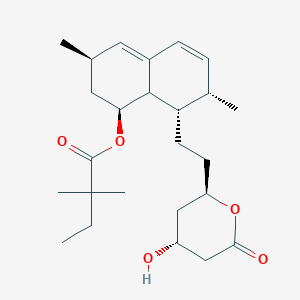
Simvastatin
Description
Historical Context and Discovery of HMG-CoA Reductase Inhibitors
The understanding that elevated plasma cholesterol concentrations represent a major risk factor for heart disease emerged in the 1950s and 1960s, initiating a quest for effective cholesterol-reducing drugs. news-medical.netnih.gov The enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase was identified as the rate-limiting enzyme in the cholesterol biosynthetic pathway, making it a prime target for therapeutic intervention. nih.govnih.govwikipedia.organimalresearch.infomdpi.com
A breakthrough occurred in 1976 when Japanese biochemist Akira Endo, working at the Sankyo Company, isolated a substance from the fungus Penicillium citrinum. news-medical.netnih.govwikipedia.orgclinicalcorrelations.orgbjcardio.co.ukescardio.orgnih.gov This substance, named compactin (or mevastatin), was identified as a competitive inhibitor of HMG-CoA reductase and was the first statin to be administered to humans. news-medical.netnih.govclinicalcorrelations.orgbjcardio.co.uk Compactin demonstrated its ability to lower plasma cholesterol in animal models such as dogs, rabbits, and monkeys. news-medical.netnih.govbjcardio.co.uk
Following this, in 1978, Alfred Alberts and his colleagues at Merck Research Laboratories independently discovered another potent HMG-CoA reductase inhibitor, lovastatin (also known as mevinolin or monacolin K), from a fermentation broth of Aspergillus terreus. news-medical.netnih.govclinicalcorrelations.orgbjcardio.co.uknih.govwikipedia.orgbionity.com Akira Endo also independently identified the same compound within a year of Alberts' discovery. news-medical.net
Evolution of Statin Research and Simvastatin's Place
The discovery of lovastatin paved the way for the development of the statin class. Lovastatin became the first statin approved by the U.S. Food and Drug Administration (FDA) on September 1, 1987, revolutionizing hypercholesterolemia treatment. news-medical.netnih.govclinicalcorrelations.orgescardio.orgnih.gov
This compound, a semi-synthetic derivative of lovastatin, was developed by Merck scientists through chemical modification, exhibiting a 2.5-fold greater activity in inhibiting HMG-CoA reductase. news-medical.netwikipedia.orgbionity.comamericanscientist.org It was patented by Merck in 1980 and subsequently approved for marketing in Sweden in 1988, followed by worldwide distribution. news-medical.netnih.govwikipedia.org
This compound's lipophilic nature, derived from its fungal origin (Aspergillus terreus), is a key characteristic that distinguishes it from some other statins. mdpi.combionity.comlaphil.comattodiagnostics.com While all statins primarily inhibit HMG-CoA reductase to lower LDL cholesterol, their potencies and specific characteristics vary. For instance, this compound has been observed to achieve an average decrease in LDL-C of approximately 35% in clinical studies. drugbank.com
Table 1: Key Milestones in HMG-CoA Reductase Inhibitor Discovery and Statin Development
| Year | Event | Key Compound(s) | Source/Organism | Reference |
| 1976 | Discovery of first HMG-CoA reductase inhibitor | Compactin (Mevastatin) | Penicillium citrinum | news-medical.netnih.govclinicalcorrelations.orgbjcardio.co.uk |
| 1978 | Discovery of Lovastatin | Lovastatin (Mevinolin, Monacolin K) | Aspergillus terreus | news-medical.netnih.govclinicalcorrelations.orgbjcardio.co.ukwikipedia.orgbionity.com |
| 1980 | This compound patented by Merck | This compound | Semi-synthetic (from Lovastatin) | wikipedia.orgbionity.com |
| 1987 | Lovastatin approved by FDA (USA) | Lovastatin | news-medical.netnih.govclinicalcorrelations.orgescardio.orgnih.gov | |
| 1988 | This compound approved for marketing (Sweden) | This compound | news-medical.netnih.gov | |
| 1994 | Scandinavian this compound Survival Study (4S) results published | This compound | nih.govanimalresearch.infowikipedia.orgamericanscientist.orgmonash.eduahajournals.orgbinasss.sa.cr |
Current Research Paradigms and Future Directions for this compound
Beyond its well-established role in lipid management, current research on this compound is increasingly focused on its "pleiotropic effects"—beneficial actions that extend beyond direct cholesterol lowering. mdpi.comlaphil.comattodiagnostics.comuspharmacist.comstanford.edunih.govbjcardio.co.ukahajournals.orgnih.govmdpi.commdpi.com These effects include anti-inflammatory, antioxidant, and antithrombotic properties, as well as improvements in endothelial function and the stabilization of atherosclerotic plaques. laphil.comattodiagnostics.comuspharmacist.comstanford.edunih.govbjcardio.co.ukahajournals.orgnih.govmdpi.com Many of these pleiotropic effects are mediated by the inhibition of isoprenoid synthesis, which impacts intracellular signaling molecules like Rho, Rac, and Cdc42. nih.govnih.gov
Table 2: Documented Pleiotropic Effects of this compound and Statins
| Effect Category | Specific Actions/Mechanisms | Reference |
| Vascular Health | Improved endothelial function, increased nitric oxide (NO) production, plaque stabilization, reduced blood viscosity, reduced inflammation within atherosclerotic plaques, reduced monocyte adherence to endothelial cells. | mdpi.comahajournals.orglaphil.comattodiagnostics.comuspharmacist.comstanford.edunih.govbjcardio.co.ukahajournals.orgnih.govmdpi.com |
| Anti-inflammatory | Reduction of inflammatory cytokines (e.g., IL-6, TNF-alpha, interferon-gamma), reduction of C-reactive protein, inhibition of NF-kB pathway. | laphil.comattodiagnostics.comuspharmacist.comnih.govmdpi.comnih.gov |
| Antioxidant | Reduction of oxidative stress. | laphil.comuspharmacist.comnih.govmdpi.com |
| Antithrombotic | Decreased platelet aggregation, inhibition of tissue factor and plasminogen activator inhibitor (PAI)-1 expression, increased tissue plasminogen activator (tPA) and thrombomodulin (TM) expression. | uspharmacist.comnih.govahajournals.org |
| Cellular Regulation | Inhibition of vascular smooth muscle proliferation, cell-cycle arrest (e.g., G1/S phase in AD-vulnerable lymphocytes), modulation of cell signaling pathways. | uspharmacist.commdpi.commdpi.com |
Ongoing research is exploring this compound's potential in various non-cardiovascular conditions:
Neurological and Psychiatric Disorders: this compound's anti-inflammatory and antioxidant properties are being investigated for potential therapeutic applications in conditions such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, depression, and schizophrenia. mdpi.comlaphil.comuspharmacist.commdpi.comwikipedia.org Some studies suggest a positive effect on cognitive performance, particularly in mild cognitive impairment or early-stage Alzheimer's disease. laphil.comwikipedia.org
Oncology: Emerging evidence suggests statins, including this compound, may exert anticancer effects through multiple mechanisms, such as inhibiting tumor cell proliferation, inducing cell death, and modulating signaling pathways like the mevalonate pathway. mdpi.comuspharmacist.comstanford.edumdpi.comnih.govfrontiersin.orgjtgga.orgnih.gov Research indicates a potential role in reducing mortality related to breast and colorectal cancer. frontiersin.orgnih.gov
Bone Regeneration: this compound has shown promise in promoting bone formation and enhancing osteogenesis by inhibiting osteoclastogenesis and promoting mineral formation in rodents. nih.govmdpi.comnovapublishers.com Novel this compound derivatives, such as KMUHC-01, are being synthesized and studied for their bone selectivity and anabolic capacity with reduced HMG-CoA reductase inhibition and cytotoxicity. mdpi.com
Future directions for this compound research emphasize elucidating its precise molecular mechanisms in these diverse applications, defining optimal dose-response relationships for specific non-lipid indications, and developing personalized treatment strategies. binasss.sa.crmdpi.comfrontiersin.orgnih.gov The development of new delivery systems, such as nanoemulsions and nanostructured lipid carriers, is also being explored to target this compound to specific tissues for novel therapeutic areas like cancer therapy and bone regeneration. novapublishers.com Large-scale, long-term prospective studies are crucial to validate the efficacy of this compound in these emerging fields. binasss.sa.crmdpi.comfrontiersin.orgnih.gov
Properties
IUPAC Name |
[(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RYMZZMVNJRMUDD-HGQWONQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC(C)(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCC(C)(C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H38O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0023581 | |
| Record name | Simvastatin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0023581 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
418.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Simvastatin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005007 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Insoluble, In water, 3.0X10-2 mg/L, temp not specified, Solubility (mg/mL): chloroform 610; DMSO 540; methanol 200; ethanol 160; n-hexane 0.15; 0.1 M HCl 0.06; polyethylene glycol-400 70; propylene glycol 30; 0.1 M NaOH 70, 1.22e-02 g/L | |
| Record name | Simvastatin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00641 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Simvastatin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7208 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Simvastatin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005007 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
White to off-white crystalline powder from n-butyl chloride + hexane | |
CAS No. |
79902-63-9 | |
| Record name | Simvastatin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=79902-63-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Simvastatin [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0079902639 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Simvastatin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00641 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | simvastatin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758706 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Simvastatin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0023581 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.115.749 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | (1S,3R,7S,8S,8aS)-1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-{2-[(4R,6R)-tetrahydro-4-hydroxy-2-oxo-2H-pyran-6-yl]ethyl}-1-naphthyl-2,2-dimethylbutyrat | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate. | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | SIMVASTATIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/AGG2FN16EV | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Simvastatin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7208 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Simvastatin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005007 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
135-138 °C, 135 - 138 °C | |
| Record name | Simvastatin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00641 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Simvastatin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7208 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Simvastatin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0005007 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanistic Research of Simvastatin
Molecular Mechanisms of Pleiotropic Effects
Cellular Signaling Pathway Modulation
NF-κB Signaling Pathway
Simvastatin has been shown to modulate the Nuclear Factor-kappa B (NF-κB) signaling pathway, a key regulator of inflammation, cell proliferation, and apoptosis. Studies indicate that this compound can suppress NF-κB activation. For instance, in a mouse model of intracerebral hemorrhage, this compound treatment significantly downregulated NF-κB expression and activity, leading to reduced cellular apoptosis by suppressing the NF-κB-mediated MyD88/TRIF signaling pathway. spandidos-publications.comnih.gov This effect was also observed in human castration-resistant prostate cancer cells, where this compound reduced NF-κB expression and synergistically induced apoptotic cell death when combined with an NF-κB inhibitor. plos.org Furthermore, this compound has been found to potentiate TNF-α-induced apoptosis by downregulating NF-κB-dependent antiapoptotic gene products, and this effect can be reversed by mevalonate, highlighting the involvement of HMG-CoA reductase inhibition. aai.org In nasopharyngeal carcinoma cells, this compound inhibited NF-κB signaling by attenuating p65 phosphorylation and decreasing the expression of NF-κB-regulated genes like cyclin D1 and Bcl-2. europeanreview.org
