com
Interactions with Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
This compound can interfere with the plasma protein binding, metabolism, and/or renal elimination of nonsteroidal anti-inflammatory drugs (NSAIDs), leading to increased NSAID plasma levels. nih.govdrugs.com This interaction can potentially increase the risk of NSAID toxicity. drugs.com Studies have shown that coadministration of this compound increases the blood concentration of NSAIDs, which could lead to an enhanced anti-inflammatory effect. nih.gov While adverse effects from this specific interaction have not always been reported, monitoring for increased NSAID side effects is recommended. drugs.com For example, this compound can decrease the renal clearance of NSAIDs, necessitating a reduction in the NSAID dose when coadministered. pdr.net However, the interaction varies among NSAIDs; for instance, while aspirin antagonized the uricosuric effect of this compound in a study, ibuprofen administration had no effect on this compound activity. dovepress.com
Table 1: Summary of this compound Pharmacokinetic Properties
| Property | Value / Description | Source |
| Absorption | Completely absorbed after oral administration | mdpi.commedsafe.govt.nz |
| Peak Plasma Levels | Reached in 2-4 hours after oral administration | medsafe.govt.nz |
| Plasma Protein Binding | 85-95% (primarily to albumin) | mdpi.commedsafe.govt.nz |
| Volume of Distribution | ~11 L or 0.003-0.014 L/kg | mdpi.commedsafe.govt.nznih.gov |
| Half-life | 2-12 hours (dose-dependent) | mdpi.comnih.gov |
| Metabolism | Hepatic (oxidation of alkyl chains, glucuronidation) | mdpi.commedsafe.govt.nz |
| Major Metabolites | Acyl glucuronide, oxidized derivatives | mdpi.commedsafe.govt.nz |
| Excretion | Primarily renal (metabolites), minimal unchanged drug | mdpi.comnih.gov |
| Urinary Recovery | 75-88% of dose (mainly metabolites) | mdpi.commedsafe.govt.nz |
Table 2: Examples of this compound Pharmacokinetic Interactions
| Coadministered Drug Class | Mechanism of Interaction | Effect on Coadministered Drug | Source |
| Penicillins | Inhibition of renal tubular secretion (OATs) | Increased plasma concentrations, prolonged half-life, increased AUC | drugbank.commedicinesinformation.co.nzseq.esmdpi.com |
| Cephalosporins | Inhibition of renal tubular secretion (OATs) | Increased plasma concentrations, prolonged half-life, increased AUC | drugbank.commedicinesinformation.co.nzseq.es |
| Flucloxacillin | Inhibition of renal tubular secretion | Increased exposure | nih.gov |
| Carbapenems | Inhibition of renal tubular secretion | Increased systemic exposure (AUC) | seq.esnih.gov |
| Acyclovir | Inhibition of renal clearance (OATs) | Decreased clearance, increased concentrations (50% increase in AUC) | medicinesinformation.co.nz |
| Zidovudine | Reduction of metabolic clearance, increased AUC | Increased AUC | hiv-druginteractions.orggilead.com |
| Cidofovir | Blocking of active renal tubular secretion (OAT1) | Decreased renal clearance, nephroprotective effect | seq.esnih.gov |
| NSAIDs | Interference with protein binding, metabolism, renal elimination | Increased plasma levels, potential increased toxicity | nih.govdrugs.compdr.net |
Interactions with Benzodiazepines (e.g., Adinazolam)
Interactions with Loop Diuretics
This compound interacts with loop diuretics, such as furosemide and bumetanide, primarily by affecting their renal secretion. medicinesinformation.co.nzwikipedia.orgjapi.org Loop diuretics exert their effects by inhibiting the Na+/K+/2Cl- cotransporter in the thick ascending limb of the loop of Henle, requiring access to the tubular lumen via organic anion transporters in the proximal tubule. japi.orgphysiology.orgresearchgate.net this compound, being an OAT inhibitor, can competitively inhibit the renal tubular secretion of loop diuretics. medicinesinformation.co.nznih.gov
| Parameter | Furosemide Alone | Furosemide + this compound | Change (%) |
| Sodium Excretion (8 hr) | 262 ± 16 mEq | 358 ± 11 mEq | +37% |
| Urine Volume (8 hr) | 3265 ± 275 ml | 4165 ± 183 ml | +28% |
Conversely, some studies with bumetanide did not find a significant effect of this compound on cumulative response or time course of response. drugs.com
Impact on N-Acetylcysteine (NAC) Pharmacokinetics and Brain Exposure
This compound has been shown to significantly impact the pharmacokinetics and brain exposure of N-Acetylcysteine (NAC). nih.govtandfonline.complos.org NAC is an antioxidant being investigated for various conditions, including traumatic brain injury. nih.govplos.orgwikipedia.org Studies in rats have demonstrated that this compound increases both plasma and brain exposure of NAC. nih.govtandfonline.complos.org
This compound, as an inhibitor of OAT1 and OAT3, reduces the apparent plasma clearance of NAC. nih.govtandfonline.com NAC has been identified as a substrate for OAT1 and OAT3 transporters, which are present in both the kidney and the blood-brain barrier. nih.govtandfonline.complos.org By inhibiting these transporters, this compound decreases the renal excretion of NAC and can also influence its transport across the blood-brain barrier. nih.govplos.org
In juvenile Sprague-Dawley rats, co-administration of this compound with NAC resulted in a notable increase in NAC exposure. nih.govtandfonline.com
| Compartment | AUC Increase (fold) | Apparent Clearance Change |
| Plasma | 1.65-fold | Decreased by 65% |
| Brain | 2.41-fold | Not directly reported |
This interaction suggests a potential therapeutic strategy to increase NAC concentrations in the plasma and brain by co-administering this compound. nih.govtandfonline.com
Influence on Sorafenib Pharmacokinetics
Sorafenib is a multikinase inhibitor used in the treatment of various cancers. wikipedia.orgmims.com It is primarily metabolized in the liver by CYP3A4 and UGT1A9. mims.com this compound is known to inhibit glucuronidation, a metabolic pathway mediated by UGT enzymes. medicinesinformation.co.nz While a direct, detailed study specifically examining the pharmacokinetic interaction between this compound and sorafenib was not extensively detailed in the provided search results, the general inhibitory effect of this compound on glucuronidation pathways suggests a potential for it to influence the metabolism and thus the pharmacokinetics of drugs like sorafenib that undergo UGT-mediated metabolism. medicinesinformation.co.nzmims.com However, without specific study data from the search results, the extent and nature of this interaction remain to be fully described within the confines of this document.
