Succimer
Description
Historical Context of Succimer (DMSA) Development and Initial Research Focus
The history of this compound research is intertwined with the search for effective antidotes to heavy metal poisoning. This compound was initially synthesized in 1949 in England mhmedical.com. Its development is linked to the broader research into dithiol compounds, following the earlier development of British anti-Lewisite (BAL; dimercaprol) during World War II as an antidote for arsenical warfare agents nih.gov.
In the late 1950s, water-soluble analogs of BAL, including DMSA and 2,3-dimercapto-1-propanesulfonic acid (DMPS), were developed in the Soviet Union and China nih.gov. Chinese scientists, in 1957, found that DMSA could effectively treat antimony poisoning resulting from an overdose of tartar emetic wikipedia.org. Early research in China demonstrated the ability of the sodium salt of this compound to significantly increase the lethal dose (LD50) of tartar emetic in mice mhmedical.com. Studies by I. E. Okonishnikova in 1962 showed a pronounced protective effect of DMSA in animal poisoning with arsenic and mercury wikipedia.org.
An early review of the Chinese experience with intravenous this compound in treating occupational lead and mercury poisoning suggested efficacy comparable to intravenous CaNa2EDTA for increasing urinary lead and to intramuscular DMPS for mercury, with seemingly low toxicity mhmedical.com. This early research, coupled with the realization that this compound could be administered orally, paved the way for further animal experiments and human trials in other parts of the world, eventually leading to its approval for clinical use in some regions mhmedical.com.
Role of this compound in Contemporary Heavy Metal Toxicology Research
In contemporary research, this compound continues to play a significant role in understanding and addressing heavy metal toxicity. Its ability to chelate metals like lead, mercury, and arsenic makes it a valuable tool in both in vitro and in vivo studies investigating the mechanisms of metal toxicity and the efficacy of chelation strategies patsnap.comnih.gov.
Research utilizes this compound to study the distribution and elimination of heavy metals in biological systems. Studies in animal models, for instance, have investigated the effectiveness of this compound in reducing metal burdens in different organs nih.govmdpi.com. While this compound is known to be effective in chelating lead from soft tissues, studies suggest it may be less effective at removing lead from bones mdpi.com. Research also explores its impact on the redistribution of metals; for example, studies in animals have shown that DMSA treatment after exposure to inorganic mercury can lead to an increased elevation of mercury into motor axons, potentially due to mobilization from non-neural tissues like the kidneys and liver mdpi.com.
Furthermore, this compound is used in research to explore the relationship between heavy metal exposure, oxidative stress, and the potential for chelation to mitigate these effects mdpi.comcellmolbiol.org. Studies have indicated that lead-induced oxidative stress responded moderately to DMSA treatment alongside a reduction in lead concentration in blood and soft tissue mdpi.com. Research suggests that DMSA, acting as both an antioxidant and a lead chelator, can significantly deplete lead from the hippocampus, potentially aiding in the recovery from lead-induced oxidative stress and apoptosis in research models mdpi.com.
Scope and Significance of this compound in Advanced Clinical and Mechanistic Studies
The significance of this compound in advanced clinical and mechanistic studies lies in its continued use as a reference chelating agent and the ongoing research to optimize chelation strategies and understand the underlying mechanisms of metal-chelator interactions.
Mechanistic studies delve into how this compound interacts with metal ions at a molecular level. The chelation process involves the formation of stable complexes where the metal ions are sequestered by the sulfhydryl groups of this compound patsnap.com. This binding reduces the biological activity and toxicity of the heavy metals and enhances their water solubility, facilitating renal excretion patsnap.com. Research indicates that in humans, most DMSA in plasma is bound to proteins, primarily albumin, through a disulfide bond, and the majority of the compound in circulation exists as mixed disulfide compounds with plasma proteins nih.govresearchgate.net. There is evidence suggesting that the mixed disulfides of cysteine may be the active chelating moiety in humans, implying that chelation might occur principally in the kidney researchgate.netnih.gov.
Advanced clinical studies, while not focused on dosage or safety in this context, utilize this compound to investigate its efficacy in reducing metal burden and its impact on various physiological endpoints in controlled research settings. For example, research has explored the use of this compound in combination with other agents or in different regimens to assess their comparative effectiveness in metal detoxification portlandpress.com. Kinetic modeling studies are also being developed to simulate the kinetics of DMSA in humans and predict its effect on blood lead concentrations, which could inform future research on optimizing chelation strategies tandfonline.comresearchgate.net.
Research findings have shown that DMSA treatment can significantly increase urinary lead excretion and reduce blood lead concentrations in lead-poisoned patients, although with substantial individual variation nih.gov. Studies in lead-exposed adult primates treated with this compound showed no measurable reduction in brain lead levels, while follow-up studies in rodents indicated that this compound treatment could significantly reduce brain lead levels, with multiple courses being more effective than one nih.gov. These findings highlight the complexity of metal distribution and the challenges in effectively removing metals from all tissues.
Research also explores the potential of modified DMSA compounds or combination therapies to improve chelation outcomes. Studies have investigated the effects of different monoalkyl esters of DMSA on lead mobilization in mice, observing varying degrees of reduction in lead concentrations compared to DMSA itself mdpi.com.
While this compound is a valuable tool in heavy metal toxicology research, ongoing studies continue to explore its limitations, such as its limited ability to access intracellular metals due to its extracellular distribution mdpi.com. This limitation drives research into newer strategies, including combination therapy with structurally different chelating agents or co-administration with antioxidants, to enhance intracellular metal removal and address oxidative stress mdpi.comcellmolbiol.org.
Properties
IUPAC Name |
2,3-bis(sulfanyl)butanedioic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C4H6O4S2/c5-3(6)1(9)2(10)4(7)8/h1-2,9-10H,(H,5,6)(H,7,8) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ACTRVOBWPAIOHC-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C(C(C(=O)O)S)(C(=O)O)S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C4H6O4S2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
304-55-2 (Parent) | |
| Record name | Dimercaptosuccinic acid | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002418146 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID10859324 | |
| Record name | 2,3-Dimercaptobutanedioic acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10859324 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
182.2 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Succimer | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014706 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.43e+00 g/L | |
| Record name | Succimer | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014706 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
CAS No. |
2418-14-6, 304-55-2 | |
| Record name | Dimercaptosuccinic acid | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=2418-14-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dimercaptosuccinic acid | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002418146 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dimercaptosuccinic acid | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14089 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | 2,3-Dimercaptosuccinic acid | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=259951 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Butanedioic acid, 2,3-dimercapto- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | 2,3-Dimercaptobutanedioic acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10859324 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2,3-dimercaptosuccinic acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.017.577 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Succimer | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014706 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
193 °C | |
| Record name | Succimer | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014706 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanistic Insights into Succimer's Chelating Activity
Molecular Interactions of Succimer with Divalent Metal Ions
The chelating activity of this compound is fundamentally driven by its molecular interaction with divalent metal ions. This interaction involves the donation of electron pairs from donor atoms within the this compound molecule to the metal ion, forming coordinate covalent bonds. jcimcr.org
Elucidation of Sulfhydryl Group Reactivity in Metal Binding
A key feature of this compound is the presence of two sulfhydryl (-SH) groups. patsnap.comnih.govpatsnap.com These thiol groups exhibit a high affinity for binding to heavy metal ions. patsnap.compatsnap.com The reactivity of these sulfhydryl groups is central to this compound's ability to sequester toxic metals. patsnap.compatsnap.com Studies have shown that these groups readily ionize upon complexation with metal cations, facilitating the binding process. wikipedia.org
Formation of Stable, Water-Soluble Chelate Complexes
Upon binding to heavy metal ions, this compound forms stable, ring-like structures known as chelates. patsnap.com The formation of these complexes is crucial for reducing the biological activity and toxicity of the metals. patsnap.com A significant aspect of this compound's mechanism is that the resulting metal-succimer complexes are water-soluble. patsnap.comnih.govwikipedia.orgdrugbank.com This increased water solubility enhances their excretion from the body, primarily through the kidneys in urine. patsnap.compatsnap.comontosight.ai This process effectively reduces the total body burden of toxic metals. patsnap.com
Research has investigated the structure of these metal complexes. For instance, studies on the complexes formed with Pb²⁺, Cd²⁺, and Hg²⁺ have indicated that the structure of the complex is dependent on the specific metal ion. nih.gov With Pb²⁺ and Cd²⁺, one oxygen and one sulfur atom appear to act as donor atoms. nih.gov In the case of Hg²⁺, both sulfur atoms function as donors. nih.gov The solubility of these metal chelates is also pH-dependent, becoming more soluble as noncoordinated sulfhydryl and carboxylic acid groups become ionized. nih.gov
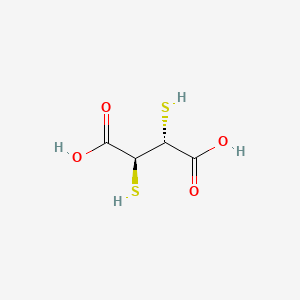
Stereochemical Aspects of Chelate Formation
This compound exists as diastereomers, with the meso isomer being the form used as a chelating agent. wikipedia.orgchemicalbook.com The this compound molecule contains two asymmetric carbon atoms, leading to the possibility of different stereoisomers. wikipedia.org The meso-2,3-dimercaptosuccinic acid structure features the two mercapto groups oriented in a specific spatial arrangement. ontosight.ai The stereochemistry of the chelating agent and the metal ion plays a role in the strength and structure of the coordination complexes formed. jcimcr.orgresearchgate.net Studies on the dimethyl ester of meso-2,3-dimercaptosuccinic acid have shown that the two methyl ester groups and the two sulfhydryl groups are in a staggered conformation, and the resulting cadmium complex forms a distorted tetrahedron with a CdS₄ kernel. nih.gov
Specificity of Metal Chelation by this compound
While this compound can chelate various metal ions, it exhibits preferential binding dynamics with certain heavy metals. This specificity is a crucial aspect of its therapeutic use, as it ideally targets toxic metals while minimizing the depletion of essential endogenous metals.
Preferential Binding Dynamics with Lead, Mercury, Arsenic, and Cadmium Ions
This compound binds with high specificity to ions of lead, mercury, cadmium, and arsenic. patsnap.comnih.govdrugbank.comnih.govrxlist.comhmdb.ca This high affinity for these toxic heavy metals is attributed to the chemical properties of the sulfhydryl groups, which have a strong tendency to bind with "soft" heavy metals like Hg²⁺ and Pb²⁺. patsnap.comwikipedia.org Research consistently highlights this compound's effectiveness in chelating lead, mercury, and arsenic, facilitating their excretion. patsnap.compatsnap.comnih.govpatsnap.comnih.govunict.itmhmedical.com
Research on Minimal Chelation of Endogenous Essential Metals (e.g., Zinc, Copper, Iron, Magnesium, Calcium)
A significant advantage of this compound over some other chelating agents is its reported minimal chelation of essential endogenous metals such as zinc, copper, iron, magnesium, and calcium at therapeutic doses. nih.govchemicalbook.comrxlist.comnih.govmhmedical.comfda.gov While some studies have indicated minor increases in the urinary excretion of zinc and copper, the effect on essential minerals is generally considered small compared to chelators like CaNa₂EDTA, which can induce more substantial excretion of these elements. rxlist.commhmedical.comfda.govoup.commedicinacomplementar.com.br
For example, studies in lead-exposed men showed that this compound treatment led to a doubling of zinc excretion but had no significant effect on the urinary elimination of iron, calcium, or magnesium. rxlist.comfda.gov Another study in heavy metal-poisoned subjects found that only plasma zinc decreased significantly after DMSA treatment, while levels of calcium, copper, iron, and magnesium were not significantly affected. oup.com However, some research in adult patients with lead poisoning noted a significant increase in urine copper and zinc excretion following DMSA administration. oup.com A study in a primate model of childhood lead exposure reported trending but non-significant increases in urinary Ca, Cu, and Fe, but not in Zn or Mg. oup.com These findings suggest some variability in the effect on essential metals, though generally considered less pronounced than with other chelating agents.