Sterol Regulatory Element-Binding Protein (SREBP) Regulation
This compound significantly impacts the regulation of Sterol Regulatory Element-Binding Proteins (SREBPs), which are transcription factors vital for cholesterol and fatty acid synthesis. By inhibiting HMG-CoA reductase, this compound lowers intracellular cholesterol levels, leading to the activation of SREBP-2. researchgate.netoncotarget.comcore.ac.uk Activated SREBP-2 then translocates to the nucleus, increasing the expression of target genes involved in cholesterol synthesis, including HMG-CoA reductase itself, and the LDL receptor, which enhances LDL clearance from plasma. core.ac.uk In the context of renal cell carcinoma, this compound has been shown to downregulate SREBP-1 expression, thereby enhancing the cytotoxic effects of sunitinib. nih.gov During fasting, this compound can activate SREBP-2-dependent autophagy, which may amplify PPARα activation and modulate HDL levels and hepatic glucose metabolism. biorxiv.org
Hypoxia-Inducible Factor 1 (HIF-1) Inhibition
This compound has been observed to inhibit Hypoxia-Inducible Factor 1 (HIF-1), a transcription factor critical for cellular responses to hypoxia, including angiogenesis and inflammation. Studies suggest that this compound can suppress the expression of the HIF-1α subunit. nih.gov For example, this compound reduced the expression of proangiogenic proteins, including HIF-1α, in hepatic stellate cells. isciii.es While some studies suggest this compound increases HIF-1α expression in endothelial cells, others indicate that statins, including this compound, can attenuate HIF-1-dependent gene expression by accelerating the ubiquitination and degradation of HIF-1α, often through isoprenoid-dependent mechanisms involving small GTP-binding proteins like Rho and Rac. frontiersin.orgoup.comoup.com
Kruppel-Like Factor 2 (KLF-2) Upregulation
This compound is a strong inducer of Kruppel-Like Factor 2 (KLF-2), an atheroprotective transcription factor predominantly expressed in endothelial cells and macrophages. researchgate.netnih.govoup.comahajournals.org Upregulation of KLF-2 by this compound contributes to its anti-inflammatory and vascular protective effects. In human peripheral blood monocyte (HPBM)-macrophages, this compound upregulated KLF-2 while downregulating pro-inflammatory genes like monocyte chemotactic protein-1 (MCP-1) and tissue factor (TF). researchgate.netoup.com This induction of KLF-2 is dependent on the inhibition of cholesterol synthesis and the Rho pathway, requiring de novo transcription involving myocyte enhancer factor 2 (MEF2) proteins. ahajournals.orgresearchgate.net KLF-2 upregulation by this compound also mediates its inhibitory effects on maladaptive cardiac remodeling by repressing TGFβ1 expression in endothelial cells. nih.govthno.org
Gene Expression and Transcriptional Regulation
Beyond specific pathways, this compound exerts broad effects on gene expression and transcriptional regulation. Its ability to inhibit HMG-CoA reductase leads to a cascade of changes that influence the transcription of numerous genes. This compound can downregulate pro-inflammatory genes, including various chemokines, and upregulate atheroprotective factors like KLF-2. researchgate.netoup.com It also affects genes involved in protein prenylation pathways and regulators of small GTPases. atsjournals.org In human breast cancer cells, this compound can suppress NF-κB and LIN28B, subsequently upregulating let-7 microRNAs. plos.org this compound has also been shown to inhibit the expression of stemness-related genes such as Oct4, Sox2, and Nanog in various cancer cell types, potentially through its effects on the cytoskeleton. spandidos-publications.com Furthermore, this compound can decrease the expression of the Farnesoid X Receptor (FXR) at both RNA and protein levels and downregulate its DNA-binding activity, which has implications for lipoprotein and carbohydrate homeostasis. nih.gov It can also inhibit the transcription of the brain-derived neurotrophic factor (BDNF). nih.gov
Post-Translational Modifications (e.g., Protein Lipidation, Prenylation)
A cornerstone of this compound's pleiotropic effects is its inhibition of protein prenylation, a post-translational modification involving the covalent attachment of isoprenoid lipids (farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP)) to proteins. scielo.org.coahajournals.orgmdpi.comualberta.ca By blocking the mevalonate pathway, this compound reduces the availability of these isoprenoid intermediates, thereby preventing the prenylation of key proteins, particularly small GTPases like Ras, Rho, and Rac. scielo.org.coahajournals.orgmdpi.comualberta.capnas.org These GTPases require prenylation for proper membrane localization and function, where they regulate diverse cellular processes including cell proliferation, differentiation, apoptosis, and cytoskeleton organization. scielo.org.coahajournals.orgjci.org Inhibition of prenylation by this compound has been linked to various effects, such as the induction of the unfolded protein response (UPR) and developmental arrest in C. elegans. pnas.org It also affects the membrane localization of proteins like Rab and RhoA GTPase and the lipidation of LC3, which can inhibit processes like influenza replication. nih.gov The disruption of these modifications contributes to this compound's anti-inflammatory, antiproliferative, and anti-thrombotic actions. jci.org
Clinical and Preclinical Research Applications and Outcomes
Cardiovascular Research
Simvastatin, a member of the statin class of drugs, has been the subject of extensive cardiovascular research. Beyond its well-established lipid-lowering effects, studies have delved into its pleiotropic (effects other than lipid-lowering) mechanisms that contribute to its cardiovascular benefits. ahajournals.orgnih.gov
Research has shown that this compound possesses anti-atherosclerotic properties that are independent of its ability to lower cholesterol. ahajournals.orgnih.gov These effects are attributed to the inhibition of the mevalonate pathway, which not only reduces cholesterol synthesis but also the production of isoprenoid intermediates. ahajournals.orgelsevier.es These isoprenoids are crucial for the function of small GTP-binding proteins like Rho and Rac, which are involved in various cellular processes contributing to atherosclerosis. nih.govahajournals.org
One of the key non-lipid-lowering mechanisms is the inhibition of vascular smooth muscle cell (VSMC) proliferation and migration. jacc.orgnih.govnih.gov In vitro studies have demonstrated that this compound markedly inhibits VSMC proliferation. jacc.orgatsjournals.org This antiproliferative effect is thought to be mediated by preventing the geranylgeranylation of the RhoA protein. atsjournals.org In vivo studies in animal models have shown that this compound reduces neointimal formation after vascular injury, a process driven by VSMC proliferation. jacc.org This effect was reversed by the addition of mevalonate, confirming the role of the HMG-CoA reductase inhibition pathway. jacc.org
Table 1: In Vivo Effects of this compound on Neointimal Formation After Vascular Injury in Rats
| Treatment Group | Neointimal Area (mm²) | Neointima-Media Ratio |
| Control | 0.266 ± 0.015 | 1.271 ± 0.074 |
| This compound (highest dose) | 0.080 ± 0.026 | 0.436 ± 0.158 |
| p-value | < 0.001 | < 0.001 |
| Data from a study on balloon injury in rat common carotid artery. jacc.org |
This compound has been shown to improve endothelial function, a critical factor in maintaining vascular health. nih.govahajournals.orgahajournals.org Endothelial dysfunction is an early event in atherosclerosis and is characterized by reduced bioavailability of nitric oxide (NO). jacc.org
Research indicates that this compound enhances endothelial function through several mechanisms. It can increase the expression and activity of endothelial nitric oxide synthase (eNOS), the enzyme responsible for producing NO in the endothelium. ahajournals.orgahajournals.orgahajournals.org This leads to increased NO production, which has vasodilatory and anti-atherosclerotic properties. ahajournals.orgahajournals.org Studies have shown that this compound treatment can improve flow-mediated dilation (FMD), a measure of endothelial function, in patients with hypercholesterolemia and rheumatoid arthritis. jacc.orgnih.gov In one study, FMD improved from a baseline of 7.7 ± 2.5% to 13.0 ± 1.4% after eight weeks of this compound treatment. nih.gov
Furthermore, this compound has been found to increase the number of circulating endothelial progenitor cells (EPCs), which are bone marrow-derived cells that can differentiate into mature endothelial cells and contribute to vascular repair. nih.govijconline.idjci.orgrevespcardiol.org In-vitro studies have shown that this compound augments the number of EPCs, and this effect is mediated through the PI3K/Akt pathway. jci.orgrevespcardiol.org In a study on diabetic rats, this compound treatment increased the levels of circulating EPCs and NO. nih.gov
Table 2: Effect of this compound on Endothelial Function and Progenitor Cells
| Study Population | Intervention | Outcome Measure | Result |
| Hypercholesterolemic patients | This compound 40 mg/day for 8 weeks | Flow-Mediated Dilation (FMD) | Increased from 7.7% to 13.0% (p=0.001) nih.gov |
| Diabetic rats | This compound treatment | Circulating Endothelial Progenitor Cells (EPCs) | Significantly increased compared to untreated diabetic rats nih.gov |
| Human Mononuclear Cells (in vitro) | This compound (1 µM) | Number of EPCs | Increased by 241% ± 102% jci.org |
Research has demonstrated that this compound can reduce the levels of inflammatory markers such as C-reactive protein (CRP) and interleukin-6 (IL-6). nih.gov It has also been shown to have a direct anti-inflammatory effect on macrophages, key immune cells in atherosclerotic plaques. oup.com this compound can downregulate the expression of inflammatory transcription factors like NF-κB and upregulate the atheroprotective transcription factor KLF-2 in macrophages. oup.com
In animal models, this compound has been shown to reduce the infiltration of inflammatory cells, such as neutrophils and macrophages, into atherosclerotic lesions and ischemic heart tissue. ahajournals.orgahajournals.orgahajournals.org This reduction in inflammatory cell accumulation is a crucial aspect of its plaque-stabilizing effects. ahajournals.org Studies have also indicated that this compound can modulate the interaction between vascular smooth muscle cells, macrophages, and activated endothelial cells, further reducing the inflammatory response in the vessel wall. nih.gov
The rupture of vulnerable atherosclerotic plaques and subsequent thrombosis are the primary causes of acute coronary syndromes. Research has focused on this compound's role in promoting plaque stability and reducing the risk of thrombosis. ahajournals.orgimrpress.com
This compound has been shown to favorably alter the composition of atherosclerotic plaques, making them more stable and less prone to rupture. ahajournals.orgslideshare.netconsensus.app It can reduce the lipid content and macrophage infiltration within plaques while increasing the collagen content, which strengthens the fibrous cap. ahajournals.orgslideshare.net In apoE-deficient mice, this compound reduced the frequency of intraplaque hemorrhage and calcification, which are markers of plaque vulnerability, independent of its lipid-lowering effects. ahajournals.org While some studies suggest statins may promote calcification, this is often seen as a stabilizing process. imrpress.com
Regarding thrombosis, this compound has been found to have antithrombotic properties. nih.govjacc.org It can reduce the expression of tissue factor, a key initiator of the coagulation cascade, in macrophages. oup.com Additionally, research has shown that this compound treatment can decrease levels of thrombin activatable fibrinolysis inhibitor (TAFI), which may enhance fibrinolysis, the process of breaking down blood clots. nih.gov
Comparative effectiveness research has been conducted to evaluate the relative benefits of this compound versus other statins, such as atorvastatin, in preventing cardiovascular events. The results of these studies have sometimes varied depending on the patient population and the specific outcomes measured.