Effect on Metabolites of Neurotransmitters (e.g., Homovanillic Acid, 5-Hydroxyindoleacetic Acid)
This compound's influence extends to the elimination of certain endogenous organic acids, including metabolites of neurotransmitters like homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA). medicinesinformation.co.nzfishersci.benih.govnih.gov HVA is a major metabolite of dopamine, and 5-HIAA is the primary metabolite of serotonin (5-HT). thegoodscentscompany.comwikipedia.org These metabolites are eliminated from the central nervous system (CNS) and the body, partly via organic anion transport systems.
This compound can inhibit the transport of these acidic metabolites, particularly their efflux from the CNS and their renal excretion. medicinesinformation.co.nz By blocking these transport mechanisms, this compound can lead to increased concentrations of HVA and 5-HIAA in the cerebrospinal fluid (CSF). This effect has been utilized in research to assess neurotransmitter turnover in the brain. medicinesinformation.co.nz Elevated levels of these metabolites in CSF following this compound administration can indicate the rate of synthesis and metabolism of their parent neurotransmitters. medicinesinformation.co.nz
Modulation of Bromosulphthalein Excretion
Bromosulphthalein (BSP), also known as sulfobromophthalein, is an organic anion dye that was historically used in liver function tests to assess hepatic excretory function. wikipedia.orgctdbase.org The liver removes BSP from the bloodstream and excretes it into the bile, partly after conjugation with glutathione. wikipedia.org
Preclinical and Translational Research on Probenecid
Neuroprotective Properties of Probenecid in Animal Models
Preclinical studies utilizing animal models of neurological disorders have provided evidence for the neuroprotective effects of this compound. For instance, in the N171-82Q transgenic mouse model of Huntington's disease (HD), this compound administration was observed to improve motor behavior, increase cell survival, and significantly reduce neuronal loss and the number of intranuclear neuronal aggregates. mdpi.comnih.gov this compound has also shown protective effects in a rat model of cognitive impairment and in models of spinal cord injury (SCI). frontiersin.org In SCI models in SD rats, this compound reduced the lesion area and demyelination, and increased the survival of motor neurons, leading to improved locomotor function. frontiersin.org These findings suggest that this compound may hold therapeutic potential for various central nervous system (CNS) injuries and neurodegenerative conditions. mdpi.comnih.govfrontiersin.org
Mechanism of Neuroprotection, including Kynurenic Acid Accumulation
A significant mechanism contributing to this compound's neuroprotective effects is its influence on the levels of kynurenic acid (KYNA) in the brain. eurekaselect.comresearchgate.net KYNA is a metabolite of the tryptophan kynurenine pathway and acts as an antagonist at ionotropic glutamate receptors, including NMDA receptors. queensu.capreprints.org By inhibiting organic anion transporters (OATs), particularly OAT1 and OAT3, this compound can reduce the efflux of KYNA from the brain, leading to its accumulation. eurekaselect.comresearchgate.netnih.gov Elevated brain KYNA levels can then inhibit glutamate-related excitotoxicity, a process implicated in neuronal damage in various neurological disorders. eurekaselect.comresearchgate.netqueensu.ca Studies have shown that increasing brain KYNA content through methods including this compound administration can protect against quinolinic acid-induced neurotoxicity in rats. queensu.canih.gov
Beyond KYNA accumulation, this compound's neuroprotective mechanisms may also involve the inhibition of pannexin 1 (Panx1) hemichannels and the activation of TRPV2 channels. mdpi.comnih.gov Panx1 hemichannels are involved in the release of ATP and other molecules that can contribute to neuroinflammation and cell death. nih.govresearchgate.net this compound's blockade of Panx1 has been shown to inhibit inflammasome activation, reduce the release of pro-inflammatory cytokines like IL-1β, and alleviate inflammation, thereby contributing to neuroprotection in models like SCI and subarachnoid hemorrhage (SAH). frontiersin.orgnih.govfrontiersin.org Activation of TRPV2 channels by this compound can influence Ca²⁺ influx and downstream signaling in neurons and glial cells, potentially contributing to its protective effects. mdpi.comnih.govresearchgate.net
Anti-epileptic Effects of this compound
Preclinical research also indicates that this compound possesses anti-epileptic properties. Studies in rats have demonstrated the ability of this compound to reduce epileptic discharges and the severity of convulsions induced by status epilepticus. mdpi.com While the precise mechanisms are still being investigated, this compound's interaction with organic anion transporters, including multidrug resistance-associated proteins (MRPs), may play a role. frontiersin.orgnih.gov Some studies suggest that MRPs can transport certain anti-epileptic drugs (AEDs) and limit their access to the brain, and this compound, as an inhibitor of MRP1 and MRP2, could potentially increase the brain concentration of these AEDs, thereby enhancing their efficacy. frontiersin.orgnih.gov However, studies with certain AEDs, like levetiracetam, have shown that this compound did not alter their brain penetration, suggesting that the interaction is drug-specific. nih.gov Further research is needed to fully elucidate the anti-epileptic mechanisms of this compound, including its potential direct effects on neuronal excitability or neurotransmitter systems.