Research findings on the impact of this compound on essential metal excretion:
| Essential Metal | Observed Effect on Excretion (compared to baseline or placebo) | Source(s) |
| Zinc | Increased (doubled or significant increase) | rxlist.commhmedical.comfda.govoup.com |
| Copper | Minor increase or significant increase | mhmedical.comoup.com |
| Iron | No significant effect or trending non-significant increase | rxlist.comfda.govoup.com |
| Magnesium | No significant effect | rxlist.comfda.govoup.com |
| Calcium | No significant effect or trending non-significant increase | rxlist.comfda.govoup.com |
Cellular and Subcellular Mechanisms of Metal Mobilization
This compound's mechanism of action involves the mobilization of heavy metals from the body by forming stable complexes that can be eliminated. While this compound is a hydrophilic compound and its capacity to access intracellular metal ions is considered weak compared to lipophilic chelators, it effectively promotes renal metal elimination by forming soluble complexes jcimcr.orgnih.gov. Studies suggest that most DMSA is found in the extracellular space, though it can cross cellular membranes to some extent nih.gov. The chelation process reduces the biological activity and toxicity of heavy metals by sequestering the metal ions patsnap.com.
Prevention of Metal-Biological Macromolecule Interactions
Heavy metals exert their toxic effects by binding to biological macromolecules such as proteins (including enzymes), nucleic acids, and lipids, often by interacting with sulfhydryl groups or displacing essential metal ions like Ca²⁺ and Zn²⁺ jcimcr.orgresearchgate.netacs.org. This compound's dithiol groups compete with these biological ligands for binding to heavy metal ions. By forming more stable complexes with the toxic metals, this compound effectively sequesters the metal ions, preventing their interaction with critical cellular components. patsnap.compatsnap.com This competitive binding reduces the ability of heavy metals to alter protein structures, inhibit enzyme functions, or disrupt cellular processes that are dependent on essential metal ions. patsnap.comjcimcr.org The stability of the this compound-metal complex is a crucial factor in its ability to prevent these interactions, as a more stable complex indicates a stronger binding affinity of this compound for the metal compared to biological ligands. jcimcr.orgnih.govfiveable.me
Reduction of Metal Toxicity at the Cellular and Enzymatic Levels
The chelation of heavy metals by this compound directly contributes to the reduction of metal toxicity at both cellular and enzymatic levels. By forming stable, water-soluble complexes, this compound lowers the concentration of free, biologically active metal ions within the body. patsnap.comdrugbank.com This reduction in free metal ions minimizes their ability to induce oxidative stress, damage cellular structures, and interfere with enzymatic activities. patsnap.comjcimcr.orgresearchgate.netujvas.com.ua
Heavy metals can inhibit enzymes by binding to their active sites, often by interacting with sulfhydryl groups or displacing essential cofactors like zinc jcimcr.orgacs.orgulisboa.pt. This compound's ability to chelate these metals reduces their availability to bind to and inactivate these critical enzymes. While some chelators can potentially interfere with metalloenzymes by binding to their essential metal cofactors, this compound is reported not to significantly chelate essential metals such as zinc, copper, or iron at therapeutic concentrations, contributing to its specificity for toxic heavy metals nih.govmhmedical.com. However, minor increases in the excretion of essential metals like zinc and copper have been noted mhmedical.com. Research has investigated the effect of chelators, including this compound, on zinc-containing enzymes like alcohol dehydrogenase (ADH), suggesting that interference might occur at higher concentrations ulisboa.pt.
Stability Constants of this compound-Metal Complexes
The stability of the complexes formed between this compound and various metal ions is a critical factor in its efficacy as a chelating agent. Higher stability constants generally indicate a stronger binding affinity between the chelator and the metal ion. Studies have determined stability constants for this compound (DMSA) with several metal ions, providing insights into its selectivity and chelating strength.
| Metal Ion | Log K (Stability Constant) | Conditions | Source |
| Bismuth(III) | 43.87 | 25°C, 0.1 M ionic strength | colab.ws |
| Lead(II) | 17.4 | 25°C, 0.1 M ionic strength | colab.ws |
| Zinc(II) | Note: Forms dimer, see text | 25°C, 0.1 M ionic strength | colab.ws |
Note: For Zinc(II), the system is dominated by a Zn₂(DMSA)₂ dimer, and no more than 20% of total zinc exists as a monomeric complex at any pH under the studied conditions colab.ws.
These stability constants highlight the strong affinity of this compound for certain heavy metals like bismuth and lead, supporting its use in treating poisoning by these elements. The relatively lower monomeric complex formation with zinc also aligns with observations that this compound does not significantly chelate essential zinc nih.govmhmedical.com.
Data from animal studies also provide insights into the effects of this compound on enzyme activity in the context of heavy metal toxicity. For example, in lead-treated rats, administration of this compound was associated with increased levels of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), which were decreased by lead exposure alone ujvas.com.ua. This suggests that this compound's action in chelating lead helps to mitigate the oxidative stress induced by the metal, thereby supporting the recovery of these enzymatic defense systems.
| Treatment Group | SOD Activity Change (vs. Lead Alone) | GPx Activity Change (vs. Lead Alone) | CAT Activity Change (vs. Lead Alone) |
| Lead Acetate + this compound | Increased (P < 0.01) | Increased (P < 0.001) | Increased (P < 0.05) |
| Lead Acetate + Leonardite | Increased | Increased | Increased |
| Lead Acetate + this compound + Leonardite | Increased | Increased | Increased |
Data derived from research findings on enzyme activity in lead-treated rats ujvas.com.ua. P-values indicate statistical significance compared to the group treated with lead acetate alone.
Pharmacological and Pharmacokinetic Investigations of Succimer Dmsa
Succimer Absorption and Bioavailability Characteristics
Following oral administration, this compound is absorbed, although the absorption is described as rapid but variable and incomplete. aap.orgfda.govnih.govdrugbank.com Peak blood concentrations are typically observed within approximately 1 to 3 hours after oral dosing in healthy adult volunteers. mhmedical.comfda.gov
Research on Gastrointestinal Absorption Dynamics and Variability
Research indicates that the gastrointestinal absorption of this compound is rapid but can be variable. aap.orgfda.govnih.govdrugbank.com Studies in healthy adult volunteers administered a single dose of 14C-succimer showed peak blood radioactivity levels between one and two hours. fda.gov On average, about 49% of the radiolabeled dose was excreted, with 39% in feces, suggesting that a significant portion represents non-absorbed drug. fda.gov This implies that the absorption is incomplete. fda.govnih.gov Chemical analysis of this compound and its metabolites in urine after a single oral dose in healthy adults showed that approximately 25% of the administered dose was excreted in the urine, with peak blood levels and urinary excretion occurring between two and four hours. fda.gov
There has been some concern that oral this compound treatment might increase the absorption of lead from the gastrointestinal tract, particularly if exposure continues during treatment. researchgate.netnih.govnih.gov A preliminary study using a stable lead isotopic tracer (204Pb) in adult male subjects suggested that GI lead absorption might be enhanced by this compound, and that this compound mediated the redistribution of lead from the circulation to other tissues. researchgate.netnih.gov However, the differences observed in this study were not statistically significant due to large within-group variability. researchgate.netnih.gov In contrast, a study using a juvenile nonhuman primate model of moderate childhood lead intoxication found that oral this compound significantly reduced the GI absorption of lead. nih.gov These discrepancies may be attributed to differences in species, age, or experimental design. researchgate.net
The following table summarizes findings on this compound absorption and excretion:
| Study Population | Dose (Oral) | Peak Blood/Radioactivity Time | Percentage Excreted in Urine (within 24h) | Percentage Excreted in Feces | Notes | Source |
| Healthy adult volunteers | 16, 32, or 48 mg/kg 14C-succimer | 1-2 hours | ~9% (as radioactivity) | ~39% | Absorption rapid but variable. fda.gov | fda.gov |
| Healthy adult volunteers | 10 mg/kg this compound | 2-4 hours | ~25% (as this compound and metabolites) | Not specified | Rapid and extensive metabolism. fda.gov | fda.gov |
| Humans | Not specified | Not specified | 10-25% | Not specified | Absorption rapid but incomplete. nih.gov | nih.gov |
| Juvenile Rhesus Monkeys | Not specified | Not specified | Increased endogenous Pb excretion | Decreased endogenous Pb excretion | Significantly reduced GI absorption of Pb compared to vehicle. nih.gov | nih.gov |
Investigations into Enterohepatic Circulation
Evidence suggests that enterohepatic circulation of this compound or its metabolites occurs. nih.govnih.gov Studies in normal adults have observed increases in plasma total DMSA concentration after meals, which were prevented by the administration of cholestyramine or neomycin. nih.gov These findings indicate that a metabolite(s) of DMSA undergoes enterohepatic circulation and that microflora are required for this reentry. nih.gov
Distribution Profile of this compound in Biological Compartments
This compound is predominantly distributed in the extracellular compartment. mhmedical.comaap.orgresearchgate.nettandfonline.comnih.govnih.gov In blood, it is largely confined to the plasma and does not significantly penetrate erythrocytes. tandfonline.com
Extracellular Distribution Dominance
Studies have shown that this compound is primarily distributed in the extracellular fluid of the body. mhmedical.comaap.orgresearchgate.nettandfonline.comnih.govnih.gov This extracellular distribution may contribute to its relatively low toxicity compared to other dithiols. nih.gov Kinetic modeling studies in healthy adult volunteers after oral administration of this compound have indicated that "total" DMSA is distributed in the body's extracellular fluid and in blood, being almost entirely distributed in the plasma and not penetrating erythrocytes. tandfonline.com The relative volume of distribution has been reported to range between 0.17 and 0.34 L/kg, further supporting that the distribution volume is mainly restricted to extracellular fluid. uu.nl
Plasma Protein Binding Dynamics (e.g., Albumin Interactions)
In the blood, this compound is extensively bound to plasma proteins, with binding exceeding 90%. mhmedical.comaap.orgsemanticscholar.org The primary protein involved in this binding is albumin. aap.orgnih.govsemanticscholar.orgresearchgate.net this compound binds to plasma proteins, mainly albumin, through a disulfide bond with cysteine. nih.govresearchgate.net This extensive protein binding means that only a very small amount of the parent drug is present as free this compound in the plasma. nih.govresearchgate.net The unbound fraction is typically considered the pharmacologically active portion of a drug, available for distribution into tissues, metabolism, and excretion. wikipedia.orgbioanalysis-zone.com However, for this compound, there is evidence suggesting that the mixed disulfides of cysteine, rather than the parent drug or protein-bound this compound, may be the active chelating moiety in vivo. mhmedical.comnih.govresearchgate.net If this is the case, chelation might occur principally in the kidney, where this compound is extensively metabolized to mixed disulfides of cysteine. nih.govresearchgate.net
Technetium Tc-99m this compound, a related compound used in renal imaging, is also distributed in plasma and bound to plasma proteins following intravenous administration, with 53% to 70% protein binding observed in humans. drugbank.com
Research on Blood-Brain Barrier Permeability and Brain Metal Mobilization
Research on the ability of this compound to cross the blood-brain barrier (BBB) and mobilize metals from the brain has yielded varying results, particularly depending on the metal involved and the study model.