A network meta-analysis of 92 trials found that across all populations, atorvastatin, fluvastatin, and this compound had the highest probability of being the most effective treatments for reducing all-cause mortality and major coronary events. nih.gov For the secondary prevention of major coronary events, atorvastatin was found to be significantly more effective than both pravastatin and this compound. nih.govheraldopenaccess.us However, in the context of primary prevention, no significant differences were observed among the individual statins in reducing deaths and major coronary events. nih.govheraldopenaccess.us
Another study focusing on patients with diabetes found that high-intensity rosuvastatin, this compound, and atorvastatin were the most effective at lowering non-HDL cholesterol. bmj.com In high-risk patients, high-intensity atorvastatin was the most effective in reducing major cardiovascular events. bmj.com Conversely, a real-world cohort study suggested that this compound users had lower long-term rates of cardiovascular events compared to atorvastatin users in a primary prevention setting, though the authors noted this could be influenced by confounding factors. dovepress.com
Table 3: Comparative Effectiveness of this compound vs. Atorvastatin for Secondary Prevention of Major Coronary Events
| Comparison | Odds Ratio (OR) | 95% Confidence Interval (CI) |
| Atorvastatin vs. This compound | 0.68 | 0.38 - 0.98 |
| Data from a network meta-analysis. nih.gov |
To achieve more aggressive lipid-lowering goals and potentially enhance cardiovascular protection, this compound has been studied in combination with other lipid-modifying agents, most notably ezetimibe. nih.govnih.gov Ezetimibe inhibits the absorption of cholesterol from the intestine, providing a complementary mechanism to the inhibition of cholesterol synthesis by this compound. nih.govahajournals.org
The landmark IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) randomized over 18,000 high-risk patients who had recently experienced an acute coronary syndrome to receive either this compound monotherapy or a combination of this compound and ezetimibe. acc.orgwikijournalclub.orgnih.gov The study found that the combination therapy was superior to this compound alone in reducing the primary composite endpoint of cardiovascular death, major coronary event, or nonfatal stroke. acc.orgwikijournalclub.orgsubstack.comnih.gov The median LDL cholesterol level was significantly lower in the combination group (53.7 mg/dL) compared to the monotherapy group (69.5 mg/dL). nih.gov The benefit was primarily driven by a reduction in non-fatal myocardial infarction and ischemic stroke. acc.orgsubstack.com
This trial provided strong evidence that adding a non-statin agent like ezetimibe to statin therapy can lead to improved cardiovascular outcomes, supporting the "lower is better" hypothesis for LDL cholesterol. ahajournals.orgwikijournalclub.org
Table 4: Key Outcomes of the IMPROVE-IT Trial
| Outcome | This compound + Ezetimibe Group | This compound Monotherapy Group | Hazard Ratio (95% CI) | p-value |
| Primary Composite Endpoint (7-year rate) | 32.7% | 34.7% | 0.936 (0.89-0.99) | 0.016 acc.orgnih.gov |
| Ischemic Stroke | 3.4% | 4.1% | 0.79 (0.67-0.94) | 0.008 acc.org |
Comparative Effectiveness Research in Cardiovascular Outcomes (e.g., vs. Atorvastatin)
Neurological Research
This compound, a lipophilic statin, has the ability to cross the blood-brain barrier, making it a subject of extensive neurological research. nih.govnih.gov Studies have explored its potential therapeutic applications in a range of neurological conditions, from brain tumors to neurodegenerative disorders. nih.govnih.gov
Neuroprotective Mechanisms
Research has identified several neuroprotective mechanisms of this compound. These include anti-inflammatory effects, modulation of receptor activity, and antioxidant properties. frontiersin.orgmdpi.come-century.us
This compound has been shown to exert anti-inflammatory effects by reducing the expression of pro-inflammatory cytokines such as TNF-alpha, IL-1beta, and IL-6 in experimental models of Parkinson's disease. e-century.us It may also inhibit the inflammatory process within nerve cells by acting on N-methyl-D-aspartate receptor 1 (NMDAR1). mdpi.com Furthermore, studies suggest that statins can reduce chronic neuroinflammation, which is considered a key mechanism for their neuroprotective effects in conditions like Alzheimer's disease. nih.gov
The compound's antioxidant properties are another key area of its neuroprotective action. In models of Parkinson's disease, this compound has demonstrated the ability to protect against oxidative damage by inhibiting the NADPH oxidase/p38 MAPK pathway and enhancing the expression of antioxidant proteins. frontiersin.org
Additionally, this compound has been found to modulate cognition-related receptors. For instance, in a rat model of Parkinson's disease, this compound treatment was associated with the attenuation of cognitive deficits through alterations of different receptors in various brain regions. e-century.us Animal studies have also indicated that this compound can protect against the loss of N-methyl-d-aspartate (NMDA) receptors. nih.gov
Research in Neurodegenerative Disorders (e.g., Alzheimer's, Parkinson's, Huntington's Disease)
This compound has been investigated for its potential role in several neurodegenerative disorders, with studies yielding mixed results. nih.govnih.gov
Alzheimer's Disease: Preclinical and epidemiological studies have suggested a potential protective effect of statins on Alzheimer's disease (AD). nih.gov Some retrospective and cross-sectional studies reported a significant reduction in the risk of dementia and AD with statin use. nih.gov Experimental evidence indicates that certain statins can cross the blood-brain barrier, influence brain cholesterol metabolism, and potentially reduce the production of amyloid-β (Aβ) peptide, a hallmark of AD. nih.gove-emj.org this compound, in particular, has been shown to reduce the levels of Aβ in both in vitro and in vivo models. neurology.org It has also been reported to enhance vascular activity in the brain, decrease neuroglia activation, and reduce dystrophic neurites induced by Aβ plaques. e-emj.org However, clinical trials have produced inconsistent findings. nih.gov One study found that while this compound affected brain cholesterol metabolism, it did not alter AD biomarkers in the cerebrospinal fluid or plasma after 12 weeks of treatment. nih.gov Another 18-month trial also showed no change in cognitive outcomes in patients with AD. neurology.org
Parkinson's Disease: Research into this compound for Parkinson's disease (PD) has also been extensive but has not yielded a definitive therapeutic role. neurologylive.comparkinsons.org.uk Epidemiological and preclinical data initially suggested a neuroprotective role for statins, with their use being associated with a lower incidence of PD. nih.govbmj.com Laboratory studies indicated that statins might protect brain cells from damaging processes involved in PD, such as inflammation, microglial activation, oxidative stress, and α-synuclein aggregation. nih.gov However, a significant clinical trial (PD-STAT) found that this compound was futile as a disease-modifying therapy for patients with moderate PD. neurologylive.comparkinsons.org.uk The trial showed that the motor scores of patients on this compound worsened slightly more than those on a placebo over 24 months. neurologylive.com
Huntington's Disease: The potential for this compound to delay the onset of movement disorders in Huntington's disease has been suggested. nih.govresearchgate.net Further research is needed to fully understand its effects in this specific neurodegenerative condition. mdpi.com
Brain Tumor Research
This compound has been investigated for its potential anti-cancer effects in brain tumors, particularly glioblastoma (GBM) and medulloblastoma. nih.govnih.gov The proposed mechanisms include inhibiting tumor cell growth and migration, inducing apoptosis (programmed cell death), and affecting key signaling pathways. iiarjournals.orgljmu.ac.uknih.gov
In glioblastoma research, studies have shown that this compound can inhibit the growth and migration of human glioma cells. iiarjournals.org It has been found to induce apoptosis in GBM cells through various mechanisms, including the inhibition of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway and caspase-3 activation. ljmu.ac.uknih.gov Research also suggests that this compound can affect GBM cells by inhibiting the Transforming Growth Factor-β (TGF-β) pathway, which is involved in angiogenesis, migration, and invasion. ljmu.ac.uk Furthermore, some studies have explored the combination of this compound with other anti-cancer agents like temozolomide, with results suggesting a synergistic effect in inducing apoptosis in GBM cells. mdpi.comjcancer.org However, the effects of this compound can be dose-dependent and complex. For instance, one in vivo study in a mouse glioblastoma model found that low-dose this compound increased necrosis and apoptosis, while high-dose this compound affected tumor vessel structure. iiarjournals.org
In medulloblastoma, another type of brain tumor, this compound has been shown to inhibit tumor growth by suppressing cancer cell proliferation and reducing the activation of the hedgehog signaling pathway. nih.gov Research has also demonstrated that this compound can induce dose-dependent apoptosis in different medulloblastoma cell lines. mdpi.com
Table 1: Summary of this compound Research in Brain Tumors
| Tumor Type | Key Research Findings | Mechanisms of Action |
|---|---|---|
| Glioblastoma (GBM) | Inhibits tumor cell growth and migration. iiarjournals.orgljmu.ac.uk | Induces apoptosis via PI3K/Akt pathway inhibition and caspase-3 activation. ljmu.ac.uknih.gov |
| Induces apoptosis and autophagy. ljmu.ac.uk | Inhibits TGF-β signaling, affecting angiogenesis, migration, and invasion. ljmu.ac.uk | |
| Dose-dependent effects on necrosis, apoptosis, and tumor vasculature. iiarjournals.org | May enhance the efficacy of other chemotherapy agents like temozolomide. mdpi.comjcancer.org | |
| Medulloblastoma | Inhibits tumor growth. nih.gov | Suppresses cancer cell proliferation. nih.gov |
| Induces dose-dependent apoptosis. mdpi.com | Reduces activation of the hedgehog signaling pathway. nih.gov |
Stroke and Intracerebral Hemorrhage Research
This compound has been studied for its potential neuroprotective effects in the context of both ischemic stroke and intracerebral hemorrhage (ICH).