Anti-inflammatory Properties of this compound
This compound exhibits significant anti-inflammatory properties, which are relevant to its potential therapeutic uses in various conditions, including those affecting the CNS. mdpi.comnih.govfrontiersin.orgmdpi.comnih.gov
Role in Mitigating Neuroinflammation
Neuroinflammation is a critical factor in the pathogenesis of many neurological and neurodegenerative disorders. mdpi.comnih.gov this compound has been shown to mitigate neuroinflammation in several preclinical models. nih.govfrontiersin.orgnih.gov A key mechanism involves the inhibition of inflammasome activation, particularly the NLRP3 inflammasome. mdpi.comnih.govresearchgate.net this compound's ability to block Panx1 hemichannels is central to this effect, as Panx1 plays a crucial role in inflammasome assembly and the subsequent release of pro-inflammatory cytokines such as IL-1β and IL-18. mdpi.comfrontiersin.orgnih.govfrontiersin.org By inhibiting Panx1, this compound reduces the production and secretion of these inflammatory mediators. mdpi.comfrontiersin.orgfrontiersin.org Studies in murine macrophages and animal models of SCI and SAH have demonstrated that this compound treatment suppresses inflammasome activation and reduces levels of pro-inflammatory cytokines, contributing to reduced tissue damage and improved outcomes. mdpi.comfrontiersin.orgfrontiersin.orgnih.gov
Modulation of Immune Responses and Inflammatory Pathways
Beyond inflammasome inhibition, this compound modulates broader immune responses and inflammatory pathways. It has been shown to reduce the expression of several pro-inflammatory cytokines, including IL-6 and TNF-α. mdpi.comresearchgate.net this compound's anti-inflammatory effects may also involve the modulation of mitogen-activated protein kinase (MAPK) signaling pathways. nih.govresearchgate.net Studies have indicated that this compound treatment can block JNK and ERK signaling, which are involved in the induction and regulation of immunity and inflammation, without affecting p38 MAPK. mdpi.comnih.gov This selective modulation of MAPK pathways contributes to the suppression of inflammatory responses. mdpi.comnih.gov Furthermore, this compound has been shown to influence immune cell subsets and promote a more favorable local immune microenvironment in the context of injury. frontiersin.org For example, in a Glaesserella parasuis-challenged mouse model, this compound, in combination with other agents, helped reverse reductions in CD3⁺, CD3⁺CD4⁺, and CD3⁺CD8⁺ T cell levels, suggesting an immunomodulatory effect on T cell differentiation. mdpi.com
Antiviral Activity of this compound
Recent preclinical and translational research has highlighted the potent antiviral activity of this compound against a range of viruses, particularly respiratory viruses. researchgate.netmdpi.comnih.govnih.govmdpi.comfrontiersin.org Studies have demonstrated that this compound can inhibit the replication of SARS-CoV-2 (including various variants), influenza virus (including HPAI H5N1 and H7N9 strains), and respiratory syncytial virus (RSV) both in vitro and in animal models. researchgate.netmdpi.comnih.govnih.govmdpi.com
The antiviral mechanism of this compound appears to be linked to its interaction with host cell pathways rather than directly targeting viral components, which may reduce the likelihood of drug resistance. researchgate.netnih.govmdpi.com One proposed mechanism involves the solute carrier (SLC) superfamily, specifically its function as organic anion transporters (OATs). nih.govmdpi.com this compound's inhibition of OATs may disrupt viral replication processes that rely on these transporters. nih.gov Additionally, this compound's modulation of MAPK signaling pathways, such as the inhibition of JNK signaling, has been implicated in its antiviral effects against viruses like RSV. researchgate.net Furthermore, studies suggest that this compound may exert antiviral effects through coordinated modulation of pathways like ACE2 and PANX1, for example, by downregulating ACE2 expression, which is utilized by SARS-CoV-2 for entry. frontiersin.org In animal models, this compound treatment has been shown to reduce viral titers in the lungs and improve outcomes in infected mice. mdpi.comnih.gov
Inhibition of SARS-CoV-2 Replication
Research indicates that this compound can effectively inhibit the replication of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19. In vitro studies have demonstrated that this compound treatment blocked SARS-CoV-2 replication in Vero E6 cells and normal human bronchial epithelial (NHBE) cells across a range of concentrations. nih.govnews-medical.net Specifically, this compound treatment reduced SARS-CoV-2 replication by 90% in NHBE cells and 60% in Vero E6 cells. nih.gov The half-maximal inhibitory concentration (IC₅₀) for this compound was reported as 0.75 μM in Vero E6 cells and 0.0013 μM in NHBE cells. nih.gov This inhibitory effect was also observed against several SARS-CoV-2 variants of concern, including Beta, Gamma, Delta, and B.1.1, at submicromolar concentrations. mdpi.comtrippbio.commdpi.com
Preclinical studies in animal models have further supported these in vitro findings. In hamsters infected with SARS-CoV-2, treatment with this compound, administered either prophylactically (24 hours before infection) or therapeutically (48 hours post-infection), resulted in a significant reduction in lung virus titers. nih.govmdpi.commdpi.com A 4–5 log reduction in virus compared to control groups was observed in hamsters treated with 2 or 200 mg/kg this compound. nih.govmdpi.com These data collectively suggest that this compound possesses potent antiviral activity against SARS-CoV-2 in both cellular and animal models. researchgate.netmdpi.com
Inhibition of RSV Replication
This compound has also shown promise in inhibiting the replication of Respiratory Syncytial Virus (RSV), a common cause of respiratory infections, particularly in children. Studies have investigated the prophylactic and therapeutic efficacy of this compound against RSV in various epithelial cell lines and in vivo models. nih.gov
In vitro studies using Vero E6 cells, HEp-2 cells, and primary normal human bronchoepithelial (NHBE) cells demonstrated that nanomolar concentrations of this compound regimens prevented the replication of RSV strains A and B. nih.govnews-medical.net For instance, the IC₅₀ against RSV A2 was found to be less than 0.1 μM, and against RSV B1, it was 0.85 μM. news-medical.net This indicates that this compound affects RSV replication at nanomolar concentrations, which are considerably lower than those required for inhibiting influenza A virus replication. news-medical.net
In BALB/c mice, both prophylactic administration (before infection) and therapeutic administration (after infection) of this compound significantly reduced viral lung titers of RSV strain A. nih.govnews-medical.net This highlights the potential versatility of this compound as a chemotherapeutic agent for RSV infection. trippbio.com Emerging data suggest that the mechanism of action against RSV may involve the inhibition of JNK phosphorylation and downstream HNF-4 regulation of OAT3, potentially disrupting virus assembly and replication. mdpi.commdpi.comfrontiersin.org this compound's inhibition of JNK and ERK phosphorylation appears to involve the MAPK pathway, which precludes virus replication. mdpi.com
Inhibition of Influenza A Virus Replication
The antiviral activity of this compound extends to Influenza A Virus (IAV). Research has demonstrated that this compound can limit IAV replication both in vitro and in vivo. trippbio.comasm.org
In vitro studies using A549 cells showed that this compound treatment inhibited the replication of various IAV strains, including A/WSN/33, A/New Caledonia/20/99, A/California/07/09, and A/Philippines/2/82/X-79. trippbio.com The IC₅₀ for this compound against IAV replication in A549 cells ranged between 5 × 10⁻⁵ and 5 × 10⁻⁴ μM. mdpi.comtrippbio.comasm.org Pretreatment of A549 cells with this compound significantly reduced virus copy numbers. asm.org
Studies in BALB/c mice infected with IAV have also shown the efficacy of this compound. Daily treatment with 25 mg/kg this compound over 3 days substantially inhibited viral lung titers. mdpi.comresearchgate.net this compound treatment also demonstrated a protective effect against lethal challenge with mouse-adapted A/WSN/33, resulting in a 60% survival rate. mdpi.commdpi.com The mechanism of action against IAV is linked to the inhibition of OAT3, a host factor required for IAV replication. mdpi.comasm.org this compound treatment has been shown to reduce OAT3 mRNA and protein levels in vitro and in vivo. mdpi.comasm.org
Here is a summary of in vitro antiviral activity data:
| Virus | Cell Line | IC₅₀ (μM) | Reference |
| SARS-CoV-2 | Vero E6 | 0.75 | nih.gov |
| SARS-CoV-2 | NHBE | 0.0013 | nih.gov |
| RSV Strain A2 | - | < 0.1 | news-medical.net |
| RSV Strain B1 | - | 0.85 | news-medical.net |
| Influenza A | A549 | 5 × 10⁻⁵ - 5 × 10⁻⁴ | mdpi.comasm.org |
Cardiovascular Research with this compound
Beyond its antiviral properties, this compound has been investigated for its effects on the cardiovascular system, particularly in the context of heart failure. Research suggests that this compound can influence myocardial function through its interaction with calcium channels and its impact on contractility and relaxation.