Some sources suggest that DMSA can cross the blood-brain barrier and is thus useful for extracting heavy metals from the brain. chemeurope.com It has been stated that this compound facilitates the urinary excretion of lead and, with sufficiently aggressive treatment, can reduce lead content in the brain. wikipedia.org Animal studies have shown that dimercaptosuccinic acid can cross the blood-brain barrier in mice. wikipedia.org
However, other research indicates that this compound does not cross the blood-brain barrier to any major extent. researchgate.net Comparative studies between this compound and calcium disodium edetate (CaNa2EDTA) in experimental models have not consistently observed an effect of chelation therapy on brain lead concentrations. researchgate.net Some animal studies have suggested that neither DMSA nor other chelating or mobilizing agents were able to ameliorate the brain burden of mercury. nih.gov
While some evidence supports this compound's ability to influence brain metal levels, particularly lead in certain models, there are conflicting findings and a lack of definitive evidence confirming significant BBB permeability and consistent brain metal mobilization in humans across all heavy metals.
Biotransformation and Metabolic Pathways of this compound
Following administration, this compound undergoes significant biotransformation. While the precise site of this metabolism is not definitively established, studies have characterized the primary metabolic products and suggested potential locations for these transformations.
Characterization of Mixed Disulfide Formation with L-Cysteine
A prominent metabolic pathway for this compound involves the formation of mixed disulfides with the amino acid L-cysteine. Chemical analysis of urine samples from individuals administered this compound has revealed that a substantial portion of the drug is excreted in these altered forms. The majority of the mixed disulfides identified consist of this compound in disulfide linkages with two molecules of L-cysteine. nih.govfda.govfda.govpharmacompass.comrxlist.com The remaining disulfides contain one L-cysteine molecule per this compound molecule. nih.govfda.govfda.govpharmacompass.comrxlist.com Research indicates that the formation of these mixed disulfides with L-cysteine is favored over the formation of cyclic disulfides of this compound itself. nih.govpharmacompass.com
Identification of Active Chelating Moiety in Vivo (e.g., this compound-Cysteine Adducts)
While this compound is the administered compound, evidence suggests that its metal-chelating activity in vivo may be primarily attributed to its metabolites, specifically the mixed disulfides formed with L-cysteine (this compound-cysteine adducts). mhmedical.combrainkart.com If these adducts are indeed the principal active form, it has been proposed that chelation might occur predominantly within the kidneys. nih.gov
Contribution of Renal and Hepatic Metabolism
This compound is known to be rapidly and extensively metabolized. fda.govmhmedical.comdrugbank.comdrugs.com Although the specific site of this biotransformation is not fully understood mhmedical.comdrugbank.com, the formation of DMSA-cysteine mixed disulfides is assumed by some research to occur in the kidney. tandfonline.com The liver is generally a primary organ for drug metabolism slideshare.netpharmaguideline.com, but there is limited clinical data available concerning the metabolism of this compound in individuals with liver impairment. fda.govfda.gov The majority of the absorbed this compound is ultimately excreted by the kidneys, primarily in its metabolized forms. fda.govdrugs.com
Excretion and Elimination Kinetics of this compound and its Metabolites
The elimination of this compound and its metabolites from the body primarily occurs via the renal pathway.
Renal Excretion Mechanisms and Routes
Absorbed this compound is predominantly excreted in the urine in the form of its metabolites. nih.govfda.govdrugbank.comdrugs.com Studies in healthy adult volunteers have shown that approximately 25% of an administered dose is excreted in the urine, with peak urinary excretion occurring between two and four hours post-administration. fda.gov Of the total amount of drug eliminated in the urine, a significant majority, approximately 90%, is in the form of altered this compound, specifically mixed this compound-cysteine disulfides, while the remaining 10% is excreted as unchanged this compound. fda.govfda.govrxlist.com The water-soluble complexes formed between this compound (or its active metabolites) and heavy metals are filtered from the bloodstream by the renal glomeruli and subsequently excreted in the urine. patsnap.com Maintaining adequate hydration is important to support the renal excretion of these chelating agents and their metal complexes. rxlist.comdrugs.com
The following table summarizes typical urinary excretion data:
| Excretion Pathway | Percentage of Total Urinary Excretion |
| Altered this compound | ~90% fda.govfda.govrxlist.com |
| Unchanged this compound | ~10% fda.govfda.govrxlist.com |
Role of Multidrug Resistance Protein 2 (Mrp2) in Renal Elimination
Experimental evidence suggests the involvement of Multidrug Resistance Protein 2 (Mrp2), an efflux transporter, in the renal elimination of this compound-metal complexes. mhmedical.combrainkart.com Mrp2 is located on the apical membrane of renal proximal tubule cells and plays a role in transporting organic anions from the cells into the tubular lumen for excretion in the urine. solvobiotech.comwikipedia.org Specifically, experimental data indicates that Mrp2 facilitates the renal excretion of mercury compounds when bound to transformed this compound. mhmedical.combrainkart.com
Apparent Elimination Half-Life Studies
Investigations into the pharmacokinetics of this compound (DMSA) have provided data on its apparent elimination half-life in humans, although findings can vary depending on the study population and methodology.
In studies involving healthy adult volunteers, the apparent elimination half-life of total DMSA (parent drug plus oxidized metabolites) in the blood has been reported to be approximately two days following a single oral dose of radiolabeled this compound at varying concentrations (16, 32, or 48 mg/kg). fda.govfda.gov In other studies of healthy adults given a single oral dose of 10 mg/kg, chemical analysis of this compound and its metabolites in urine indicated that approximately 25% of the dose was excreted in urine, with peak levels occurring between two and four hours. fda.govfda.gov Of the urinary excretion, about 90% was in the form of mixed this compound-cysteine disulfides, while 10% was eliminated unchanged. fda.govfda.gov
A pharmacokinetic modeling study in healthy adult volunteers reported an initial distribution phase half-life of about 25 minutes and an elimination phase half-life of approximately 4.3 hours for total DMSA plasma concentrations after oral administration of 10 mg/kg. tandfonline.comtandfonline.comuu.nl This study noted that the observed data sometimes showed an apparent postprandial peak in plasma DMSA concentration, occurring around 11 hours after administration, which was attributed to a fat-rich dinner activating an enterohepatic cycle. tandfonline.comtandfonline.comuu.nl
Comparisons of pharmacokinetics between different populations have revealed variations in elimination half-life. A study comparing healthy adults, lead-poisoned adults, and lead-poisoned children found differences in the elimination half-life of total DMSA. nih.gov The elimination half-life was longer in children with lead poisoning (3.0 ± 0.2 hours) compared to adults with lead poisoning (1.9 ± 0.4 hours) and healthy adults (2.0 ± 0.2 hours). nih.gov This study also indicated that renal clearance of DMSA and its metabolites was greater in healthy adults than in either adults or children with lead poisoning, suggesting that impaired renal clearance may contribute to the longer half-life observed in poisoned individuals, particularly children. nih.gov
Another source indicates that DMSA has a half-life of 2–3 hours in the blood. taylorandfrancis.com
The differences observed in reported half-life values across studies may be influenced by factors such as the specific analytical method used (measuring parent drug vs. total radiolabeled material or total DMSA including metabolites), the study population (healthy volunteers vs. poisoned patients, adults vs. children), the route and form of administration, and the pharmacokinetic model applied.