In ischemic stroke , research has suggested that statins, including this compound, may reduce the incidence of ischemic strokes. nih.gov A large-scale study, the Heart Protection Study, found that this compound therapy reduced the rate of ischemic strokes by about a quarter. nih.gov The proposed mechanisms for this protective effect are pleiotropic, extending beyond cholesterol-lowering to include anti-inflammatory, antioxidant, and antithrombotic effects. mdpi.comnih.gov However, clinical trials on the use of this compound in the acute phase of ischemic stroke have yielded mixed results regarding safety and efficacy, with some studies raising concerns about an increased risk of infection despite potential neurological improvement. researchgate.net
In the context of intracerebral hemorrhage (ICH) , research has explored the effects of this compound on recovery and brain injury. Animal studies have shown that this compound treatment can lead to significant improvements in neurological recovery, a decrease in tissue loss, and an increase in neurogenesis when administered after an ICH. nih.gov It has also been found to protect the integrity of the blood-brain barrier (BBB), reduce edema, and improve cerebral blood flow in the acute phase after an ICH in experimental models. scirp.org Mechanistically, this compound may attenuate brain edema and reduce cell death by suppressing the NF-κB-mediated MyD88/TRIF signaling pathway. spandidos-publications.com While some studies suggest a potential for worse outcomes with statin therapy in ICH patients, multiple retrospective studies have indicated favorable functional outcomes and reduced mortality with the continuation of statin therapy after an ICH. nih.gov
Multiple Sclerosis Research
This compound has been investigated as a potential treatment for secondary progressive multiple sclerosis (SPMS), primarily due to its anti-inflammatory and potential neuroprotective properties. mstrust.org.ukmscanada.ca
An initial phase II trial (MS-STAT) involving 140 people with SPMS showed encouraging results. mscanada.camssociety.org.uk Participants who received a high dose of this compound for two years had a 43% reduction in brain atrophy (shrinkage) compared to those who received a placebo. mstrust.org.ukmscanada.ca The this compound group also showed slower changes in disability scores. mscanada.ca These promising findings led to a larger phase III trial, MS-STAT2. mstrust.org.uk
However, the results of the MS-STAT2 trial, which included nearly 1,000 participants with SPMS, were disappointing. mstrust.org.ukmultiplesclerosisnewstoday.com The trial found no evidence that this compound had an effect on slowing disability progression compared to a placebo. mstrust.org.uknationalmssociety.org Despite the lack of efficacy in slowing progression, the treatment was found to be safe and well-tolerated. mstrust.org.ukmultiplesclerosisnewstoday.com
Table 2: Key Clinical Trials of this compound in Multiple Sclerosis
| Trial | Phase | Number of Participants | Key Findings |
|---|---|---|---|
| MS-STAT | II | 140 | 43% reduction in brain atrophy in the this compound group compared to placebo. mstrust.org.ukmscanada.ca |
| Slower disability progression in the this compound group. mscanada.ca | |||
| MS-STAT2 | III | ~1000 | No significant difference in slowing disability progression between this compound and placebo. mstrust.org.ukmultiplesclerosisnewstoday.com |
| This compound was found to be safe and well-tolerated. mstrust.org.ukmultiplesclerosisnewstoday.com |
Effects on Neurogenesis and Neuroplasticity
Research indicates that this compound can positively influence neurogenesis and neuroplasticity, processes crucial for brain repair and function.
Studies have shown that this compound promotes adult hippocampal neurogenesis. nih.govnih.gov It has been found to enhance the proliferation of intermediate precursor cells in the subgranular zone of the dentate gyrus, leading to an increased number of newborn neurons. nih.gov The mechanism behind this appears to be the enhancement of Wnt/β-catenin signaling, a pathway crucial for cell proliferation and differentiation. nih.govnih.govresearchgate.net This effect is thought to be mediated by the inhibition of isoprenoid synthesis rather than through cholesterol reduction. nih.govnih.gov
In models of traumatic brain injury (TBI), both this compound and atorvastatin have demonstrated the ability to enhance neurogenesis in the dentate gyrus. mdpi.com This increase in neurogenesis has been associated with several positive outcomes, including:
Increased expression of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF). mdpi.com
Reduced neuronal death in the hippocampus. mdpi.com
Improved spatial learning. mdpi.com
Furthermore, the inhibition of farnesylation, a process affected by this compound, has been linked to the enhancement of long-term potentiation between neurons in mice, a key component of neuroplasticity and learning. mdpi.com
Oncology Research
This compound, a compound primarily known for its lipid-lowering properties, has been the subject of extensive preclinical and clinical research for its potential applications in oncology. Investigations have revealed that its anticancer effects are not merely a consequence of cholesterol reduction but stem from pleiotropic effects that modulate various cellular and molecular pathways integral to cancer progression. frontiersin.orgnih.gov
Antitumor Mechanisms (e.g., Apoptosis, Cell Cycle Arrest)
This compound exerts its antitumor effects through several core mechanisms, primarily by inducing apoptosis (programmed cell death) and causing cell cycle arrest in cancer cells. nih.govoncotarget.com These actions are fundamentally linked to its inhibition of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in the mevalonate pathway. oncotarget.comarchivesofmedicalscience.com The depletion of downstream products of this pathway, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), is crucial for these anticancer activities. archivesofmedicalscience.comspandidos-publications.com
Apoptosis Induction: this compound promotes apoptosis by altering the balance of pro- and anti-apoptotic proteins. nih.govspandidos-publications.com Research has shown that it upregulates the expression of pro-apoptotic proteins like Bax and Bak, while simultaneously down-regulating the anti-apoptotic protein Bcl-2. nih.govspandidos-publications.comresearchgate.net This shift in the Bax/Bcl-2 ratio disrupts the mitochondrial membrane potential, leading to the release of cytochrome c and the subsequent activation of a cascade of caspases, including caspase-3, -8, and -9, which are the executioners of apoptosis. spandidos-publications.comresearchgate.net
Cell Cycle Arrest: A predominant effect of this compound on cancer cells is the induction of cell cycle arrest, most commonly in the G0/G1 phase. oncotarget.comspandidos-publications.come-century.us This halt in proliferation is achieved by modulating the expression of key cell cycle regulators. e-century.us this compound has been observed to downregulate the expression of cyclin D1 and cyclin-dependent kinases (CDKs), such as CDK2 and CDK4. spandidos-publications.come-century.us Concurrently, it increases the expression of CDK inhibitors like p21 and p27, which effectively block the progression of the cell cycle from the G1 to the S phase. archivesofmedicalscience.come-century.us
Table 1: Antitumor Mechanisms of this compound
| Mechanism | Key Molecular Targets/Pathways | Outcome | Supporting Citations |
|---|---|---|---|
| Apoptosis Induction | Upregulation of Bax; Downregulation of Bcl-2; Activation of Caspase-3, -8, -9 | Programmed cell death of cancer cells | nih.govspandidos-publications.comresearchgate.net |
| Cell Cycle Arrest | Downregulation of Cyclin D1, CDK2, CDK4; Upregulation of p21, p27 | Inhibition of cancer cell proliferation at G0/G1 phase | archivesofmedicalscience.comspandidos-publications.come-century.us |
Inhibition of Proliferation, Angiogenesis, and Metastasis
Beyond inducing apoptosis and cell cycle arrest, this compound actively inhibits other critical processes required for tumor growth and spread: proliferation, angiogenesis, and metastasis. mdpi.com
Inhibition of Proliferation: The inhibition of the mevalonate pathway is central to this compound's anti-proliferative effects. nih.gov By blocking the synthesis of FPP and GGPP, this compound prevents the post-translational lipid modification (prenylation) of small GTP-binding proteins like Ras and Rho. frontiersin.orgarchivesofmedicalscience.com These proteins are essential signal transducers in pathways that drive cell proliferation, such as the MAPK/ERK pathway. mdpi.com Their inactivation disrupts downstream signaling, leading to a decrease in cell proliferation. archivesofmedicalscience.commdpi.com
Inhibition of Angiogenesis: Angiogenesis, the formation of new blood vessels, is vital for supplying tumors with nutrients and oxygen. This compound has demonstrated anti-angiogenic properties by reducing the expression of pro-angiogenic factors, most notably Vascular Endothelial Growth Factor (VEGF). frontiersin.orgarchivesofmedicalscience.com It can also suppress angiogenesis by activating AMP-activated protein kinase (AMPK), which in turn blocks the hypoxia-inducible factor-1α (HIF-1α), a key regulator of pro-angiogenic factors. frontiersin.orgnih.gov
Inhibition of Metastasis: Metastasis, the spread of cancer cells to distant sites, is a major cause of cancer-related mortality. mdpi.com this compound can inhibit the key steps of metastasis, including cell migration and invasion. oncotarget.comdovepress.com This is achieved by interfering with the function of the Rho family of proteins, which control the cytoskeletal rearrangements necessary for cell movement. spandidos-publications.com Furthermore, this compound has been shown to decrease the expression and activity of matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9, which are enzymes that degrade the extracellular matrix, allowing cancer cells to invade surrounding tissues. spandidos-publications.comresearchgate.netufmg.br
Cancer Stemness Modulation
Emerging evidence suggests that this compound can target cancer stem cells (CSCs), a subpopulation of tumor cells responsible for tumor initiation, recurrence, and resistance to therapy. spandidos-publications.com Studies have shown that this compound can reduce the population of CSCs, often identified by markers like CD44. frontiersin.orgspandidos-publications.com
The proposed mechanism involves the disruption of the mevalonate pathway, which appears to be crucial for maintaining the "stem-like" state. spandidos-publications.com By inhibiting the synthesis of GGPP, this compound interferes with the function of proteins like RhoA, which are involved in signaling pathways that regulate stemness, such as the Hippo/YAP pathway. spandidos-publications.combioscientifica.com Research indicates that this compound can downregulate the expression of key stemness-related genes, including Oct4, Sox2, and Nanog. spandidos-publications.com By targeting CSCs, this compound may help to overcome chemoresistance and reduce the likelihood of tumor relapse. spandidos-publications.com
Tumor Microenvironment Remodeling
This compound can influence the tumor microenvironment (TME), which is the complex ecosystem of cells, signaling molecules, and extracellular matrix that surrounds a tumor and plays a critical role in its development. frontiersin.orgaacrjournals.org
This compound has been shown to have immunomodulatory effects. frontiersin.org It can promote the repolarization of tumor-associated macrophages (TAMs) from the pro-tumoral M2 phenotype to the anti-tumoral M1 phenotype. frontiersin.org This shift can enhance the anti-cancer immune response. Additionally, some studies suggest statins can improve the function of cytotoxic T cells and natural killer (NK) cells, further bolstering the body's ability to fight the tumor. frontiersin.org this compound may also affect other components of the TME, such as cancer-associated fibroblasts (CAFs), which are known to support tumor growth. researchgate.net
Research in Specific Cancer Types (e.g., Breast Cancer, Lung Cancer, Melanoma, Colon Cancer)
The antitumor effects of this compound have been investigated across a wide range of cancer types in preclinical models.