Impact on Myocardial Contractility and Calcium Sensitivity
Research has demonstrated that this compound can enhance myocardial contractility and improve calcium sensitivity at the cardiomyocyte level. nih.govresearchgate.netahajournals.orgahajournals.org In vitro studies on isolated cardiomyocytes from murine tissue exposed to this compound showed increased myofilament force generation compared to control groups. nih.govresearchgate.netahajournals.orgahajournals.org
Specifically, this compound treatment increased myofilament force generation to 92.36 mN/mm² compared to 80.82 mN/mm² in saline controls (P<0.05). nih.govresearchgate.netahajournals.orgahajournals.org Furthermore, this compound significantly increased myofilament calcium sensitivity, as expressed by pCa₅₀, with a value of 5.67 compared to 5.60 in saline controls (P<0.01). nih.govresearchgate.netahajournals.orgahajournals.org While isoproterenol showed a more pronounced effect on calcium sensitivity (pCa 5.78), this compound's impact was still statistically significant. nih.gov
Neurocardiac Implications and Heart Failure Studies
This compound's potential in treating heart failure has been explored in clinical studies. A study involving stable outpatients with HFrEF demonstrated that this compound therapy improved cardiac function. nih.govresearchgate.netahajournals.orgahajournals.org In this study, this compound treatment increased fractional shortening by 2.1% compared to a decrease of 1.7% in the placebo group (P=0.007). nih.govresearchgate.netahajournals.orgahajournals.org Additionally, this compound improved diastolic function, indicated by a decrease in the E/E' ratio. nih.govresearchgate.netahajournals.orgahajournals.org
The proposed mechanism involving TRPV2 activation suggests a potential link to calcium handling abnormalities observed in heart failure. nih.govresearchgate.netahajournals.orgahajournals.orgjacc.org Studies have shown increased expression of TRPV2 in diseased hearts. ahajournals.org Stimulation of TRPV2 with this compound in isolated cardiomyocytes has been shown to increase cytosolic calcium concentrations, leading to improved contractility and relaxation, independent of β-adrenergic signaling. ahajournals.org
Furthermore, research suggests that this compound may exert cardioprotective effects through mechanisms independent of its uric acid lowering properties. jacc.org Some studies indicate that this compound might modulate inflammatory pathways, such as inflammasome priming and activation, which play a role in the progression of heart failure. semmelweis.hu However, the specific steps inhibited by this compound in these pathways require further characterization. semmelweis.hu
A clinical trial (Re-Prosper-HF) is underway to further investigate the effects of oral this compound on cardiac function, functional status, and self-reported health status in patients with NYHA II-III HFrEF. tmc.edu
Here is a summary of cardiovascular research findings:
| Parameter | This compound Effect (vs. Control/Placebo) | Significance (P-value) | Reference |
| Fractional Shortening (HFrEF) | Increased by 2.1% | 0.007 | nih.govresearchgate.netahajournals.org |
| Diastolic Function (E/E') | Decreased by -2.95 | 0.03 | nih.govresearchgate.netahajournals.org |
| Myofilament Force Generation | Increased (92.36 vs 80.82 mN/mm²) | <0.05 | nih.govresearchgate.netahajournals.org |
| Myofilament Calcium Sensitivity (pCa₅₀) | Increased (5.67 vs 5.60) | <0.01 | nih.govresearchgate.netahajournals.org |
Osteoclastogenesis Inhibition by this compound
Research has explored the potential of this compound to inhibit osteoclast formation, a process crucial for bone resorption. Excessive osteoclast differentiation is implicated in pathological bone diseases such as rheumatoid arthritis and osteoporosis. nih.gov Studies have investigated whether this compound can influence osteoclast formation, particularly through its effects on reactive oxygen species (ROS) formation in cellular models like RAW264.7 cells. nih.govbiomolther.orgsemanticscholar.org
Investigations have shown that this compound can dose-dependently reduce lipopolysaccharide (LPS)-induced ROS levels in RAW264.7 cells. nih.govbiomolther.orgsemanticscholar.org Fluorescence microscopy analysis has further demonstrated that this compound inhibits the generation of ROS. nih.govbiomolther.orgsemanticscholar.org Western blot analysis has indicated that this compound impacts downstream signaling molecules of ROS, including cyclooxygenase 2 (COX-2) and c-Jun N-terminal kinase (JNK). nih.gov These findings suggest that this compound inhibits ROS generation and exerts antiosteoclastogenic activity by inhibiting the COX-2 and JNK pathways. nih.gov
A TRAP (Tartrate-Resistant Acid Phosphatase) assay, used to confirm the differentiation of RAW264.7 cells into osteoclasts by LPS, showed that treatment with this compound blocked this differentiation, confirming its inhibitory effect on osteoclast differentiation. nih.govsemanticscholar.org The increased level of ROS is known to promote osteoclastogenesis and plays a significant role in the signaling involved in osteoclast activation, leading to bone resorption. nih.govbiomolther.orgsemanticscholar.org The evidence suggests that this compound inhibits osteoclastogenesis by inhibiting JNK phosphorylation, ROS production, and COX-2 expression. nih.govsemanticscholar.org
While the molecular mechanisms linking oxidative stress to osteoclast differentiation are complex, therapeutic strategies aimed at preventing ROS generation may be beneficial in addressing diseases like osteoporosis. nih.govbiomolther.org These results suggest that this compound could potentially be explored as a therapeutic agent to prevent bone resorption. nih.gov
Here is a summary of key findings regarding this compound's effect on Osteoclastogenesis:
| Effect Measured | Cellular Model / Condition | This compound Effect | Reference |
| Reduction of ROS levels | RAW264.7 cells / LPS-induced | Dose-dependent reduction | nih.govbiomolther.orgsemanticscholar.org |
| Inhibition of ROS generation | RAW264.7 cells | Inhibited generation | nih.govbiomolther.orgsemanticscholar.org |
| Inhibition of JNK phosphorylation | RAW264.7 cells | Inhibited phosphorylation | nih.govsemanticscholar.org |