Here is a summary of some reported apparent elimination half-life data:
| Study Population | Form Measured | Apparent Elimination Half-Life | Notes | Source |
| Healthy Adult Volunteers | Radiolabeled material | ~2 days | Following single oral dose (16-48 mg/kg) | fda.govfda.gov |
| Healthy Adult Volunteers | Total DMSA (plasma) | ~4.3 hours | Elimination phase (oral 10 mg/kg) | tandfonline.comtandfonline.comuu.nl |
| Healthy Adults | Total DMSA | 2.0 ± 0.2 hours | Following oral administration | nih.gov |
| Adults with Lead Poisoning | Total DMSA | 1.9 ± 0.4 hours | Following oral administration | nih.gov |
| Children with Lead Poisoning | Total DMSA | 3.0 ± 0.2 hours | Following oral administration | nih.gov |
| General (Blood) | DMSA | 2–3 hours | taylorandfrancis.com |
Interactive table:
Clinical Research on Succimer Efficacy in Heavy Metal Intoxication Management
Succimer in Lead Poisoning Research
Studies on Reduction of Blood Lead Concentrations
Research has consistently shown that this compound significantly increases urine lead elimination and reduces blood lead concentrations in patients with lead poisoning. nih.govtandfonline.comoup.com The reduction in blood lead levels is dose-dependent. tandfonline.comfda.gov Over a 5-day treatment course, mean daily urine lead excretion can increase by 5- to 20-fold compared to baseline, and blood lead concentrations can fall to 50% or less of pretreatment levels, although individual responses vary. nih.govtandfonline.com Maximum enhancement of urine lead elimination typically occurs with the first dose. nih.govtandfonline.com
In a dose-ranging study involving pediatric patients aged 2 to 7 years with blood lead levels between 30-49 µg/dL, different this compound doses resulted in varying reductions in mean blood lead levels over 5 days: 78% reduction with 350 mg/m² every 8 hours, 63% with 233 mg/m² every 8 hours, and 42% with 116 mg/m² every 8 hours. fda.gov These doses corresponded to approximately 10, 6.7, and 3.3 mg/kg, respectively. fda.gov
A large multicenter, placebo-controlled randomized trial (Treatment of Lead-Exposed Children - TLC Trial) involving children with blood lead levels of 20-44 µg/dL found that this compound-treated children experienced an abrupt drop in blood lead levels compared to placebo-treated children. nih.gov The mean blood lead level in the this compound group during the 6 months after treatment initiation was 4.5 µg/dL lower than in the placebo group. nih.gov
| Study Type | Patient Population (Age, BLL) | This compound Dose/Regimen | Observed Effect on Blood Lead Levels | Reference |
| Dose-ranging study | Pediatric (2-7 yrs, 30-49 µg/dL) | 350 mg/m² q8h for 5 days (~10 mg/kg) | 78% reduction in mean blood lead levels over 5 days | fda.gov |
| Dose-ranging study | Pediatric (2-7 yrs, 30-49 µg/dL) | 233 mg/m² q8h for 5 days (~6.7 mg/kg) | 63% reduction in mean blood lead levels over 5 days | fda.gov |
| Dose-ranging study | Pediatric (2-7 yrs, 30-49 µg/dL) | 116 mg/m² q8h for 5 days (~3.3 mg/kg) | 42% reduction in mean blood lead levels over 5 days | fda.gov |
| Randomized, placebo-controlled trial (TLC) | Pediatric (12-33 mo, 20-44 µg/dL) | Up to three courses | Mean BLL 4.5 µg/dL lower than placebo over 6 months | nih.gov |
| Clinical Study | Adults (≥ 50 µg/dL) | 30 mg/kg/day for ≥ 5 days | Significantly reduced blood lead concentrations (mean decline to 55.4% of pre-treatment) | oup.com |
| Non-controlled clinical trials | Children (31-69 µg/dL) | High-dose DMSA over 5 days | Reduced mean blood lead levels by 61-77% over 5 days | aafp.org |
Mobilization of Lead from Soft Tissues and Bone Stores
This compound promotes the excretion of lead, thereby reducing body stores. medscape.com While it effectively reduces blood lead levels, studies suggest that this compound's efficacy in removing lead from brain tissues may lag behind and be smaller in magnitude compared to its effect on blood lead levels. oup.comresearchgate.netnih.gov This is an important consideration as blood lead levels are often used clinically to estimate lead reductions in the brain. researchgate.netnih.gov
Following chelation therapy, including with this compound, a rebound increase in blood lead concentration can occur. cncb.ac.cnnih.govtandfonline.comfda.govwho.intwho.int This rebound is attributed to the redistribution of lead from bone and soft tissue stores back into the blood as the concentration in the blood re-equilibrates. nih.govtandfonline.comfda.govwho.intwho.intresearchgate.net Studies in rats have detected this rebound in blood lead levels after this compound regimens, but not in brain lead levels. researchgate.netnih.gov Substantial amounts of lead are eliminated into the urine, suggesting mobilization from bone or soft tissues. avma.org
Impact on Lead-Induced Biochemical Alterations (e.g., δ-aminolevulinic acid dehydratase activity)
Lead exposure is known to inhibit the activity of enzymes involved in heme synthesis, such as δ-aminolevulinic acid dehydratase (ALAD). nih.govaoemj.orgunc.edu This inhibition leads to the accumulation of δ-aminolevulinic acid. nih.govaoemj.org Research indicates that this compound can help reverse lead-induced alterations in biochemical markers. nih.gov Both this compound and its analogue, monoisoamyl DMSA (MiADMSA), have been shown to increase blood ALAD activity and glutathione (GSH) levels towards normal in lead-exposed rats. nih.gov The most prominent effect on blood ALAD activity was observed when MiADMSA was co-administered with alpha-lipoic acid. nih.gov this compound reverses the adverse metabolic effects of lead on heme synthesis while increasing urinary lead output. cncb.ac.cn
Comparative Efficacy Studies with Other Lead Chelating Agents (e.g., CaNa2EDTA, BAL)
Comparative studies have evaluated the efficacy of this compound against other chelating agents like calcium disodium edetate (CaNa₂EDTA) and dimercaprol (BAL). This compound is an oral agent, offering an advantage over parenteral chelators like CaNa₂EDTA and BAL, which require injection. cncb.ac.cnmedscape.commhmedical.comaafp.orgessentialmeds.orgindianpediatrics.net
In two small, non-controlled clinical trials comparing this compound and CaNa₂EDTA in children with initial blood lead levels of 31 to 69 µg/dL, high-dose this compound reduced mean blood lead levels by 61% to 77% over five days, while CaNa₂EDTA treatment resulted in about a 45% decline. aafp.org A retrospective study comparing children treated with BAL and CaNa₂EDTA versus children treated with this compound and CaNa₂EDTA found a comparable reduction in post-treatment blood lead levels between the two groups. aafp.org
In a study involving adults with blood lead concentrations >40 µg/dL, both this compound and intravenous CaNa₂EDTA significantly reduced blood lead levels and the prevalence of clinical symptoms. nih.govresearchgate.net this compound had a greater impact on reducing blood lead concentrations (p = .005), while CaNa₂EDTA had a greater impact on lead mobilization (p = .04). nih.govresearchgate.net However, when comparing equimolar doses, CaNa₂EDTA was found to be more effective than this compound (p < .001). nih.govresearchgate.net
This compound is generally considered to have a more favorable adverse effect profile compared to other lead chelators and causes less urinary loss of essential minerals. essentialmeds.orgindianpediatrics.net
| Chelating Agent | Administration Route | Impact on Blood Lead Reduction (Comparative) | Impact on Lead Mobilization (Comparative) | Notes | Reference |
| This compound | Oral | Comparable to or greater than CaNa₂EDTA in some studies; less effective than equimolar CaNa₂EDTA | Less impact than CaNa₂EDTA in one study | Oral administration advantage; less loss of essential minerals | aafp.orgessentialmeds.orgindianpediatrics.netnih.govresearchgate.net |
| CaNa₂EDTA | Parenteral (IV/IM) | Comparable to or less than this compound in some studies; more effective than equimolar this compound | Greater impact than this compound in one study | Requires parenteral administration; risk of renal toxicity; may cause zinc depletion | oup.comaafp.orgnih.govresearchgate.net |
| BAL | Parenteral (IM) | Used in combination with CaNa₂EDTA for high BLLs | Chelates lead in the brain | Recommended for severe cases, often with CaNa₂EDTA | aafp.org |
Research on Therapeutic Regimen Optimization for Sustained Metal Reduction and Prevention of Lead Rebound
Research has explored different this compound therapeutic regimens to optimize sustained lead reduction and prevent the rebound in blood lead levels that can occur after treatment. cncb.ac.cnnih.govtandfonline.comfda.govresearchgate.net Initial clinical studies often involved a 5-day course of treatment. nih.govtandfonline.com Subsequently, longer regimens (19 to 26 days) were introduced with the aim of blunting the blood lead rebound. nih.govtandfonline.comresearchgate.net
Studies suggest that repeated courses of this compound, such as 30 mg/kg/day for at least 5 days, can be equally efficacious in enhancing urine lead excretion and reducing blood lead concentrations. nih.govtandfonline.com Including a treatment-free period of at least 1 week between courses may allow for the redistribution of lead from bone to soft tissues and blood. nih.govtandfonline.comresearchgate.net In cases of more severe poisoning, there is evidence that administering this compound for more than 5 days can be beneficial. nih.govtandfonline.com
Following therapy, monitoring for blood lead level rebound is recommended, typically by measuring levels at least once weekly until stable. nih.govfda.gov The severity of the initial intoxication and the rate and degree of rebound should guide the frequency of monitoring. fda.gov Studies have shown that after a 5-day course of 350 mg/m² (10 mg/kg) every 8 hours, mean lead levels rebounded and plateaued at 60-85% of pretreatment levels two weeks after therapy. fda.gov This rebound plateau was somewhat higher with lower this compound doses and with intravenous CaNa₂EDTA. fda.gov
| Regimen Type | Duration | Potential Benefit | Outcome regarding Rebound | Reference |
| Short course | 5 days | Effective initial reduction in blood lead and increased excretion | Significant rebound observed | nih.govtandfonline.com |
| Extended course | 19-26 days | Introduced to potentially blunt rebound | May help prevent or blunt rebound | nih.govtandfonline.comresearchgate.net |
| Repeated courses | Multiple 5+ day courses with breaks | Equally efficacious in lead reduction and excretion | Break allows for redistribution before subsequent chelation | nih.govtandfonline.comresearchgate.net |
This compound in Mercury Poisoning Research
Research has investigated the efficacy of this compound in addressing mercury poisoning, examining its impact on blood concentrations, organ mobilization, and its effectiveness against different mercury species.
Efficacy in Reducing Blood Mercury Concentrations
Studies have shown that this compound can lead to decreases in blood mercury concentrations. In clinical reports concerning ingestion and dermal exposure to inorganic mercury, both unithiol and this compound significantly increased urine mercury excretion and decreased blood mercury levels. tandfonline.comtandfonline.com In cases of elemental mercury and mercury vapor poisoning, thiol chelators like unithiol and this compound have demonstrated increases in urinary mercury and decreases in blood mercury. tandfonline.comtandfonline.com
A randomized clinical trial evaluating this compound in children with background mercury exposures, initially designed for lead-exposed children, measured mercury in blood samples before and after treatment. The study found that the adjusted mean organic mercury concentration in the this compound group relative to the placebo group decreased from 99% at baseline to 82% after three courses of treatment. nih.govnih.gov However, this reduction appeared to result from the prevention of an age-related increase in the this compound group rather than a direct reduction in mercury levels. nih.govnih.gov This suggests limited efficacy of this compound chelation for low-level organic mercury exposure in children. nih.govnih.gov
Mobilization of Mercury from Specific Organs (Kidney, Brain)
Research indicates that this compound's ability to mobilize mercury from organs varies depending on the timing of administration and the specific organ. In animal studies involving mercuric chloride injection, this compound decreased mercury content in the kidney and blood when therapy was initiated within a day of exposure. tandfonline.comtandfonline.com However, if there was a delay in chelator administration, the therapy was less effective, and brain mercury levels did not improve. tandfonline.comtandfonline.com This highlights a limitation of this compound, and unithiol, in tissue penetration. tandfonline.com
For elemental mercury and mercury vapor poisoning, thiol chelators like unithiol and this compound have shown reductions in kidney and total body mercury. tandfonline.comtandfonline.com However, neither this compound nor unithiol have demonstrated an effect on brain mercury concentrations after mercury vapor exposure. tandfonline.comtandfonline.com This inability to cross the blood-brain barrier appears to limit their effectiveness in directly removing mercury from the brain and other target tissues like the liver and muscle, particularly after chronic exposure where mercury has distributed into these organs. tandfonline.comtandfonline.com
Studies on Chelation of Inorganic vs. Organic Mercury Species
This compound has shown varying efficacy depending on the form of mercury involved. For inorganic mercury, this compound is considered to have clinical utility in treating human poisoning and is protective against the acute lethal and nephrotoxic effects of mercuric salts in animal models, increasing urinary mercury excretion in both animals and humans. mhmedical.com DMSA is used for both inorganic and organic mercurials. medscape.com
With organic mercuries, such as methylmercury and ethylmercury, thiol chelators including unithiol and this compound have demonstrated increases in urinary mercury and, in some instances, decreases in blood mercury. tandfonline.com Despite these changes in mercury concentration, clinical improvement in patients has been limited or absent in numerous reported cases, with some patients experiencing continued deterioration. tandfonline.com This limited clinical efficacy with organic mercury likely reflects the in vivo demethylation of organomercuries in the brain and the inability of this compound to cross the blood-brain barrier. tandfonline.com Ethylmercury, which has a shorter half-life and greater difficulty crossing the blood-brain barrier than methylmercury, has shown limited or no effect on neurotoxicity when treated with unithiol and this compound in humans. tandfonline.com
Comparative Studies with Other Mercury Chelators (e.g., Unithiol)
Comparative studies have evaluated this compound alongside other chelating agents for mercury. Unithiol (DMPS) and this compound (DMSA) are water-soluble analogs of BAL (dimercaprol) and are currently used as chelating agents in mercury poisoning. nih.govresearchgate.net DMPS is recommended as a first-line chelating agent for chronic and acute inorganic mercury cases in some regions and is considered similar to DMSA in efficacy and redistribution properties. cehn.org However, DMPS is considered more effective than this compound at removing mercury from kidney cells. cehn.org Neither DMPS nor this compound are effective at removing mercury from the brain. cehn.org
In animal studies involving mercuric chloride injection, unithiol decreased mercury content in all organs, whereas this compound only decreased kidney and blood concentrations when administered promptly. tandfonline.comtandfonline.com If administration was delayed, unithiol was also only effective in reducing blood and kidney mercury, similar to this compound, with no significant effect on other target organs. tandfonline.com
In a recent animal model of methylmercury exposure during pregnancy, this compound was effective in reducing maternal and fetal mercury burden, although unithiol appeared to be somewhat more potent in that setting. mhmedical.com While this compound has replaced penicillamine in some instances due to its strong metal-mobilizing capacity and lower side effects, DMPS is often considered the chelator of choice for chronic or mild mercury toxicity due to its ease of use, good efficacy, and safety profile. medscape.comnih.gov
This compound in Arsenic and Cadmium Poisoning Research
This compound's role in managing arsenic and cadmium poisoning has also been investigated.