Breast Cancer: this compound has been shown to inhibit the proliferation of breast cancer cells, including triple-negative breast cancer (TNBC) subtypes. spandidos-publications.comnih.gov It induces apoptosis by modulating Bcl-2 family proteins and activating caspases. spandidos-publications.com It also suppresses invasion by downregulating the expression of genes like PTTG1 and the activity of MMPs. spandidos-publications.comfrontiersin.org
Lung Cancer: In non-small cell lung cancer (NSCLC) models, this compound induces G1 cell cycle arrest and apoptosis. archivesofmedicalscience.comresearchgate.net It has been shown to inhibit tumor growth and metastasis by suppressing pathways like MAPK/ERK and downregulating MMPs. researchgate.netmdpi.com
Melanoma: Research indicates that this compound can induce apoptosis in melanoma cells while sparing normal cells. researchgate.netspandidos-publications.com It inhibits tumor growth and improves survival in murine models, and its effects are linked to the inhibition of Rho-dependent processes and the downregulation of growth factors. researchgate.netspandidos-publications.commdpi.com
Colon Cancer: this compound has been found to inhibit the proliferation and migration of colon cancer cells and promote apoptosis. ufmg.brwjgnet.com Studies suggest these effects are mediated through the modulation of pathways like EGFR/RhoA and the induction of ferroptosis. wjgnet.comfrontiersin.org
Table 2: Preclinical Research Findings of this compound in Specific Cancer Types
| Cancer Type | Key Findings | Observed Mechanisms | Supporting Citations |
|---|---|---|---|
| Breast Cancer | Inhibition of proliferation, invasion, and metastasis; Induction of apoptosis. | G0/G1 cell cycle arrest, caspase activation, suppression of PTTG1 and MMPs. | spandidos-publications.comnih.govfrontiersin.orgwaocp.org |
| Lung Cancer | Inhibition of proliferation and tumor growth; Induction of apoptosis. | G1 cell cycle arrest, inhibition of MAPK/ERK pathway, downregulation of MMPs. | archivesofmedicalscience.comresearchgate.netmdpi.com |
| Melanoma | Inhibition of tumor growth and metastasis; Induction of apoptosis. | Inhibition of Rho-dependent pathways, downregulation of growth factors. | researchgate.netspandidos-publications.commdpi.com |
| Colon Cancer | Inhibition of proliferation and migration; Promotion of apoptosis. | Modulation of EGFR/RhoA pathway, induction of ferroptosis. | ufmg.brwjgnet.comfrontiersin.org |
Combination Therapy Strategies in Cancer
A significant area of research is the use of this compound in combination with standard anticancer treatments, such as chemotherapy, radiotherapy, and targeted therapies. aacrjournals.org The rationale is that this compound may sensitize cancer cells to these treatments, thereby enhancing their efficacy. nih.gov
Chemotherapy: this compound has shown synergistic effects when combined with various chemotherapeutic agents, including doxorubicin and cisplatin. archivesofmedicalscience.comwaocp.org In some cases, it appears to enhance the cytotoxic effects of these drugs, allowing for potentially lower and less toxic doses. waocp.org For instance, in metastatic colorectal cancer, the addition of this compound to a XELOX and bevacizumab regimen was found to be feasible and showed comparable efficacy to the standard treatment alone. e-crt.org
Radiotherapy: Preclinical studies suggest that this compound can act as a radiosensitizer. nih.gov In esophageal cancer, for example, it was found to enhance radiosensitivity by inducing the tumor suppressor PTEN and inhibiting the PI3K/Akt pathway. mdpi.com
Targeted Therapy: this compound has been investigated for its ability to overcome resistance to tyrosine kinase inhibitors (TKIs) in lung cancer. aacrjournals.org Studies in patient-derived models showed that combining this compound with TKIs could overcome acquired resistance and lead to greater tumor reduction. aacrjournals.org It has also been shown to enhance responses to immune checkpoint blockade in models of head and neck cancer. bmj.com
Table 3: Examples of this compound Combination Strategies in Oncology Research
| Combination Agent/Therapy | Cancer Type | Observed Outcome | Supporting Citations |
|---|---|---|---|
| Doxorubicin | Breast Cancer | Synergistic cytotoxic effect in MCF-7 cells. | waocp.org |
| Cisplatin | Lung Cancer | Beneficial effects demonstrated in squamous cell carcinoma. | archivesofmedicalscience.com |
| Radiotherapy | Colorectal Cancer, Esophageal Cancer | Enhanced sensitivity of cancer cells to radiation. | mdpi.comnih.gov |
| Tyrosine Kinase Inhibitors (TKIs) | Lung Adenocarcinoma | Overcame acquired resistance to TKIs. | aacrjournals.org |
| Immune Checkpoint Blockade (anti-PD-1) | Head and Neck Cancer | Enhanced tumor growth delay and survival. | bmj.com |
Immunological and Inflammatory Disease Research
This compound has demonstrated a range of immunomodulatory and anti-inflammatory effects in preclinical and clinical research, independent of its lipid-lowering properties. These effects are being investigated for their therapeutic potential in a variety of inflammatory and autoimmune conditions.
Modulating Inflammatory Cytokines (e.g., TNF-α, IL-6, CRP)
This compound has been shown to modulate the production of several key inflammatory cytokines. In hypercholesterolemic patients, treatment with this compound resulted in a significant decrease in serum levels of C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). ahajournals.orgnih.gov Specifically, one study observed a distinct reduction of CRP and TNF-α in both asymptomatic men with high cholesterol and men with coronary heart disease; however, IL-6 levels only decreased in the group with markedly high cholesterol. nih.gov Another study in hypercholesterolemic patients found that this compound treatment led to a significant decrease in serum levels of IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1). ahajournals.org The reduction in these pro-inflammatory cytokines suggests a direct anti-inflammatory action of this compound. ahajournals.orgjacc.org
In preclinical models, this compound has also demonstrated the ability to suppress inflammatory cytokine production. For instance, in a rat model of lipopolysaccharide-induced inflammation, this compound pretreatment significantly suppressed the peak levels of TNF-α and IL-1β. sav.sk However, the effect on IL-6 was not observed in this particular study. sav.sk In a mouse model of sepsis, this compound treatment reduced the levels of TNF-α, IL-6, IL-1β, and macrophage migration inhibitory factor (MIF) in the peritoneal cavity. nih.gov These findings from various studies highlight the potential of this compound to mitigate inflammatory responses by downregulating the expression and production of key pro-inflammatory cytokines. ahajournals.orgsav.sknih.govoup.com
Table 1: Effect of this compound on Inflammatory Cytokines
| Cytokine | Effect Observed in Human Studies | Effect Observed in Animal Models |
|---|---|---|
| TNF-α | Decreased serum levels nih.govjacc.org | Decreased production sav.sknih.govscielo.br |
| IL-6 | Decreased serum levels ahajournals.orgnih.gov | Decreased production nih.govscielo.br |
| CRP | Decreased serum levels nih.govnih.gov | Not extensively reported in these studies |
| IL-1β | Decreased monocyte expression jacc.org | Decreased production sav.sknih.govscielo.br |
| IL-8 | Decreased serum levels ahajournals.org | Increased release ex vivo nih.gov |
| MCP-1 | Decreased serum levels ahajournals.org | Not extensively reported in these studies |
T-cell Modulation and Lymphocyte Activity
Studies have also explored the impact of this compound on different T-cell subsets. For example, this compound has been shown to induce the expression of Foxp3, a key transcription factor for regulatory T cells (Tregs), which play a crucial role in maintaining immune tolerance. nih.govnih.gov This suggests that this compound may promote an anti-inflammatory environment by enhancing the activity of Tregs. nih.gov Furthermore, some research suggests that this compound can influence the balance between T-helper 1 (Th1) and T-helper 2 (Th2) responses, potentially suppressing Th1-mediated inflammation. archivesofmedicalscience.com In a murine model of allergic asthma, a Th2-driven condition, this compound treatment was associated with a reduction in IL-4 and IL-5 levels, key Th2 cytokines. aai.org Conversely, other studies indicate a potential for this compound to suppress Th2 responses. researchgate.net In a study on dendritic cells, this compound was found to promote Th2-type responses through the induction of the chitinase family member Ym1. pnas.org
Research in Autoimmune Diseases (e.g., Uveitis, Rheumatoid Arthritis)
The immunomodulatory properties of this compound have led to research into its potential therapeutic role in autoimmune diseases such as uveitis and rheumatoid arthritis. elsevier.esrusimmun.ru
In the context of non-infectious uveitis, an inflammatory condition of the eye, statins have shown promise. elsevier.esrusimmun.rumdpi.com Animal models of experimental autoimmune uveoretinitis have demonstrated that statins can reduce the clinical and histological signs of inflammation and inhibit the recruitment of T lymphocytes into the retina. nih.gov Observational studies in humans have also suggested a protective effect of statins against the development of uveitis. elsevier.esnih.gov A pilot study investigating this compound as an adjunct to conventional therapy for non-infectious uveitis found that it was associated with better control of inflammation and improved visual acuity. tandfonline.com
In research related to rheumatoid arthritis, statins have been shown to inhibit the production of pro-inflammatory cytokines by synovial cells. elsevier.es In vitro studies have demonstrated that this compound can suppress the production of pro-inflammatory cytokines by T-cell contact-activated macrophages, highlighting its potential to modulate the inflammatory processes central to rheumatoid arthritis. researchgate.net
Immunomodulation in Sepsis and Acute Inflammatory Conditions
This compound's immunomodulatory effects are also being investigated in the context of sepsis and other acute inflammatory conditions. frontiersin.orgahajournals.org Sepsis is characterized by a dysregulated systemic inflammatory response to infection. frontiersin.orgnih.gov
Preclinical studies using animal models of sepsis have shown that this compound can improve survival rates. frontiersin.orgahajournals.orgnih.gov In a murine model of sepsis induced by cecal ligation and puncture (CLP), prophylactic treatment with this compound was found to increase animal survival. frontiersin.orgnih.gov This was associated with a modulation of the immune response, including an increase in the activation and proliferation of CD4+ T cells and an increase in the production of IL-6 and MCP-1, suggesting a shift towards an effective antimicrobial response rather than a purely anti-inflammatory effect. frontiersin.org Another study showed that treatment with this compound after the onset of sepsis also improved survival in a CLP mouse model. ahajournals.org Furthermore, this compound has been shown to reduce the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in animal models of sepsis. nih.govscielo.br In a mouse model of acute inflammation induced by Staphylococcus aureus α-toxin, this compound was found to inhibit leukocyte rolling, adherence, and transmigration, key steps in the inflammatory cascade. ahajournals.org
Metabolic Pathways Research (Beyond Cholesterol)
Beyond its well-established role in cholesterol synthesis, research has begun to explore the effects of this compound on other metabolic pathways, particularly amino acid metabolism.
Amino Acid Metabolism
Metabolomic studies have revealed that this compound treatment can influence amino acid metabolism. frontiersin.orgplos.orgnih.gov A study using a metabolomics platform to analyze the effects of this compound in participants found that the metabolic signature of drug exposure included changes in essential amino acids. plos.orgnih.gov The metabolites affected were enriched for the amino acid degradation pathway. plos.orgnih.gov
Fatty Acid Metabolism
This compound's influence extends beyond cholesterol synthesis to affect various aspects of fatty acid metabolism. Research indicates that by inhibiting HMG-CoA reductase, statins may increase the availability of acetyl-CoA for other metabolic processes, including fatty acid synthesis. ahajournals.org Studies in hypercholesterolemic men have shown that this compound treatment alters the composition of serum fatty acids. ahajournals.orgahajournals.org Specifically, it decreases the proportions of palmitic acid, linoleic acid, and α-linolenic acid, while increasing the proportions of γ-linolenic acid, dihomo-γ-linolenic acid, and arachidonic acid. ahajournals.orgahajournals.org These changes suggest that this compound enhances the activity of fatty acid elongase and desaturase enzymes, promoting the formation of long-chain polyunsaturated fatty acids. ahajournals.orgahajournals.org
In animal models, this compound has demonstrated effects on lipid accumulation. In high-fat diet-induced hyperlipidemic rats, this compound administration significantly inhibited lipid accumulation in the liver and reduced lipid deposition in both adipocytes and hepatocytes. frontiersin.org Conversely, in mice with skeletal muscle PGC-1α overexpression, this compound led to an increased accumulation of lipid droplets in the skeletal muscle. mdpi.com It has been suggested that statins may activate peroxisome proliferator-activated receptor-α (PPAR-α), which boosts the liver's uptake of fatty acids and promotes their beta-oxidation, thereby reducing the availability of fatty acids. unito.it
Table 1: Effect of this compound on Serum Fatty Acid Proportions
| Fatty Acid | Change Reported | Suggested Mechanism | Citation |
|---|---|---|---|
| Palmitic Acid | ▼ 2.0% Decrease | Altered fatty acid metabolism | ahajournals.orgahajournals.org |
| Linoleic Acid | ▼ 5.3% Decrease | Increased conversion to derivatives | ahajournals.orgahajournals.org |
| α-Linolenic Acid | ▼ 6.8% Decrease | Increased conversion to derivatives | ahajournals.orgahajournals.org |
| γ-Linolenic Acid | ▲ 11.1% Increase | Increased Δ6-desaturase activity | ahajournals.orgahajournals.org |
| Dihomo-γ-linolenic Acid | ▲ 4.2% Increase | Increased fatty acid elongase activity | ahajournals.orgahajournals.org |
| Arachidonic Acid | ▲ 14.2% Increase | Increased Δ5-desaturase activity | ahajournals.orgahajournals.org |
Nucleotide, Carbohydrate, Cofactor, and Vitamin Metabolism
This compound's metabolic influence includes complex interactions with carbohydrate, cofactor, and vitamin pathways.