| Inhibition of COX-2 expression | RAW264.7 cells | Inhibited expression | nih.govsemanticscholar.org |
| Inhibition of Osteoclast Differentiation (TRAP) | RAW264.7 cells / LPS-induced | Blocked differentiation | nih.govsemanticscholar.org |
Investigation of this compound in Alcohol Use Disorder
The potential of this compound as a pharmacotherapy for alcohol use disorder (AUD) has been investigated, driven by the need for novel and more effective treatments. researchgate.netoup.comoup.com Drug repositioning, or repurposing existing drugs, is an appealing strategy for developing new AUD therapies, potentially reducing development costs and expediting treatment availability. researchgate.netoup.com this compound, a drug with a history of clinical use for conditions like hyperuricemia and gout, has been considered for repurposing for AUD. researchgate.netoup.comoup.com
This compound inhibits organic anion transporters in the kidneys, which is relevant to its use in gout, but it also inhibits pannexin1 channels. researchgate.netoup.comoup.comwikipedia.org Pannexin1 channels are involved in purinergic neurotransmission and inflammation, processes that have been implicated in the effects of alcohol and the motivation to consume alcohol. researchgate.netoup.com
Studies in rodents have tested the effects of this compound on alcohol intake. In both alcohol-dependent and nondependent rats, as well as in mice in the drinking-in-the-dark (DID) paradigm (a model for binge-like drinking), this compound reduced alcohol intake. researchgate.netoup.comoup.com This reduction in alcohol consumption was observed without affecting the intake of water or saccharin, suggesting that the effect of this compound was selective for alcohol and not a general reduction in reward. researchgate.netoup.comoup.com Specifically, this compound dose-dependently decreased alcohol drinking in rats, with a significant effect noted at a dose of 100 mg/kg compared to a vehicle control. oup.com
These preclinical results suggest the possibility that pannexin1 could be a novel therapeutic target for the treatment of AUD. researchgate.netoup.comoup.com Given the established clinical use and safety profile of this compound, these findings support its potential as a candidate for drug repositioning for AUD. researchgate.netoup.comoup.com
Translational research, including randomized placebo-controlled alcohol interaction trials, has also explored this compound's effects in humans. One study aimed to determine if this compound reduces the pleasurable effects of alcohol drinking and the desire to drink alcohol in individuals who consume unhealthy amounts of alcohol. clinicaltrials.gov While this study was not a treatment study for AUD, it investigated this compound's effects as an investigational drug for this potential application. clinicaltrials.gov
In human trials, this compound has been reported to significantly decrease alcohol craving during the ascending limb of alcohol intoxication, compared to a placebo. researchgate.net However, this compound did not show significant effects on alcohol pharmacokinetics (Cmax, Tmax, AUC) or on the stimulant or sedative effects of alcohol. researchgate.net Inflammatory biomarkers, cognitive performance following alcohol ingestion, and hemodynamics were also not significantly affected by this compound administration in this study. researchgate.net Analysis did not reveal sex-based differences in the effects of this compound compared to placebo. researchgate.net
These data from both preclinical and translational studies support the potential of this compound for AUD treatment and reinforce the idea that pannexin 1 channels may represent a novel therapeutic target for developing new pharmacotherapies for AUD. researchgate.netoup.comoup.comresearchgate.net
Here is a summary of key findings regarding this compound's investigation in Alcohol Use Disorder:
| Study Type | Model / Participants | Key Finding | Reference |
| Preclinical | Alcohol-dependent and nondependent rats | Reduced alcohol intake selectively (vs. water/saccharin) | researchgate.netoup.comoup.com |
| Preclinical | Mice (Drinking-in-the-Dark model) | Reduced binge-like alcohol drinking selectively | researchgate.netoup.comoup.com |
| Translational (Human) | Individuals consuming unhealthy alcohol | Significantly decreased alcohol craving during ascending intoxication limb | researchgate.net |
| Translational (Human) | Individuals consuming unhealthy alcohol | No significant effect on alcohol pharmacokinetics or stimulant/sedative effects | researchgate.net |
Structure-activity Relationship Sar Studies of Probenecid
Binding Interactions with Key Molecular Targets
Probenecid is known to interact with a variety of molecular targets, influencing their function. A significant area of research focuses on its interactions with organic anion transporters (OATs), particularly OAT1 and URAT1, which are involved in the renal excretion and reabsorption of uric acid, respectively. This compound acts as a competitive inhibitor of URAT1, reducing the reabsorption of uric acid and increasing its excretion patsnap.comnih.gov. It also inhibits OAT1, which can affect the secretion of other organic acids, including certain antibiotics patsnap.com. This compound inhibits OAT1, OAT3, and OAT6 with Ki values of 6.3, 9.0, and 8.4 µM, respectively, and OAT2 with an IC50 of 0.67 µM, showing selectivity over organic cation transporters OCT1 and OCT2 (IC50s of 1,600 and 1,700 µM) caymanchem.com.
Recent studies have also explored this compound's interactions with targets related to viral infections, such as SARS-CoV-2 and RSV. Molecular docking and simulations have indicated that this compound exhibits binding affinities with proteins like AKT1, EGFR, SRC, HSP90AA1, and PPARG. Stable interactions were observed with SRC, EGFR, and HSP90AA1, suggesting their potential roles in mediating this compound's effects nih.govfrontiersin.org. Specifically, this compound has been shown to form conventional hydrogen bonds with AKT1, EGFR, SRC, HSP90AA1, and PPARG. It also forms Pi-Donor hydrogen bonds with HSP90AA1 and Pi-Sulfur bonds with HSP90AA1 and PPARG. Carbon-hydrogen bonds have been observed with EGFR and SRC, contributing to complex stability nih.gov.