Efficacy in Arsenic Detoxification
Animal experiments have established that prompt treatment with dithiol chelators, including DMSA (this compound), can prevent the detrimental effects of inorganic arsenic. researchgate.net These chelators enhance arsenic excretion. researchgate.net Controlled animal experiments support a therapeutic role for this compound in the prompt treatment of acute poisoning by inorganic arsenic salts. researchgate.net Its efficacy declines or disappears if treatment is delayed. researchgate.net
Clinical studies have also examined this compound's effect on arsenic excretion. In a comparative evaluation of this compound and CaNa2 EDTA in workers exposed to lead, arsenic, and cadmium, this compound demonstrated a much better chelating effect on arsenic compared to CaNa2 EDTA, as measured by arsenic excretion in urine. researchgate.netlongdom.org
Data from this study comparing the effect of this compound and CaNa2 EDTA on arsenic excretion in urine is presented below:
| Treatment Group | Arsenic in Urine Before Treatment (µmol/l) | Arsenic in Urine After Treatment (µmol/l) |
| This compound | [Data from source 9 or 18 if available] | [Data from source 9 or 18 if available] |
| CaNa2 EDTA | [Data from source 9 or 18 if available] | [Data from source 9 or 18 if available] |
Note: Specific numerical data for arsenic concentrations before and after treatment with this compound and CaNa2 EDTA was indicated as being in Figure 3 of source researchgate.net and source longdom.org, but the exact values were not extracted in the text snippets. The table structure is provided to represent where such data would be placed if available.
This compound is protective against the acute lethal effects of arsenic in animal models and may have potential utility in acute human arsenic poisoning. mhmedical.com Studies suggest that monoesters of DMSA may be preferred over DMSA diesters due to their higher efficacy against arsenic intoxication. nih.gov
Regarding cadmium poisoning, studies suggest that this compound may not be the drug of choice. ijpsonline.com While the structure of this compound chelates of lead and cadmium are similar in vitro, a randomized trial using blood samples from a study designed for treating lead poisoning found that this compound did not lower blood cadmium in children with background exposure. nih.govresearchgate.net One potential reason for this lack of efficacy is that this compound is mainly distributed in the extracellular space, while cadmium is mostly bound intracellularly to metallothionein. nih.gov
Cadmium Mobilization Studies
Research has explored the efficacy of this compound and its derivatives in mobilizing cadmium from the body. Studies in mice with aged in vivo cadmium deposits compared the effectiveness of two newly synthesized esters of meso-2,3-dimercaptosuccinic acid, di(2'-methoxyethyl) meso-2,3-dimercaptosuccinate (MEDMS) and di(2'-ethoxyethyl) meso-2,3-dimercaptosuccinate (EEDMS), with 2,3-dimercaptopropan-1-ol (BAL) nih.gov. Both MEDMS and EEDMS demonstrated superiority over BAL in reducing the whole body burden of cadmium. EEDMS resulted in approximately a 26% reduction, while MEDMS achieved about a 20% reduction, compared to BAL's approximately 12% reduction under similar dosage regimens nih.gov.
Further studies on monoalkyl esters of meso-2,3-dimercaptosuccinic acid in mice examined their ability to mobilize cadmium one week after administration of cadmium chloride nih.gov. The relative whole body cadmium mobilization increased with the number of carbon atoms in the alkyl group of the monoester up to C5, after which it decreased nih.gov. The monoisoamyl ester was identified as the most effective in removing cadmium from both the liver and kidneys nih.gov. This monoester also demonstrated effectiveness in mobilizing cadmium from aged deposits in the liver and kidneys when administered orally, marking it as the first reported compound capable of achieving this nih.gov.
Research on Neurodevelopmental and Cognitive Outcomes in Chelation Therapy
Research has investigated the impact of this compound chelation therapy on neurodevelopmental and cognitive outcomes, particularly in the context of lead exposure.
Impact on Lead-Induced Cognitive Deficits in Animal Models
Animal models, such as studies in rats, have been instrumental in evaluating the potential of this compound to alleviate lead-induced cognitive deficits nih.govnih.govescholarship.orgnih.govresearchgate.net. Early lead exposure in rats has been shown to produce lasting impairments in learning, attention, inhibitory control, and arousal regulation, mirroring the dysfunctions observed in lead-exposed children nih.govnih.gov.
A study using a rodent model of early childhood lead exposure found that this compound treatment of lead-exposed rats significantly improved learning, attention, and arousal regulation nih.govnih.gov. The efficacy of the treatment varied depending on the level of lead exposure and the specific functional deficit nih.govnih.gov. This research suggested that it might be possible to identify a this compound treatment protocol that could improve cognitive outcomes in lead-exposed children nih.govnih.gov.
Another study in rodents demonstrated that this compound chelation therapy could alleviate certain types of lead-induced behavioral and cognitive dysfunction nih.gov. This suggested that if a this compound treatment regimen could achieve a substantial reduction in brain lead levels in humans, a functional benefit might be derived nih.gov. Studies in rodents indicated that this compound treatment significantly reduced brain lead levels, with two courses of treatment being more effective than one nih.gov. However, reductions in blood lead levels were found to be a relatively poor predictor of reductions in brain lead levels in both primate and rodent studies nih.gov.
Studies on Learning, Attention, and Arousal Regulation following Chelation
Studies in lead-exposed rats have specifically examined the effects of this compound chelation on learning, attention, and arousal regulation nih.govnih.govresearchgate.net. This compound treatment was effective in normalizing the heightened reactivity to errors and reward omission observed in lead-exposed animals researchgate.net. This provided evidence that this compound chelation can significantly lessen the lasting neurobehavioral dysfunction caused by early lead exposure researchgate.net.
In lead-exposed rats, this compound treatment improved performance in a selective attention task, particularly by normalizing the disruptive effect of prior errors researchgate.netresearchgate.net. The this compound-only group (rats not exposed to lead but treated with this compound) showed impaired performance in the selective attention task compared to controls researchgate.net.
Findings on Cognitive Benefit at Lower Lead Exposure Levels in Clinical Trials
Clinical trials have investigated the cognitive benefits of this compound in children with lower lead exposure levels. The Treatment of Lead-Exposed Children (TLC) trial was a randomized, double-blind, placebo-controlled study that evaluated this compound in children with blood lead levels between 20 and 44 µg/dL aap.orgresearchgate.netresearchgate.netresearchgate.net. Despite this compound effectively lowering average blood lead levels for approximately six months, the trial found no significant benefit in cognitive, behavioral, and neuromotor endpoints at age 7 years aap.orgresearchgate.net. At 36 months of follow-up, children given this compound had a mean IQ score that was 1 point lower than the placebo group, and their behavior was rated as slightly worse by a parent, although these differences were not statistically significant researchgate.netnih.gov. Scores on the Developmental Neuropsychological Assessment were slightly better in the this compound group, but again, the differences were small and not statistically significant researchgate.netnih.gov.
The TLC trial concluded that treatment with this compound lowered blood lead levels but did not improve scores on tests of cognition, behavior, or neuropsychological function in children with blood lead levels below 45 µg/dL researchgate.netnih.gov. Given that this compound is considered as effective as other available lead chelators, these findings suggested that chelation therapy might not be indicated for children with these blood lead levels researchgate.netnih.gov.
Toxicological Research and Safety Profile of Succimer
Organ-Specific Toxicological Investigations
Studies have investigated the potential for succimer to induce toxicity in specific organs, including the liver, kidneys, and the hematological system.
Hepatic Effects and Aminotransferase Elevations
Clinical trials and studies have reported transient elevations in serum aminotransferase levels, specifically alanine aminotransferase (ALT) and aspartate aminotransferase (AST), during this compound therapy. nih.govoup.comrxlist.comresearchgate.netfda.gov In clinical trials involving children with lead poisoning, serum aminotransferase elevations occurred in a percentage of this compound-treated subjects, although levels significantly exceeding the upper limit of normal were rare. nih.gov For instance, in one study, ALT levels above 5 times the upper limit of normal were observed in less than 1% of treated children, and no child discontinued therapy due to liver test abnormalities. nih.gov Another study in adults reported asymptomatic ALT elevations (less than 3 times the upper limit of normal) in a percentage of patients treated with this compound, which resolved upon cessation of therapy. nih.gov While transient increases in transaminase activity have been noted in a percentage of patients, clinically significant liver injury with jaundice has not been commonly linked to this compound use. nih.govresearchgate.net Monitoring of serum AST and ALT is recommended at baseline and periodically during treatment, with dose reduction or temporary cessation advised if levels exceed 5 times the upper limit of normal. nih.govrxlist.comfda.gov
Renal Effects and Tubular Pathologies
This compound and its metabolites are primarily eliminated by the kidneys. fda.govdrugbank.commhmedical.com Research, particularly in animal models, has explored the potential for this compound to affect renal function and structure. In a 28-day toxicity study, dogs receiving higher doses of this compound showed lower urinary specific gravity and an increase in renal tubular regenerative hyperplasia. fda.gov A chronic 6-month oral toxicity study in dogs also reported treatment-related renal tubule epithelial changes at higher doses, which correlated with increased kidney weights. fda.gov Nephropathy was not observed at lower doses in these studies. fda.gov
Studies using technetium-99m-dimercaptosuccinic acid ((99m)Tc-DMSA), a radiolabeled form of this compound used for renal imaging, provide insights into its interaction with renal tubules. drugbank.comnih.govopenmedscience.com These studies suggest that (99m)Tc-DMSA is filtered in the glomeruli and subsequently undergoes endocytosis in the proximal tubules, a process that can be affected in patients with tubular disorders. nih.gov Decreased renal uptake of (99m)Tc-DMSA has been observed in patients with proximal tubular disorders, even with normal creatinine clearance, suggesting that its uptake is sensitive to changes in renal tubular function. nih.govnih.gov While these studies primarily relate to the diagnostic use of radiolabeled this compound, they highlight the interaction of this compound with renal tubular cells.