Carbohydrate Metabolism: Research has yielded varied results regarding this compound's impact on glucose homeostasis. In-vitro studies on human myotubes show that this compound can reduce glucose uptake and oxidation in a dose-dependent manner. plos.org Both the lactone and active acid forms of this compound have been found to decrease glycolysis and mitochondrial ATP production in primary human muscle cells. nih.gov However, the two forms show differential effects on glucose storage; the lactone form increases glycogen synthesis, whereas the acid form impairs it. nih.gov
Clinical and animal studies present a conflicting picture. Some studies report that this compound may increase plasma glucose levels and decrease insulin sensitivity. scielo.brmdpi.comfrontiersin.org The proposed mechanisms include the inhibition of glucose-induced insulin secretion. scielo.brscielo.br This could be due to a reduction in the synthesis of coenzyme Q10 (CoQ10), which is essential for ATP production required for insulin release. e-dmj.org In contrast, other studies have reported no significant effect or even an improvement in glycemia. scielo.brscielo.br
Cofactor and Vitamin Metabolism: The impact of this compound on cofactors and vitamins is also an area of active investigation with some contradictory findings. As an inhibitor of the mevalonate pathway, this compound inherently reduces the synthesis of CoQ10, an essential component of the mitochondrial electron transport chain. e-dmj.orgahajournals.orgfrontiersin.org Studies have confirmed that this compound treatment can lower CoQ10 levels in muscle tissue and endothelial cells. ahajournals.orgmdpi.com
Regarding fat-soluble vitamins, one study in hypercholesterolemic patients found that 8 weeks of this compound treatment significantly increased the lipid-corrected plasma levels of retinol (vitamin A), α-tocopherol (vitamin E), and γ-tocopherol, while levels of CoQ10 and carotenoids remained unchanged. nih.gov Conversely, other sources suggest that statins may interfere with the absorption of fat-soluble vitamins (A, D, E, and K) and reduce the production of vitamin K2, which is also synthesized via the mevalonate pathway. ivdrip.uk The effect on vitamin D is particularly controversial, with different studies reporting either increased or decreased levels in patients taking statins. ameliaheartcenter.com
Table 2: Summary of this compound's Effects on Various Metabolites
| Metabolite/Process | Observed Effect | Context/Study Type | Citation |
|---|---|---|---|
| Glucose Uptake | ▼ Decreased | Human myotubes | plos.org |
| Glycogen Synthesis | ▼ Decreased (acid form) | Primary human muscle cells | nih.gov |
| Insulin Sensitivity | ▼ Decreased | Some human and animal studies | scielo.brmdpi.com |
| Coenzyme Q10 (CoQ10) | ▼ Decreased | Muscle tissue, endothelial cells | ahajournals.orgmdpi.com |
| Vitamin A (Retinol) | ▲ Increased (lipid-corrected) | Hypercholesterolemic patients | nih.gov |
| Vitamin E (Tocopherol) | ▲ Increased (lipid-corrected) | Hypercholesterolemic patients | nih.gov |
| Vitamin D | conflicted reports | General patient populations | ameliaheartcenter.com |
Gut Microbiota Interactions
This compound interacts with the gut microbiota in a bidirectional manner; the drug can alter the composition of the gut microbiome, and the microbes can, in turn, metabolize the drug. frontiersin.orgfrontiersin.orgnih.gov In hyperlipidemic rats, this compound treatment was found to reduce microbial diversity and significantly remodel the composition of the fecal bacterial community. frontiersin.orgmdpi.com Notably, administration of this compound led to an increase in the abundance of certain bacteria, such as Ruminococcaceae and Lactobacillus. frontiersin.orgfrontiersin.org These alterations in the gut microbiota are believed to contribute to the lipid-lowering efficacy of this compound. mdpi.comfrontiersin.org
Research also shows that gut bacteria can metabolize this compound. plos.org In vitro studies demonstrate that this compound is subject to bioaccumulation within bacterial cells and can be biotransformed by bacterial enzymes, with the lactone ring being a likely site for reactions like hydrolysis. frontiersin.orgnih.gov This microbial metabolism could be an underlying mechanism for the varied bioavailability and therapeutic effects of this compound observed among individuals. frontiersin.orgnih.gov However, compared to other statins like fluvastatin, this compound's effect on altering the gut bacterial composition was found to be relatively static in one in vitro fermentation study. plos.org
Table 3: Reported Changes in Gut Microbiota with this compound Treatment
| Bacterial Group | Change in Abundance | Model System | Citation |
|---|---|---|---|
| Ruminococcaceae | ▲ Increased | High-fat diet-fed rats | frontiersin.orgfrontiersin.org |
| Lactobacillus | ▲ Increased | High-fat diet-fed rats | frontiersin.orgfrontiersin.org |
| Overall Diversity | ▼ Decreased | High-fat diet-fed rats | mdpi.com |
Novel Therapeutic Applications and Research Areas
Uterine Fibroids
A novel area of research is the potential application of this compound for the treatment of uterine fibroids (leiomyomas), the most common benign tumors of the female reproductive system. utmb.edu Preclinical studies have shown that this compound exhibits anti-tumor effects on uterine fibroid cells. utmb.eduresearchgate.net Research has demonstrated that this compound impedes the growth of human uterine fibroid cells by inhibiting a critical step in a molecular pathway that promotes cell proliferation. utmb.edu Furthermore, it has been shown to halt the progression of existing tumor cells and trigger calcium-dependent cell death (apoptosis) in fibroid tumor cells. utmb.edu
Observational studies in humans have found that statin use is associated with a lower risk of developing uterine fibroids and experiencing fibroid-related symptoms such as menorrhagia and pelvic pain. researchgate.net Based on these promising preclinical findings, a phase II, double-blind, placebo-controlled clinical trial was initiated to formally evaluate the efficacy of this compound in reducing the size of uterine fibroids in women scheduled for surgery. clinicaltrials.govdrugbank.com This research signals a potential new, non-hormonal therapeutic strategy for managing uterine fibroids. researchgate.net
Table 4: Research Findings on this compound for Uterine Fibroids
| Finding | Mechanism of Action | Study Type | Citation |
|---|---|---|---|
| Inhibits fibroid cell growth | Inhibition of ERK phosphorylation pathway | In vitro (human cells) | utmb.edu |
| Induces cell death | Triggers calcium-dependent apoptosis | In vitro (human cells) | utmb.edu |
| Decreases risk of fibroids | Not specified | Observational human study | researchgate.net |
| Reduces fibroid-related symptoms | Not specified | Observational human study | researchgate.net |
| Under investigation to reduce fibroid size | Add-on to ongoing medical management | Phase II Clinical Trial | clinicaltrials.govdrugbank.com |
Bone Turnover and Neovascularization
This compound has been identified as a potent modulator of bone metabolism, with research highlighting its dual capacity to promote bone formation and inhibit bone resorption. mdpi.comdovepress.com The anabolic effect on bone is largely attributed to its ability to stimulate the differentiation of osteoblasts, the cells responsible for bone formation. mdpi.comnih.gov This is achieved by upregulating the expression of key osteogenic factors, including Bone Morphogenetic Protein-2 (BMP-2) and osteocalcin. dovepress.comnih.govnih.gov Simultaneously, this compound inhibits bone resorption by reducing the activity and number of osteoclasts, the cells that break down bone tissue. mdpi.comnih.gov
A meta-analysis of randomized controlled trials confirmed that statins increase levels of the bone formation biomarker osteocalcin (OC) while decreasing levels of bone resorption markers such as C-terminal telopeptide of type I collagen (CTX) and N-terminal telopeptide (NTX). jst.go.jp
Crucially, the processes of bone regeneration and blood vessel formation (neovascularization) are tightly linked. This compound also promotes neovascularization by increasing the gene expression of Vascular Endothelial Growth Factor (VEGF) in osteoblastic cells. mdpi.comnih.govoup.com This stimulation of new blood vessel growth is vital for supplying oxygen and nutrients to support the reconstruction of bone tissue. mdpi.com
Table 5: Effects of this compound on Bone Turnover and Neovascularization
| Process | Effect | Key Mediators | Citation |
|---|---|---|---|
| Bone Formation | |||
| Osteoblast Differentiation | ▲ Stimulated | BMP-2, Osteocalcin | dovepress.comnih.govnih.gov |
| Bone Formation Marker (OC) | ▲ Increased | Increased osteoblast activity | jst.go.jp |
| Bone Resorption | |||
| Osteoclast Activity | ▼ Inhibited | Reduced osteoclast number/fusion | mdpi.comnih.gov |
| Bone Resorption Markers (CTX, NTX) | ▼ Decreased | Reduced osteoclast activity | jst.go.jp |
| Neovascularization | |||
| Angiogenesis | ▲ Promoted | VEGF | mdpi.comnih.govoup.com |
Pharmacogenomics and Inter-individual Variability Research
Genetic Polymorphisms and Statin Response
Genetic variations can significantly alter the pharmacokinetics and pharmacodynamics of simvastatin, leading to different treatment outcomes. Key genes implicated in this variability include those responsible for transporting the drug into the liver, metabolizing it, and regulating lipid levels.