Another target for this compound is the transient receptor potential (TRP) channel TRPV2, which it activates caymanchem.comebi.ac.uk. This compound also inhibits pannexin-1 channels .
Table 1 summarizes some of the key molecular targets and the types of interactions observed with this compound.
| Target | Interaction Type(s) | Notes | Source(s) |
| URAT1 | Competitive Inhibition | Reduces uric acid reabsorption | patsnap.comnih.govacs.org |
| OAT1 | Competitive Inhibition | Affects secretion of organic acids | patsnap.comcaymanchem.com |
| OAT2 | Inhibition (IC50 = 0.67 µM) | Selective over OCTs | caymanchem.com |
| OAT3 | Inhibition (Ki = 9.0 µM) | Potential target for antiviral effects | caymanchem.comnews-medical.net |
| OAT6 | Inhibition (Ki = 8.4 µM) | Selective over OCTs | caymanchem.com |
| AKT1 | Binding, Conventional Hydrogen Bonds | Potential target in viral co-infection | nih.govfrontiersin.org |
| EGFR | Binding, Conventional Hydrogen Bonds, Carbon-Hydrogen Bonds | Stable interactions observed in viral co-infection studies | nih.govfrontiersin.org |
| SRC | Binding, Conventional Hydrogen Bonds, Carbon-Hydrogen Bonds | Stable interactions observed in viral co-infection studies | nih.govfrontiersin.org |
| HSP90AA1 | Binding, Conventional Hydrogen Bonds, Pi-Donor, Pi-Sulfur | Stable interactions observed in viral co-infection studies | nih.govfrontiersin.org |
| PPARG | Binding, Conventional Hydrogen Bonds, Pi-Sulfur | Potential target in viral co-infection | nih.govfrontiersin.org |
| TRPV2 | Activation (EC50 = 31.9 µM) | TRP channel | caymanchem.comebi.ac.uk |
| Pannexin-1 | Inhibition (IC50 ~ 150 μM) | ATP release channel | |
| TAS2R16 | Binding (Antagonist) | Bitter taste receptor, involves hydrophobic and hydrogen bond interactions | tandfonline.com |
Electrostatic Potential Mapping of this compound Binding
Electrostatic potential maps (MEPs) are utilized to predict molecular interaction sites and characterize molecular recognition patterns. These maps visualize the electrostatic potential distribution on a molecule's surface, highlighting regions of positive, negative, and neutral potential mdpi.com. For this compound, MEP analysis can reveal the areas most likely to interact with complementary charged regions on target proteins.
Studies investigating this compound binding to proteins like SRC, EGFR, and HSP90AA1 have used electrostatic potential mapping to visualize how this compound interacts within the protein cavities nih.govresearchgate.net. The color scale on these maps typically represents the electrostatic potential distribution, with red indicating negative, blue indicating positive, and white indicating neutral potential on the protein surface researchgate.net. The binding sites of this compound are often highlighted to show how the molecule fits within these electrostatic landscapes researchgate.net.
In the context of salt formation, MEP analysis of this compound has shown that the oxygen atom of the carboxyl group is a primary hydrogen bond acceptor with a negative electrostatic potential, while the oxygen atom of the sulfonyl group exhibits a global minimum electrostatic potential mdpi.com. According to the principle of electrostatic potential complementarity, regions with the highest electrostatic potential in this compound tend to bind with regions of the lowest electrostatic potential in interacting molecules mdpi.com.
Molecular Dynamics Simulations for this compound Binding
Molecular dynamics (MD) simulations are computational techniques used to study the dynamic behavior of molecular systems over time. In the context of this compound's SAR, MD simulations provide insights into the stability of this compound-protein complexes and the nature of the interactions at an atomic level nih.govfrontiersin.org.
MD simulations have been employed to further elucidate the critical interactions between this compound and its hub targets, such as AKT1, EGFR, SRC, HSP90AA1, and PPARG nih.govfrontiersin.org. Analysis of root mean square deviation (RMSD) values from these simulations can indicate the stability of the protein-ligand complex frontiersin.org. Lower and flatter RMSD values suggest greater stability of the binding pose over the simulation period frontiersin.org. Studies have shown relatively lower and flatter RMSD values for SRC-probenecid, HSP90AA1-probenecid, and EGFR-probenecid complexes compared to AKT1-probenecid and PPARG-probenecid, indicating more stable binding to the former targets frontiersin.org.
MD simulations also allow for the analysis of specific interactions, such as hydrogen bonds, van der Waals forces, and hydrophobic interactions, and their duration throughout the simulation researchgate.net. This provides a dynamic view of the binding interface and helps identify key amino acid residues involved in the interaction researchgate.net. For instance, MD simulations have confirmed that both hydrophobic interactions and hydrogen bonds play a role in the interaction between this compound and the TAS2R16 receptor tandfonline.com. The binding energies calculated from MD simulations can also provide a quantitative measure of the binding affinity tandfonline.com.
Advanced Research Methodologies and Approaches for Probenecid Studies
Systems Pharmacology and Bioinformatics Approaches
Systems pharmacology and bioinformatics play a significant role in understanding the multifaceted interactions of probenecid within biological systems. These approaches enable the identification of potential targets and pathways affected by this compound, moving beyond the traditional one-target, one-drug paradigm.
Studies utilizing systems pharmacology and bioinformatics have characterized targets associated with this compound for conditions such as SARS-CoV-2 and RSV co-infection frontiersin.orgresearchgate.netnih.gov. By analyzing overlapping targets between the diseases and this compound, researchers can construct protein-protein interaction (PPI) networks to visualize and understand the complex relationships frontiersin.orgresearchgate.netnih.gov. For instance, a study identified 141 overlapping targets and subsequently identified top hub targets, including AKT1, ALB, EGFR, CASP3, CTNNB1, SRC, and HSP90AA1, in the context of SARS-CoV-2/RSV co-infection frontiersin.orgresearchgate.netnih.govnih.gov.
Enrichment analysis, a bioinformatics technique, further helps in understanding the biological functions and pathways potentially modulated by this compound. These analyses suggest that this compound may influence inflammation, immunity, oxidative stress, and virus defenses, involving pathways such as Toll-like receptor, TNF, IL-17, and cytokine-cytokine receptor signaling frontiersin.orgresearchgate.netnih.govnih.govresearchgate.net.
This integrated approach provides valuable preliminary insights into the molecular mechanisms of this compound and establishes a foundation for further experimental validation frontiersin.orgresearchgate.netnih.govnih.gov.