Hematological Effects (e.g., Thrombocytopenia, Neutropenia)
This compound has been associated with certain hematological effects, including thrombocytopenia (low platelet count) and neutropenia (low neutrophil count). rxlist.comwikipedia.orguspharmacist.compatsnap.comhealthline.com Reduced platelet counts were noted in some dogs in chronic toxicity studies, although group means were not always statistically different from controls. fda.gov The mechanism in dogs was suggested to potentially be autoimmune-mediated, a finding common in dogs but not typically seen in other species like rats, which did not develop thrombocytopenia in chronic dosing studies. fda.gov
In clinical use, neutropenia is considered a less common but potentially serious side effect of this compound. patsnap.com It is characterized by a reduced number of neutrophils, which are crucial for fighting infections. patsnap.comhealthline.com Monitoring of complete blood counts is recommended during this compound treatment. rxlist.com Treatment interruption may be necessary if the absolute neutrophil count falls below a certain threshold. rxlist.com Thrombocytopenia has also been reported as an adverse effect in clinical settings. uspharmacist.comphebra.com
Impact on Endogenous Essential Mineral Homeostasis
This compound's chelating properties extend beyond heavy metals like lead, and research has examined its impact on the excretion dynamics of essential endogenous minerals such as zinc and copper.
Research on Zinc Excretion Dynamics
Studies consistently indicate that this compound treatment leads to an increase in urinary zinc excretion. mhmedical.comoup.comrxlist.comoup.comfda.govnih.gov In dose-ranging studies in adults, zinc excretion doubled during this compound treatment. rxlist.comfda.gov Compared to other chelating agents like CaNa2EDTA, this compound's effect on zinc excretion is generally considered smaller. fda.govfda.gov CaNa2EDTA can induce a significantly greater increase in urinary zinc excretion. fda.govfda.gov While some studies in primates and humans have shown statistically significant increases in urinary zinc excretion with this compound, others have reported trending but non-significant increases or have noted that the effect is not clinically significant. oup.comresearchgate.netoup.comnih.gov One study in heavy metal-poisoned subjects found that only plasma zinc decreased significantly among several essential elements monitored. oup.com
Research on Copper Excretion Dynamics
The effect of this compound on copper excretion has also been investigated, with varying findings across studies. Some research indicates that this compound treatment can cause an increase in urinary copper excretion. oup.comoup.com A study in adult patients with lead poisoning observed a mean increase of 2.4-fold in urine copper excretion compared to baseline values during this compound chelation. oup.com Similarly, a study in primates showed that a lower this compound dosing regimen led to statistically significant increases in urinary copper excretion. oup.com However, other studies have reported trending but non-significant increases in copper excretion or have concluded that the increase is not to a clinically important extent. researchgate.netoup.com Some comparative studies with CaNa2EDTA highlight that while both chelators can increase copper excretion, the effect of this compound is generally less pronounced. fda.govfda.gov
| Organ System | Effect | Research Findings |
| Hepatic | Aminotransferase Elevations | Transient elevations (ALT/AST) reported; rarely above 5x ULN; generally resolve upon cessation. nih.govoup.comrxlist.comresearchgate.netfda.gov |
| Renal | Tubular Pathologies | Renal tubular regenerative hyperplasia and epithelial changes observed in animal studies at higher doses. fda.gov |
| Hematological | Thrombocytopenia, Neutropenia | Reduced platelet counts in animal studies; reversible neutropenia reported in clinical use. fda.govrxlist.comuspharmacist.compatsnap.com |
| Essential Mineral | Excretion Dynamics with this compound | Comparative Notes (vs. CaNa2EDTA) |
| Zinc | Increased urinary excretion; zinc excretion doubled in some studies. oup.comrxlist.comoup.comfda.govnih.gov | Generally smaller effect on zinc excretion compared to CaNa2EDTA. fda.govfda.gov |
| Copper | Increased urinary excretion observed in some studies. oup.comoup.com | Generally less pronounced effect on copper excretion compared to CaNa2EDTA. fda.govfda.gov |
Studies on Minimal Effects on Iron, Calcium, and Magnesium Excretion
Studies have investigated the impact of this compound on the urinary excretion of essential minerals such as iron, calcium, and magnesium. Research indicates that this compound has no significant effect on the urinary elimination of iron, calcium, or magnesium. rxlist.comfda.govfda.gov While this compound effectively increases the urinary excretion of lead, its effect on endogenous minerals like calcium, iron, and magnesium is considered insignificant compared to other chelating agents such as CaNa2EDTA. mhmedical.comfda.govwikidoc.orgmedscape.com Some studies in healthy adult volunteers and primate models have reported minor or non-significant increases in the urinary excretion of certain essential elements, including trending but non-significant increases in urinary calcium, copper, and iron in one study, and marginally non-significant increases in zinc excretion in another. mhmedical.comoup.comresearchgate.net Multivariate analyses in a primate model indicated a significant increase in the cumulative total excretion of essential elements over the first five days of treatment when considered collectively, although not for any single element individually in all cases. researchgate.net
Preclinical Safety Assessments and Animal Model Toxicology
Preclinical safety assessments of this compound have been conducted using various animal models to evaluate its toxicity profile. These studies provide valuable insights into potential adverse effects before clinical use. biomimx.comselvita.com
Acute Toxicity Studies
Acute toxicity studies typically involve administering a single high dose of a substance to animals to determine immediate adverse effects and the dose at which lethality occurs. fda.gov this compound has demonstrated low acute oral toxicity in preclinical studies. Oral median lethal doses (LD50) in rodents have been reported in excess of 3.6 g/kg and over 5011 mg/kg in mice. drugbank.comwikidoc.orgfda.gov Doses of 2300 mg/kg in rats and 2400 mg/kg in mice produced signs of toxicity including ataxia, convulsions, and labored respiration, and frequently resulted in death. drugbank.comfda.gov
Chronic Toxicity Studies in Animal Models
Chronic toxicity studies assess the effects of prolonged and repeated exposure to a substance over an extended period, often exceeding 90 days or longer in animal models. criver.comoecd.orgnih.govoecd.org In a 28-day toxicity study in dogs, those receiving 30 and 100 mg/kg/day of this compound showed lower urinary specific gravity and an increase in renal tubular regenerative hyperplasia. wikidoc.orgfda.gov However, no renal toxicity was observed in dogs given 50 mg/kg/day. wikidoc.orgfda.gov Long-term animal studies evaluating the carcinogenic potential of this compound have not been conducted. fda.gov
Developmental and Reproductive Toxicology Research
Developmental and reproductive toxicology (DART) research investigates the potential adverse effects of a substance on fertility, embryonic development, fetal development, and postnatal development. frontiersin.orgpremierconsulting.comcriver.comdrugdiscoverynews.com Studies in pregnant mice have shown this compound to be teratogenic and fetotoxic when administered subcutaneously during organogenesis at doses ranging from 410 to 1640 mg/kg/day. fda.gov In a developmental study in rats, maternal toxicity and deaths occurred at doses of 720 mg/kg/day or more during organogenesis, with 510 mg/kg/day being the highest tolerable dose in pregnant rats. rxlist.comfda.govfda.gov Impaired development of reflexes was noted in the pups of dams receiving 720 mg/kg/day. rxlist.comfda.govfda.gov this compound did not show adverse effects on fertility and reproductive performance in rats at doses up to 510 mg/kg/day in males and 100 mg/kg/day in females. fda.govfda.gov Studies in non-lead-exposed rodents have also indicated that this compound treatment can produce lasting adverse neurobehavioral effects. nih.gov
Immunological and Hypersensitivity Reactions Research
Research has explored the potential for this compound to cause immunological and hypersensitivity reactions. Clinical experience with this compound is limited, and the full spectrum and incidence of these reactions are not completely determined. fda.gov Hypersensitivity reactions and dermatologic reactions, including rash, have been associated with this compound treatment. rxlist.com Rashes have been reported in approximately 4% of patients, with some cases necessitating discontinuation of therapy. rxlist.comfda.gov Allergic reactions, such as urticaria and angioedema, have also been reported, particularly upon repeated administration. fda.gov
Research on Gastrointestinal Disturbances as Adverse Effects
Gastrointestinal disturbances are among the most commonly reported adverse effects associated with this compound treatment. mhmedical.compatsnap.com These can include symptoms such as anorexia, nausea, vomiting, and diarrhea. mhmedical.com In clinical experience, gastrointestinal symptoms have been observed in about 10% of patients. fda.gov These effects are generally mild but can be bothersome. patsnap.com They may occur due to irritation of the stomach and intestinal lining. patsnap.com
Data Table: Summary of Selected Preclinical Toxicity Findings
| Study Type | Animal Model | Dose Range (mg/kg/day) | Key Findings | Source |
| Acute Oral Toxicity (LD50) | Rodents | >3600 | Low acute oral toxicity. wikidoc.orgfda.gov | wikidoc.orgfda.gov |
| Acute Oral Toxicity (Lethal Doses) | Rat | 2300 | Ataxia, convulsions, labored respiration, death. drugbank.comfda.gov | drugbank.comfda.gov |
| Acute Oral Toxicity (Lethal Doses) | Mouse | 2400, >5011 | Ataxia, convulsions, labored respiration, death. drugbank.comfda.gov Oral LD50 >5011 mg/kg. drugbank.com | drugbank.comfda.gov |
| 28-day Toxicity | Dog | 30, 100 | Lower urinary specific gravity, increased renal tubular regenerative hyperplasia. wikidoc.orgfda.gov | wikidoc.orgfda.gov |
| 28-day Toxicity | Dog | 50 | No renal toxicity noted. wikidoc.orgfda.gov | wikidoc.orgfda.gov |
| Developmental Toxicity (Organogenesis) | Pregnant Mice | 410-1640 | Teratogenic and fetotoxic effects. fda.gov | fda.gov |
| Developmental Toxicity (Organogenesis) | Pregnant Rats | 720+ | Maternal toxicity and deaths. rxlist.comfda.govfda.gov | rxlist.comfda.govfda.gov |
| Developmental Toxicity (Organogenesis) | Pregnant Rats | 510 | Highest tolerable dose; impaired reflex development in pups at higher dose (720 mg/kg/day). rxlist.comfda.govfda.gov | rxlist.comfda.govfda.gov |
| Fertility and Reproductive Performance | Rats (Male) | Up to 510 | No adverse effects. fda.govfda.gov | fda.govfda.gov |
| Fertility and Reproductive Performance | Rats (Female) | Up to 100 | No adverse effects. fda.govfda.gov | fda.govfda.gov |
Data Table: Incidence of Selected Adverse Effects in Clinical Experience
| Adverse Effect | Incidence (%) | Source |
| Gastrointestinal Symptoms | ~10 | fda.gov |
| Rashes | ~4 | rxlist.comfda.gov |
| Elevated Serum Transaminases | ~10 | fda.gov |
Advanced Research Methodologies and Translational Challenges in Succimer Studies
In Vitro and Cellular Mechanism Studies for Succimer Action
In vitro and cellular studies are fundamental to elucidating the precise mechanisms by which this compound exerts its chelating effects. This compound is a water-soluble compound characterized by two sulfhydryl groups (-SH) that are crucial for its activity. patsnap.com These sulfhydryl groups exhibit a high affinity for binding to "soft" heavy metal ions, including lead (Pb²⁺), mercury (Hg²⁺), and arsenic (As³⁺). patsnap.comwikipedia.org The mechanism involves the formation of strong, stable, water-soluble complexes, or chelates, with these metal ions. patsnap.comdrugbank.com This chelation process sequesters the metal ions, thereby reducing their biological activity and toxicity. patsnap.com The resulting this compound-metal complexes are more water-soluble than the free metals, which facilitates their excretion from the body, primarily through the kidneys. patsnap.com
In vitro studies have also been instrumental in identifying potential interactions and effects of this compound on laboratory tests. For instance, in vitro studies have shown that this compound can cause false positive results for ketones in urine when using nitroprusside reagents. wikidoc.orgrxlist.comfda.gov Additionally, it has been observed to lead to falsely decreased measurements of serum uric acid and creatinine phosphokinase (CPK) in in vitro settings. wikidoc.orgrxlist.comfda.gov
Animal Models in this compound Research
Animal models play a critical role in the preclinical assessment of this compound's efficacy and toxicological profile before translation to human studies. Various animal species, particularly rodents and non-human primates, are utilized to investigate different aspects of this compound's pharmacology and toxicology.