The SLCO1B1 gene encodes the organic anion transporting polypeptide 1B1 (OATP1B1), a transporter protein that facilitates the uptake of this compound and its active acid form into hepatocytes, the primary site of action. hee.nhs.ukumich.edu Genetic variants that reduce the function of this transporter can lead to decreased hepatic uptake of this compound, resulting in higher plasma concentrations of the drug and an increased risk of muscle-related side effects (myopathy). hee.nhs.uknih.govgoffinmoleculartechnologies.com
The most studied variant is the c.521T>C (rs4149056) single nucleotide polymorphism (SNP), where a cytosine (C) replaces a thymine (T) at position 521. goffinmoleculartechnologies.comgbcbiotech.com This substitution leads to the Val174Ala amino acid change and reduced transporter function. oup.com
Research findings indicate a strong association between the c.521C allele and this compound-induced myopathy. gbcbiotech.com
Individuals carrying at least one copy of the C allele (TC genotype) have a significantly increased risk of myopathy compared to those with the normal TT genotype. nih.govarkansasbluecross.com The odds ratio for myopathy is approximately 4.5 per copy of the C allele. gbcbiotech.com
Patients with the CC genotype exhibit the highest risk. nih.govjppres.com Studies have shown that patients with the SLCO1B1 521CC genotype had a significantly higher risk of myopathy and rhabdomyolysis compared to those with the TT genotype. jppres.com
The presence of the c.521T>C variant leads to increased plasma levels, area under the plasma concentration curve (AUC), and maximum plasma concentration (Cmax) of this compound. jppres.com Patients with the TC and CC genotypes showed 71% and 248% higher concentrations of this compound acid, respectively, compared to carriers of the TT genotype. jppres.com
The Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG) have issued guidelines recommending alternative statins or dose adjustments for individuals with decreased or poor SLCO1B1 function (i.e., carriers of the c.521C allele) to mitigate the risk of myopathy. hee.nhs.uknih.govpharmgkb.org
Table 1: Impact of SLCO1B1 c.521T>C Genotype on this compound Response
Genotype Phenotype (OATP1B1 Function) Effect on this compound Plasma Concentration Associated Myopathy Risk TT (Wild-type) Normal Function Normal Baseline Risk TC (Heterozygous) Decreased Function Increased (e.g., ~2.6-fold higher risk at 40mg/day) jppres.com Significantly Increased [3, 8] CC (Homozygous) Poor Function Substantially Increased (e.g., up to 248% higher SVA) hee.nhs.uk Highest Risk (e.g., ~18% cumulative risk with 80mg dose)
This compound is primarily metabolized in the liver and intestines by the cytochrome P450 3A4 (CYP3A4) and, to a lesser extent, CYP3A5 enzymes. numberanalytics.complos.org Genetic variations in these enzymes can alter the rate of this compound metabolism, thereby affecting its plasma concentration and efficacy. plos.orgnih.gov
CYP3A4: The CYP3A422 allele (rs35599367) is associated with reduced CYP3A4 enzyme activity. jppres.comnumberanalytics.com Carriers of this allele tend to have higher plasma concentrations of this compound. jppres.comresearchgate.net One study found that the bioavailability of this compound was 49% higher in individuals with the CYP3A41/22 genotype compared to CYP3A41/1 individuals. nih.gov However, studies on whether this translates to a better lipid-lowering response have produced conflicting results. researchgate.netpharmgkb.org
CYP3A5: The CYP3A53 allele (rs776746) results in a non-functional protein. plos.org Individuals who are homozygous for this allele (CYP3A53/*3) are considered "non-expressors." Conversely, carriers of the CYP3A51 allele produce a functional enzyme. Some studies have shown that CYP3A5 expressors (carriers of CYP3A51) have lower this compound concentrations and may experience a reduced lipid-lowering effect compared to non-expressors. plos.orgscielo.br Combined genotypes of CYP3A4 and CYP3A5 have been shown to significantly affect this compound plasma concentrations. plos.orgnih.gov
CYP2C9: While CYP2C9 is the primary metabolizing enzyme for fluvastatin, its role in this compound metabolism is considered minor. umich.edunih.govsci-hub.se Therefore, variants in the CYP2C9 gene, such as CYP2C93, have a more pronounced effect on the pharmacokinetics of fluvastatin than on this compound. sci-hub.seingentaconnect.com
ATP-binding cassette (ABC) transporters are involved in the efflux of drugs and their metabolites from cells. Variants in the genes encoding these transporters can influence the pharmacokinetics and response to this compound.
ABCB1: This gene encodes P-glycoprotein (P-gp), an efflux transporter. pharmgkb.org Some studies suggest that variants in ABCB1, such as C3435T (rs1045642) and G2677T/A (rs2032582), are associated with variability in the cholesterol-lowering effect of this compound. oup.comnih.govpharmgkb.org For instance, one study found that male carriers of the C3435T variant had a significantly smaller reduction in LDL cholesterol. oup.com Another study reported that homozygous carriers of the C allele at rs1045642 had a better reduction in non-HDL cholesterol. nih.gov However, other research has found no significant effects of some ABCB1 variants on this compound pharmacokinetics, indicating that its role may be complex or less critical than that of SLCO1B1. ingentaconnect.com
ABCG2: The ABCG2 gene encodes the breast cancer resistance protein (BCRP), another efflux transporter. nih.govcpicpgx.org The c.421C>A (p.Gln141Lys, rs2231142) variant results in a loss-of-function protein. cpicpgx.orgnih.gov This variant has been shown to significantly increase the plasma concentrations of several statins, most notably rosuvastatin. cpicpgx.orgnih.govpharmgkb.org While some clinical trials have shown that this variant can affect the pharmacokinetics of this compound, its impact is generally considered less pronounced than its effect on rosuvastatin. ingentaconnect.comnih.gov
The apolipoprotein A5 (APOA5) gene plays a crucial role in triglyceride metabolism. nih.govplos.org Genetic variants in this locus have been linked to variations in the lipid-lowering response to statin therapy.
A study investigating the APOA5 promoter SNP rs662799 (-1131T>C) found that this genotype influenced the efficacy of this compound. nih.gov Specifically, changes in HDL cholesterol and triglycerides were affected by the APOA5 genotype in subjects treated with this compound. nih.govbiorxiv.org Carriers of the -1131C allele (AG or GG genotypes) showed a significantly smaller reduction in LDL cholesterol in response to statin treatment compared to those with the common TT genotype. pharmgkb.orgtandfonline.com
ABCB1 and ABCG2 Gene Variants
Predictive Biomarkers for Efficacy and Response Variation
Identifying biomarkers that can predict a patient's response to this compound is a key goal of pharmacogenomic and metabolomic research. Such markers could help clinicians select the most appropriate therapy and dose for individual patients, maximizing efficacy while minimizing risks.
The genetic polymorphisms detailed above, particularly in SLCO1B1, serve as important predictive biomarkers for this compound-associated myopathy risk. nih.govgoffinmoleculartechnologies.com Beyond genetics, metabolomics studies have identified several small molecules whose baseline levels or changes during treatment could predict the drug's efficacy.
A metabolomics study profiled participants before and after six weeks of this compound treatment and identified several potential biomarkers: plos.org
Baseline Predictors: The pre-treatment plasma levels of certain metabolites were found to predict the magnitude of the LDL-C lowering response. Lower baseline levels of xanthine, 2-hydroxyvaleric acid, succinic acid, and stearic acid were correlated with a greater LDL-C reduction. plos.org
Response Correlates: The change in levels of specific metabolites after treatment correlated with the LDL-C response. These included cystine and intermediates of the urea cycle such as ornithine, citrulline, and lysine. plos.org
Drug Exposure Signature: this compound treatment was also found to significantly alter plasma levels of alpha-tocopherol, lauric acid, and several amino acids, including threonine and phenylalanine. plos.org
In the context of cancer research, studies have explored biomarkers to predict the anti-neoplastic effects of statins. One such study identified that the expression of Voltage-Dependent Anion Channel 1 (VDAC1) was positively correlated with this compound response in ovarian cancer cells, while Low-Density Lipoprotein Receptor Adaptor Protein 1 (LDLRAP1) was negatively correlated. mdpi.comresearchgate.net These findings suggest that VDAC1 and LDLRAP1 could serve as predictive biomarkers for statin efficacy in specific therapeutic areas beyond hyperlipidemia. mdpi.com
Table 2: Potential Predictive Biomarkers for this compound Response
Biomarker Category Biomarker Name Association with this compound Response Source Type Genetic SLCO1B1 c.521T>C Predicts risk of myopathy and higher plasma concentrations. nih.gov Pharmacogenomics CYP3A4*22 / CYP3A5*3 Associated with higher plasma concentrations; variable effect on efficacy. [1, 27] Pharmacogenomics ABCB1 / ABCG2 Variants Associated with variability in cholesterol-lowering effect. [5, 7] Pharmacogenomics APOA5 rs662799 Influences changes in triglycerides and HDL-C. scielo.br Pharmacogenomics Metabolic (Baseline) Xanthine Lower levels predict better LDL-C response. oup.com Metabolomics 2-hydroxyvaleric acid Lower levels predict better LDL-C response. oup.com Metabolomics Succinic acid Lower levels predict better LDL-C response. oup.com Metabolomics Stearic acid Lower levels predict better LDL-C response. oup.com Metabolomics Fructose Among metabolites separating "good" and "poor" responders. oup.com Metabolomics Molecular (Cancer) VDAC1 Positive correlation with this compound response. nih.gov Transcriptomics LDLRAP1 Negative correlation with this compound response. nih.gov Transcriptomics
Methodological Considerations in Simvastatin Research
Clinical Trial Design and Implementation
Clinical trials evaluating simvastatin are meticulously designed to assess its efficacy and impact on various health outcomes. These trials often feature rigorous methodologies, including double-masking, randomization, and multicenter participation, to ensure the reliability and generalizability of their findings plos.orgbmj.comnih.govresearchgate.netclinicaltrialsregister.eunih.gov. Key aspects of their implementation involve defining primary and secondary outcome measures, selecting appropriate participant populations, and monitoring compliance and adverse events plos.orgnih.govclinicaltrialsregister.eunih.govnih.gov.
Randomized Controlled Trials (RCTs) represent the gold standard in clinical research, providing robust evidence for the causal effects of interventions. This compound has been extensively investigated in numerous RCTs across a spectrum of conditions.
Key this compound RCTs and Findings:
| Study Focus | Study Design | Participants | Key Findings | Citation |
| Age-Related Macular Degeneration (AMD) Progression | Double-masked, randomized, placebo-controlled | 114 participants (53-91 years) with intermediate AMD | This compound (40 mg/day) significantly decreased AMD progression risk by 2-fold (OR 0.43). The effect was more pronounced in bilateral intermediate AMD (OR 0.23) and those with the CFH gene CC (Y402H) genotype (OR 0.08). | plos.org |
| Alzheimer's Disease (AD) | Randomized, double-blind, placebo-controlled | 406 individuals with mild to moderate AD | This compound lowered lipid levels but showed no effect on ADAS-Cog score or other secondary cognitive outcome measures over 18 months. | nih.gov |
| Multiple Sclerosis (MS-STAT Phase 2) | Double-blind, randomized, controlled, Phase 2 | Not specified (secondary progressive MS patients) | This compound (80 mg/day) reduced brain atrophy rate and was associated with beneficial effects on cognitive and disability outcomes over two years. | biorxiv.orgnih.gov |
| Multiple Sclerosis (MS-STAT2 Phase 3) | Randomized, multicenter, double-blind, parallel-group, Phase 3 | 235 participants with secondary progressive MS | Designed to determine if this compound (80 mg/day) can slow the time to confirmed disability progression (CDP) based on EDSS score. Initial findings suggest it is unlikely to slow the rate of progression of Parkinson's disease, leading to a recommendation against further trials for this purpose. | bmj.complymouth.ac.uk |
| Psoriasis Severity | Meta-analysis of 5 RCTs | 223 patients (128 received statins) | This compound significantly improved Psoriasis Area and Severity Index (PASI) values (Mean Difference = 3.70, 95% CI: 2.52–4.89, p < 0.001). | archivesofmedicalscience.com |
| Hypertension | Double-blind, parallel, placebo-controlled | 79 hypertensive patients | This compound (40 mg) significantly lowered 24-hour diastolic blood pressure (2.8 mmHg), daytime systolic blood pressure (4.2 mmHg), and daytime diastolic blood pressure (3.1 mmHg), particularly in patients with higher baseline cholesterol levels. | researchgate.net |
| Periodontal Maintenance Therapy | Double-masked, randomized, controlled | Patients with persistent 6–9 mm periodontal pockets | Local application of this compound improved clinical attachment level (CAL), reduced probing depth (PD), and decreased bleeding on probing (BOP). | nih.gov |
These trials demonstrate the rigorous approach to evaluating this compound's effects, from its primary lipid-lowering capabilities to its potential pleiotropic effects in diverse disease contexts. For instance, the Aggrastat to Zocor (A to Z) trial compared intensive and moderate statin therapy after acute coronary syndromes, with this compound being a component of the therapy nih.gov.