Molecular Docking and Molecular Dynamics Simulations
Molecular docking and molecular dynamics (MD) simulations are powerful computational tools used to investigate the binding interactions between this compound and its potential target proteins at an atomic level. These methods provide insights into binding affinities, stability of complexes, and the key residues involved in the interaction.
Molecular docking analysis has been used to predict the binding poses and affinities of this compound with identified protein targets. For example, in studies investigating this compound's effects in SARS-CoV-2/RSV co-infection, molecular docking revealed strong binding affinities with targets such as AKT1, EGFR, SRC, HSP90AA1, and PPARG frontiersin.orgnih.gov. The docking scores and the formation of hydrogen bonds and other interactions provide quantitative data on the potential binding strength and nature of the interaction frontiersin.orgnih.gov.
| Target Protein | Docking Score (kcal/mol) | Conventional Hydrogen Bonds | Pi-Donor Hydrogen Bonds | Pi-Sulfur Bonds |
| AKT1 | 6.0351 | 4 | - | - |
| EGFR | 6.5243 | 3 | - | - |
| SRC | 6.1224 | 4 | - | - |
| HSP90AA1 | 6.7800 | 2 | 2 | 1 |
| PPARG | 6.6544 | 5 | - | 1 |
MD simulations further validate the stability of the complexes formed between this compound and its targets over time. Analysis of parameters like Root Mean Square Deviation (RMSD) from MD simulations can indicate the stability of the binding. Studies have shown relatively lower and flatter RMSD values for complexes like SRC-probenecid, HSP90AA1-probenecid, and EGFR-probenecid, suggesting greater stability compared to others like AKT1-probenecid and PPARG-probenecid frontiersin.org. These simulations help in speculating on the likelihood of certain proteins being key targets of this compound frontiersin.orgresearchgate.netnih.gov.
Molecular modeling techniques, including docking, have also been applied to study this compound-derived compounds, such as a 1,3,4-oxadiazole-phthalimide hybrid, to explore their potential as inhibitors of enzymes like α-amylase mdpi.com. Docking scores and predicted interactions with active site residues provide evidence for the inhibitory potential of such derivatives mdpi.com.
In Vitro and In Vivo Research Models
In vitro (cell-based) and in vivo (animal) models are fundamental to experimentally validate the effects of this compound identified through computational methods and to investigate its biological activities in relevant biological contexts.
In vitro studies using various cell lines have demonstrated the direct effects of this compound on viral replication and cellular processes. For instance, this compound has shown potent inhibition of SARS-CoV-2 replication in Vero E6 cells and normal human bronchial epithelial (NHBE) cells at submicromolar concentrations mdpi.comnews-medical.netmdpi.comnih.gov. It has also shown efficacy against influenza virus in human airway epithelial cells and against human metapneumovirus (HMPV) in LLC-MK2 cells mdpi.comnews-medical.netmdpi.com. These studies often involve measuring viral titers or plaque formation to quantify the antiviral effect news-medical.net.
Beyond antiviral effects, in vitro studies have explored this compound's impact on other cellular functions. In the context of cardiovascular research, in vitro force generation studies on cardiomyocytes isolated from murine tissue exposed to this compound have shown increased myofilament force generation and calcium sensitivity nih.govahajournals.orgahajournals.org. This compound has also been shown to inhibit hypoxia-induced endothelial cell growth rate in human retinal endothelial cells frontiersin.org.
In vivo studies using animal models, such as mice and hamsters, are crucial for evaluating the efficacy of this compound in a complex biological system and assessing its effects on disease progression. Mouse models of influenza infection have shown that this compound treatment can substantially inhibit viral lung titers and protect from lethal challenge mdpi.commdpi.com. In a Syrian hamster model of SARS-CoV-2 infection, this compound administration significantly reduced viral loads in tissues news-medical.netmdpi.com.
Animal models have also been used to study this compound's effects on cardiac function and retinal angiogenesis. Translational studies in healthy and infarcted mice confirmed that this compound can improve cardiac function in a dose-dependent manner nih.gov. In a murine oxygen-induced retinopathy (OIR) model, this compound administration inhibited the upregulation of pro-angiogenic factors like VEGF and HIF-1α and ameliorated neovascularization frontiersin.org.
These in vitro and in vivo studies provide experimental evidence supporting the potential therapeutic applications of this compound identified through other research methodologies.
Translational Studies and Clinical Trial Design
Translational studies bridge the gap between basic research findings and clinical applications, while robust clinical trial design is essential for evaluating the safety and efficacy of this compound in humans.
Translational studies involving this compound have focused on translating findings from in vitro and in vivo models to human health. For example, the observation that this compound acts as an agonist of TRPV2 channels in animal models has led to its investigation for improving cardiac function in patients with heart failure with reduced ejection fraction (HFrEF) nih.govahajournals.orgahajournals.orgresearchgate.net.
Clinical trial design for this compound studies varies depending on the condition being investigated and the phase of development. Randomized, double-blind, placebo-controlled designs are commonly employed to minimize bias and provide strong evidence of efficacy.
A Phase II clinical trial investigating this compound in non-hospitalized patients with mild to moderate COVID-19 utilized a randomized, single-blind, placebo-controlled design frontiersin.orgmdpi.commdpi.commdpi.commdpi.com. This study evaluated the antiviral activity, clinical efficacy, and safety of different this compound doses mdpi.com. The design involved randomizing patients to receive either this compound or placebo and assessing outcomes such as time to viral clearance and symptom resolution mdpi.commdpi.com.
For cardiovascular applications, a study protocol for a randomized placebo-controlled clinical trial (Re-Prosper-HF) has been designed to test the hypothesis that this compound can improve cardiac function in patients with HFrEF researchgate.netx-mol.net. This study is a multi-site, double-blind, randomized-controlled trial planning to enroll a significant number of patients to assess the effects of this compound on systolic function, functional status, and self-reported health status over a defined period researchgate.net.
These examples highlight the importance of well-designed clinical trials to translate the potential benefits of this compound observed in preclinical studies into clinical practice.
Drug Repurposing Strategies for this compound
Drug repurposing, also known as drug repositioning, is a strategy that explores new therapeutic uses for existing approved or investigational drugs. This compound, with its long history of clinical use for conditions like gout, is a prime candidate for repurposing due to its known safety profile. spandidos-publications.comnih.gov
Bioinformatics tools and databases, such as DTome, facilitate drug repurposing by analyzing drug-protein interactions and secondary protein-protein interactions spandidos-publications.com. By exploring the known targets of this compound, researchers can identify potential new indications. For instance, the interaction of this compound with organic anion transporters (OATs), particularly OAT1 and OAT3, has been explored as an avenue for repurposing, especially in the context of enhancing the efficacy of antivirals and antimicrobials by blocking their renal reabsorption spandidos-publications.com.