Utilization of Rodent Models (Rats, Mice) for Efficacy and Toxicological Assessments
Rodent models, such as rats and mice, are widely used in this compound research for both efficacy and toxicological assessments. Studies in rodents have investigated this compound's ability to reduce lead levels in blood and tissues, as well as its potential effects on neurobehavioral outcomes following heavy metal exposure.
Research in lead-exposed rats has demonstrated that this compound treatment can be effective in normalizing certain behavioral deficits. For example, a study in rats exposed to lead during their early life showed that a course of this compound treatment was effective in normalizing heightened reactivity to errors and reward omission. researchgate.netnih.gov This suggests that this compound chelation can potentially mitigate lasting neurobehavioral dysfunction induced by early lead exposure. researchgate.netnih.govnih.gov However, these studies also highlight potential risks, as this compound treatment in rats not previously exposed to lead produced lasting cognitive and affective dysfunction. nih.gov
Studies in rodents have also explored the efficacy of this compound in reducing brain lead levels. While some studies in primates showed no significant reduction in brain lead concentrations after a 19-day course, a study in rodents exposed to lead demonstrated that a three-week course of this compound significantly reduced both blood and brain lead concentrations compared to a vehicle. researchgate.netnih.gov Two courses of this compound were found to be significantly more efficacious at reducing brain lead levels than one in this rodent model. nih.gov However, reductions in blood lead levels were not always a strong predictor of reductions in brain lead levels in these rodent studies. nih.gov
Toxicological assessments in rodents have provided information on the potential adverse effects of this compound. This compound has been reported to have low acute oral toxicity in rodents, with median lethal doses in excess of 3.6 g/kg. wikidoc.orgrecordatirarediseases.com However, higher doses (e.g., 2300 mg/kg in rats and 2400 mg/kg in mice) produced signs of toxicity including ataxia, convulsions, and labored respiration, and frequently resulted in death. drugbank.comfda.govrecordatirarediseases.com Developmental toxicity studies in rats have shown that this compound can cause maternal toxicity and deaths at certain doses during organogenesis, and impaired reflex development was noted in the pups of dams receiving higher doses. recordatirarediseases.comfda.gov Studies in pregnant mice have also indicated that this compound can be teratogenic, potentially mediated through disturbed maternal/fetal copper metabolism. nih.gov
Studies using rodent models of retained lead fragments have investigated whether this compound chelation can mobilize lead from these sources. In one rodent model, this compound chelation on day 1 led to significant blood lead reductions, and fragment lead was not mobilized into the blood initially. escholarship.org However, with continued chelation and over a period post-chelation, blood lead levels rebounded with mobilization of lead from the fragments, suggesting the need for long-term monitoring in cases of retained fragments. escholarship.org
Application of Non-Human Primate Models for Absorption and Retention Studies
Non-human primate models are valuable for studying the absorption and retention of this compound and chelated metals, providing data that is often more directly translatable to humans than rodent data. Studies in juvenile non-human primates have been conducted to investigate the effects of oral this compound chelation on the gastrointestinal absorption and whole-body retention of lead. researchgate.netresearchgate.netnih.gov
Contrary to concerns that this compound might increase gastrointestinal lead absorption, a study in a juvenile non-human primate model of moderate childhood lead intoxication found that oral this compound significantly reduced the gastrointestinal absorption of lead. researchgate.netnih.gov This study utilized a stable lead isotopic tracer methodology to track lead absorption and retention. researchgate.netresearchgate.netnih.gov this compound treatment in these primates also significantly increased the urinary excretion of endogenous lead. researchgate.netnih.gov While this compound reduced the whole-body retention of endogenous lead by approximately 10%, a significant portion (77%) of the administered internal dose of the lead tracer was still retained in the body after 5 days of treatment. researchgate.netnih.gov
Studies in adult primates exposed to lead have also assessed the efficacy of this compound in reducing brain lead levels. A single 19-day course of this compound in these models did not measurably reduce brain lead levels compared to control groups. researchgate.netnih.gov This contrasts with some findings in rodent models, highlighting potential species differences in the distribution and removal of chelated lead from the brain. researchgate.netnih.gov
Clinical Trial Design and Methodological Considerations
Translating findings from preclinical studies to human application requires rigorous clinical trial designs. Randomized controlled trials (RCTs) and placebo-controlled designs are considered the gold standard for evaluating the efficacy and safety of interventions like this compound.
Design and Outcomes of Randomized Controlled Trials and Placebo-Controlled Designs
Randomized controlled trials are crucial for minimizing bias and providing robust evidence of treatment effects. paho.org In placebo-controlled trials, participants are randomly assigned to receive either the active treatment (this compound) or a placebo that is identical in appearance but lacks the active compound. paho.org Blinding, where participants and researchers are unaware of the treatment assignment, further helps to reduce bias. paho.org
A notable example of a large-scale randomized, placebo-controlled, double-blind clinical trial of this compound is the Treatment of Lead-Exposed Children (TLC) trial. nih.govnih.govresearchgate.netaap.org This multicenter trial enrolled children with blood lead levels between 20 and 44 micrograms/dL to assess the effects of this compound treatment on cognitive, behavioral, and physical development. nih.govnih.govresearchgate.net Children were randomized to receive up to three courses of this compound or placebo and were followed for several years. nih.govnih.govresearchgate.net
Outcomes from the TLC trial provided important insights into the effectiveness of this compound in children with moderately elevated blood lead levels. While this compound treatment effectively lowered mean blood lead levels during the initial phase of the trial compared to placebo, this reduction did not translate into significant improvements in scores on standardized tests of cognition, behavior, or neuropsychological function at the 36-month follow-up. nih.govresearchgate.netaap.org The mean IQ score in the this compound group was slightly lower than in the placebo group at 36 months, and parental ratings indicated slightly worse behavior in the this compound group, although these differences were not statistically significant. nih.govresearchgate.net Some beneficial neuromotor effects, such as improved postural balance and functional locomotion, were observed in a subset of children from the TLC trial. nih.gov
Other controlled studies have also investigated this compound's efficacy in different populations and for different heavy metals. While this compound has been associated with increased urinary excretion of mercury and arsenic in limited patient numbers, controlled clinical studies specifically for mercury or arsenic poisoning are limited. wikidoc.orgrecordatirarediseases.com A randomized trial examining this compound chelation for low-level organic mercury exposure in children found limited efficacy in reducing blood mercury concentration. nih.gov
Biomarker Identification and Validation for Treatment Efficacy and Safety
Biomarkers play a vital role in drug development and clinical trials, serving as indicators of treatment efficacy and safety. bio-rad.comwisecube.ai Biomarkers can be used to select patients for trials, monitor treatment response, and predict outcomes. bio-rad.comwisecube.ai The identification and validation of reliable biomarkers are crucial for the efficient and accurate assessment of this compound's effects.
In the context of this compound treatment for heavy metal poisoning, blood lead levels are a primary and widely used biomarker for assessing exposure and monitoring the effectiveness of chelation in reducing the body burden of lead. nih.govnih.govresearchgate.netaap.orgmhmedical.com The ability of this compound to increase the urinary excretion of chelated metals is another key indicator of its activity, and urinary metal levels are often measured in research studies. patsnap.comdrugbank.comwikidoc.orgfda.govresearchgate.netnih.govoup.commhmedical.com
Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling and Simulation in Chelation Therapy
Pharmacokinetic/pharmacodynamic (PK/PD) modeling and simulation play a crucial role in understanding the behavior of this compound within the body and predicting its effects in chelation therapy. Kinetic models can potentially assist clinicians in managing cases of lead poisoning uu.nlnih.govresearchgate.net. While several models exist to simulate lead kinetics, the ability to predict the effect of chelation therapy on lead levels has been a subject of research uu.nlnih.govresearchgate.net.
One approach involves integrating a kinetic this compound model into existing physiologically based pharmacokinetic (PBPK) lead models to create a Chelation Lead Therapy (CLT) model uu.nlnih.govtandfonline.com. Such models aim to predict blood lead concentrations during and after this compound treatment uu.nlnih.govtandfonline.com. A this compound pharmacokinetic model has been developed, comprising a gut and a systemic compartment, and validated using data from healthy volunteers uu.nl. This model does not distinguish between the parent this compound compound and its metabolites in terms of lead chelation uu.nl.
Studies have shown that a CLT model integrating a this compound kinetic model into a PBPK lead model can accurately simulate blood lead concentrations in patients poisoned by lead, particularly when the duration of exposure is known uu.nlnih.govtandfonline.com. The model's predictions for urinary lead excretion have shown variability, being successfully predicted in some patients but poorly in others uu.nlnih.govtandfonline.com.
Further research is needed to assess the applicability of these models in clinical practice tandfonline.com. Extending CLT models to include kinetic models for other lead-chelating agents could help determine the effects of different chelators on lead distribution throughout the body tandfonline.com.
Challenges in Translational Research and Clinical Implementation
Translational research, aiming to bridge findings from preclinical studies to clinical applications, faces significant challenges in the context of this compound and chelation therapy for lead poisoning. The "Lost in Translation" problem, where preclinical research fails to replicate in human clinical trials, is widely recognized researchgate.netosf.io. This can stem from various factors, including inherent differences between species, methodological disparities, and limitations in study design researchgate.netosf.ionih.gov.
Discrepancies between Animal and Human Study Outcomes
Significant discrepancies have been observed between outcomes in animal studies and human clinical trials involving this compound. While animal models, particularly in rodents and non-human primates, are valuable for assessing the efficacy and safety of potential therapies, translating these findings directly to humans can be challenging due to fundamental differences in genetic background, developmental span, and organ structure and function researchgate.netosf.ionih.gov.