Observational studies and database research provide valuable insights into the real-world usage patterns and long-term effects of this compound, complementing the controlled environment of RCTs. These studies often leverage large administrative databases, such as managed care claims data pfizer.comnih.gov.
For example, a large observational study utilizing data from over 186,000 patients in a U.S. managed care claims database investigated statin persistence, finding that new users of atorvastatin were significantly more likely to remain on their medication compared to those taking this compound pfizer.com. Another observational study, using claims data from 92 U.S. managed care plans, assessed cardiovascular outcomes in 98,471 hypertensive patients without prior cardiovascular disease who initiated atorvastatin or this compound therapy. This study concluded that atorvastatin was associated with a significantly lower risk of subsequent cardiovascular events compared to this compound, even after adjusting for clinical and demographic confounders nih.gov.
Real-World Evidence (RWE) studies offer a practical perspective on how this compound is used and performs in routine clinical practice, beyond the strict inclusion and exclusion criteria of traditional RCTs.
Mechanistic studies integrated within clinical trials aim to elucidate the biological pathways and molecular changes underlying a drug's observed clinical effects. This approach helps to understand how a treatment works, rather than just if it works.
A notable example is the application of mechanistic models to the MS-STAT Phase 2 clinical trial of this compound in secondary progressive multiple sclerosis. This research investigated the hypothesized pathways linking this compound to clinical outcomes. The findings suggested that the beneficial effects of this compound on reducing the rate of brain atrophy and slowing disability progression were independent of its serum cholesterol-lowering effects biorxiv.orgnih.gov. This compound was found to directly affect motor functioning and indirectly by slowing atrophy rates, with a weaker effect on visuospatial memory mediated by reduced atrophy rates nih.gov. These studies demonstrate the utility of computational models, such as structural equation models, in uncovering the causal architecture of treatment effects in complex diseases nih.gov.
Real-World Evidence Studies
Preclinical Models and In Vitro Studies
Preclinical models and in vitro studies are fundamental to understanding the basic biological and pharmacological properties of this compound before or in parallel with human clinical trials. These studies allow for controlled investigations into cellular and molecular mechanisms, as well as initial assessments of efficacy in living organisms.
Overview of Preclinical and In Vitro Research:
| Study Type | Model System | Key Findings | Citation |
| In Vitro Studies | |||
| Metabolic Profiling | Rat liver microsomes (RLMs), isolated perfused rat liver hepatocytes (RLHs) | Identified 29 this compound metabolites, including new phase-I metabolites (exomethylene this compound acid, monohydroxy SVA, dihydrodiol SVA). | mdpi.com |
| Uterine Fibroids | Human and rat leiomyoma cells | This compound induced dose-dependent apoptosis and inhibited proliferation (significant at 5 and 10 μM); decreased Akt signaling pathway phosphorylation. | nih.gov |
| Post-Traumatic Elbow Contracture | Collagen gel contraction assay (fibroblasts/myofibroblasts) | This compound (≥10 μM) prevented gel contraction, partly by decreasing cell viability (at 100 μM). | frontiersin.org |
| Mantle Cell Lymphoma (MCL) | MCL cell cultures | This compound impaired MCL proliferation, triggered caspase-independent, ROS-mediated cell death, and inhibited migration/invasion by suppressing AKT/mTOR signaling pathway. | mdpi.com |
| Anti-cancer effects (general) | Various cancer cell lines | This compound inhibits cancer cell growth by inducing apoptosis and inhibiting cell cycle progression through various cell signaling pathways. | oncotarget.com |
Animal models, particularly mice and rats, are widely used in this compound research to investigate its effects in a whole-organism context, often focusing on its anti-inflammatory and immunomodulatory properties, especially in models where cholesterol-lowering effects might be less prominent aai.org.
Key Animal Model Studies with this compound:
| Animal Model | Condition Studied | Key Findings | Citation |
| Mice, Rats, Rabbits (Meta-analysis) | Cholesterol lowering, cardiovascular disease | Statins lowered total cholesterol: rabbits (-30%), mice (-20%), rats (-10%). Reduction was greater in animals on a high-cholesterol diet. Statins reduced infarct volume but showed inconsistent effects on blood pressure or overall survival. | nih.govresearchgate.net |
| Ovarian Cancer (Mouse xenograft model) | Tumor growth and metastasis | This compound (3 mg/kg/day) significantly inhibited orthotropic xenograft growth, decreased VEGF levels in serum and tumor tissues, and inhibited MAPK and AKT/mTOR pathways. | oncotarget.com |
| Murine Lupus Model (gld.apoE−/− mice) | Autoimmune disease, atherosclerosis | This compound (0.125 mg/kg/day) decreased atherosclerotic lesion area by ~25%, reduced lymphadenopathy, splenomegaly, and ANA titer. It also decreased apoptotic cells in lymph nodes, without altering plasma cholesterol levels in these specific mice. | aai.org |
| Post-Traumatic Elbow Contracture (Rat injury model) | Joint fibrosis and cartilage damage | This compound modestly reduced capsule fibrosis and lessened cartilage damage, particularly in the anterior region of the joint. | frontiersin.org |
| Fragile X Syndrome (Fmr1-/y mouse model) | Neurological phenotypes | This compound did not correct excessive hippocampal protein synthesis or reduce audiogenic seizures, in contrast to lovastatin, suggesting differential mechanisms of action between statins in this model. | eneuro.org |
| Uterine Leiomyoma (Patient-derived xenograft mouse model) | Tumor growth | This compound (20 μg/gm body weight/day) inhibited tumor growth and decreased the expression of the proliferation marker Ki67 in xenograft tumor tissue. | nih.gov |
| Diet-Induced Hypercholesterolemia (Rat supraspinatus tendon model) | Tendon mechanical and histological properties | Three months of this compound treatment (20 mg/kg) improved tendon biomechanical properties (increased cross-sectional area, decreased modulus at insertion) and histological properties (more spindle-shaped cells in midsubstance). | nih.gov |
| Mantle Cell Lymphoma (Chick embryo chorioallantoic membrane xenograft model) | Tumor growth and metastasis | This compound significantly inhibited MCL tumor growth and the infiltration of chick embryo's bone marrow and spleen by tumor B cells. | mdpi.com |
These preclinical and animal studies are crucial for identifying potential therapeutic avenues, understanding the molecular underpinnings of this compound's effects, and guiding the design of subsequent clinical investigations.
Q & A
Q. What experimental designs are commonly used to optimize simvastatin formulations, and how do they ensure robustness?
Researchers employ statistical experimental designs such as D-optimal design and full-factorial design to evaluate critical variables in formulation development. For example, D-optimal design was applied to assess HPLC method robustness for this compound purity testing, analyzing variables like mobile phase composition, pH, and column temperature. This design minimizes experimental runs while maximizing predictive accuracy, with coefficients of determination (R² > 0.95) confirming model validity . Similarly, full-factorial designs optimize transdermal patches by testing factors like croscarmellose sodium concentration and compression force, enabling predictive polynomial equations for dissolution profiles .
Q. What are standard methodologies for quantifying this compound in pharmacokinetic studies?
High-performance liquid chromatography (HPLC) remains the gold standard. A validated protocol includes monitoring retention time, theoretical plates, and peak asymmetry. For instance, a study using Modde® software demonstrated HPLC robustness across variable ranges, with resolution of critical impurity pairs (e.g., A/I, E/F) ensuring accurate quantification . Dialysis techniques are also used to purify this compound in lipid nanoparticles, with ANOVA confirming no significant difference in free drug removal after 15 vs. 30 minutes (p > 0.05) .
Q. How are clinical trial endpoints selected for evaluating this compound’s efficacy in cardiovascular disease?
Primary endpoints often include carotid intima-media thickness (CIMT) and LDL cholesterol reduction . In a 24-month trial, this compound combined with ezetimibe reduced LDL by 16.5% (p < 0.01) but showed no significant CIMT improvement compared to monotherapy, highlighting the complexity of linking lipid metrics to clinical outcomes . Subgroup analyses in diabetic patients with coronary heart disease prioritized major CHD events (RR = 0.45, p = 0.002) and total mortality (RR = 0.57, p = 0.087) as key endpoints .
Advanced Research Questions
Q. How can researchers address contradictions between this compound’s biochemical efficacy and clinical outcomes?
Contradictions, such as LDL reduction without atherosclerosis improvement, require subgroup analysis and meta-regression . For example, in familial hypercholesterolemia trials, this compound + ezetimibe reduced LDL and CRP but did not alter CIMT progression, suggesting pleiotropic effects may not translate to structural benefits. Researchers must stratify data by risk factors (e.g., diabetes, baseline CRP) and apply multivariate models to isolate confounding variables . PRISMA-guided systematic reviews further contextualize contradictions by aggregating data across study designs .
Q. What advanced statistical models are used to optimize this compound delivery systems?
Response surface methodology (RSM) with Box-Behnken designs is widely used. A study optimizing transdermal patches employed a three-factor, three-level design to model this compound permeation, achieving a quadratic model with R² = 0.9948. Derringer’s desirability function predicted optimal permeation (78.77 µg/cm²) at specific poloxamer and D-limonene concentrations . Similarly, Design Expert® software analyzes dissolution data via ANOVA and multiple linear regression, identifying significant interactions between formulation variables .
Q. How can researchers investigate this compound’s pleiotropic effects in non-cardiovascular contexts, such as neuroprotection or bone metabolism?
Preclinical models use immunohistochemistry and longitudinal MRI to assess mechanisms. In experimental periodontitis, this compound reduced alveolar bone loss by downregulating RANKL (p < 0.05) and upregulating osteoprotegerin (OPG), confirmed via immunofluorescence . For neuroprotection, rodent ICH models combined MRI (susceptibility-weighted imaging) with neurobehavioral tests, showing this compound reduced tissue loss by 28% (p = 0.0003) and enhanced neurogenesis .
Methodological Considerations
Q. How should researchers design studies to evaluate this compound’s antioxidant and anti-inflammatory properties?
- Oxidative stress markers : Measure gingival glutathione (GSH) and malonaldehyde (MDA) levels, as done in periodontitis models .
- Cytokine profiling : Use ELISA or multiplex assays for IL-1β, TNF-α, and IL-10 in tissue homogenates.
- Gene/protein expression : Apply qPCR and Western blotting for iNOS, MMPs, and BMP-2 .
- Statistical rigor : Two-way ANOVA with post-hoc tests (e.g., Tukey’s) to compare treatment groups.
Q. What are best practices for handling this compound’s low aqueous solubility in formulation studies?
- Surfactant optimization : Increase poloxamer concentration to enhance solubility, as demonstrated in solid lipid nanoparticles (SLNs) .
- Organic co-solvents : Use isopropyl alcohol (IPA) in nanodispersions, balancing solubility and toxicity .
- Dissolution testing : USP Apparatus II (paddle method) in phosphate buffer (pH 7.4) at 100 rpm, with sampling at 5–30 minutes .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