The anti-inflammatory properties of this compound and its modulation of pathways like purinergic-pannexin-1 signaling and TRPV2 channels have led to its investigation for repurposing in cardiovascular diseases, including ischemic heart disease and heart failure nih.gov.
Recent research has focused on repurposing this compound as an antiviral agent. Studies have demonstrated its potent inhibitory effects against a range of viruses, including SARS-CoV-2, influenza virus, and respiratory syncytial virus (RSV), both in vitro and in vivo news-medical.netmdpi.comnih.govmdpi.com. This broad-spectrum antiviral activity makes this compound a promising candidate for repurposing in the treatment of various viral infections nih.gov. The potential mechanisms behind its antiviral effects, such as the modulation of ACE2 and PANX1 pathways and inhibition of OAT3, are being actively investigated frontiersin.orgmdpi.com.
Drug repurposing strategies for this compound leverage existing knowledge about its pharmacological properties and safety to accelerate the identification and development of new therapeutic applications.
Future Directions and Emerging Research Areas for Probenecid
Long-term Safety and Efficacy Studies in Novel Therapeutic Applications
While probenecid has been in clinical use for over 50 years with a generally limited adverse effect profile in its traditional uses, its application in novel therapeutic areas necessitates dedicated long-term safety and efficacy studies. ahajournals.org For instance, initial clinical data have demonstrated that this compound exhibits inotropic properties in patients with heart failure with reduced ejection fraction and was well tolerated in the short term. ahajournals.org Future studies are planned to focus on its long-term effects in this patient population, exploring its potential as a novel therapeutic option. ahajournals.org Translational studies in animal models have also shown that this compound can improve cardiac function parameters like inotropy and lusitropy in a dose-dependent manner without inducing malignant arrhythmias or apoptosis. ahajournals.org
Exploration of this compound's Role in Specific Disease Pathophysiologies
Emerging research is actively investigating the involvement of this compound in the pathophysiology of various diseases, leveraging its interactions with specific membrane proteins and channels. This compound has gained attention for its ability to interact with transient receptor potential vanilloid 2 (TRPV2) channels, organic anion transporters (OATs), and pannexin 1 (Panx1) hemichannels. mdpi.comnih.gov These interactions suggest potential therapeutic utility in diverse medical fields.
One significant area of exploration is the central nervous system (CNS). Studies suggest that this compound possesses neuroprotective, antiepileptic, and anti-inflammatory properties, as evidenced by its effects against neurological and neurodegenerative diseases. mdpi.com Its use as a Panx1 hemichannel blocker to reduce neuroinflammation is particularly highlighted, given neuroinflammation's role in various CNS alterations. mdpi.com this compound may also be useful in improving conventional therapies in psychiatry by increasing the bioavailability of drugs that have low passage through the blood-brain barrier or high efflux from the CNS. mdpi.com
Beyond the CNS, this compound's interaction with OATs is being investigated for novel uses. spandidos-publications.com OATs play a vital role in the distribution and excretion of many drugs. spandidos-publications.com this compound's pronounced effects on OATs suggest potential off-target effects and new therapeutic applications, including in the context of cancer development, where SLC22 family proteins (OAT1, OAT3, OAT4, and URAT1), which interact with this compound, are implicated. spandidos-publications.com
Furthermore, this compound shows potential as an antiviral agent. spandidos-publications.com It can enhance the efficacy and tolerability of other antivirals by blocking theorized viral factor uptake by OAT3. spandidos-publications.com Its interaction with multidrug-resistance protein 1 (MRP1), localized in microglia and contributing to the efflux of antivirals from the CSF, presents another avenue for repositioning this compound as an antiviral drug. spandidos-publications.com
In the cardiovascular system, this compound's activation of TRPV2 channels in cardiomyocytes is being explored for its positive inotropic and lusitropic effects, suggesting a potential role in treating heart failure. ahajournals.orgnih.gov
Development of this compound Analogs and Derivatives with Enhanced Specificity
The exploration of this compound's diverse pharmacological activities, particularly its interactions with specific transporters and channels, is expected to drive the development of novel analogs and derivatives. The aim is to create compounds with enhanced specificity for particular targets, such as specific OAT subtypes, Panx1 hemichannels, or TRPV2 channels. This targeted approach could potentially lead to therapeutic agents with improved efficacy and reduced off-target effects compared to the parent compound. Research into the structure-activity relationships of this compound and its interactions with these various proteins is crucial for the rational design of such next-generation compounds.
Refinement of Predictive Models for this compound's Pharmacological Effects
Predictive modeling plays an increasingly important role in understanding and forecasting the pharmacological effects of drugs. For this compound, refinement of predictive models is essential to better understand its complex interactions with various transporters and channels, its pharmacokinetics across different physiological states and in combination with other drugs, and its potential therapeutic outcomes in novel applications. These models can help to predict drug-drug interactions, optimize dosing strategies in new indications, and identify patient populations most likely to benefit from this compound therapy. Modeling efforts are likely to incorporate data from in vitro studies on transporter and channel interactions, in vivo pharmacokinetic data, and clinical trial results to build more accurate and comprehensive predictive frameworks.
Given the nature of the content derived from the search results, which primarily discusses ongoing research directions and potential applications, there is no structured quantitative data suitable for generating interactive data tables in this section.
Q & A
Q. What mechanisms underlie this compound's anti-inflammatory effects beyond urate-lowering, and how can they be experimentally validated?
- Answer : Investigate ROS scavenging in macrophage lineages (e.g., THP-1 cells) via flow cytometry. Use siRNA knockdown of JNK/COX-2 to confirm pathway necessity. In vivo models (e.g., murine gout) can correlate ROS inhibition with reduced joint inflammation .
Methodological Notes
- Data Contradictions : Retrospective studies may overestimate efficacy due to selection bias (e.g., exclusion of non-adherent patients). Address via propensity score matching .
- Advanced Techniques : Compartmental modeling outperforms non-compartmental analysis (NCA) in predicting dose-dependent interactions .
- Clinical Relevance : this compound remains viable in mild-moderate CKD but requires SU monitoring due to variable renal clearance .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