Studies in animal models have investigated the extent to which this compound reduces body lead, particularly in the brain, and whether reductions in blood lead accurately reflect changes in brain lead nih.govnih.gov. Results from juvenile primate studies indicated that this compound treatment accelerated lead elimination, but was only marginally more effective than stopping lead exposure alone nih.gov. Studies in lead-exposed adult primates showed that a single course of this compound did not measurably reduce brain lead levels compared to controls nih.govnih.gov. In contrast, rodent studies with one or two courses of this compound showed significant reductions in brain lead levels, with two courses being more effective than one nih.govnih.gov. These studies also highlighted that reductions in blood lead levels were relatively poor predictors of reductions in brain lead levels nih.govnih.gov.
Furthermore, some animal studies have suggested that this compound chelation therapy can alleviate certain lead-induced behavioral and cognitive dysfunctions nih.govnih.govresearchgate.netnih.govresearchgate.net. For instance, studies in lead-exposed rats demonstrated improvements in learning, attention, and arousal regulation following this compound treatment nih.govnih.govresearchgate.net. However, these findings contrast with outcomes from comprehensive human clinical trials, such as the Treatment of Lead-Exposed Children (TLC) trial, which did not find significant neurodevelopmental benefits in children with moderate blood lead levels (20-44 µg/dL) despite this compound effectively lowering blood lead levels for a period aap.orgresearchgate.net. The failure to demonstrate benefits in the human trial might be attributed to the relatively small and transient reduction in blood lead levels achieved with the this compound regimen used, and consequently, an even smaller reduction in brain lead aap.orgnih.gov.
An unexpected finding in some animal studies is that this compound treatment in the absence of lead exposure produced lasting adverse neurobehavioral effects, including cognitive dysfunction nih.govnih.govnih.govnih.govresearchgate.netnih.gov. This highlights potential risks associated with administering this compound to individuals without elevated tissue lead levels nih.govnih.govnih.govnih.govresearchgate.netnih.gov.
Future Directions and Emerging Research Avenues for Succimer Dmsa
Exploration of Novel Applications Beyond Traditional Heavy Metal Chelation
Beyond its well-established use in treating poisoning by lead, mercury, and arsenic, the unique properties of succimer are being investigated for other applications. wikipedia.orgdrugbank.com
Radiopharmaceutical Applications (e.g., Technetium-99m this compound for Renal Imaging)
One significant area of expanded application is in nuclear medicine, specifically as a radiopharmaceutical agent. Technetium-99m (⁹⁹mTc) this compound (⁹⁹mTc-DMSA) is a widely used diagnostic tool for renal imaging. openmedscience.comdrugbank.com When labeled with the radioisotope technetium-99m, this compound exhibits specific uptake and retention in the renal cortex, allowing for scintigraphic evaluation of renal parenchymal disorders, assessment of differential renal function, and detection of conditions like acute pyelonephritis. openmedscience.comdrugbank.comappliedradiology.com This application is particularly valuable in pediatric patients for evaluating congenital anomalies and the impact of urinary tract infections on kidney function. openmedscience.comappliedradiology.com The binding of ⁹⁹mTc-succimer to renal tubular cells is believed to involve its affinity for sulfhydryl groups. openmedscience.com The use of ⁹⁹mTc-succimer provides detailed anatomical images and functional assessment of the kidneys with minimal invasiveness and low radiation exposure due to the short half-life of ⁹⁹mTc. openmedscience.comnih.gov
Potential in Other Toxicological Exposures
Research is also exploring the potential of this compound in mitigating the effects of other toxicological exposures beyond the classic heavy metals. While the primary focus remains on lead, mercury, and arsenic, its chelating properties and influence on oxidative stress pathways suggest potential in other scenarios where metal involvement or oxidative damage is a factor. drugbank.comcellmolbiol.org Studies have investigated its effects in animal models of toxicity from other metals and metalloids, sometimes in combination with other agents. nih.govresearchgate.net
Development of Modified this compound Analogs and Advanced Delivery Systems
Efforts are underway to develop modified this compound analogs and advanced delivery systems to potentially enhance its efficacy, reduce side effects, or improve targeting. The goal is to overcome some of the limitations of the parent compound, such as its ability to access intracellular metal stores or potential gastrointestinal effects. taylorandfrancis.com Research into advanced delivery systems, such as incorporating this compound into nanoparticles, aims to achieve targeted delivery and potentially sustained release, which could reduce the required dose and minimize systemic toxicity. taylorandfrancis.comoup.comresearchgate.net Studies on nanoparticle-based delivery systems incorporating DMSA have shown promise in directing compounds to specific organs like the lungs. oup.com
Research into Combination Therapies with Antioxidants and Other Therapeutic Agents
Given that heavy metal toxicity often involves oxidative stress, research is actively exploring the benefits of combining this compound chelation with antioxidants and other therapeutic agents. cellmolbiol.org The rationale is that a multi-modal approach addressing both metal burden and the resulting oxidative damage could lead to improved outcomes, particularly in mitigating neurotoxicity and restoring biochemical functions. nih.govresearchgate.net Studies in animal models have investigated the co-administration of this compound with antioxidants like vitamin C, vitamin E, melatonin, or N-acetylcysteine, demonstrating potential for enhanced depletion of metals from certain tissues and better restoration of antioxidant defense systems. nih.govresearchgate.net This approach aims to provide a more comprehensive therapeutic strategy against the multifaceted pathology of heavy metal poisoning. researchgate.net
Addressing Research Gaps in Long-Term Outcomes, Neuroprotection, and Optimal Intervention Strategies
Despite its established use, several research gaps remain regarding this compound. There is a continued need for research into the long-term outcomes of chelation therapy, particularly concerning potential neuroprotective effects and the reversal of cognitive or developmental deficits caused by early heavy metal exposure. chemicalbook.comnih.govresearchgate.net While some animal studies suggest a potential for this compound to lessen neurobehavioral dysfunction, large-scale human trials have yielded less conclusive results regarding long-term cognitive benefits. nih.govresearchgate.net Further research is needed to determine the optimal intervention strategies, including the timing, duration, and frequency of this compound administration, especially in cases of chronic low-level exposure or in vulnerable populations. researchgate.netnih.gov Understanding the optimal chelation-free period between treatment courses is also an area requiring further investigation to balance efficacy with potential lead redistribution from bone. nih.gov
Q & A
Basic Research Question: What experimental designs are most effective for evaluating succimer’s efficacy in reducing lead burden across tissues?
Methodological Answer:
Preclinical studies should employ factorial designs with multiple exposure levels (e.g., low, moderate, high Pb) and chelation regimens (e.g., single vs. repeated this compound courses). Tissue-specific lead quantification (blood, brain, liver, bone) via ICP-MS or isotope tracing is critical, as blood Pb reductions may not correlate with brain Pb clearance . Longitudinal sampling post-treatment is essential to assess rebound effects, as endogenous Pb redistribution from bone can negate short-term benefits . Rodent and primate models with controlled Pb exposure windows (e.g., developmental vs. adult stages) are recommended to mimic pediatric cases .
Advanced Research Question: How do contradictory findings on this compound’s neurocognitive benefits in human trials versus animal models inform mechanistic hypotheses?
Methodological Answer:
Human trials (e.g., TLC trial) showing no neurocognitive improvement despite blood Pb reductions suggest this compound may fail to address Pb-induced neurotoxicity once irreversible damage occurs . Animal models, however, demonstrate partial recovery in attention and arousal regulation when this compound is administered during active Pb exposure . Researchers should investigate:
- Temporal factors : Optimal treatment windows via time-series analyses in Pb-exposed rodents.
- Biomarkers : Synaptic plasticity markers (e.g., BDNF, NMDA receptors) to differentiate reversible vs. permanent neural damage .
- Dose-response : Multi-arm trials comparing standard this compound regimens (30 mg/kg/day) with extended protocols to enhance brain Pb clearance .
Basic Research Question: What are the standardized protocols for assessing this compound’s impact on essential trace elements?
Methodological Answer:
Urinary excretion profiles (Ca, Zn, Mg, Cu) should be analyzed via ICP-MS during and after this compound treatment. Primate studies recommend 24-hour urine collection over 5–19 days to capture acute vs. chronic effects . Multivariate ANOVA is critical to distinguish this compound-induced diuresis from baseline variability. For pediatric studies, paired serum and urine samples pre-/post-treatment are necessary to monitor Zn depletion, which may exacerbate Pb toxicity .
Advanced Research Question: Why does this compound fail to reduce blood cadmium (Cd) in children with background exposure, despite structural similarity to Pb chelates?
Methodological Answer:
Cd’s higher affinity for metallothionein and preferential renal accumulation limits this compound’s accessibility. Researchers should:
- Compare molecular dynamics : DFT simulations to evaluate this compound-Cd binding kinetics versus Pb .
- Conduct tissue-specific analyses : Liver/kidney Cd levels in this compound-treated animal models using neutron activation or synchrotron XRF .
- Explore combination therapies : Co-administration with EDTA or DMPS to enhance Cd mobilization, as seen in Pb/Cd co-exposure models .
Basic Research Question: How should researchers address confounding variables in clinical trials evaluating this compound’s long-term safety?
Methodological Answer:
- Control groups : Include Pb-unexposed cohorts receiving this compound to isolate treatment-related adverse effects (e.g., cognitive deficits in rats ).
- Blinding : Double-blind designs with placebo-controlled arms to mitigate caregiver bias in behavioral assessments .
- Covariates : Adjust for nutritional status (e.g., iron deficiency), which modulates Pb absorption and this compound efficacy .
Advanced Research Question: What explains the paradoxical improvement in postural balance but not IQ scores in this compound-treated children?
Methodological Answer:
Motor function (e.g., static balance) may recover via cerebellar Pb clearance, whereas cortical deficits (executive function) persist due to irreversible synaptic pruning. Researchers should:
- Use diffusion tensor imaging (DTI) : To map white matter integrity in this compound-treated vs. placebo groups .
- Apply task-specific neuropsychological batteries : E.g., NEPSY-II for visuomotor coordination vs. WISC-V for IQ .
- Analyze Pb deposition patterns : LA-ICP-MS on postmortem brain tissues to correlate regional Pb levels with functional outcomes .
Basic Research Question: What pharmacokinetic parameters are critical for optimizing this compound dosing regimens?
Methodological Answer:
- Absorption : Monitor plasma this compound-cysteine disulfide levels via HPLC-MS to assess bioavailability (~25% of oral dose excreted unchanged ).
- Half-life : Serial blood sampling over 4–24 hours post-dose to model elimination kinetics (Tmax = 2–4 hours ).
- Pediatric adjustments : Allometric scaling based on body surface area, as neonates exhibit delayed renal clearance .
Advanced Research Question: How can preclinical data guide the use of this compound in combination therapies for severe Pb poisoning (BLL > 100 µg/dL)?
Methodological Answer:
Case studies suggest this compound + CaNa2EDTA reduces encephalopathy risk compared to BAL + EDTA . Researchers should:
- Evaluate synergistic chelation : Sequential administration (this compound first to mobilize soft-tissue Pb, followed by EDTA for bone Pb) .
- Quantify urinary Pb : Atomic absorption spectrometry to compare monotherapy vs. combination efficacy .
- Model rebound kinetics : Post-treatment Pb redistribution using compartmental PK/PD models .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


